Abstract
Reactive oxygen species (ROS) present in cigarette smoke (CS) are thought to contribute to the development of COPD. Although CS-ROS can hardly enter airway epithelial cells, and certainly not the circulation, systemic levels of ROS have been found to be elevated in COPD patients. We hypothesize that lipophilic components present in CS can enter airway epithelial cells and increase intracellular ROS production by disturbing mitochondrial function. Different airway epithelial cells were exposed to CS extract (CSE), hexane-treated CSE (CSE without lipophilic components), gaseous-phase CS, and water-filtered CS (gaseous-phase CS without ROS). Mitochondrial membrane potential (Δψm) and ATP levels were assessed using the bronchial epithelial cell line Beas-2b. ROS generation measured directly by DCF fluorescence and indirectly by measuring free thiol groups (-SH) upon exposure to CS was assessed using lung alveolar epithelial cells devoid of functional mitochondria (A549-ρ0), with normal A549 cells serving as controls. In Beas-2b cells, CSE (4 h) caused a dose-dependent decrease in Δψm and ATP levels, whereas hexane-treated CSE did not. DCF fluorescence in A549 cells increased in response to CSE, whereas this was not the case in A549-ρ0 cells. Exposure of A549 cells to CS resulted in a rapid decrease in free -SH, whereas exposure to ROS-depleted CS only resulted in a delayed decrease. This delayed decrease was less pronounced in A549-ρ0 cells. Lipophilic components in CS disturb mitochondrial function, which contributes to increased intracellular generation of ROS. Our results are of importance in understanding the systemic effects of smoking observed in patients with COPD.
Keywords: mitochondria, epithelial cells, reactive oxygen species, chronic obstructive pulmonary disease
smoking cigarettes is a major risk factor for the development of chronic obstructive pulmonary disease (COPD) (2, 24). Cigarette smoke (CS) contains more than 4,000 chemicals (5), including reactive oxygen species (ROS) such as superoxide (O2•−) and hydroxyl radicals (•OH), which are present in high concentrations in the gaseous phase. ROS in CS will primarily react with antioxidants in the epithelial lining fluid covering airway epithelial cells and their plasma cell membranes, causing direct damage (13). However, these ROS are not capable of diffusing through the plasma membranes of these cells and therefore are not capable of entering the circulation (1, 6, 19). Despite this, there is considerable evidence for induction of systemic oxidative stress by CS, as certified by high circulating levels of malondialdehyde (MDA) and low plasma levels of vitamin C and reduced glutathione (GSH) in smokers and patients with COPD (7, 35, 36). Such systemic oxidative stress may result in tissue injury with apoptotic and necrotic cell death, muscle dysfunction, organ failure, and maintenance of systemic inflammation, all potentially of importance in the development and progression of COPD (29, 30, 37).
Whereas the gaseous-phase ROS in CS are hardly able to enter the cells and are thus unlikely to pass the airway epithelial barrier, lipophilic components in CS, including phenolic compounds, aldehydes, and polycyclic aromatic hydrocarbons, can easily pass the cell and enter the systemic circulation (26, 38). We hypothesize that once inside cells, lipophilic components disturb mitochondrial function, leading to increased mitochondrial ROS formation.
The potential importance of mitochondrial ROS metabolism in COPD may be best illustrated by the fact that many antioxidants that are supposed to be important in the pathophysiology of COPD are linked to mitochondria. These include GSH, thioredoxin, superoxide dismutase, and, also, as we recently demonstrated, heme-oxygenase-1 (HO-1) (32). The primary function of mitochondria is production of ATP, a process linked to the action of the electron transfer chain (ETC). Disturbance of the respiratory chain by lipophilic components may enhance leakage of single electrons from the ETC. These electrons that leak from the ETC can be accepted by oxygen, converting them into O2•−, a very potent free radical (15, 21).
In this study, we aimed to investigate whether lipophilic components present in CS result in 1) disturbance of mitochondrial function and 2) increased mitochondrial ROS formation. To assess potential disturbance of mitochondrial function, we used the bronchial epithelial cell line Beas-2b, and to investigate whether mitochondria are involved in increased ROS production upon exposure to CS, we made use of alveolar epithelial cells devoid of functional mitochondria (A549-ρ0), with normal A549 cells serving as controls.
MATERIALS AND METHODS
Chemicals.
BSA, DTNB, hexane, l-cysteine, PMA, sodium pyruvate, Triton X-100, uridine, and vitamin C were obtained from Sigma-Aldrich Chemie (Zwijndrecht, The Netherlands). 5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethyl-benzimidazolyl-carbocyanine iodide (JC-1), 5- (and 6-)chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester (DCF), and ethidium bromide were purchased from Invitrogen (Breda, The Netherlands).
Cell culture and preparation of ρ0 cells.
The human bronchial epithelial cell line (Beas-2b) and the human alveolar type II epithelium-like adherent cell line (A549) were purchased from American Type Culture Collection (Manassas, VA). Cells were grown in RPMI 1640 with 25 mM HEPES, l-glutamine (BioWitthaker, Verviers, Belgium) supplemented with 10% heat-inactivated fetal calf serum (BioWhittaker), and 20 μg/ml gentamycin (Centafarm Services, Etten-Leur, The Netherlands). Cells were grown in 25-cm2 plastic culture flasks (Costar, Cambridge, MA) and six-well tissue cell culture plates (Costar) at 37°C in an atmosphere of 5% CO2 until 80–90% confluency was reached. Before the experiments, cells were incubated for 16 h in serum-free RPMI 1640 media. A549-ρ0 cells generated by chemical elimination of mitochondrial DNA were kindly provided by Navdeep Chandel (Dept. of Medicine, Univ. of Chicago, IL). These cells are not competent to carry out normal electron transport or ATP synthesis and must rely solely on ATP derived from anaerobic glycolysis (8). Briefly, A549-ρ0 cells were prepared by long-term growth in medium supplemented with ethidium bromide (50 ng/ml), sodium pyruvate (110 μg/ml), and uridine (100 μg/ml). The lack of a functional ETC in these cells was examined with the potentiometric fluorescent dye JC-1.
Preparation of CS extract.
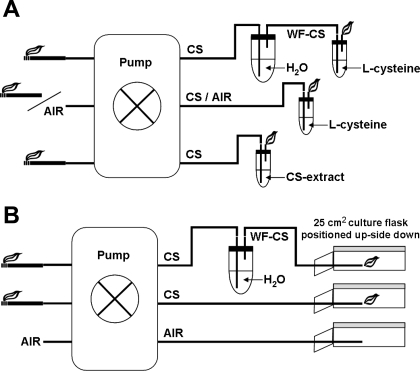
Preparation of CS extract (CSE) was performed according to the methods as represented in Fig. 1A and as previously described (32, 37). Briefly, Kentucky 2R4F research-reference filtered cigarettes (The Tobacco Research Institute, Univ. of Kentucky, Lexington, KY) were smoked using a peristaltic pump. Just before the experiments, the filters were cut from the cigarettes. Each cigarette was smoked in 5 min with a 17-mm butt remaining. Two cigarettes were bubbled through 25 ml of cell growth medium, and this solution was regarded as 100% strength CSE.
Fig. 1.
Gaseous-phase cigarette smoke (CS) exposure system scheme. A: preparation of CS extract and the exposure of l-cysteine to air, CS, or water-filtered CS (WF-CS). B: exposure of airway epithelial cells to air, CS, and WF-CS.
Hexane extraction of CSE.
Extraction of lipophilic components from CSE was performed using a two-step hexane extraction procedure. Briefly, 1 vol of CSE was added to 2 vol of hexane. The vial was tightly closed and fastened on a rocking platform for 5 min. Thereafter, the mixture was centrifuged at low speed (2,000 rpm) for 2 min to separate the aqueous and organic phase. The hexane phase, containing the lipophilic components, was removed by careful suction with needle and pump or syringe. The previous step was then repeated.
Preparation of lipophilic components from CSE.
Extraction of lipophilic components from CSE was performed using a one-step hexane extraction procedure. Briefly, 25 ml of CSE was added to 25 ml of hexane. The vial was tightly closed and fastened on a rocking platform for 5 min. Thereafter, the mixture was centrifuged at low speed (2,000 rpm) for 2 min to separate the aqueous and organic phases. The hexane phase, containing the lipophilic components, was evaporated, and the lipophilic components were resuspended in 50 μl of DMSO. This solution was regarded as 100% strength lipophilic components.
Fluorescent monitoring of the mitochondrial membrane potential.
Beas-2b cells were incubated for 4 h with 5%, 10%, and 20% CSE or hexane-treated CSE (Hx-CSE). At the end of the incubation period, cells were loaded with the potentiometric fluorescent dye JC-1 (4 μM) for 10 min in an incubator. At the end of the incubation period, cells were washed with PBS and measured on a Perkin Elmer Victor3 V fluorescent plate reader (Groningen, The Netherlands).
Luminescence monitoring of intracellular ATP levels.
Beas-2b cells were incubated for 4 h with 5%, 10%, and 20% CSE or Hx-CSE. At the end of the incubation period, intracellular ATP levels were quantified by treating the cells with 0.1% (vol/vol) Triton X-100. ATP levels were measured using the Enliten ATP assay from Promega (Leiden, The Netherlands) and a Berthold microplate Luminometer (Berthold Detection Systems, Pforzheim, Germany).
DCF fluorescence microscopy analyses of intracellular ROS.
ROS generation was determined using the fluorescent probe DCF. A549 cells and A549-ρ0 cells were grown in six-well tissue culture plates and treated with CSE (20%) or PMA (10 ng/ml) for 4 h. Cells were washed twice with PBS and then loaded for 30 min with DCF (2.5 μg/ml) at 37°C in the dark. At the end of the incubation period, cells were washed twice with PBS, and images were acquired with a Leica DM IL inverted fluorescence microscope (Rijswijk, The Netherlands).
Exposure of l-cysteine to different oxidizing agents.
A solution of l-cysteine (150 μM) was exposed to air, 0, 0.1, 0.5, 1.0, and 1.5 mM H2O2, the gaseous phase of CS (with and without 1 mM vitamin C), and the gaseous phase of water-filtered CS (WF-CS) as represented in Fig. 1A. Briefly, 25 ml of solution was placed in a 50-ml Falcon tube (BD Biosciences, Alphen aan den Rijn, The Netherlands) at 37°C. Kentucky 2R4F research-reference cigarettes were smoked using a peristaltic pump. Just before the experiments, filters were cut from the cigarettes. Each cigarette was smoked in exactly 5 min at a flow rate of 8 l/h and bubbled through the l-cysteine solution. This solution was used immediately for the experimental procedures as described below. WF-CS was produced with the same peristaltic pump, but the smoke was first passed through a receptacle of water (1 l) before it was bubbled through the l-cysteine solution. Air, produced with the same peristaltic pump, but without a cigarette, was used as a negative control under the same conditions.
Exposure of airway epithelial cells to CS and WF-CS.
Epithelial cells were exposed to air, the gaseous phase of CS, or the gaseous phase of WF-CS as represented in Fig. 1B and as described previously (38). Briefly, A549 cells were grown in 25-cm2 plastic culture flasks as described above. Just before the experiments, medium was removed, and the culture flask was positioned upside down, allowing a direct contact of smoke with epithelial cells. Kentucky 2R4F research-reference cigarettes were smoked using a peristaltic pump. Just before the experiments, filters were cut from the cigarettes. Each cigarette was smoked in exactly 5 min at a rate of 8 l/h. Gaseous-phase CS or WF-CS was directly distributed inside the culture flasks by blowing the smoke inside through a small plastic tube. After the exposure, cells were washed with PBS, lysed by one freeze-thaw cycle in 2.5 ml of pure H2O, and analyzed. Air was used as negative control under the same conditions as CS.
Quantitative determination of free thiol groups.
Total protein concentration in cell culture was determined by the Bradford method, using BSA as standard (Bio-Rad Laboratories, The Netherlands). Ellman's reagent was used for the determination of free thiol groups in cell culture and a cell-free solution of l-cysteine. Ellman's reagent (12 mM DTNB) was added to the lysed cells or l-cysteine solution to a final concentration of 6 mM DTNB followed by a 10-min incubation. Thereafter, samples were centrifuged at 1,000 g for 5 min. The supernatant was used in the assay and measured at 405 nm in a Biotek EL808 microplate reader (Abcoude, The Netherlands). The amount of free thiol groups was calibrated against a standard curve of l-cysteine.
Statistical analysis.
Calculations were performed using Prism 4 for Windows (GraphPad Software, San Diego, CA). Statistical analysis was performed using repeated-measures ANOVA (see Figs. 2 and 4). Comparisons between different experimental groups were performed with Dunnett's multiple comparison test (see Fig. 5). P < 0.05 was considered significant. Results are presented as means ± SD unless otherwise mentioned.
RESULTS
Lipophilic components in CSE disturb mitochondrial function.
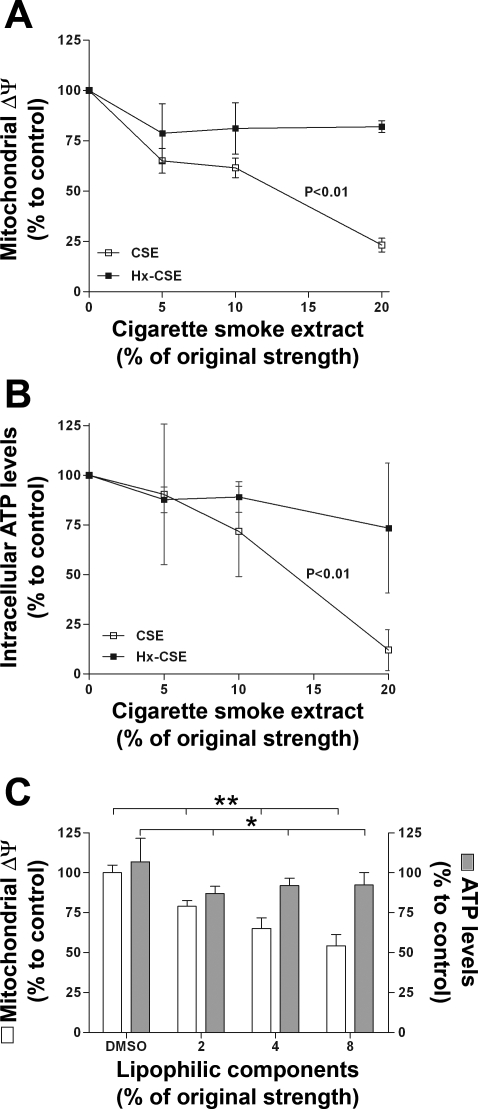
To investigate the acute effects of lipophilic components on mitochondrial function, we examined whether exposure of Beas-2b cells to CSE or Hx-CSE affects the mitochondrial membrane potential (Δψm) and intracellular ATP levels. Exposure of Beas-2b cells to increasing concentrations of CSE for 4 h caused a significant dose-dependent decrease of Δψm (Δ = −76.9 ± 3.4% , P < 0.01; Fig. 2A) and ATP levels (Δ = −88.0 ± 10.2%, P < 0.01; Fig. 2B). Compared with CSE, Hx-CSE-exposed Beas-2b cells displayed a significantly attenuated decrease in Δψm and ATP levels. To confirm that lipid-soluble components are responsible for the acute effects on mitochondrial function, the hexane phase, containing the lipophilic components, was evaporated and the lipophilic components resuspended in DMSO. Exposure of Beas-2b cells to increasing concentrations of lipophilic components for 4 h caused a significant dose-dependent decrease of Δψm (Δ = −45.7 ± 3.5% , P < 0.01; Fig. 2C) and a significant decrease in ATP levels (P < 0.05; Fig. 2C).
Fig. 2.
Cigarette smoke extract (CSE) induces a loss of mitochondrial function in human bronchial epithelial cell line (Beas-2b). Effects of increasing concentrations of CSE and hexane-treated CSE (Hx-CSE) on mitochondrial membrane potential (Δψm; A) and intracellular ATP levels (B) in Beas-2b exposed for 4 h (n = 3) are shown. C: effects of increasing concentrations of lipophilic components on Δψm and intracellular ATP levels in Beas-2b exposed for 4 h (n = 4) are shown. P values given above the CSE lines represent the significance for the decline of Δψm and ATP levels. *P < 0.05 for comparison between the lipophilic components and DMSO control experiment. **P < 0.01 for comparison between the lipophilic components and DMSO control experiment.
Functional mitochondria are essential in CSE-induced ROS generation.
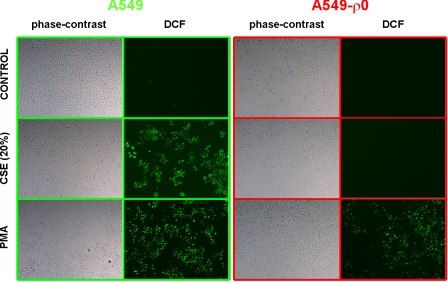
After the observation made in Beas-2b cells above, we set out to further investigate mitochondrial function. Therefore, we used alveolar epithelial cells devoid of functional mitochondria by treatment with ethidium bromide (A549-ρ0 cells), with normal A549 cells serving as controls. A549-ρ0 cells are characterized by lacking a Δψm. This was confirmed with the potentiometric probe JC-1 (data not shown). The fluorescent dye DCF was used to detect the levels of intracellular ROS. Both cell lines (A549 and A549-ρ0 cells) were exposed to CSE or PMA for 4 h. A549 cells stimulated with CSE showed an increase in DCF fluorescence, whereas A549-ρ0 cells did not. PMA, which interferes with calcium, protein kinase C metabolism, and NADPH-oxidase activity, respectively, was used as a positive control (39). Addition of PMA resulted in A549 cells as well as A549-ρ0 cells with an increase in DCF fluorescence compared with control (Fig. 3). These results suggest that a functional mitochondrial ETC is necessary to produce ROS in response to CSE.
Fig. 3.
CSE induces reactive oxygen species (ROS) generation in human alveolar epithelial cells (A549). Effects of CSE (20%) or PMA (10 ng/ml) on ROS generation in A549 and alveolar epithelial cells lacking a mitochondrial membrane potential (A549-ρO) are shown. Live cells were loaded with 2′,7′-dichlorodihydrofluorescein diacetate (DCF) before being imaged on an inverted phase-contrast and fluorescence microscope. Representative images selected from randomly chosen fields are shown.
Gaseous-phase CS can be depleted of its ROS.
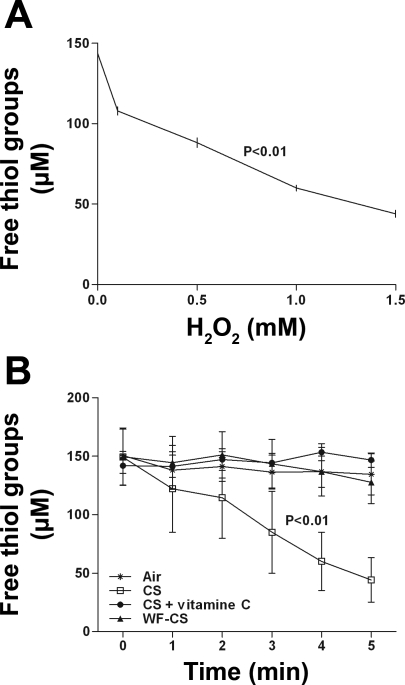
The thiol-specific interaction between l-cysteine and free radicals induced by gaseous-phase CS was studied by the DTNB assay that measures free thiol content. l-cysteine is easily oxidized by ROS. Results showed that free thiol groups of l-cysteine (150 μM) were almost fully oxidized within minutes after exposure of increasing concentrations of H2O2 (Δ = −99.5 ± 2.1 μM, P < 0.01; Fig. 4A). In Fig. 4B, we showed that CS significantly decreases free thiol groups of l-cysteine in solution, thereby indirectly showing that CS contains ROS (Δ = −105.1 ± 18.9 μM, P < 0.01; Fig. 4B). Treatment with the antioxidant vitamin C caused a significant attenuation of the effects of CS. Interestingly, WF-CS did not alter free thiol groups of l-cysteine, indicating that ROS is easily removed from CS by a single passage through water.
Fig. 4.
Effects of different oxidizing agents on the level of free thiol groups of l-cysteine in solution. A: incubation of 150 μM l-cysteine with increasing concentrations of H2O2 in the absence of light. B: control air (Air), CS, CS + vitamin C, and WF-CS are shown. Data (n = 4) are expressed as mean values ± SD. Free thiol groups were studied using Ellman's reagent. P values given above the lines in A and B represent the significance for the total decline of free thiol groups.
Mitochondrial ROS generation oxidizes free thiol groups in epithelial cells.
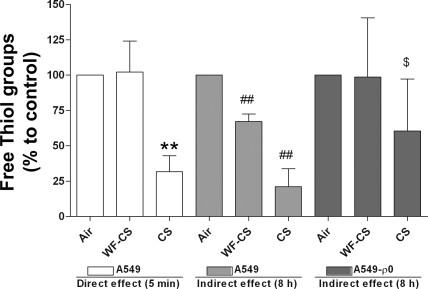
To investigate the effect of CS on intracellular ROS generation, A549 cells were exposed for 5 min to gaseous-phase CS and WF-CS. CS significantly decreases free thiol content in airway epithelial cells compared with air control, proving that gaseous-phase CS contains ROS (CS Δ = −68.3 ± 5.5%, P < 0.01; Fig. 5). WF-CS initially showed no effect on free thiol content, showing that this smoke does not contain ROS. However, 8 h after incubation of the cells with WF-CS, a significant decline of free thiol groups was observed, indicating an intracellular generation of ROS (WF-CS Δ = −41.1 ± 2.5%, P < 0.01; Fig. 5). To test the involvement of mitochondria in this “indirect” ROS generation, A549-ρ0 cells were exposed to gaseous-phase CS and WF-CS. Eight hours after incubation of A549-ρ0 cells, no difference in free thiol content was observed between A549-ρ0 exposed to WF-CS and A549-ρ0 exposed to air. Furthermore, A549-ρ0 cells were less responsive to CS compared with the A549 cells. These results suggest that mitochondria play a role in CS-induced intracellular oxidative stress.
Fig. 5.
Effects of gaseous-phase CS and gaseous-phase WF-CS on free thiol groups in human alveolar epithelial cells (A549) and A549-ρ0. Free thiol groups in A549 cells were measured after 5 min and 8 h using Ellman's reagent. Free thiol groups in A549-ρ0 were measured after 8 h using Ellman's reagent. Data are expressed as mean values ± SD. WF-CS = exposure to 1 cigarette first passed through a receptacle of water; CS = exposure to 1 cigarette directly distributed inside the culture flasks. **P < 0.01 for CS vs. control (A549, 5 min); ##P < 0.01 for WF-CS or CS vs. control (A549, 8 h); $P < 0.05 for CS vs. control (A549-ρ0, 8 h).
DISCUSSION
ROS present in gaseous-phase CS are thought to be important factors for the development of COPD (28). In patients with COPD, increased systemic markers of oxidative stress have been shown, despite the fact that the ROS present in the CS hardly enter the circulation or pass cellular membranes. In the current study, we investigated the effects of CS on mitochondrial ROS generation and demonstrated that next to a “direct” ROS effect, from the gaseous phase of CS, an indirect intracellular ROS generation was observed that depends on disturbance of functional mitochondria by lipophilic components in CS.
There is a very strong physiological link between mitochondria, which are located in the cytoplasm of all eukaryotic cells, and the human respiration. The lungs extract oxygen, which is essential for mitochondria to generate ATP, necessary for life, by oxidative phosphorylation. Under physiological conditions, oxygen-based radicals such as O2•− and •OH are byproducts of the oxidative phosphorylation. To ensure an appropriate defense against these byproducts, mitochondria are balanced with enzymatic and non-enzymatic antioxidant systems. If the amount of ROS increases or if antioxidant defense decreases, oxidative stress will be enhanced. Direct damage to mitochondria can result in a feed-forward process, whereby mitochondrial injury causes additional damage (17, 25). It is well established that smoking cigarettes contributes to an imbalance between ROS and antioxidant defenses (4).
CS is a complex aerosol that can be separated into a gas and particulate phase, whereas many substances are partitioned between these two phases. Both phases contain high levels of reactive components and radicals. In the gaseous phase, high levels of ROS are found. ROS are highly reactive due to the presence of unpaired valence shell electrons, which make them incapable to diffuse through the respiratory membranes (1, 6, 19). Therefore, gaseous-phase ROS only cause local damage in the lung, especially at the lipid bilayer of the epithelial cells, and decrease the antioxidant content in the epithelial lining fluid (28, 40).
In the particulate phase of CS, lipophilic components are present. Lipophilic components are able to pass the lipid bilayer. Recent reports have shown that components present in CSE, which does not contain ROS, are able to pass through the membranes of cells and subsequently disturb mitochondria (20, 37). Highly reactive components like polycyclic aromatic hydrocarbons, aldehydes, phenols, heavy metals, and amines are lipophilic candidates that easily enter the cell and disturb mitochondria (18, 31). We recently showed that two examples of these highly reactive lipophilic components, acrolein and crotonaldehyde, indeed easily enter the cell and react very fast with antioxidant proteins like glutathione (38). Our data now demonstrate that the lipophilic fraction present in CSE is responsible for a decrease in Δψm, ATP, and concomitant generation of mitochondrial ROS. A recent study provides evidence that CSE resulted in a dose-dependent inhibition of complex I and II activities. This inhibition was accompanied by decreases in Δψm and ATP production (37). In this study, we provide evidence that a functional ETC in mitochondria is essential in CS-induced ROS generation. This is in agreement with other studies showing that blocking a functional ETC leads to enhanced generation of ROS (9, 15). Not only pulmonary cells, but also circulating cells, can be affected in this way (20).
Gaseous-phase CS contains a higher percentage of lipophilic components than CSE. To investigate these lipophilic components in gaseous-phase CS without the influence of ROS, a water filter or so-called “waterpipe” was used to remove ROS from the gaseous phase of CS. The effects of the lipophilic components, still present in the gaseous phase of WF-CS, did not immediately oxidize free thiol groups in airway epithelial cells. This may indicate that these components first disturb mitochondrial function and second induce an indirect burst of intracellular ROS resulting in oxidation of free thiol groups. Free thiol groups play an important role in the defense against ROS. It is possible that A549-ρ0 cells have a more efficient scavenger system, due to the lack of natural mitochondrial ROS production, which is suggested by higher free thiol groups after exposure to CS. If so, the mitochondrial malfunction may not solely be the cause of the decrease in free thiol groups observed in A549 cells. Other sources of ROS could be present, and they would have been more efficiently neutralized in A549-ρ0 cells due to a more active scavenger system. Healthy cells are generally found in a reduced state. We recently showed that differences in cell viability and the relative content of free thiol groups influence the ability to recover from oxidative stress (33). In the same study, it was observed that cell degeneration was correlated with a marked depletion of intracellular free thiol groups. An alternative interpretation for the reduction in free thiol groups in A549 cells at 8 h after exposure, as presented in Fig. 5, could therefore be that the CS-induced mitochondrial dysfunction is associated with depletion of free thiol groups as a consequence of degeneration of the cells.
It is known that organic compounds from air pollution or diesel exhaust induce generation of ROS in airway epithelial cells (3, 16). A recent study by Kelsen et al. (14) implicates that exposure to cigarette smoke interferes with protein folding in the endoplasmic reticulum. Organic and inorganic oxidants are able to pass membranes of cells and induce an unfolded protein response that is able to alter expression of a variety of genes involved in antioxidant defense, inflammation, energy metabolism, protein synthesis, apoptosis, and cell cycle regulation. These oxidants can act as an electron acceptor for electrons inside the cell. Once they enter the cell and receive or accept an electron, they become a reactive oxidant. Biological membranes are highly impermeable to water and ions. It is therefore unlikely that components with unpaired electrons pass the epithelial barrier, but can enter the cell in its reductive form. ROS in the gaseous phase of CS and ROS generated by mitochondria in response to exposure to CS may both be of importance in the pathogenesis of COPD. This concept is strengthened by the fact that smokers not only develop respiratory diseases, but also systemic disease. A chronic inflammatory response, loss of skeletal muscle mass (muscle wasting), loss of body weight, and accelerated atherosclerosis have all been observed in smoking COPD patients (11, 23). Interestingly, the vastus lateralis muscles from COPD patients shift from oxidative type I to glycolytic type II fibers (12). The glycolytic metabolism produces less ATP per mole of glucose than oxidative metabolism. The functional consequences are reflected in significant changes in skeletal muscle energy metabolism and accelerated acidification, indicating that mitochondrial function is disturbed (27). In addition, lipophilic components inside the gaseous phase of cigarette smoke easily enter the blood stream facilitating systemic effects by influencing organs distal from the lungs, e.g., heart, liver, kidney, pancreas, etc. (10, 22, 26, 34).
In conclusion, in this study we showed that mitochondria are essential in the generation of intracellular ROS in response to CS. The current data strengthen the idea that ROS in the gaseous phase of CS and generated by the mitochondria themselves can attenuate both local and systemic injury in smokers, leading to the development of COPD.
GRANTS
This work was supported by the Dutch Kidney Foundation (M. van der Toorn, Co1.1923), the Jan Kornelis de Cock Foundation (M. van der Toorn, D. Slebos), and National Heart, Lung, and Blood Institute Grants R01-HL-6234, R01-HL-079904, and R01-HL-55330 (A. M. K. Choi).
Acknowledgments
We thank Harold de Bruin for valuable technical support.
REFERENCES
- 1.Afri M, Gottlieb HE, Frimer AA. Superoxide organic chemistry within the liposomal bilayer, part II: a correlation between location and chemistry. Free Radic Biol Med 32: 605–618, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J 22: 672–688, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Bonvallot V, Baeza-Squiban A, Baulig A, Brulant S, Boland S, Muzeau F, Barouki R, Marano F. Organic compounds from diesel exhaust particles elicit a proinflammatory response in human airway epithelial cells and induce cytochrome p450 1A1 expression. Am J Respir Cell Mol Biol 25: 515–521, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Bowler RP, Barnes PJ, Crapo JD. The role of oxidative stress in chronic obstructive pulmonary disease. COPD 1: 255–277, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Brunnemann KD, Hoffmann D. Analytical studies on tobacco-specific N-nitrosamines in tobacco and tobacco smoke. Crit Rev Toxicol 21: 235–240, 1991. [DOI] [PubMed] [Google Scholar]
- 6.Brzezinska AK, Lohr N, Chilian WM. Electrophysiological effects of O2*- on the plasma membrane in vascular endothelial cells. Am J Physiol Heart Circ Physiol 289: H2379–H2386, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Calikoglu M, Unlu A, Tamer L, Ercan B, Bugdayci R, Atik U. The levels of serum vitamin C, malonyldialdehyde and erythrocyte reduced glutathione in chronic obstructive pulmonary disease and in healthy smokers. Clin Chem Lab Med 40: 1028–1031, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Chandel NS, Schumacker PT. Cells depleted of mitochondrial DNA (rho0) yield insight into physiological mechanisms. FEBS Lett 454: 173–176, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Millan-Ward E, Kong J, Israels SJ, Gibson SB. Mitochondrial electron-transport-chain inhibitors of complexes I and II induce autophagic cell death mediated by reactive oxygen species. J Cell Sci 120: 4155–4166, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Chen Z, Boreham J. Smoking and cardiovascular disease. Semin Vasc Med 2: 243–252, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Gosker HR, Wouters EF, van der Vusse GJ, Schols AM. Skeletal muscle dysfunction in chronic obstructive pulmonary disease and chronic heart failure: underlying mechanisms and therapy perspectives. Am J Clin Nutr 71: 1033–1047, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Gosker HR, Zeegers MP, Wouters EF, Schols AM. Muscle fibre type shifting in the vastus lateralis of patients with COPD is associated with disease severity: a systematic review and meta-analysis. Thorax 62: 944–949, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoshino Y, Mio T, Nagai S, Miki H, Ito I, Izumi T. Cytotoxic effects of cigarette smoke extract on an alveolar type II cell-derived cell line. Am J Physiol Lung Cell Mol Physiol 281: L509–L516, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Kelsen SG, Duan X, Ji R, Perez O, Liu C, Merali S. Cigarette smoke induces an unfolded protein response in the human lung: a proteomic approach. Am J Respir Cell Mol Biol 38: 541–550, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Koopman WJ, Verkaart S, Visch HJ, van der Westhuizen FH, Murphy MP, van den Heuvel LW, Smeitink JA, Willems PH. Inhibition of complex I of the electron transport chain causes O2-.-mediated mitochondrial outgrowth. Am J Physiol Cell Physiol 288: C1440–C1450, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Li N, Sioutas C, Cho A, Schmitz D, Misra C, Sempf J, Wang M, Oberley T, Froines J, Nel A. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ Health Perspect 111: 455–460, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J, Killilea DW, Ames BN. Age-associated mitochondrial oxidative decay: improvement of carnitine acetyltransferase substrate-binding affinity and activity in brain by feeding old rats acetyl-l-carnitine and/or R-α-lipoic acid. Proc Natl Acad Sci USA 99: 1876–1881, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu X, Cai J, Kong H, Wu M, Hua R, Zhao M, Liu J, Xu G. Analysis of cigarette smoke condensates by comprehensive two-dimensional gas chromatography/time-of-flight mass spectrometry I acidic fraction. Anal Chem 75: 4441–4451, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Mao GD, Poznansky MJ. Electron spin resonance study on the permeability of superoxide radicals in lipid bilayers and biological membranes. FEBS Lett 305: 233–236, 1992. [DOI] [PubMed] [Google Scholar]
- 20.Miro O, Alonso JR, Jarreta D, Casademont J, Urbano-Marquez A, Cardellach F. Smoking disturbs mitochondrial respiratory chain function and enhances lipid peroxidation on human circulating lymphocytes. Carcinogenesis 20: 1331–1336, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Nohl H, Gille L, Kozlov A, Staniek K. Are mitochondria a spontaneous and permanent source of reactive oxygen species? Redox Rep 8: 135–141, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Orth SR, Ritz E, Schrier RW. The renal risks of smoking. Kidney Int 51: 1669–1677, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Oudijk EJ, Lammers JW, Koenderman L. Systemic inflammation in chronic obstructive pulmonary disease. Eur Respir J, Suppl 46: 5s–13s, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med 163: 1256–1276, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Pieczenik SR, Neustadt J. Mitochondrial dysfunction and molecular pathways of disease. Exp Mol Pathol 83: 84–92, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Prokopczyk B, Hoffmann D, Bologna M, Cunningham AJ, Trushin N, Akerkar S, Boyiri T, Amin S, Desai D, Colosimo S, Pittman B, Leder G, Ramadani M, Henne-Bruns D, Beger HG, El-Bayoumy K. Identification of tobacco-derived compounds in human pancreatic juice. Chem Res Toxicol 15: 677–685, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Rabinovich RA, Bastos R, Ardite E, Llinas L, Orozco-Levi M, Gea J, Vilaro J, Barbera JA, Rodriguez-Roisin R, Fernandez-Checa JC, Roca J. Mitochondrial dysfunction in COPD patients with low body mass index. Eur Respir J 29: 643–650, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Rahman I The role of oxidative stress in the pathogenesis of COPD: implications for therapy. Treat Respir Med 4: 175–200, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Rahman I, MacNee W. Role of oxidants/antioxidants in smoking-induced lung diseases. Free Radic Biol Med 21: 669–681, 1996. [DOI] [PubMed] [Google Scholar]
- 30.Remels AH, Gosker HR, van der Velden J, Langen RC, Schols AM. Systemic inflammation and skeletal muscle dysfunction in chronic obstructive pulmonary disease: state of the art and novel insights in regulation of muscle plasticity. Clin Chest Med 28: 537–552, vi, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Rustemeier K, Stabbert R, Haussmann HJ, Roemer E, Carmines EL. Evaluation of the potential effects of ingredients added to cigarettes. Part 2: chemical composition of mainstream smoke. Food Chem Toxicol 40: 93–104, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Slebos DJ, Ryter SW, van der Toorn M, Liu F, Guo F, Baty CJ, Karlsson JM, Watkins SC, Kim HP, Wang X, Lee JS, Postma DS, Kauffman HF, Choi AM. Mitochondrial localization and function of heme oxygenase-1 in cigarette smoke-induced cell death. Am J Respir Cell Mol Biol 36: 409–417, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Smit-de Vries MP, van der TM, Bischoff R, Kauffman HF. Resistance of quiescent and proliferating airway epithelial cells to H2O2 challenge. Eur Respir J 29: 633–642, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Smith CJ, Fischer TH. Particulate and vapor phase constituents of cigarette mainstream smoke and risk of myocardial infarction. Atherosclerosis 158: 257–267, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Tkacova R, Kluchova Z, Joppa P, Petrasova D, Molcanyiova A. Systemic inflammation and systemic oxidative stress in patients with acute exacerbations of COPD. Respir Med 101: 1670–1676, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Tsuchiya M, Asada A, Kasahara E, Sato EF, Shindo M, Inoue M. Smoking a single cigarette rapidly reduces combined concentrations of nitrate and nitrite and concentrations of antioxidants in plasma. Circulation 105: 1155–1157, 2002. [DOI] [PubMed] [Google Scholar]
- 37.van der Toorn M, Slebos DJ, de Bruin HG, Leuvenink HG, Bakker SJ, Gans RO, Koeter GH, van Oosterhout AJ, Kauffman HF. Cigarette smoke induced blockade of the mitochondrial respiratory chain switches lung epithelial cell apoptosis into necrosis. Am J Physiol Lung Cell Mol Physiol 292: L1211–L1218, 2007. [DOI] [PubMed] [Google Scholar]
- 38.van der Toorn M, Smit-de Vries MP, Slebos DJ, de Bruin HG, Abello N, van Oosterhout AJ, Bischoff R, Kauffman HF. Cigarette smoke irreversibly modifies glutathione in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 293: L1156–L1162, 2007. [DOI] [PubMed] [Google Scholar]
- 39.van Klaveren RJ, Roelant C, Boogaerts M, Demedts M, Nemery B. Involvement of an NAD(P)H oxidase-like enzyme in superoxide anion and hydrogen peroxide generation by rat type II cells. Thorax 52: 465–471, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wright DT, Cohn LA, Li H, Fischer B, Li CM, Adler KB. Interactions of oxygen radicals with airway epithelium. Environ Health Perspect 102, Suppl 10: 85–90, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]