Abstract
Soluble adenylyl cyclase toxins, such as Pseudomonas aeruginosa exoY, generate a cAMP pool that retracts cell borders. However, the cytoskeletal basis by which this cAMP signal retracts cell borders is not known. We sought to determine whether activation of chimeric, soluble adenylyl cyclase I/II (sACI/II) reorganizes either microtubules or peripheral actin. Endothelial cells were stably transfected with either green fluorescent protein-labeled α-tubulin or β-actin, and then infected with adenovirus to express sACI/II. Forskolin, which stimulates both the endogenously expressed transmembrane adenylyl cyclases and sACI/II, induced cell retraction accompanied by the reorganization of peripheral microtubules. However, cortical filamentous-actin (f-actin) did not reorganize into stress fibers, and myosin light-chain-20 phosphorylation was decreased. Isoproterenol, which activates endogenous adenylyl cyclases but does not activate sACI/II, did not induce endothelial cell gaps and did not influence microtubule or f-actin architecture. Thus, sACI/II generates a cAMP signal that reorganizes microtubules and induces cell retraction, without inducing f-actin stress fibers. These findings illustrate that endothelial cell gap formation can proceed without f-actin stress fiber formation, and provide mechanistic insight how bacterial adenylyl cyclase toxins reorganize the cytoskeleton to induce cell rounding.
Keywords: cAMP, actin, cytoskeleton, exoY, stress fibers
camp is a ubiquitous second messenger that exerts diverse, cell-specific signaling functions. In vascular endothelium, membrane-associated cAMP concentrations stabilize the cortical filamentous-actin (f-actin) rim, and facilitate junctional integrity necessary to strengthen barrier function (6, 11, 22, 33, 43). The principal endothelial cell adenylyl cyclase that catalyzes cAMP production is inhibited by submicromolar calcium concentrations (11, 45). Inflammatory agonists that increase cytosolic calcium therefore reduce membrane-associated cAMP, and this decrease in cAMP is necessary for the cell-cell border retraction that increases tissue edema (31, 44). β-adrenergic agonists, such as epinephrine and isoproterenol, increase membrane-cAMP; increased cAMP reverses the permeability that accompanies inflammation (8, 12, 21, 31, 43, 45). Thus, maintenance of membrane-associated cAMP is a critical determinant of endothelial cell barrier integrity (11, 31).
A cAMP gradient exists between the plasma membrane, where cAMP is typically synthesized, and the bulk cytosol (36, 51). Maintaining this gradient is essential to the physiological signaling role of cAMP. When soluble bacterial enzymes, such as exoY from Pseudomonas aeruginosa, synthesize cAMP within the cytosol rather than at the plasma membrane, the resulting cAMP signal induces cell rounding with retraction of cell-cell borders (40, 52). These actions of exoY can be mimicked using a forskolin activated, soluble chimeric mammalian enzyme, soluble adenylyl cyclase I/II (sACI/II), which also localizes within the cytosol (39). So while it is evident that cytosolic adenylyl cyclase activity induces endothelial cell retraction, the intracellular cAMP cytoskeletal target(s) that mediate this effect are unknown.
F-actin and microtubules dynamically interact to control cell shape (5, 19, 25, 27, 29). The cortical f-actin rim stabilizes adherens and focal adhesion junctional integrity and interacts with microtubules near the cell periphery (13, 15, 19, 28, 42). F-actin and microtubule reorganization must be coordinated for cell retraction to occur. Inflammatory agonists realign cortical f-actin into stress fibers, and simultaneously disassemble microtubules (1, 2, 4, 23, 34, 47–49). Stabilizing either f-actin or microtubules attenuates intercellular gaps induced by inflammatory agonists (34, 35, 46). Directly disrupting microtubules using nocodazole also realigns f-actin into stress fibers and induces endothelial cell gap formation (5, 50). Moreover, administration of vinca alkaloids, which disrupt microtubules, causes pulmonary edema in breast cancer patients (9). We, therefore, sought to determine whether activation of soluble adenylyl cyclase activity reorganizes microtubules and realigns cortical f-actin into stress fibers. Our results indicate that activation of sACI/II preferentially reorganizes microtubules without realigning f-actin into stress fibers. These results identify for the first time a unique signaling paradigm in which the production of a cytosolic cAMP pool uncouples microtubule reorganization from stress fiber formation in the course of endothelial cell retraction.
MATERIALS AND METHODS
Isolation and culture of pulmonary microvascular endothelial cells.
Pulmonary microvascular endothelial cells (PMVECs) were isolated, cultured, and passaged in endothelial cell (EC) media (DMEM+ 10% FBS+ 100 U/ml penicillin+ 100 U/ml streptomycin) using a method previously described (44).
Retrovirus constructs and infection.
Retroviral vectors encoding AcGFP1-β-actin and AcGFP1-α-tubulin fusions under control of cytomegalovirus promoter were constructed by inserting fragments encoding Pcmv-AcGFP1-β-actin and Pcmv-AcGFP1-α-tubulin from pAcGFP1-β-actin and pAcGFP1-α-tubulin plasmids (Clontech, Mountain View, CA), respectively, into puromycin resistance-encoding retroviral vector pMA1629 (pBABEpuro derivative, M. Alexeyev, unpublished data). Retroviral supernatants were generated by standard techniques in Phoenix ampho packaging cell line. Retroviral supernatants supplemented with 4 μg/ml polybrene were added to 30–40% confluent PMVECs, centrifuged at 2,000 rpm for 30 min at 20°C, and incubated for 40 to 48 h to allow for retroviral integration. Subsequently, retroviral supernatants were replaced with EC media containing puromycin (15 μg/ml; Sigma-Aldrich, St. Louis, MO) to select for cells expressing the construct. Live cell confocal imaging was performed in the absence or presence of forskolin (10 μmol/l; Tocris, Ellisville, MO) or isoproterenol (1 μmol/l; Sigma-Aldrich) using PerkinElmer Ultraview RS-3 spinning disk confocal microscope (Nikon ECLIPSE TE2000) at an excitation wavelength of 488 nm and an emission wavelength of 510 nm.
Adenovirus construct and infection.
Recombinant adenoviruses encoding soluble ACI/II were generated using a method previously described (17). PMVECs stably expressing green fluorescent protein (GFP)-actin or GFP-tubulin were seeded on 25-mm circle microscope glass coverslips (Fisher Scientific, Pittsburgh, PA) in 6-well plates and grown to 70% to 90% confluence. Cells were infected with adenovirus expressing soluble ACI/II construct at multiplicity of infection 10:1 for 36 to 48 h; then, live-cell confocal imaging was performed in the absence or presence of forskolin (10 μmol/l) or isoproterenol (1 μmol/l) using PerkinElmer Ultraview RS-3 spinning disk confocal microscope at an excitation wavelength of 488 nm and an emission wavelength of 510 nm.
Confocal fluorescence microscopy.
Live-cell confocal fluorescent images were acquired using PerkinElmer Ultraview RS-3 spinning disk confocal microscope (Nikon ECLIPSE TE2000) at an excitation wavelength of 488 nm and emission wavelength of 510 nm. Confocal images (of 0.4-μm sections through cells) were captured using a charge-coupled device camera (Hamamatsu ORCA ER) for every 1 min for the first 5 min without drug treatment, and for another 30 min with forskolin (10 μmol/l) or isoproterenol (1 μmol/l), then all the confocal images were compressed into time-lapsed movies using PerkinElmer Ultraview software.
Microtubule Western blot analysis.
Soluble fractions of microtubules from forskolin (10 μmol/l) -treated or vehicle control-treated PMVECs expressing soluble ACI/II were isolated using a method previously described (26). Briefly, cells were rinsed with PBS, extracted with soluble microtubule-extraction buffer (80 mmol/l PIPES, pH 6.8, 1 mmol/l EGTA, 1 mmol/l MgCl2 with 25% glycerol and 0.5% TritonX-100) for 2 min, supernatant containing soluble tubulin was collected, cells were rinsed with additional extraction buffer, and supernatant was pooled with the original. Soluble proteins were suspended in 1× SDS-PAGE sample buffer (Invitrogen, Carlsbad, CA), resolved in 4–12% Bis-Tris gel (Invitrogen), transferred to nitrocellulose paper (Bio-Rad, Hercules, CA), and subjected to Western blot analysis using mouse monoclonal antibody to depolymerized β-tubulin (Covance, Berkeley, CA) or mouse monoclonal antibody to polymerized β-tubulin (Covance). Primary antibody binding to immunoreactive proteins was detected using horseradish peroxidase-labeled goat anti-mouse IgG (Santa Cruz Biotechnology, Santa Cruz, CA). Immunoreactive bands were detected using enhanced chemiluminescence detection system, according to manufacturer's protocol (Pierce, Rockford, IL).
Live-cell tubulin labeling.
PMVECs expressing sACI/II were treated with vehicle control or forskolin (10 μmol/l) for 30 min. Cells were washed with Kreb's buffer containing 2 mmol/l Ca+2, stained with 250 nmol/l tubulin Tracker Green Reagent (Molecular Probes, Eugene, OR), incubated for 30 min at 37°C-5% CO2, rinsed 3 times with Kreb's buffer containing 2 mmol/l Ca+2, and viewed using PerkinElmer Ultraview RS-3 spinning disk confocal microscope at excitation wavelength of 488 nm and emission wavelength of 510 nm.
Tau phosphorylation Western blot analysis.
PMVECs expressing sACI/II were treated with vehicle control, forskolin (10 μmol/l), or isoproterenol (1 μmol/l) for 30 min. After 30 min of treatment, cells were washed with PBS, scraped in prewarmed 1× SDS-PAGE sample buffer for 2 min and boiled for 10 min. The whole cell protein extracts suspended in 1× SDS-PAGE sample buffer were separated in 10% Bis-Tris gel, transferred to nitrocellulose paper, and subjected to Western blot analysis using rabbit polyclonal antibody to phosphorylated-Ser214 residue of tau (Invitrogen). Primary antibody binding to immunoreactive proteins was detected using horseradish peroxidase-labeled goat anti-rabbit IgG (Santa Cruz Biotechnology). Immunoreactive bands were detected using enhanced chemiluminescence detection system according to manufacturer's protocol (Pierce).
Phalloidin staining and confocal microscopy.
PMVECs expressing sACI/II were treated with vehicle control or forskolin (10 μmol/l) for 30 min. Likewise, PMVECs were treated with forskolin (10 μmol/l) and thrombin (10 U/ml; cat. no. T-5772, lot # 105K7555, from rat plasma; Sigma-Aldrich) as negative and positive controls, respectively. After 30 min of treatment, cells were washed with prewarmed PBS, fixed in 1–2% paraformaldehyde for 10 min at room temperature, permeabilized in 0.1% Triton-X-100 in PBS for 3–5 min, stained with 100–150 nmol/l Alexa Fluor 635-congugated-phalloidin (Molecular Probes) in PBS for 20 min and washed with PBS. PerkinElmer Ultraview RS-3 spinning disk confocal system was used to image fixed cells at an excitation wavelength of 633 nm and an emission wavelength of 647 nm.
Myosin light-chain Western blot analysis.
PMVECs expressing sACI/II were treated with vehicle control or forskolin (10 μmol/l) for 30 min. Similarly, PMVECs were treated with forskolin (10 μmol/l) and thrombin (10 U/ml) as negative and positive controls for myosin light chain (MLC) phosphorylation, respectively. After 30 min of treatment, cells were washed with PBS, scraped in prewarmed 1× SDS-PAGE sample buffer for 2 min and boiled for 10 min. The whole cell protein extracts suspended in 1× SDS-PAGE sample buffer were separated in 4–12% Bis-Tris gel, transferred to nitrocellulose paper, and subjected to Western blot analysis using mouse monoclonal antibody to phosphorylated-Ser19 residue of MLC (Cell Signaling Technology, Danvers, MA) or rabbit polyclonal antibody to phosphorylated-Thr18/Ser19 residues of MLC (Cell Signaling Technology). Primary antibody binding to immunoreactive proteins was detected using horseradish peroxidase-labeled donkey anti-mouse or goat anti-rabbit IgG (Santa Cruz Biotechnology). Immunoreactive bands were detected using enhanced chemiluminescence detection system according to manufacturer's protocol (Pierce).
Hydraulic conductance (Lp) measurement.
PMVECs were grown on the top of porous filters in Snapwell Polystyrene plates (Fisher Scientific) that allow for the measurement of fluid filtration at different transmembrane pressures. These cells were infected with an adenovirus expressing sACI/II construct and incubated for 36–48 h. The confluent monolayers of cells that were expressing sACI/II were mounted on a Ussing-type chamber to measure filtration rate by timing fluid movement in a calibrated micropipette at a specific transmembrane pressure controlled by reservoir height. The transmembrane pressure and the filtration rate (corrected for surface area) were used to calculate hydraulic conductance in the presence or absence of forskolin (10 μmol/l), as previously described (32).
Statistical analysis.
Unpaired two-tailed t-test with Welch's correction was used for comparison between two groups. A value of P < 0.05 was considered statistically significant. GraphPad Prism software was used for the statistical analysis. Data are presented as means ± SD.
RESULTS
Activation of sACI/II reorganizes microtubules in PMVECs.
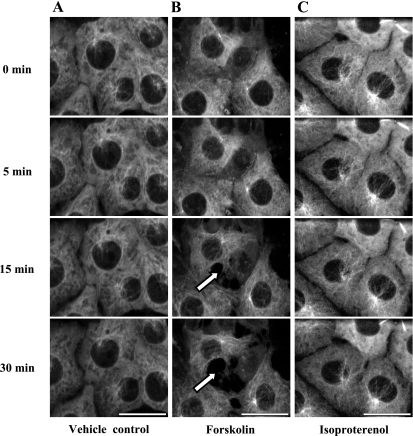
We initially sought to determine whether sACI/II activation reorganizes microtubules. To test this idea, PMVECs were infected with a GFP-tubulin-expressing retrovirus (Fig. 1A), and cells expressing the construct were selected to homogeneity based upon their puromycin resistance. Elaborate microtubule structures emanating from centrosomes were resolved in GFP-tubulin-expressing PMVECs (Fig. 1B). The microtubule networks visualized using GFP fluorescence resembled the endogenous microtubule pattern previously detected in these cells (data not shown), and while microtubule structures dynamically reorganized over a 30-min time course, they did not grossly change their architecture. We next stimulated endogenously expressed adenylyl cyclases (ACs) using forskolin and isoproterenol. Forskolin directly activates transmembrane ACs, whereas isoproterenol activates the β2-adrenergic receptor in endothelium, and β2-rececptor activation leads to Gαs stimulation of transmembrane ACs. Neither forskolin (Fig. 1C) nor isoproterenol (Fig. 1D) induced a gross change in microtubule patterning. Thus, activation of endogenously expressed transmembrane ACs does not change the global organization of microtubules in PMVECs over a 30-min time course.
Fig. 1.
Stimulation of membrane adenylyl cyclase increases membrane-localized cAMP that does not disrupt microtubules in pulmonary microvascular endothelial cells (PMVECs). PMVECs were infected with a retrovirus to stably express GFP-α-tubulin. Live cell confocal imaging was performed for the first 5 min without treatment, and for another 30 min with forskolin (10 μmol/l) or isoproterenol (1 μmol/l). A: schematic representation of a retroviral construct that stably expresses GFP-α-tubulin. Microtubules did not reorganize in control- (B, n = 3), forskolin- (C, n = 5), or isoproterenol- (D, n = 5) treated cells. The scale bars represent 25 μm.
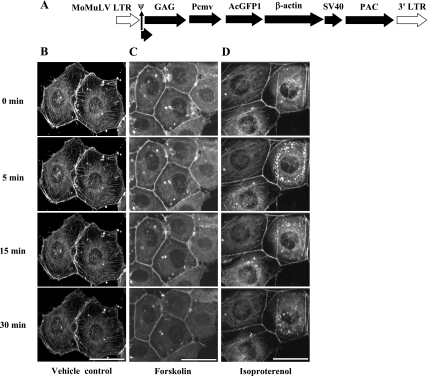
To determine whether soluble AC activity produces a cAMP pool that reorganizes microtubule architecture, GFP-tubulin-expressing PMVECs were infected with an adenovirus to express the sACI/II construct. Live-cell confocal imaging was performed in the presence or absence of forskolin. Peripheral microtubule networks remained intact over the 30-min time course in control experiments (Fig. 2A). Forskolin treatment, which simultaneously activates both transmembrane ACs and sACI/II, reorganized peripheral microtubules and induced gaps between adjacent cells within 15 min (Fig. 2B). This effect was progressive, peaking at the 30-min time point. Despite reorganizing peripheral microtubules, forskolin stimulation of sACI/II did not appear to abolish microtubule nucleation at the centrosome. Isoproterenol does not stimulate the sACI/II when it is expressed in cells, and it serves as a treatment control (17, 39). As in time controls, isoproterenol did not grossly alter microtubule morphology (Fig. 2C). Thus, activation of sACI/II increases a cytosolic cAMP pool that reorganizes microtubules important for interendothelial cell gap formation.
Fig. 2.
Forskolin stimulation of sACI/II increases a cytosolic cAMP pool that reorganizes microtubules sufficient to disrupt PMVEC barrier function. PMVECs that are stably expressing GFP-α-tubulin were infected with an adenovirus expressing the sACI/II. Live cell confocal imaging was performed for 35 min in control cells and in forskolin (10 μmol/l) or isoproterenol (1 μmol/l) treated cells. A: in vehicle control cells, microtubules were not reorganized, and endothelial barrier was not disrupted (n = 4). B: in forskolin-treated cells, peripheral microtubule networks were reorganized, and endothelial cell gap formation (arrows denote endothelial barrier disruption as a consequence of reorganization of peripheral microtubule networks, n = 9). C: in isoproterenol-treated cells, microtubules were not reorganized, and the endothelial barrier was not disrupted (n = 5). The scale bars represent 25 μm.
To further examine whether activation of sACI/II reorganizes peripheral microtubules, we measured fluorescence intensity of microtubule polymers specifically at cell-cell borders in PMVECs expressing sACI/II. Fluorescence intensity decreased modestly in time controls due to photobleaching (Fig. 3, A and C). However, as illustrated in Fig. 3A, peripheral microtubule structures remained intact. In forskolin-treated cells, fluorescence intensity of microtubule polymers was decreased significantly compared with control cells (Fig. 3, B and C). The decrease in fluorescence intensity of microtubule polymers in forskolin activated sACI/II expressing cells was due to endothelial gap formation, suggesting that activation of sACI/II, but not transmembrane ACs, generates a cAMP pool that reorganizes microtubules important for interendothelial cell gap formation.
Fig. 3.
Forskolin stimulation of sACI/II increases cytosolic cAMP that depolymerizes microtubules. PMVECs that are stably expressing GFP-α-tubulin were infected with an adenovirus expressing the sACI/II. Live cell confocal imaging was performed for 35 min in the absence (A) or presence (B) of forskolin (10 μmol/l). Western blot analysis was performed in these cells for depolymerized and polymerized tubulin, and live-cell tubulin labeling using Tubulin Tracker Green Reagent (250 nmol/l) was performed in vehicle-treated or forskolin (10 μmol/l)-treated PMVECs expressing sACI/II. A: in vehicle control cells, microtubules remained distributed throughout the cell, included at sites of cell-cell borders, over the 30-min time frame (n = 4). B: in forskolin-treated cells, fluorescence intensity of microtubules decreased significantly, in association with endothelial gap formation (n = 9). C: total fluorescence intensity decreased in control cells due to photobleaching over a 30-min time frame. Forskolin stimulation of sACI/II significantly decreased fluorescence intensity of microtubules, demonstrating microtubule reorganization and gap formation. *P < 0.05. D: forskolin stimulation of sACI/II increased depolymerized tubulin and decreased polymerized tubulin compared with control cells (n = 4). Summary is shown in E. *P < 0.05. F: forskolin stimulation of sACI/II decreased polymerized tubulin compared with control cells. The scale bars represent 25 μm. G: whole cell lysates were used to determine whether activation of sACI/II results in Tau-Ser214 phosphorylation. Whereas forskolin (10 μmol/l) increased Tau-Ser214 phosphorylation, isoproterenol (1 μmol/l) was without effect.
We next sought to determine whether forskolin activation of sACI/II depolymerizes microtubules. PMVECs expressing sACI/II were treated with either vehicle control or forskolin for 30-min, lysed, and lysates subjected to Western blot analysis for depolymerized and polymerized microtubules. Forskolin increased depolymerized, and decreased polymerized, tubulin, compared with control cells (Fig. 3, D and E). These findings support the idea that activation of sACI/II increases a cytosolic cAMP pool that reorganizes peripheral microtubules.
To confirm that our observations using GFP-labeled tubulin were representative of the reorganization that occurs in endogenously expressed microtubules, we labeled endogenous microtubules with Tubulin Tracker Green Reagent. The sACI/II expressing PMVECs were treated with vehicle control or forskolin for 30 min. In sACI/II-expressing cells, forskolin reduced polymerized microtubule polymers and disrupted the endothelial barrier, just as we observed in GFP-tubulin expressing cells (Fig. 3F). These observations indicate that activation of sACI/II increases a cytosolic cAMP pool that not only reorganizes expressed GFP-labeled microtubules, but also reorganizes endogenous microtubules.
PKA phosphorylation of tau, on serine 214 (Tau-Ser214) results in microtubule disassembly (14). We, therefore, examined whether forskolin activation of sACI/II promotes Tau-Ser214 phosphorylation. sACI/II expressing cells were treated with vehicle control, forskolin, or isoproterenol for 30 min, lysed, and Tau-Ser214 phosphorylation status was examined. Whereas forskolin increased Tau-Ser214 phosphorylation, isoproterenol was without effect. These findings suggest that activation of sACI/II results in PKA phoshorylation of Tau-Ser214, which promotes microtubule disassembly (14).
Forskolin activation of sACI/II is reversible. To determine whether activation of sACI/II irreversibly, or reversibly, disassembles microtubules, we stimulated sACI/II-expressing cells with forskolin for 30 min and then washed out the agonist and examined microtubule organization for 3 h. As is seen in Fig. 4A, forskolin reorganized microtubules, as observed in Fig. 2B. Over 3 h, however, a recovery of normal microtubule architecture was seen, illustrating the reversible nature of this cAMP-dependent signal.
Fig. 4.
Activation of sACI/II reversibly reorganizes microtubules necessary for intercellular gap formation. PMVECs were infected with an adenovirus expressing sACI/II, and the endogenous microtubule organization was examined using Tubulin Tracker Green Reagent (250 nmol/l). A: forskolin (10 μmol/l) stimulation of sACI/II reorganized endogenous microtubules and resulted in gap formation. Forskolin washout resulted in recovery of peripheral microtubule architecture over a 3-h time course. B: PMVECs were pretreated with paclitaxel (100 nmol/l) for 3 h, and then stimulated with forskolin (10 μmol/l) for 30 min. Paclitaxel stabilized microtubule structures and prevented forskolin from reorganizing microtubules and inducing intercellular gaps. The scale bars represent 25 μm. Arrows denote intercellular gaps.
We examined whether microtubules represent a centrally important cAMP effector necessary for gap formation. In this case, paclitaxel was administered to sACI/II-expressing cells for a 3-h time course before forskolin was applied. Paclitaxel stabilized microtubules in control cells and prevented forskolin from reorganizing microtubules (Fig. 4B). Paclitaxel also prevented forskolin from inducing interendothelial cell gaps, indicating that microtubule reorganization is essential for activation of sACI/II to disrupt the endothelial cell barrier.
Activation of sACI/II does not reorganize the cortical actin rim into stress fibers.
We next examined whether gaps induced by activation of sACI/II are due, in part, to stress fiber formation, or whether the cortical actin rim remains intact. To test this idea, we prepared retrovirus to express GFP-actin. PMVECs were infected with the GFP-actin-expressing retrovirus, and cells expressing the construct were selected to homogeneity (Fig. 5A). Live-cell confocal imaging of GFP fluorescence revealed a dense peripheral actin rim, similar to the endogenous actin rim previously observed in these cells using phalloidin staining (4, 6, 22, 34, 35, 43). Cortical actin was stable and did not dynamically reorganize over a 30-min time course (Fig. 5B). Neither forskolin (Fig. 5C) nor isoproterenol (Fig. 5D) disrupted the cortical actin rim. Thus, activation of membrane ACs does not reorganize peripheral actin into stress fibers, and likely strengthens it, as previously suggested by studies using phalloidin staining (6, 22, 43).
Fig. 5.
Stimulation of transmembrane adenylyl cyclase increases membrane-localized cAMP that does not disrupt cortical actin cytoskeletal networks in PMVECs. PMVECs were infected with a retrovirus to stably express GFP-β-actin. Live cell confocal imaging was performed for the first 5 min without treatment, and for another 30 min with forskolin (10 μmol/l) or isoproterenol (1 μmol/l). A: schematic representation of a retroviral construct that stably expresses a GFP-β-actin. Cortical actin cytoskeletal networks were not disrupted in control- (B, n = 3), forskolin- (C, n = 5), or isoproterenol (D, n = 5) -treated cells. The scale bars represent 25 μm.
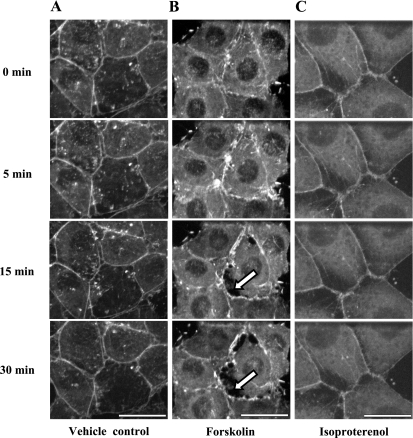
To determine whether soluble AC activity produces a cAMP pool that disrupts peripheral actin, GFP-actin-expressing PMVECs were infected with an adenovirus to express the sACI/II construct. sACI/II-expressing PMVECs displayed normal junctional integrity, as well as a typical cortical actin rim, over a 30-min time course in control experiments (Fig. 6A). In contrast, forskolin induced gaps between adjacent cells that were prominent by 15 min, and that peaked by 30 min (Fig. 6B). Despite inducing interendothelial cell gaps, activation of sACI/II did not overtly disrupt the cortical actin rim, and it did not induce stress fiber formation. As in time control experiments, isoproterenol (Fig. 6C) neither disrupted the endothelial cell barrier, nor reorganized the cortical actin rim. Thus, activation of sACI/II increases a cytosolic cAMP pool that does not disrupt cortical actin cytoskeletal networks and still induces endothelial gap formation.
Fig. 6.
Forskolin stimulation of sACI/II increases cytosolic cAMP, and induces endothelial gap formation without disrupting cortical actin cytoskeletal networks. PMVECs that are stably expressing GFP-actin were infected with an adenovirus expressing the sACI/II. Live cell confocal imaging was performed for the first 5 min without treatment, and for another 30 min in the presence of forskolin (10 μmol/l) or isoproterenol (1 μmol/l). A: in vehicle control cells, cortical actin cytoskeletal networks were not disrupted, and endothelial gaps were not formed (n = 3). B: in forskolin-treated cells, cortical actin cytoskeletal networks were not disrupted, but endothelial cell gaps were formed (arrows denote endothelial gaps, n = 7). C: in isoproterenol-treated cells, cortical actin cytoskeletal networks were not disrupted, and endothelial gaps were not formed (n = 6). The scale bars represent 25 μm.
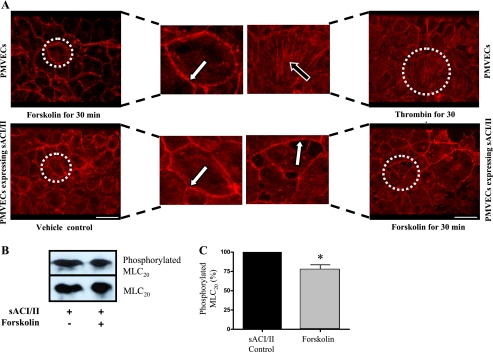
To further examine whether activation of sACI/II reorganizes the cortical actin rim into stress fibers, we labeled f-actin in sACI/II-expressing cells using phalloidin staining. Either vehicle control or forskolin was added to cells for 30-min, and the actin architecture was examined. As in our studies using GFP-actin, forskolin induced gaps between adjacent cells, but it did not reorganize cortical actin into stress fibers (Fig. 7A). Thrombin was used as a positive control in PMVECs that were not expressing sACI/II, as it is widely recognized to induce stress fiber formation (34). Whereas thrombin treatment eliminated the cortical actin rim, and induced stress fiber formation, forskolin treatment was without effect in cells lacking sACI/II expression (Fig. 7A). Thus, activation of sACI/II generates a cytosolic cAMP pool that induces endothelial cell gap formation (39), without reorganizing cortical actin into stress fibers.
Fig. 7.
Forskolin stimulation of sACI/II increases cytosolic cAMP and induces endothelial gap formation without reorganizing cortical actin cytoskeletal networks into stress fibers. PMVECs were treated with forskolin (10 μmol/l) or thrombin (10 U/ml) for 30 min. Similarly, PMVECs expressing sACI/II were treated with or without forskolin (10 μmol/l) for 30 min. Cells were subjected to phalloidin staining to assess actin stress fibers, as well as to Western blot analysis to evaluate MLC20 phosphorylation. A: forskolin stimulation of sACI/II induces endothelial gap formation without disrupting cortical actin cytoskeletal networks and reorganizing them into stress fibers. Thrombin induces endothelial gap formation by disrupting cortical actin cytoskeletal networks and reorganizing them into stress fibers (positive control). Forskolin-treated PMVECs and control PMVECs expressing sACI/II neither form endothelial gaps nor reorganize cortical actin into stress fibers (negative controls, n = 3). The scale bars are equal to 25 μm. B: forskolin decreased MLC20 phosphorylation in PMVECs expressing sACI/II (n = 3). Summary data are shown in C. *P < 0.05.
We examined whether activation of sACI/II decreases MLC20 phosphorylation. sACI/II-expressing cells were treated with vehicle control or forskolin for 30-min, and MLC20 phosphorylation was examined. As shown in Fig. 7, B and C, forskolin stimulation decreases MLC20 phosphorylation, providing a potential explanation for why gaps form in endothelial cells without concomitant stress fiber formation.
Activation of sACI/II increases endothelial permeability.
We next examined whether gaps formed between adjacent PMVECs were sufficient to increase permeability. Hydraulic conductance (Lp) measurements were performed in PMVECs expressing sACI/II in the presence or absence of forskolin. Hydraulic conductance measures water permeability for any transmembrane pressure. Forskolin increased the hydraulic conductance in PMVECs, compared with controls (Fig. 8). These results validate the idea that sACI/II increases a cytosolic cAMP pool that induces endothelial gaps and increases permeability.
Fig. 8.
Forskolin stimulation of sACI/II increases cytosolic cAMP and increases endothelial permeability. PMVECs were infected with an adenovirus expressing the sACI/II. Hydraulic conductance (Lp) measurements were performed in the absence (control cells) or presence of forskolin (10 μmol/l). Lp was significantly higher in forskolin-treated cells compared with control cells, indicating increased endothelial permeability in forskolin-treated PMVECs expressing sACI/II. *P < 0.05.
DISCUSSION
Cell shape is dynamically controlled by interactions between f-actin and microtubules (25, 27, 29). In endothelium, a cortical actin rim stabilizes the junctional complexes, such as the cadherin-catenin complex, which are necessary to maintain barrier function (13, 28). Microtubules extend from the centrosome in anterograde fashion to abut the cortical actin rim, where they indirectly interact with f-actin and also stabilize cell shape (15, 42). cAMP that is synthesized at the plasma membrane strengthens the cortical actin rim, and promotes the indirect interaction between cortical actin and peripheral microtubules (6, 11, 22, 24, 33, 37, 38, 41, 43). In stark contrast to these actions of transmembrane adenylyl cyclases, exoY, and sACI/II synthesize cAMP within the bulk cytosol, away from the plasma membrane (39, 40). cAMP synthesis within the cytosol retracts endothelial cell borders (39, 40). Our present studies examined whether cytosolic cAMP synthesis initiates f-actin or microtubule reorganization as a necessary prerequisite to endothelial cell gap formation. Whereas sACI/II activation resulted in Tau-Ser214 phosphorylation and reorganization of peripheral microtubules at sites of cell retraction, f-actin did not reorganize from a peripheral actin rim into centripetally directed stress fibers. Our findings illustrate the preferential targeting of cAMP that is synthesized within the cytosol to microtubule structures and demonstrate that cell border retraction can occur in the absence of concomitant stress fiber formation.
Microtubule disassembly is sufficient to retract endothelial cell borders (2, 50), and therefore it is not surprising that sACI/II-induced microtubule reorganization was accompanied by gap formation and increased permeability. However, cAMP control of microtubule architecture remains poorly understood. Activation of transmembrane adenylyl cyclases inhibits nocodozole-induced microtubule disassembly (3). The mechanism for such a protective effect of membrane-localized cAMP is unknown, although it is not likely due to direct PKA phosphorylation of microtubules or their associated proteins. Tau and microtubule-associated proteins (MAP) stabilize microtubules (16). The direct PKA phosphorylation of tau and MAP(s) release them from microtubules, resulting in microtubule disassembly into α/β heterodimers (18). Indeed, our recent findings indicate that tau is not typically PKA phosphorylated following the direct activation of transmembrane adenylyl cyclases (14). Yet, when tau is PKA phosphorylated on Ser214, it is released from microtubules, allowing their disassembly. We now provide evidence that activation of sACI/II results in Tau-Ser214 that enables microtubule reorganization leading to gap formation.
Recent discovery of the ubiquitously expressed mammalian soluble adenylyl cyclase 10 (AC10) provides a putative cAMP source that may regulate tau phosphorylation status and, hence, microtubule architecture (7, 10, 53). AC10 localizes to the centrosome and its associated microtubules and has been incriminated in control of microtubule organization during cell division (53). Thus, the sACI/II-dependent reorganization of microtubules described presently may be indicative of an important physiological role for AC10 during mitosis. In addition, since sACI/II and exoY act similarly to disrupt the endothelial cell barrier (39, 40), our present findings suggest that exoY may hijack this microtubule regulatory control mechanism as a means of increasing endothelial cell permeability.
While activation of sACI/II reorganized microtubules, it did not reorganize the cortical actin rim into stress fibers. This was a surprising observation, since both microtubule disassembly and stress fiber formation are thought to be necessary for cell retraction to occur (1–6, 50). Nocodazole-induced microtubule disassembly is accompanied by stress fiber formation (5, 50), and neurohumoral inflammatory mediators that disassemble microtubules also promote stress fiber formation (1, 2, 4, 6, 34, 35, 48, 49). In the latter case, either stabilizing microtubules or inhibiting stress fiber formation attenuates inflammatory mediator-induced endothelial cell gaps, suggesting both processes are essential for cell retraction to proceed. We confirmed this idea in this study, as thrombin acutely disassembled peripheral actin and induced stress fibers. Indeed, our observations represent the first evidence that microtubule reorganization in the absence of concomitant stress fiber formation is sufficient to retract endothelial cell borders.
We considered how sACI/II activation prevents stress fiber formation. Microtubule disassembly activates myosin light-chain kinase, which phosphorylates MLC20 and promotes actomyosin interaction (50). In addition, RhoA activates Rho kinase, which phosphorylates and inactivates myosin-associated phosphatase, resulting in increased MLC20 phosphorylation (4, 5). cAMP opposes the actions of both myosin light-chain kinase and RhoA; PKA directly phosphorylates both myosin light-chain kinase and RhoA, and decreases MLC20 phosphorylation (3, 20, 23, 30, 33). In our experiments, sACI/II activation decreased MLC20 phosphorylation, providing an explanation for why stress fibers did not form. It is interesting that in control cells, neither forskolin nor isoproterenol reduced MLC20 phosphorylation (data not shown). Indeed, in our present studies, forskolin only decreased MLC20 phosphorylation in cells expressing the sACI/II. There is controversy regarding whether myosin light-chain kinase, or RhoA, represents a physiologically relevant cAMP/PKA effector in vivo. Our findings would support the idea that cAMP generated by the sACI/II more effectively inhibits MLC20 phosphorylation than does cAMP generated by transmembrane ACs. Thus, sACI/II generates cAMP in a location that is readily accessible to the cell's contractile machinery.
It is not clear how microtubule reorganization in the absence of stress fiber formation allows for loss of cell-cell adhesion. Indeed, the cortical actin rim coordinates adherens and tight junction proteins to stabilize junctional strength. Realignment of cortical actin into stress fibers is accompanied by coordinated loss of cell-cell junctional integrity. It will be essential to determine whether microtubule reorganization is uniquely coupled to a subset of junctional proteins that do not require loss of the cortical actin rim to initiate gap formation.
In summary, activation of sACI/II is sufficient to induce endothelial cell retraction and increase permeability. cAMP that is generated from sACI/II reorganizes microtubules, and inhibits stress fiber formation. Indeed, interendothelial cell gap formation can proceed in the absence of stress fiber formation.
GRANTS
This work was supported by National Institutes of Health grants HL-60024 and HL-66299 (to T. Stevens) and by American Heart Association Greater Southeast Affiliate Predoctoral Fellowship 0715456B (to N. Prasain).
Acknowledgments
We thank Drs. James Parker and Judy King, and Ms. Linn Ayers and Anna Buford, for their contribution to the development of this work, and Dr. Carmen W. Dessauer for providing the sACI/II construct.
REFERENCES
- 1.Birukova AA, Birukov KG, Adyshev D, Usatyuk P, Natarajan V, Garcia JG, Verin AD. Involvement of microtubules and Rho pathway in TGF-β1-induced lung vascular barrier dysfunction. J Cell Physiol 204: 934–947, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Birukova AA, Birukov KG, Smurova K, Adyshev D, Kaibuchi K, Alieva I, Garcia JG, Verin AD. Novel role of microtubules in thrombin-induced endothelial barrier dysfunction. FASEB J 18: 1879–1890, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Birukova AA, Liu F, Garcia JG, Verin AD. Protein kinase A attenuates endothelial cell barrier dysfunction induced by microtubule disassembly. Am J Physiol Lung Cell Mol Physiol 287: L86–L93, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Birukova AA, Smurova K, Birukov KG, Kaibuchi K, Garcia JG, Verin AD. Role of Rho GTPases in thrombin-induced lung vascular endothelial cells barrier dysfunction. Microvasc Res 67: 64–77, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Birukova AA, Smurova K, Birukov KG, Usatyuk P, Liu F, Kaibuchi K, Ricks-Cord A, Natarajan V, Alieva I, Garcia JG, Verin AD. Microtubule disassembly induces cytoskeletal remodeling and lung vascular barrier dysfunction: role of Rho-dependent mechanisms. J Cell Physiol 201: 55–70, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Birukova AA, Zagranichnaya T, Fu P, Alekseeva E, Chen W, Jacobson JR, Birukov KG. Prostaglandins PGE(2) and PGI(2) promote endothelial barrier enhancement via PKA- and Epac1/Rap1-dependent Rac activation. Exp Cell Res 313: 2504–2520, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buck J, Sinclair ML, Schapal L, Cann MJ, Levin LR. Cytosolic adenylyl cyclase defines a unique signaling molecule in mammals. Proc Natl Acad Sci USA 96: 79–84, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carson MR, Shasby SS, Shasby DM. Histamine and inositol phosphate accumulation in endothelium: cAMP and a G protein. Am J Physiol Lung Cell Mol Physiol 257: L259–L264, 1989. [DOI] [PubMed] [Google Scholar]
- 9.Cattan CE, Oberg KC. Vinorelbine tartrate-induced pulmonary edema confirmed on rechallenge. Pharmacotherapy 19: 992–994, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, Buck J. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science 289: 625–628, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Cioffi DL, Moore TM, Schaack J, Creighton JR, Cooper DM, Stevens T. Dominant regulation of interendothelial cell gap formation by calcium-inhibited type 6 adenylyl cyclase. J Cell Biol 157: 1267–1278, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Comerford KM, Lawrence DW, Synnestvedt K, Levi BP, Colgan SP. Role of vasodilator-stimulated phosphoprotein in PKA-induced changes in endothelial junctional permeability. FASEB J 16: 583–585, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Conacci-Sorrell M, Zhurinsky J, and Ben-Ze'ev A. The cadherin-catenin adhesion system in signaling and cancer. J Clin Invest 109: 987–991, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Creighton J, Zhu B, Alexeyev M, Stevens T. Spectrin-anchored phosphodiesterase 4D4 restricts cAMP from disrupting microtubules and inducing endothelial cell gap formation. J Cell Sci 121: 110–119, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Dejana E Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol 5: 261–270, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Desai A, Mitchison TJ. Microtubule polymerization dynamics. Annu Rev Cell Dev Biol 13: 83–117, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Dessauer CW, Gilman AG. Purification and characterization of a soluble form of mammalian adenylyl cyclase. J Biol Chem 271: 16967–16974, 1996. [DOI] [PubMed] [Google Scholar]
- 18.Diviani D, Scott JD. AKAP signaling complexes at the cytoskeleton. J Cell Sci 114: 1431–1437, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol 91: 1487–1500, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Essler M, Staddon JM, Weber PC, Aepfelbacher M. Cyclic AMP blocks bacterial lipopolysaccharide-induced myosin light chain phosphorylation in endothelial cells through inhibition of Rho/Rho kinase signaling. J Immunol 164: 6543–6549, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Farrukh IS, Gurtner GH, Michael JR. Pharmacological modification of pulmonary vascular injury: possible role of cAMP. J Appl Physiol 62: 47–54, 1987. [DOI] [PubMed] [Google Scholar]
- 22.Fukuhara S, Sakurai A, Sano H, Yamagishi A, Somekawa S, Takakura N, Saito Y, Kangawa K, Mochizuki N. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol Cell Biol 25: 136–146, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia JG, Davis HW, Patterson CE. Regulation of endothelial cell gap formation and barrier dysfunction: role of myosin light chain phosphorylation. J Cell Physiol 163: 510–522, 1995. [DOI] [PubMed] [Google Scholar]
- 24.Goeckeler ZM, Wysolmerski RB. Myosin phosphatase and cofilin mediate cAMP/cAMP-dependent protein kinase-induced decline in endothelial cell isometric tension and myosin II regulatory light chain phosphorylation. J Biol Chem 280: 33083–33095, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Goode BL, Drubin DG, Barnes G. Functional cooperation between the microtubule and actin cytoskeletons. Curr Opin Cell Biol 12: 63–71, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Howell B, Deacon H, Cassimeris L. Decreasing oncoprotein 18/stathmin levels reduces microtubule catastrophes and increases microtubule polymer in vivo. J Cell Sci 112: 3713–3722, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Klymkowsky MW Weaving a tangled web: the interconnected cytoskeleton. Nat Cell Biol 1: E121–E123, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Lampugnani MG, Zanetti A, Breviario F, Balconi G, Orsenigo F, Corada M, Spagnuolo R, Betson M, Braga V, Dejana E. VE-cadherin regulates endothelial actin activating Rac and increasing membrane association of Tiam. Mol Biol Cell 13: 1175–1189, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee TY, Gotlieb AI. Microfilaments and microtubules maintain endothelial integrity. Microsc Res Tech 60: 115–127, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Liu F, Verin AD, Borbiev T, Garcia JG. Role of cAMP-dependent protein kinase A activity in endothelial cell cytoskeleton rearrangement. Am J Physiol Lung Cell Mol Physiol 280: L1309–L1317, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Moore TM, Chetham PM, Kelly JJ, Stevens T. Signal transduction and regulation of lung endothelial cell permeability. Interaction between calcium and cAMP. Am J Physiol Lung Cell Mol Physiol 275: L203–L222, 1998. [DOI] [PubMed] [Google Scholar]
- 32.Parker JC, Stevens T, Randall J, Weber DS, King JA. Hydraulic conductance of pulmonary microvascular and macrovascular endothelial cell monolayers. Am J Physiol Lung Cell Mol Physiol 291: L30–L37, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Patterson CE, Lum H, Schaphorst KL, Verin AD, Garcia JG. Regulation of endothelial barrier function by the cAMP-dependent protein kinase. Endothelium 7: 287–308, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Phillips PG Thrombin-induced alterations in endothelial cell cytoarchitectural and functional properties. Semin Thromb Hemost 20: 417–425, 1994. [DOI] [PubMed] [Google Scholar]
- 35.Phillips PG, Lum H, Malik AB, Tsan MF. Phallacidin prevents thrombin-induced increases in endothelial permeability to albumin. Am J Physiol Cell Physiol 257: C562–C567, 1989. [DOI] [PubMed] [Google Scholar]
- 36.Rich TC, Fagan KA, Nakata H, Schaack J, Cooper DM, Karpen JW. Cyclic nucleotide-gated channels colocalize with adenylyl cyclase in regions of restricted cAMP diffusion. J Gen Physiol 116: 147–161, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodionov V, Yi J, Kashina A, Oladipo A, Gross SP. Switching between microtubule- and actin-based transport systems in melanophores is controlled by cAMP levels. Curr Biol 13: 1837–1847, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Sato T, Sato-Harada R, Takano M, Kato S, Saburi S, Harada A. Localization of cAMP-dependent protein kinase in the actin and microtubule cytoskeletons in mouse hippocampal neurons. Neurosci Lett 325: 83–86, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Sayner SL, Alexeyev M, Dessauer CW, Stevens T. Soluble adenylyl cyclase reveals the significance of cAMP compartmentation on pulmonary microvascular endothelial cell barrier. Circ Res 98: 675–681, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Sayner SL, Frank DW, King J, Chen H, VandeWaa J, Stevens T. Paradoxical cAMP-induced lung endothelial hyperpermeability revealed by Pseudomonas aeruginosa ExoY. Circ Res 95: 196–203, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Skold HN, Norstrom E, Wallin M. Regulatory control of both microtubule- and actin-dependent fish melanosome movement. Pigment Cell Res 15: 357–366, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Small JV, Kaverina I. Microtubules meet substrate adhesions to arrange cell polarity. Curr Opin Cell Biol 15: 40–47, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Stelzner TJ, Weil JV, O'Brien RF. Role of cyclic adenosine monophosphate in the induction of endothelial barrier properties. J Cell Physiol 139: 157–166, 1989. [DOI] [PubMed] [Google Scholar]
- 44.Stevens T, Creighton J, Thompson WJ. Control of cAMP in lung endothelial cell phenotypes. Implications for control of barrier function. Am J Physiol Lung Cell Mol Physiol 277: L119–L126, 1999. [DOI] [PubMed] [Google Scholar]
- 45.Stevens T, Nakahashi Y, Cornfield DN, McMurtry IF, Cooper DM, Rodman DM. Ca(2+)-inhibitable adenylyl cyclase modulates pulmonary artery endothelial cell cAMP content and barrier function. Proc Natl Acad Sci USA 92: 2696–2700, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suzuki S, Bing H, Sugawara T, Matsuda Y, Tabata T, Hoshikawa Y, Saijo Y, Kondo T. Paclitaxel prevents loss of pulmonary endothelial barrier integrity during cold preservation. Transplantation 78: 524–529, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Troyer DA, Bouton A, Bedolla R, Padilla R. Tyrosine phosphorylation of focal adhesion kinase (p125FAK): regulation by cAMP and thrombin in mesangial cells. J Am Soc Nephrol 7: 415–423, 1996. [DOI] [PubMed] [Google Scholar]
- 48.van Nieuw Amerongen GP, Draijer R, Vermeer MA, van Hinsbergh VW. Transient and prolonged increase in endothelial permeability induced by histamine and thrombin: role of protein kinases, calcium, and RhoA. Circ Res 83: 1115–1123, 1998. [DOI] [PubMed] [Google Scholar]
- 49.van Nieuw Amerongen GP, van Delft S, Vermeer MA, Collard JG, van Hinsbergh VW. Activation of RhoA by thrombin in endothelial hyperpermeability: role of Rho kinase and protein tyrosine kinases. Circ Res 87: 335–340, 2000. [DOI] [PubMed] [Google Scholar]
- 50.Verin AD, Birukova A, Wang P, Liu F, Becker P, Birukov K, Garcia JG. Microtubule disassembly increases endothelial cell barrier dysfunction: role of MLC phosphorylation. Am J Physiol Lung Cell Mol Physiol 281: L565–L574, 2001. [DOI] [PubMed] [Google Scholar]
- 51.Willoughby D, Wong W, Schaack J, Scott JD, Cooper DM. An anchored PKA and PDE4 complex regulates subplasmalemmal cAMP dynamics. EMBO J 25: 2051–2061, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yahr TL, Vallis AJ, Hancock MK, Barbieri JT, Frank DW. ExoY, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III system. Proc Natl Acad Sci USA 95: 13899–13904, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zippin JH, Chen Y, Nahirney P, Kamenetsky M, Wuttke MS, Fischman DA, Levin LR, Buck J. Compartmentalization of bicarbonate-sensitive adenylyl cyclase in distinct signaling microdomains. FASEB J 17: 82–84, 2003. [DOI] [PubMed] [Google Scholar]