Abstract
IL-6 overexpression protects mice from hyperoxic acute lung injury in vivo, and treatment with IL-6 protects cells from oxidant-mediated death in vitro. The mechanisms of protection, however, are not clear. We characterized the expression, localization, and regulation of Bax, a proapoptotic member of the Bcl-2 family, in wild-type (WT) and IL-6 lung-specific transgenic (Tg+) mice exposed to 100% O2 and in human umbilical vein endothelial cells (HUVEC) treated with H2O2 and IL-6. In control HUVEC treated with H2O2 or in WT mice exposed to 100% O2, a marked induction of Bax translocation and dimerization was associated with increased JNK and p38 kinase activity. In contrast, specific JNK or p38 kinase inhibitors or treatment with IL-6 inhibited Bax mitochondrial translocation and apoptosis of HUVEC. IL-6 Tg+ mice exposed to 100% O2 exhibited enhanced phosphatidylinositol 3-kinase (PI3K)/Akt kinase and increased serine phosphorylation of Bax at Ser184 compared with WT mice. The PI3K-specific inhibitor LY-2940002 blocked this IL-6-induced Bax phosphorylation and promoted cell death. Furthermore, IL-6 potently blocked hyperoxia- or oxidant-induced Bax insertion into mitochondrial membranes. Thus IL-6 functions in a cytoprotective manner, in part, by suppressing Bax translocation and dimerization through PI3K/Akt-mediated Bax phosphorylation.
Keywords: apoptosis, mitochondria, endothelial cells, JNK, cytokine, Bcl-2
supplemental O2 for enhancement of O2 delivery is an essential part of therapy for cardiopulmonary diseases; however, delivery of a high fraction (>65%) of inspired supplemental O2 for prolonged periods has harmful effects and may cause acute lung injury. Hyperoxic lung injury is associated with an epithelial and endothelial cell death response that involves both necrosis and apoptosis and an enhanced alveolar capillary protein leak that leads to protein-rich fluid in the alveolar space (38, 39). Cytokines such as IL-11 and IL-6 confer significant protection from hyperoxic acute lung injury (HALI). This protective response appears to result from the ability of the IL-6-type cytokines to inhibit hyperoxia-induced cell death without causing major alterations in lung antioxidants (38, 39, 41). The molecular mechanisms of these protective effects have not been fully elucidated.
Bax is a proapoptotic member of the Bcl-2 family of proteins, which are important regulators of apoptosis. In nonapoptotic cells, Bax is generally found in the cytosol; however, once the cell is exposed to an apoptotic stimulus, such as H2O2, Bax translocates to the mitochondria (1, 34) to form Bax oligomers. Oligomerized Bax induces apoptosis by forming large pores in the mitochondrial membrane, which results in the loss of mitochondrial membrane potential and the release of cytochrome c into the cytosol (5, 6, 21, 34). Protection from hyperoxic lung injury is associated with increased Bcl-XL, which blocks Bax-activated cell death during oxidative stress (37). The mechanisms underlying Bax translocation, however, are not fully understood.
Exposure of cells to stress, such as staurosporine, H2O2 (4, 21), etoposide (2), or UV irradiation, frequently activates JNK and p38 kinases (mitogen-activated protein kinase) (30) to induce cell death. Recent studies indicate that Bax and Bak are required for JNK-induced apoptosis and that Bax remains inactive on exposure of JNK-deficient fibroblasts to environmental stress. In addition, overexpression of active JNK fails to induce apoptosis in Bax/Bak-deficient fibroblasts (24, 44). H2O2-induced JNK- and p38 kinase-mediated phosphorylation of Bax leads to its activation and mitochondrial translocation and to apoptosis of human cancer cells (21).
IL-6 is a pleiotropic cytokine that activates multiple signal transduction pathways such as the JAK/STAT pathway (18), the Ras/ERK pathway (18), and the phosphatidylinositol 3-kinase (PI3K)/Akt pathway via gp130 tyrosine phosphorylation (16, 17). The roles of the JAK/STAT and Ras/ERK pathways in the biological effects of IL-6 have been extensively studied; however, the role of PI3K/Akt in IL-6 signaling is less clear. Akt is a Ser/Thr protein kinase that resides within the cytosol in an inactive state. After stimulation of cells with growth factors and cytokines, Akt becomes activated and phosphorylates downstream target molecules to induce the expression and regulation of antiapoptosis proteins (15). Mounting evidence suggests involvement of PI3K/Akt in IL-6-dependent survival and proliferative responses in several types of cancer cells (15, 19).
Activation of the PI3K and Akt/protein kinase B-related cell survival pathway regulates several survival factors such as IL-7 (11), cAMP (33), and granulocyte/macrophage colony-stimulating factor (GM-CSF) (10) through inhibition of Bax translocation to the mitochondria and, thus, inhibition of apoptosis. Recent studies suggest that phosphorylation of Bax at Ser184 by Akt maintains Bax in an inactive state in the cytoplasm, preventing translocation to the mitochondria (10, 43).
This study focuses on the mechanism by which IL-6 modulates Bax activation in oxidative stress. Our data suggest that IL-6-induced antiapoptotic stimuli lead to the activation of Akt and Ser184 phosphorylation of Bax. This phosphorylation of Bax promotes its sequestration to the cytoplasm and inhibits its ability to translocate to mitochondrial membranes, which inhibits its proapoptotic functions.
MATERIALS AND METHODS
Antibodies and reagents.
The PI3K inhibitor LY-294002 was obtained from Sigma (St. Louis, MO); [32P]orthophosphate and [γ-32P]ATP from Perkin-Elmer (Waltham, MA); recombinant human IL-6, anti-Bax antibody, antibody 6A7 (which recognizes only the active form of Bax), and anti-human Bax antibodies from R & D Biosystems (Minneapolis, MN); anti-phosphoserine monoclonal antibodies from Alexis (San Diego, CA); active Akt protein from Upstate Biotechnology (Lake Placid, NY); mouse anti-Bax monoclonal antibody, p38 kinase, phosphorylated JNK (p-JNK), JNK, 60-kDa heat shock protein (HSP-60), and horseradish peroxidase (HRP)-conjugated secondary antibodies from Santa Cruz Biotechnology (Santa Cruz, CA); and cytochrome c and phosphorylated ASK1 antibodies from Cell Signaling (Beverly, MA). All other reagents were obtained from Sigma (St. Louis, MO). Green fluorescent protein (GFP)-WT, GFP-S184A, and GFP-S184E Bax cDNAs were kindly provided by Dr. David A. Hildeman (10) (Cincinnati Children's Hospital Medical Center, Cincinnati, OH) and Dr. Richard J. Youle (29) (National Institutes of Health, Bethesda, MD). GFP served as a control and was used to track transfection efficiency.
Mice.
IL-6 lung-specific overexpression transgenic (IL-6 Tg+) mice were generated on a C57BL/6 background using the Clara cell 10-kDa protein (CC10) promoter, as previously described (8). In all cases, transgene− littermates served as controls. For terminal procedures, animals were euthanized using intramuscular injection of xylazine-ketamine. The Institutional Animal Care and Use Committee at the Massachusetts General Hospital at Harvard Medical School approved all protocols involving mice.
O2 exposure.
Four 6-wk-old mice (50% male and 50% female) were placed in cages in an airtight chamber (50 × 50 × 30 cm) and exposed to 100% O2 for 72 h. Controls were not exposed to hyperoxia. The O2 concentration in the chamber was monitored with an O2 analyzer (Vascular Technology, Chelmsford, MA), as described previously (38, 39).
Immunoprecipitation.
Immunoprecipitation was performed using the Seize Classic Mammalian Immunoprecipitation Kit (Pierce Biotechnology, Rockford, IL). Briefly, protein (200 μg) from human umbilical vein endothelial cells (HUVEC) or lung homogenates was incubated with anti-Bax (10 μg) at 4°C overnight with agitation. For control immunoprecipitation, we used IgG (data not shown). The protein-antibody complex was further incubated with protein A/G-agarose gel (50 μl) for 2 h. The protein was washed with immunoprecipitation buffer [50 mM sodium acetate buffer (pH 5.0), 500 mM NaCl, 0.1% SDS, 1% NP-40, and 0.02% sodium azide], and the protein bound to the beads was eluted with 0.1 M glycine-HCl buffer (pH 2.5) that had been neutralized to pH 7.5 with 1.0 M Tris (pH 7.5) and then subjected to Western blot analysis, as described earlier (10, 22, 43).
Cell culture.
Since cytoprotection plays an important role in IL-6-induced protection from oxidant-mediated cellular injury, a biologically relevant system in which cytoprotective effects could be evaluated independently of inflammation and other host responses is important. Elevated levels of H2O2 have been found in the expired gas and urine of patients with acute respiratory distress syndrome (25). Thus we characterized the effects of H2O2 on cultured cells. Primary HUVEC, human pulmonary microvascular endothelial cells (HPMVEC), and small airway epithelial cells (SAEC; Clonetics, San Diego, CA) were grown on 35 × 10 mm tissue culture petri dishes in basal medium supplemented with growth factors and antibiotics according to the manufacturer's instructions (Cambrex Bioscience, Walkersville, MD). Lung-derived HPMVEC and SAEC were used to determine the mechanism of IL-6-induced protection against HALI. Confluent cell cultures were treated with IL-6 (100 ng/ml), as described previously (41). After 24 h of IL-6 exposure, the medium was removed and replaced with standard growth medium with various concentrations of H2O2. After an additional 1-h incubation period, the cells were analyzed by Western blotting. On the basis of previous findings (41), the concentration of H2O2 used in vitro to induce cell death was 1.5 mM. At 1.5 mM H2O2, Bax translocation and oligomerization occur in our cell experimental system.
Transfection.
The pcDNA3 plasmids bearing GFP-WT, GFP-S184A, or GFP-S184E Bax cDNA were transfected into HUVEC or SAEC using Lipofectamine 2000-plus according to the manufacturer's instructions (Invitrogen, Carlsbad, CA). Cells were cultured to 90% confluence in six-well plates and transfected with a total of 4 μg of plasmid constructs. For determination of the transfection efficiency, fluorescence was checked using an inverted fluorescence microscope (see Supplemental Fig. 1 in the online version of this article). Cells were harvested 36–48 h after transfection, and cell lysates were used for protein assays. Medium was changed every 12 h, as described elsewhere (22).
Silencing of Akt expression by RNA interference.
Human Akt small interfering RNA (siRNA) was obtained from Santa Cruz Biotechnology. HUVEC were transfected with Akt siRNA using Lipofectamine 2000 according to the manufacturer's instructions. Nontargeted β-galactosidase siRNA was used as control siRNA. The levels of Akt expression were analyzed by Western blotting using an Akt antibody. Specific silencing of the targeted Akt gene was confirmed by at least three independent experiments.
Trypan blue exclusion.
Cell viability assays were performed as described elsewhere (41).
Western blot analysis.
After stimulation with IL-6 (100 ng/ml), HUVEC were lysed in lysis buffer [20 mM Tris·HCl (pH 7.4), 150 mM NaCl, 0.5% Triton X-100, 100 μM sodium vanadate, 1 mM dithiothreitol, 5 mg/ml leupeptin, and 1 mM phenylmethylsulfonyl fluoride] and then centrifuged at 15,000 g at 4°C for 15 min (22, 41). Protein from mouse lungs was extracted by the same method after homogenization of the whole lung. After centrifugation, supernatants were boiled in 6× SDS reducing sample buffer and subjected to SDS-PAGE. Proteins were then transferred onto polyvinylidene difluoride membranes, as described previously (13, 22). The membranes were blocked in Tris-buffered saline [20 mM Tris·HCl (pH 7.5) and 150 mM NaCl] with 0.1% Tween 20 (TBS-T) containing 5% skim milk and then probed with the appropriate primary antibodies overnight at 4°C. Antibodies to the following proteins were used: Bax, Akt, phosphorylated Akt (p-Akt), JNK, p-JNK, p38, phosphorylated p38 (p-p38), HSP-60, β-actin, and GAPDH. The membranes were washed with TBS-T, incubated with HRP-conjugated anti-rabbit antibody for 1.5 h at room temperature, and then washed twice with TBS-T for 15 min. Blots were visualized using 20× LumiGLO reagent and 20× peroxide according to the manufacturer's instructions (Cell Signaling Technology) with Kodak Biomax MR film (Eastman Kodak, Rochester, NY).
Phosphorylation of Bax in vitro.
Endogenous Bax was immunoprecipitated from HUVEC or lung homogenates with agarose-conjugated Bax antibody. Precipitated Bax was resuspended in a kinase assay buffer containing 1 μCi of [γ-32P]ATP, 10 μM ATP, 75 mM MgCl2, 1 mM dithiothreitol, and 1 μM NaF in Tris buffer (pH 7.5) and incubated with purified active Akt at 30°C. The reaction was terminated by addition of SDS sample buffer and boiling. Phosphorylation of Bax was determined by autoradiography following SDS-PAGE, as described earlier (10, 43).
Metabolic labeling, immunoprecipitation, and Western blot analysis.
Cells were washed with supplemented phosphate-free RPMI medium and metabolically labeled with [32P]orthophosphoric acid for 60 min. After treatment with the stimulus, cells were washed with ice-cold 1× PBS and lysed in RIPA buffer. Bax was immunoprecipitated as described above. The samples were subjected to SDS-PAGE, transferred to a nitrocellulose membrane, and exposed to Kodak X-Omat film at −80°C. Western blot analysis with an anti-Bax antibody was performed on the same blot, and the bands were developed using an enhanced chemiluminescence kit, as described previously (10, 43).
Phosphoamino acid analysis.
HUVEC were metabolically labeled with [32P]orthophosphoric acid for 2 h and treated with IL-6 (100 ng/ml) for 1 h. 32P-labeled Bax was immunoprecipitated using an agarose-conjugated Bax antibody. Proteins were separated by SDS-PAGE and transferred to a polyvinylidene difluoride membrane. The slices containing Bax membrane were excised, and phosphoamino acid analysis was performed as described previously (9, 10, 43). Ninhydrin staining and autoradiography were employed to determine the location of the phosphoamino acids.
Bax oligomerization and cross-linking.
Bax oligomerization in cells and isolated mitochondria was analyzed as described previously (20, 43). Briefly, isolated mitochondria or the digitonin-insoluble membrane fractions of the cells or lung homogenates were incubated with 5 mM bismaleimidohexane for 1 h at room temperature with agitation to facilitate cross-linking. The cross-linked samples were subjected to Western blot analysis of Bax, as described above.
Cellular fractionation.
Cells were fractionated into cytosolic and membrane-bound organellar fractions with digitonin (20), which, at low concentrations, selectively permeabilizes the plasma membrane without solubilizing mitochondria. Briefly, cells were incubated with 0.05% digitonin in an isotonic buffer containing 300 mM sucrose, 10 mM HEPES, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, and 1 mM EGTA (pH 7) for 5 min at room temperature. The extracts were collected as cytosolic fractions. The digitonin-insoluble fraction was washed with isotonic sucrose buffer and further dissolved in 2% SDS buffer as a membrane-bound organellar fraction enriched with mitochondria. These two fractions were analyzed for Bax and cytochrome c by immunoblotting (10, 14, 20, 22, 41, 43).
Isolation of mitochondria.
Mitochondria were isolated from HUVEC or total lung homogenates with use of the Mitochondria Isolation Kit (Pierce, Rockford, IL) according to the manufacturer's instructions. The mitochondrial fraction was identified on the basis of high expression of cytochrome c (40).
Statistics.
Values are means ± SD (n ≥ 3). Statistical differences between two groups were determined using one- and two-way ANOVA, and individual group means were then compared by Student's unpaired t-test. P < 0.05 was considered significantly different. In all experiments, unless otherwise noted, n is equal to at least six animals from three separate experiments.
RESULTS
Oxidant stress induces Bax translocation from the cytosol to the mitochondrial membrane and dimerization in HUVEC.
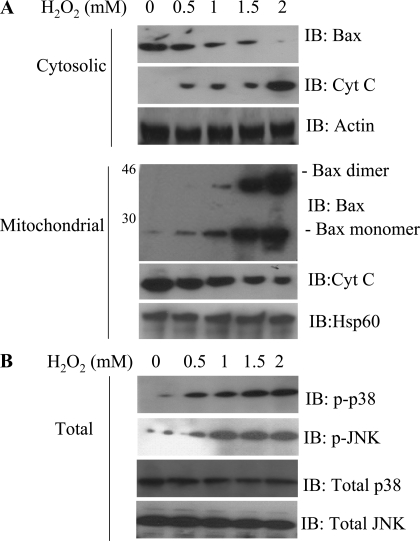
To assess whether oxidant stress stimulates Bax translocation, HUVEC were treated with H2O2 for 1 h and then subjected to subcellular fractionation. In unstimulated HUVEC, Bax was localized to the cytosol. After H2O2 treatment, Bax translocated to the mitochondrial membrane (Fig. 1); this response was time dependent and reached a maximum after 1 h (data not shown). An important event for mitochondrial disruption by proapoptotic Bcl-2 family proteins is the oligomerization of these molecules within the outer mitochondrial membranes. Thus we examined the oligomerization status of Bax in the mitochondrial membrane-bound organellar fraction. To preserve protein-protein interactions and the membrane-bound organellar fractions, the cells were treated with cross-linking agents before the proteins were extracted for immunoblot analysis of Bax. In the control cells, minimal Bax oligomerization was observed compared with the H2O2-treated cells (Fig. 1). H2O2 induced Bax oligomerization in a dose-dependent manner. In response to H2O2 treatment, cytochrome c was released from the mitochondria into the cytosol (Fig. 1A).
Fig. 1.
Oxidant stress-induced Bax translocation and dimerization in human umbilical vein endothelial cells (HUVEC). A: HUVEC were exposed to 0.5–2 mM H2O2 for 1 h. Equal amounts of cytosolic and mitochondrial proteins were separated on SDS-PAGE and then subjected to immunoblot (IB) analysis for Bax, cytochrome c (Cyt C), and actin. Note deletion of Bax from cytosol (top) and translocation to mitochondrial fraction (bottom) in H2O2-treated cells. B: Bax translocation was associated with activated JNK/p38 kinase activities, as indicated by phosphorylated kinases (p-p38 and p-JNK). Each blot is representative of ≥3 similar evaluations.
Recent studies (4, 21) suggest a role for JNK/p38 in Bax-mediated apoptosis. Inasmuch as JNK and p38 kinase are involved in various cell death stimuli, we investigated whether H2O2 activates these cell death-related protein kinases in HUVEC. Phosphorylation of JNK and p38 kinase were increased after H2O2 treatment (Fig. 1), whereas the levels of total JNK and p38 kinase were comparable in treated and untreated cells. These results suggest that activation of JNK and p38 kinase correlates with Bax translocation and cytochrome c release.
IL-6 and specific inhibitors of JNK and p38 kinase inhibited Bax mitochondrial translocation and apoptosis of HUVEC.
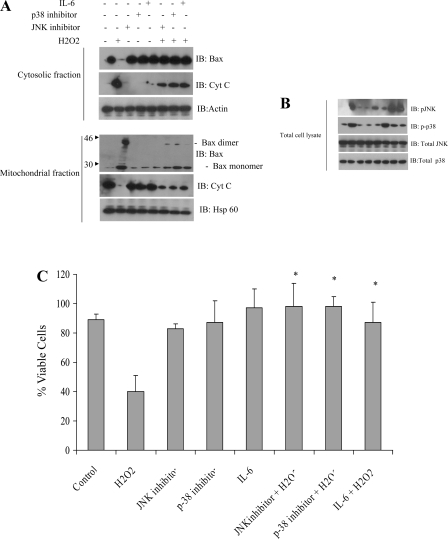
Since we previously showed that IL-6 protected against H2O2-induced cell death (2), we examined the effects of IL-6 on H2O2-induced Bax translocation and cytochrome c release in HUVEC. Mitochondrial and cytosolic Bax in HUVEC lysates were assessed after incubation with or without IL-6 for 24 h and subsequent treatment with or without H2O2 (1.5 mM) for 1 h. IL-6 diminished the H2O2-induced translocation and oligomerization of Bax (Fig. 2A). Bax was present as monomers in control cells (lane 1) and oligomerized after H2O2 treatment (lane 2).
Fig. 2.
IL-6 or specific inhibitors of JNK and p38 kinase significantly inhibit Bax mitochondrial translocation and apoptosis of HUVEC. A: HUVEC were exposed to H2O2 (1.5 mM) for 1 h in the presence or absence of JNK inhibitor, p38 inhibitor, or IL-6. Cells were harvested, and equal amounts of cytosolic and mitochondrial proteins were separated via SDS-PAGE and immunoblot analysis for Bax, cytochrome c, and actin. IL-6 and inhibitors of JNK or p38 kinase significantly inhibited Bax mitochondrial translocation. Actin and heat shock protein 60 (HSP-60) were used as loading controls in cytosolic and mitochondrial fractions, respectively. B: Western blot analysis of indicated proteins from HUVEC grown with or without JNK inhibitor, p38 kinase inhibitor, and IL-6 in the presence or absence of 1.5 mM H2O2. Note phosphorylation of JNK/p38 kinase pathway in response to H2O2. C: HUVEC were treated with H2O2 in the absence or presence of 20 μM JNK inhibitor or p38 kinase inhibitor for 1 h, and cell death rates were determined by trypan blue exclusion. Inhibitors of JNK or p38 kinase, as well as IL-6, significantly protected cells from H2O2-induced cell death. Experiments are representative of ≥4 similar evaluations. *Significantly different from H2O2 (P < 0.005).
To further examine the role of JNK and p38 in H2O2-induced Bax translocation and dimerization, the specific inhibitors SP-600125 and SB-203580 were used to effectively inhibit the activation of JNK and p38 kinase, respectively. After exposure to H2O2 for 1 h, the level of Bax in the cytosol decreased markedly, and the mitochondrial content of Bax increased (Fig. 2A, top). In addition, the levels of cytochrome c in the cytosol and mitochondria were inversely related to Bax after H2O2 treatment (Fig. 2A, bottom). Pretreatment of cells with SP-600125 or SB-203080 efficiently inhibited H2O2-induced translocation of Bax and cytochrome c release (Fig. 2A, bottom, lanes 6 and 7, respectively). These results confirm the involvement of activated JNK and p38 kinase in the increased mitochondrial translocation of Bax and subsequent release of cytochrome c into the cytoplasm to invoke the cell death response (Fig. 2A). In addition, IL-6 suppressed activation of p38 kinase. SP-600125 and SB-203580 (20 μM) effectively inhibited the activation of JNK and p38 kinase, respectively (Fig. 2B). Interestingly, active JNK (p-JNK) was not inhibited by IL-6. In fact, IL-6 enhanced H2O2-induced JNK activation. These findings suggest that IL-6 (100 ng/ml) suppresses Bax translocation and dimerization and subsequent cytochrome c release through specific inhibition of p38. In addition, inhibitors of JNK and p38 significantly reduced the H2O2-mediated cell death rates (Fig. 2C).
IL-6 lung-specific overexpression inhibits hyperoxia-induced Bax translocation from the cytosol to the mitochondrial membrane, as well as Bax dimerization, in vivo.
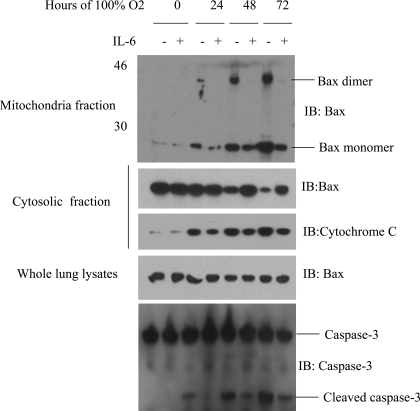
Our in vitro results suggest that IL-6-mediated protection occurs through inhibition of Bax translocation. To further investigate the mechanism of IL-6-induced protection against hyperoxic lung injury, we subjected lung lysates from IL-6 Tg+ mice to subcellular fractionation before and after exposure to 100% O2 and assessed protein levels using Western blot analysis. Impressive differences in the levels of Bax translocation and dimerization were noted between WT and IL-6 Tg+ mice (Fig. 3). At baseline, the levels of Bax protein were similar in WT and IL-6 Tg+ mice; however, exposure to 100% O2 for 72 h increased the levels of Bax mitochondrial translocation and dimerization in WT mice compared with IL-6 Tg+ mice, whereas the levels of the dimerized active form of Bax increased with exposure to 100% O2 in WT, but not IL-6 Tg+, mice (Fig. 3). Dimerization of Bax results in its translocation, mitochondrial dysfunction, and apoptosis (11). Our findings in our Tg+ mice show that IL-6 inhibits Bax dimerization. Furthermore, after exposure to 100% O2, cytochrome c release from the mitochondria into the cytosol was suppressed in IL-6 Tg+ mice compared with WT mice (Fig. 3). Thus IL-6 overexpression suppresses hyperoxia-induced Bax translocation and inhibits Bax dimerization and cytochrome c release.
Fig. 3.
Inhibition of Bax mitochondrial translocation and cytochrome c release after hyperoxia exposure in IL-6-overexpressing mice generated on a background using Clara cell 10-kDa protein (CC10). Membrane-bound organellar fractions were extracted from lung protein homogenates and cross-linked using bismaleimidohexane. Samples were then subjected to immunoblotting for Bax, cytochrome c, and caspase-3. Experiments are representative of ≥3 similar evaluations.
IL-6 suppresses H2O2-induced JNK- and p38 kinase-mediated activation of Bax.
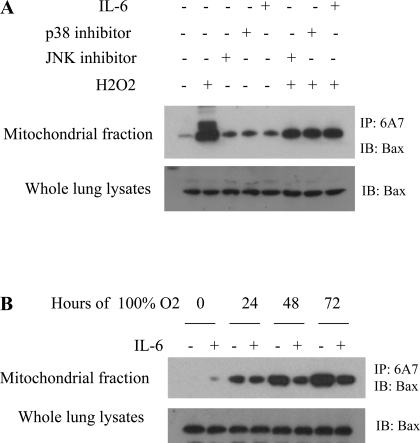
In healthy cells, Bax is inactive and retained in the cytoplasm by its binding partners. After a cell death stimulus, phosphorylation of Bax by stress-activated kinases, such as JNK and p38, leads to exposure of its NH2 terminus (activation) and a conformational change. The availability of the COOH-terminal hydrophobic transmembrane domain is important for translocation of activated Bax to the mitochondria to initiate mitochondrion-dependent apoptosis (21). To determine whether IL-6 has an effect on H2O2-induced JNK- and p38 kinase-mediated activation of Bax, we immunoprecipitated Bax with an antibody (6A7) that selectively recognizes only active Bax (open form) with an exposed NH2-terminal domain. We then probed the precipitates for Bax protein by Western blotting. Interestingly, treatment with IL-6, p38 kinase inhibitor, or JNK inhibitor suppressed H2O2-induced JNK- and p38 kinase-mediated Bax conformational change and, thereby, inhibited Bax translocation (Fig. 4A). Moreover, hyperoxia-induced Bax conformational change and subsequent Bax activation was inhibited in IL-6 Tg+, but not WT, mice (Fig. 4B).
Fig. 4.
IL-6 suppresses oxidative stress-induced Bax conformational change (activation) in vitro and in vivo. A: HUVEC were exposed to 1.5 mM H2O2 with and without JNK inhibitor, p38 inhibitor, or IL-6, and cell lysates were immunoprecipitated with the 6A7 antibody, which selectively recognizes the exposed NH2-terminal domain of active Bax. Western blotting with a Bax antibody was performed. B: mice were exposed to hyperoxia for 0–72 h. Extracted lung protein homogenates were immunoprecipitated (IP) with the 6A7 antibody. IL-6 inhibited hyperoxia-induced Bax conformational change. Each blot is representative of ≥3 similar evaluations.
IL-6 induces Bax phosphorylation.
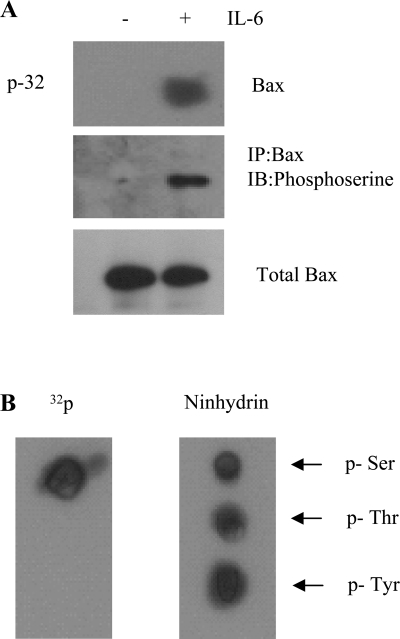
Recent reports suggest that Bax phosphorylation via PI3K/Akt activation results in markedly decreased proapoptotic activity of Bax (10, 43). Since IL-6 can potently induce activation of PI3K/Akt (14), which is considered a Bax kinase (10, 43), this cytokine may, in fact, regulate the function of Bax via a mechanism involving phosphorylation by PI3K/Akt. HUVEC were metabolically labeled with [32P]orthophosphoric acid and treated with IL-6 for 4 h. Then serine phosphorylation was analyzed with an anti-phosphoserine antibody after immunoprecipitation of Bax with an agarose-conjugated Bax antibody. IL-6 effectively stimulated serine phosphorylation of Bax (Fig. 5A). Phosphoamino acid analysis confirmed that IL-6-induced Bax phosphorylation occurred at serine sites (Fig. 5B).
Fig. 5.
IL-6 stimulates serine phosphorylation of Bax. A: HUVEC or small airway epithelial cells (SAEC) were labeled with [32P]orthophosphate for 6 h and then incubated in the presence or absence of IL-6 for 4 h. After cell lysis, Bax was immunoprecipitated with an agarose-conjugated Bax antibody and probed with anti-phosphoserine antibody. 32P labeling was analyzed by autoradiography. B: positions of phosphoserine (p-Ser), phosphothreonine (p-Thr), and phosphotyrosine (p-Tyr) were analyzed by ninhydrin staining and compared with internal standards. Bax band indicates that IL-6 induced Bax phosphorylation at serine residue(s). Each blot is representative of ≥3 similar evaluations.
IL-6 induces Bax phosphorylation at Ser184 and inactivates the proapoptotic function of Bax.
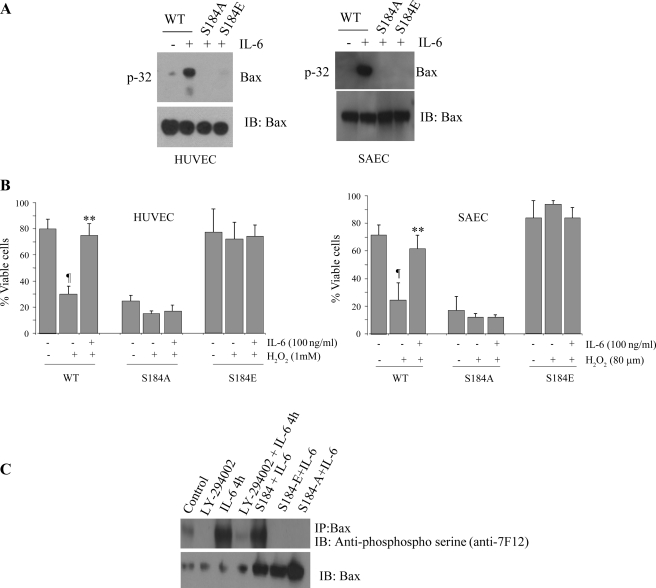
To determine whether IL-6 induces Bax phosphorylation at Ser184, we transfected mutant (S184A, or S184E) or WT Bax cDNAs into HUVEC or SAEC. After 48 h, transfected cells were metabolically labeled with [32P]orthophosphoric acid and treated with IL-6 (100 ng/ml), and the phosphorylation of Bax was analyzed. IL-6 induced phosphorylation of WT, but not S184A or S184E mutant, Bax (Fig. 6A), suggesting that IL-6 stimulates Bax phosphorylation exclusively at Ser184. Moreover, transfection of the nonphosphorylatable S184A mutant Bax resulted in more cell death than in HUVEC or SAEC transfected with WT Bax (Fig. 6B). Thus IL-6 increases the survival of cells expressing WT, but not S184A mutant, Bax. By contrast, the phosphomimetic S184E Bax exhibited no apoptotic activity. IL-6 exerted no additional survival effect in HUVEC or SAEC expressing the S184E mutant Bax (Fig. 6B). These findings reveal that IL-6-induced Ser184 phosphorylation or genetically mimicking Ser184 phosphorylation (i.e., S184E) results in abrogation of the proapoptotic function of Bax.
Fig. 6.
IL-6-induced Bax phosphorylation at Ser184 results in Bax inactivation. A: pcDNA3 plasmids bearing green fluorescent protein (GFP)-WT, GFP-S184A, or GFP-S184E were transfected into HUVEC or SAEC using Lipofectamine 2000. After transfection, cells were metabolically labeled with [32P]orthophosphoric acid and treated with IL-6 for 60 min. Bax was immunoprecipitated with an agarose-conjugated Bax antibody. Phosphorylation of Bax was determined by autoradiography (left). Western blot analysis with an anti-Bax antibody was performed to confirm and quantify Bax protein (right). IL-6 induced phosphorylation of wild-type (WT) Bax but not Bax in which the serine residue was manipulated. B: pcDNA3 plasmids bearing GFP-WT, GFP-S184A, or GFP-S184E were transfected into HUVEC or SAEC using Lipofectamine 2000. After 48 h, cells were treated with H2O2 in the absence or presence of IL-6 for 24 h. Cell viability was analyzed. Nonphosphorylatable S184A Bax mutant was more susceptible to H2O2-induced cell death. Values are means ± SD of 3 determinations. Significantly different from H2O2: ¶P < 0.05; **P < 0.01. C: in HUVEC cells, Bax was immunoprecipitated with an agarose-conjugated Bax antibody. Phosphorylation of Bax was determined by Western blotting with anti-phosphoserine antibody 7F12. Each blot is representative of ≥3 similar evaluations.
To further examine PI3K/Akt-dependent Bax serine phosphorylation, we pretreated HUVEC with the specific inhibitor LY-294002 before addition of IL-6 (100 ng/ml). Samples were lysed, immunoprecipitated with anti-Bax antibody, subjected to SDS-PAGE, and probed with anti-phosphoserine antibody. HUVEC treated with IL-6 exhibited a significant increase in Bax serine phosphorylation compared with control cells (Fig. 6C). This phosphorylation was inhibited in the presence of the PI3K inhibitor, supporting a role for the PI3K pathway in IL-6-induced Bax phosphorylation (Fig. 6C).
Akt is activated by IL-6 and directly phosphorylates Bax.
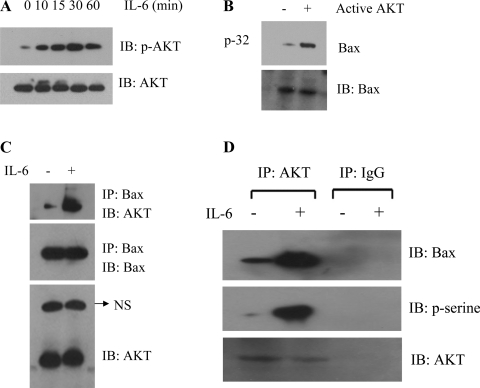
We next assessed the ability of IL-6 to activate Akt in primary HUVEC. Cells were treated with IL-6 (100 ng/ml) for up to 60 min. Phosphorylation of Akt was analyzed by Western blotting with a phospho-specific Akt antibody. IL-6 potently activated Akt in a time-dependent manner (Fig. 7A). To determine whether Akt directly phosphorylates endogenous Bax, Bax protein from HUVEC was immunoprecipitated and incubated with purified active Akt in a kinase assay buffer containing [32P]ATP. Active Akt, indeed, directly phosphorylated Bax in vitro (Fig. 7B). Next, to assess a potential direct role for Akt as a physiological Bax kinase, the interaction of Akt and Bax was determined by immunoprecipitation with anti-Bax and analyzed by Western blotting. A mouse antibody against human Bax, a rabbit antibody against human Akt, and HRP-conjugated anti-rabbit antibodies were used. Results indicate an interaction of Bax with Akt, which was enhanced in the presence of IL-6 (Fig. 7C). These findings suggest that IL-6-activated Akt may directly phosphorylate and inactivate Bax in cells.
Fig. 7.
Akt is activated by IL-6, and active Akt directly phosphorylates Bax in vitro. A: HUVEC were treated with IL-6 for 0–60 min. Cells were harvested, washed, and lysed in detergent buffer. Phosphorylation of Akt was analyzed by Western blotting with the phospho-specific Akt S473 antibody. B: endogenous Bax was immunoprecipitated from HUVEC and incubated with purified active Akt in an in vitro kinase assay. Phosphorylation of Bax was determined by autoradiography (top). Western blot analysis was performed to confirm and quantify Bax protein (bottom). C: endogenous Bax was immunoprecipitated from HUVEC and probed for Akt and Bax. NS, nonspecific bond. Each blot is representative of ≥4 similar evaluations.
The PI3K-specific inhibitor LY-2940002 blocks IL-6-induced Bax phosphorylation and promotes apoptosis.
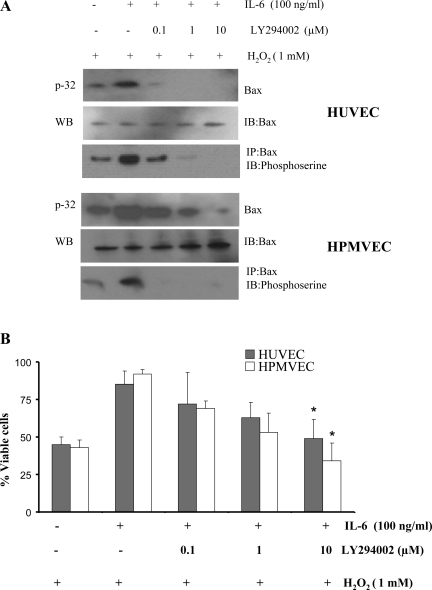
HUVEC or HPMVEC were metabolically labeled with [32P]orthophosphoric acid and treated with IL-6 in the absence and presence of various concentrations of LY-2940002 for 60 min. Bax phosphorylation was analyzed with a phosphoserine antibody. LY-2940002 blocked IL-6-induced Bax phosphorylation in a dose-dependent manner in HUVEC and HPMVEC (Fig. 8A). Importantly, LY-2940002 inhibited IL-6-induced protection against H2O2 and promoted apoptosis in HUVEC and HPMVEC (Fig. 8B). These results demonstrate that inhibition of Bax phosphorylation may reverse the proapoptotic function of Bax.
Fig. 8.
PI3K/Akt-specific inhibitor LY-294002 blocks IL-6-induced Bax phosphorylation and enhances apoptosis. A: HUVEC or human pulmonary microvascular endothelial cells (HPMVEC) were metabolically labeled with [32P]orthophosphoric acid and treated with 1 mM H2O2 in the absence or presence of increasing concentrations of LY-294002 for 60 min. Phosphorylation of Bax was analyzed. Each blot is representative of ≥3 similar evaluations. B: HUVEC or HPMVEC were treated with H2O2 in the absence or presence of IL-6 or increasing concentrations of LY-294002 for 48 h. Cell viability was assessed. LY-294002 inhibited IL-6-induced protection against H2O2-induced cell death. Values are means ± SD of 3 determinations. *Significantly different from IL-6-treated cells without LY-294002 (P < 0.005).
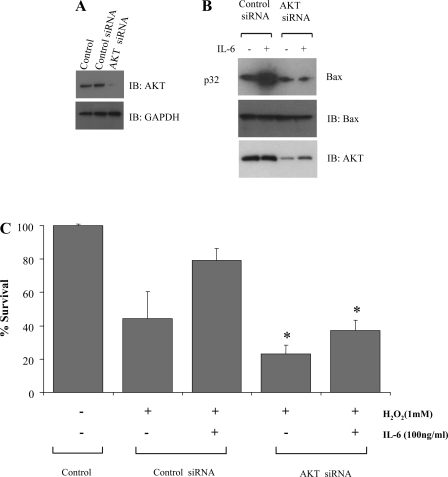
Silencing of Akt expression by RNA interference blocks IL-6-stimulated Bax phosphorylation and suppresses protection against oxidant stress.
Our results strongly suggest that Akt functions as an IL-6-activated Bax kinase in endothelial cells (Fig. 8). To confirm whether Akt is essential for IL-6-enhanced Bax phosphorylation, HUVEC were transfected with Akt siRNA and incubated with 1 mM H2O2 in the presence or absence of IL-6 (100 ng/ml). Akt siRNA efficiently and specifically blocked Akt expression in HUVEC, whereas control siRNA had no effect (Fig. 9, A and B). Importantly, specific depletion of Akt expression by RNA interference suppressed IL-6-induced Bax phosphorylation (Fig. 9B) and significantly enhanced cell death after treatment of cells with H2O2 (Fig. 9C). These results suggest that Akt is a required kinase for IL-6-stimulated Bax phosphorylation and protection of HUVEC.
Fig. 9.
Silencing of Akt expression by RNA interference blocks IL-6-stimulated Bax phosphorylation and suppresses protection against oxidant stress. A: control small interfering RNA (siRNA) or Akt siRNA was transfected into HUVEC cells using Lipofectamine 2000. Expression of Akt was determined by Western blotting using an Akt antibody. B: HUVEC expressing Akt siRNA or control siRNA were metabolically labeled with [32P]orthophosphoric acid and treated with IL-6 (100 ng/ml). Phosphorylation of Bax was analyzed by autoradiography. Each blot is representative of ≥4 similar evaluations. C: HUVEC transfected with Akt siRNA or control siRNA were subjected to oxidant stress (1 mM H2O2) in the absence or presence of IL-6 (100 ng/ml) for 48 h. Cell viability was assessed. Values are means ± SD of 3 determinations. *Significantly decreased compared with control siRNA (P < 0.005).
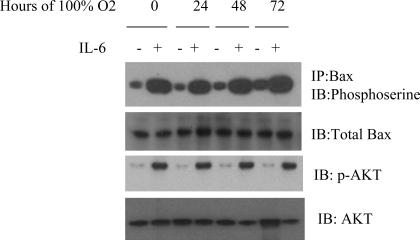
IL-6 overexpression induces phosphorylation of Bax and Akt in mice.
Our in vitro results suggest that IL-6-mediated protection may function via induction of Akt-mediated Bax phosphorylation. To explore this possibility in mice, IL-6 Tg+ and WT mice were exposed to hyperoxia, and Bax phosphorylation was examined with an anti-phosphoserine antibody after immunoprecipitation of Bax. Phosphorylation of Bax was observed in IL-6 Tg+ mice in room air and hyperoxia, but not in WT mice (Fig. 10). In addition, enhanced Bax phosphorylation was associated with enhanced Akt phosphorylation in IL-6-overexpressing, but not WT, mice (Fig. 10). Thus IL-6 protection from hyperoxia correlates with Bax and Akt phosphorylation.
Fig. 10.
IL-6 overexpression induces Bax phosphorylation in room air and 100% O2. Lung protein homogenates were immunoprecipitated with anti-Bax antibody and assessed for serine phosphorylation with anti-phosphoserine antibody 7F12, which is associated with enhanced phospho-Akt (p-Akt). IL-6 mice exhibited significantly enhanced phosphorylation of Bax and Akt compared with WT mice in room air and in hyperoxia. Each blot is representative of ≥3 similar evaluations.
DISCUSSION
These studies demonstrate that the JNK/p38 kinase pathway is activated in endothelial cells in response to H2O2. This activation induces Bax translocation and dimerization on the mitochondrial membrane. Furthermore, the p38 kinase death-signaling pathway is inhibited by IL-6 treatment in HUVEC. Inhibition of p38 kinase is a major mediator of the cytoprotective effects of IL-6 in endothelial cells. In addition, the PI3K/Akt survival pathway is activated in the lungs of IL-6 Tg+ mice and in endothelial cells exposed to IL-6. Activation of Akt by IL-6 induces phosphorylation of Bax at Ser184 and inhibits Bax translocation and dimerization on the mitochondrial membrane. This blockade of activation of the proapoptotic factor Bax is a key event in IL-6-induced protection against HALI. Together, these studies are the first to demonstrate that IL-6-induced Akt activates Ser184 phosphorylation of Bax, which inhibits its proapoptotic functions.
Exposure of cells to oxidant stress results in activation of the JNK/p38 signaling pathway (21), which regulates Bcl-2 family proteins, including Bax, a proapoptotic family member (24, 42). Bax translocation and cytochrome c release are inhibited in JNK1/JNK2-deficient cells (36), and Bax phosphorylation by JNK/p38 activates Bax translocation in cancer cells (21). H2O2 treatment activates the JNK and p38 kinase pathways, both of which are suppressed in cells treated with IL-6.
A limitation of the present study is the use of H2O2 and the choice of H2O2 concentrations, which may not be a perfect model for oxidative stress. H2O2 was chosen, because 1) it directly confers oxidant injury on cells in culture, and oxidant injury is an essential feature in the pathogenesis of HALI, and 2) it is a surrogate for the oxidants induced in vivo by 100% O2. In our previous studies, we quantified a clear dose response for H2O2-induced cell death and used H2O2 as an easily reproducible and quantifiable oxidant and oxidative stress-inducing agent (41), a hallmark of hyperoxia-induced lung injury. The mechanism of IL-6-induced protection is difficult to address in a cell-specific manner in vivo; however, we used SAEC and HPMVEC to provide insight into the IL-6-induced protection against O2-mediated lung injury. A limitation of our approach is that the mechanism of IL-6-induced protection is difficult to address in a cell-specific manner in vivo; however, we used SAEC and HPMVEC to provide insight into the IL-6-induced protection against O2-mediated lung injury.
Treatment of cells with a specific JNK or p38 kinase inhibitor efficiently inhibited H2O2-induced Bax conformational change, which exposes its NH2-terminal domain and renders the protein activated. These findings are consistent with previous studies and confirm the important role of JNK and/or p38 kinase in Bax activation through conformational change for translocation and cell death (24). Recent studies suggest that Thr167 is a critical amino acid that can be phosphorylated by stress-activated JNK and/or p38 kinase in cancer cell lines (21). Bax is activated by hyperoxia-activated protein kinases that induce mitochondrial translocation and apoptosis, which is in agreement with other reports (21). Importantly, IL-6 inhibits Bax translocation, as well as p38, but activates JNK. According to previous reports, JNK activation may be transient in IL-6-treated endothelial cells (3). Consistent with this notion, p-JNK was significantly reduced in the IL-6 Tg+ mice (22).
Recent studies indicate that transgenic overexpression of GM-CSF also confers protection in HALI (32). This cytokine induces Akt-mediated Bax phosphorylation at Ser184 and inhibits its proapoptotic function. Phosphorylation of Bax at Ser184 leads to a conformational change in the COOH-terminal tail, which is crucial for insertion of Bax into the mitochondrial membrane. In contrast, Akt-mediated phosphorylation of Bax at Ser184 resulted in retention of Bax in the cytoplasm after treatment of cells with GM-CSF or nicotine (10, 43). Our data demonstrate that, similar to GM-CSF overexpression, IL-6 overexpression protects mice from HALI through PI3K/Akt-mediated Bax phosphorylation at Ser184. In addition, inhibition of the PI3K/Akt pathway by LY-294002 (Fig. 8A) led to decreased IL-6-induced phosphorylation of Bax. This finding supports the theory that cytoplasmic Bax is maintained in a phosphorylated state. Importantly, LY-294002 does not totally abolish IL-6-induced protection, perhaps because IL-6 has diverse functions and IL-6-mediated protection can involve multiple pathways.
IL-6 protection against oxidant-mediated (100% O2 or H2O2 treatment) lung injury works, in part, by inhibiting Bax activation and mitochondrial translocation before apoptosis. In the nonapoptotic state, Bax is retained in the cytoplasm by its binding proteins (24); however, after activation, Bax forms oligomers that lead to release of cytochrome c from mitochondria (20). IL-6-induced constitutive STAT3 activation contributes to Bcl-2 gene expression and correlates with inhibition of apoptosis (12, 23). Another possible mechanism of IL-6-induced Bax protection may be retention of cytosolic Bax, which then heterodimerizes with antiapoptotic Bcl-2 (40) and Bcl-XL (31). In addition, Bcl-2 directly protects against hyperoxia-induced apoptosis through inhibition of the mitochondria-dependent pathway (26). It has been reported that IL-6 has inverse effects on apoptosis of fibroblasts in pulmonary fibrosis (27), where IL-6 enhanced Fas-induced apoptosis and expression of Bax in normal fibrosis but inhibited apoptosis and induced expression of Bcl-2 in idiopathic pulmonary fibrosis. Importantly, a recent study suggests that endothelial cells are a source of antiapoptotic IL-6 (28). Our findings show that IL-6 is cytoprotective for endothelial and epithelial cells in the setting of oxidant-mediated cell injury.
Our results suggest that the major role of IL-6 in blocking oxidant/hyperoxia-induced Bax translocation is through its ability to suppress JNK/p38 activation. These results, however, do not exclude the possibility that IL-6 may also suppress cell death through other pathways that are not dependent on JNK/p38 activation. Alternatively, oxidant-mediated cell death may proceed through an alternative cell death pathway that does not involve mitochondrial disruption and apoptosis. IL-6-type cytokines inhibit apoptotic cell death in a number of experimental systems via alteration of the PI3K/Akt pathway. Active Akt has been shown to inhibit mitochondrial membrane permeability (7) and the proapoptotic activity of Bax by retaining Bax in the cytosol through phosphorylation (10). IL-6 induced activation of Akt, and purified active Akt directly phosphorylated endogenous Bax in vitro (Fig. 7). Furthermore, treatment of cells with the PI3K/Akt inhibitor LY-294002 inhibited IL-6-induced Akt-mediated Bax phosphorylation. Thus Akt functions as a physiological IL-6-activated Bax kinase.
The inability of the Bax S184E mutant to translocate to the mitochondrial membrane (29, 43) correlated with enhanced cell viability (Fig. 6A). Consistent with earlier reports (43), this suggests that activation of PI3K/Akt is required for retention of Bax in the cytosol and inhibition of apoptosis due to blockade of Bax translocation. As a result, IL-6-stimulated, Akt-induced Bax phosphorylation is a likely mechanism by which Bax is retained in the cytosol. Thus IL-6-type cytokine-induced protection of endothelial cells is mediated, at least in part, via Bax phosphorylation and the PI3K-dependent signal transduction pathway. Alternatively, IL-6 may reduce cell death through different mechanisms, including the inhibition of superoxide production (35) and upregulation of Bcl-2 family proteins (23). IL-6 induces Bcl-2 expression, resulting in cytoprotection in hyperoxia, which is mediated by alterations in the interactions between Bak and mitofusion proteins (40). Furthermore, Ser184 phosphorylation of Bax may yield a conformational change that enhances binding with antiapoptotic Bcl-2 proteins to form heterodimers (10).
In summary, our studies demonstrate that IL-6 promotes survival of human endothelial cells and protects against hyperoxia-induced lung injury by a novel mechanism involving inactivation of the proapoptotic function of Bax via its phosphorylation. IL-6 activates the PI3K/Akt pathway, which, in turn, induces Bax phosphorylation at Ser184 in the COOH-terminal hydrophobic transmembrane domain. IL-6 also suppresses the H2O2-induced JNK- and p38 kinase-mediated Bax conformational change of Bax (activation). Phosphorylated Bax is inactive and fails to translocate into mitochondrial membranes. Thus IL-6-induced Bax phosphorylation apparently sequesters Bax in the cytosol and destabilizes the Bax protein to effectively abolish the proapoptotic activity of this protein. This blockade of Bax translocation likely contributes to the IL-6-induced cell protection from O2 toxicity. These findings provide insight into the molecular processes underlying IL-6 protection against hyperoxia-induced lung injury and further support a pivotal role of Bax in oxidant-mediated lung injury. Taken together, our results suggest that regulators or inhibitors of Bax translocation and its signaling pathways may serve as potent treatment targets for HALI.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant RO1 HL-074859 (to A. B. Waxman).
Supplementary Material
REFERENCES
- 1.Ahmad KA, Iskandar KB, Hirpara JL, Clement MV, Pervaiz S. Hydrogen peroxide-mediated cytosolic acidification is a signal for mitochondrial translocation of Bax during drug-induced apoptosis of tumor cells. Cancer Res 64: 7867–7878, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Anderson SM, Reyland ME, Hunter S, Deisher LM, Barzen KA, Quissell DO. Etoposide-induced activation of c-Jun N-terminal kinase (JNK) correlates with drug-induced apoptosis in salivary gland acinar cells. Cell Death Differ 6: 454–462, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Andreozzi F, Laratta E, Procopio C, Hribal ML, Sciacqua A, Perticone M, Miele C, Perticone F, Sesti G. Interleukin-6 impairs the insulin signaling pathway, promoting production of nitric oxide in human umbilical vein endothelial cells. Mol Cell Biol 27: 2372–2383, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arany I, Megyesi JK, Kaneto H, Tanaka S, Safirstein RL. Activation of ERK or inhibition of JNK ameliorates H2O2 cytotoxicity in mouse renal proximal tubule cells. Kidney Int 65: 1231–1239, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong JS Mitochondrial membrane permeabilization: the sine qua non for cell death. Bioessays 28: 253–260, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer 2: 647–656, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev 13: 2905–2927, 1999. [DOI] [PubMed] [Google Scholar]
- 8.DiCosmo B, Geba G, Picarella D, Elias JA, Rankin JA, Stripp B, Whitsett JA, Flavell RA. Expression of interleukin-6 by airway epithelial cells. Effects on airway inflammation and hyperreactivity in transgenic mice. Chest 107 Suppl: 131S, 1995. [DOI] [PubMed] [Google Scholar]
- 9.Frankel SK, Van Linden AA, Riches DW. Heterogeneity in the phosphorylation of human death receptors by p42mapk/erk2. Biochem Biophys Res Commun 288: 313–320, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Gardai SJ, Hildeman DA, Frankel SK, Whitlock BB, Frasch SC, Borregaard N, Marrack P, Bratton DL, Henson PM. Phosphorylation of Bax Ser184 by Akt regulates its activity and apoptosis in neutrophils. J Biol Chem 279: 21085–21095, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Gross A, Jockel J, Wei MC, Korsmeyer SJ. Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J 17: 3878–3885, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haga S, Terui K, Zhang HQ, Enosawa S, Ogawa W, Inoue H, Okuyama T, Takeda K, Akira S, Ogino T, Irani K, Ozaki M. Stat3 protects against Fas-induced liver injury by redox-dependent and -independent mechanisms. J Clin Invest 112: 989–998, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He CH, Waxman AB, Lee CG, Link H, Rabach ME, Ma B, Chen Q, Zhu Z, Zhong M, Nakayama K, Nakayama KI, Homer R, Elias JA. Bcl-2-related protein A1 is an endogenous and cytokine-stimulated mediator of cytoprotection in hyperoxic acute lung injury. J Clin Invest 115: 1039–1048, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He Y, Zhang W, Zhang R, Zhang H, Min W. SOCS1 inhibits tumor necrosis factor-induced activation of ASK1-JNK inflammatory signaling by mediating ASK1 degradation. J Biol Chem 281: 5559–5566, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Hideshima T, Nakamura N, Chauhan D, Anderson KC. Biologic sequelae of interleukin-6 induced PI3-K/Akt signaling in multiple myeloma. Oncogene 20: 5991–6000, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Hirano T, Ishihara K, Hibi M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene 19: 2548–2556, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Hirano T, Nakajima K, Hibi M. Signaling mechanisms through gp130: a model of the cytokine system. Cytokine Growth Factor Rev 8: 241–252, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Horvath CM The Jak-STAT pathway stimulated by interleukin 6. Sci STKE 2004: TR9, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Hsu JH, Shi Y, Frost P, Yan H, Hoang B, Sharma S, Gera J, Lichtenstein A. Interleukin-6 activates phosphoinositol-3′ kinase in multiple myeloma tumor cells by signaling through RAS-dependent and, separately, through p85-dependent pathways. Oncogene 23: 3368–3375, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Jiang M, Pabla N, Murphy RF, Yang T, Yin XM, Degenhardt K, White E, Dong Z. Nutlin-3 protects kidney cells during cisplatin therapy by suppressing Bax/Bak activation. J Biol Chem 282: 2636–2645, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim BJ, Ryu SW, Song BJ. JNK- and p38 kinase-mediated phosphorylation of Bax leads to its activation and mitochondrial translocation and to apoptosis of human hepatoma HepG2 cells. J Biol Chem 281: 21256–21265, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Kolliputi N, Waxman AB. IL-6 cytoprotection in hyperoxic acute lung injury occurs via SOCS-1-induced ASK-1 degradation. Am J Respir Cell Mol Biol 40: 314–324, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kovalovich K, Li W, DeAngelis R, Greenbaum LE, Ciliberto G, Taub R. Interleukin-6 protects against Fas-mediated death by establishing a critical level of anti-apoptotic hepatic proteins FLIP, Bcl-2, and Bcl-xL. J Biol Chem 276: 26605–26613, 2001. [DOI] [PubMed] [Google Scholar]
- 24.Lei K, Nimnual A, Zong WX, Kennedy NJ, Flavell RA, Thompson CB, Bar-Sagi D, Davis RJ. The Bax subfamily of Bcl2-related proteins is essential for apoptotic signal transduction by c-Jun NH2-terminal kinase. Mol Cell Biol 22: 4929–4942, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matthay MA, Geiser T, Matalon S, Ischiropoulos H. Oxidant-mediated lung injury in the acute respiratory distress syndrome. Crit Care Med 27: 2028–2030, 1999. [DOI] [PubMed] [Google Scholar]
- 26.Metrailler-Ruchonnet I, Pagano A, Carnesecchi S, Ody C, Donati Y, Barazzone Argiroffo C. Bcl-2 protects against hyperoxia-induced apoptosis through inhibition of the mitochondria-dependent pathway. Free Radic Biol Med 42: 1062–1074, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Moodley YP, Misso NL, Scaffidi AK, Fogel-Petrovic M, McAnulty RJ, Laurent GJ, Thompson PJ, Knight DA. Inverse effects of interleukin-6 on apoptosis of fibroblasts from pulmonary fibrosis and normal lungs. Am J Respir Cell Mol Biol 29: 490–498, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Moreno A, Villar ML, Camara C, Luque R, Cespon C, Gonzalez-Porque P, Roy G, Lopez-Jimenez J, Bootello A, Santiago ER. Interleukin-6 dimers produced by endothelial cells inhibit apoptosis of B-chronic lymphocytic leukemia cells. Blood 97: 242–249, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Nechushtan A, Smith CL, Hsu YT, Youle RJ. Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J 18: 2330–2341, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oh SW, Mukhopadhyay A, Svrzikapa N, Jiang F, Davis RJ, Tissenbaum HA. JNK regulates lifespan in Caenorhabditis elegans by modulating nuclear translocation of forkhead transcription factor/DAF-16. Proc Natl Acad Sci USA 102: 4494–4499, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 74: 609–619, 1993. [DOI] [PubMed] [Google Scholar]
- 32.Paine R, Wilcoxen SE, Morris SB, Sartori C, Baleeiro CE, Matthay MA, Christensen PJ. Transgenic overexpression of granulocyte macrophage-colony stimulating factor in the lung prevents hyperoxic lung injury. Am J Pathol 163: 2397–2406, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Putcha GV, Deshmukh M, Johnson EM Jr. BAX translocation is a critical event in neuronal apoptosis: regulation by neuroprotectants, BCL-2, and caspases. J Neurosci 19: 7476–7485, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, Korsmeyer SJ. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science 300: 135–139, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Shingu M, Isayama T, Yasutake C, Naono T, Nobunaga M, Tomari K, Horie K, Goto Y. Role of oxygen radicals and IL-6 in IL-1-dependent cartilage matrix degradation. Inflammation 18: 613–623, 1994. [DOI] [PubMed] [Google Scholar]
- 36.Varfolomeev EE, Ashkenazi A. Tumor necrosis factor: an apoptosis JuNKie? Cell 116: 491–497, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Vitiello PF, Staversky RJ, Keng PC, O'Reilly MA. PUMA inactivation protects against oxidative stress through p21/Bcl-XL inhibition of Bax death. Free Radic Biol Med 44: 367–374, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ward NS, Waxman AB, Homer RJ, Mantell LL, Einarsson O, Du Y, Elias JA. Interleukin-6-induced protection in hyperoxic acute lung injury. Am J Respir Cell Mol Biol 22: 535–542, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Waxman AB, Einarsson O, Seres T, Knickelbein RG, Warshaw JB, Johnston R, Homer RJ, Elias JA. Targeted lung expression of interleukin-11 enhances murine tolerance of 100% oxygen and diminishes hyperoxia-induced DNA fragmentation. J Clin Invest 101: 1970–1982, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waxman AB, Kolliputi N. IL-6 protects against hyperoxia induced mitochondrial damage via BCL-2 induced BAK interactions with mitofusions. Am J Respir Cell Mol Biol. In press. [DOI] [PMC free article] [PubMed]
- 41.Waxman AB, Mahboubi K, Knickelbein RG, Mantell LL, Manzo N, Pober JS, Elias JA. Interleukin-11 and interleukin-6 protect cultured human endothelial cells from H2O2-induced cell death. Am J Respir Cell Mol Biol 29: 513–522, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Cell Biol 19: 142–149, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Xin M, Deng X. Nicotine inactivation of the proapoptotic function of Bax through phosphorylation. J Biol Chem 280: 10781–10789, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Yamamoto K, Ichijo H, Korsmeyer SJ. BCL-2 is phosphorylated and inactivated by an ASK1/Jun N-terminal protein kinase pathway normally activated at G2/M. Mol Cell Biol 19: 8469–8478, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.