Abstract
In many organs, integrins and cadherins are partly regulated by Hox genes, but their interactions in airway morphogenesis and congenital lung diseases are unknown. We previously showed that the Hox protein HoxB5 is abnormally increased in bronchopulmonary sequestration (BPS) and congenital cystic adenomatoid malformation (CCAM), congenital lung lesions with abnormal airway branching. We now report on α2-, α3-, and β1-integrin and E-cadherin expression in normal human lung and in BPS and CCAM tissue previously shown to have abnormal HoxB5 expression and on the relationship of cell adhesion molecule expression to Hoxb5 regulation. α2-, α3-, and β1-integrins and E-cadherin expression in normal human lung and BPS and CCAM were evaluated using Western blot and immunohistochemistry. Fetal mouse lung fibroblasts with Hoxb5-specific siRNA downregulation were evaluated for α2-integrin protein levels by Western blot. Compared with normal human lung, a previously undetected α2-integrin isoform potentially lacking essential cytoplasmic sequences was significantly increased in BPS and CCAM, and α2-integrin spatial and cellular expression was more intense. E-cadherin protein levels were also significantly increased, whereas α3 increased in CCAM compared with canalicular, but not with alveolar, stage lung. β1-integrin levels were unchanged. We conclude that in BPS and CCAM, altered α2-integrin cytoplasmic signaling contributes to abnormal cellular behavior in these lung lesions. Aberrant cell adhesion molecule and Hox protein regulation are likely part of the mechanism involved in the development of BPS and CCAM.
Keywords: α2-integrin, α3-integrin, E-cadherin, Hox proteins
integrins are heterodimeric transmembrane receptor proteins consisting of one α- and one β-subunit that bind to ECM proteins and other cells to mediate “outside-in” and “inside-out” signaling between cells and the cellular environment. More than 14 α- and 8 β-subunits have been described with ligand specificity depending on the specific α/β receptor complex and the tissue type. These integrin receptors affect cell-cell signaling through interactions with other integrins and with one or more specific ligands, including but not limited to laminins, fibronectin, tenascin-C, vitronectin, entactin, and collagen (19, 27). Integrins also influence the activity of focal adhesion kinase (FAK), other membrane-bound cell adhesion molecules such as E-cadherin, and growth factor receptors including epidermal growth factor (EGFR), fibroblast growth factor (FGFR), vascular endothelial growth factor, and transforming growth factor-β. Through these many interactions and through interactions with the actin cytoskeleton, integrins help control cell migration, cellular orientation, and differentiation, as well as cell growth and cell survival (5, 7, 8, 19, 29, 33, 52, 65).
In the lung, like in many other organs, integrins help regulate organ morphogenesis during development and later in life contribute to tissue homeostasis (53). Integrins are located both in lung mesodermal and epithelial cells where distinct temporal, cellular, and regional expression domains of different integrins are poised to influence airway branching and lung cell fate. Several integrins have distinct patterns of expression during lung development suggesting unique roles for different integrin receptor complexes at different times in development and in different regions within the developing lung (16, 67). β1-integrin is the preferred partner for α2- and α3-integrin in lung with specificity being determined by different cell types in which the integrins are expressed (33). α2β1-integrin is localized to mesodermal and epithelial cells of branching airway tips during mouse and human lung development and is also expressed in cells involved in remodeling of extracellular matrix that are entering the later stages of cell differentiation (17, 66). Mice deficient in α2-integrin have abnormal and simplified mammary gland branching morphogenesis with decreased numbers of branch points per terminal duct, whereas detailed developmental evaluation of airway branching in these mice has not been reported (11). α3-integrin, however, is only expressed in established airway branches but not at airway branch points. α3-integrin has a vital role in lung morphogenesis with α3-deficient mice having severely retarded airway branching beyond the mainstem bronchi with the epithelium resembling proximal bronchial epithelium (columnar cells) instead of cuboidal epithelium usually found in terminal bronchioles (20, 36).
Integrins interact with and influence the activity of other membrane-bound cell adhesion molecules including E-cadherin (69). Similar to α3-integrin, E-cadherin is expressed in airway epithelial cells but not at airway branching tips, consistent with E-cadherin's roles in adherens junctions to stabilize cell-cell contacts and in establishing and maintaining apico-basal polarity of epithelial cells. E-cadherin, like integrins, interact through their cytoplasmic effector proteins, catenins, and with actin filaments influencing cell motility. Furthermore, the extracellular domains of some cadherins also interact with FGFRs resulting in E-cadherin receptor cleavage and altered cell adhesion. Consistent with this finding, decreased E-cadherin expression leads to loss of cell-cell adhesion and increased cell migration, an abnormality that has been associated with cancer progression and cell metastasis (24, 25, 48). In vitro blockade of E-cadherin function in embryonic mouse lung organ cultures alters epithelial organization and lumen formation of new airway branches, whereas increased E-cadherin expression prevents in vitro budding of lung endoderm (25, 40).
It is clear from these studies and others that normal lung branching morphogenesis requires precise spatial and temporal distribution of cell adhesion receptors such as integrins and E-cadherin to help coordinate intracellular and intercellular changes in communication between lung mesenchymal and epithelial cells (12, 17, 19, 24, 25). Branching morphogenesis is altered in the congenital lung lesions bronchopulmonary sequestration (BPS) and congenital cystic adenomatoid malformation (CCAM). These lung lesions cause significant morbidity and mortality in infants due to associated respiratory distress, lung hypoplasia, fetal hydrops, and pulmonary infections. BPS is caused by aberrant formation of an accessory lung diverticulum that has budded from the primitive foregut in the embryonic period of lung development (5–6 wk of human gestation), resulting in either an intralobar or extralobar anatomical position. CCAMs are “adenomatous” overgrowths of terminal bronchioles that develop into cyst-like structures of varying sizes and airway pathology that develop during the first 5–10 wk of human development and are classified as type 0 to IV based on tissue histology, airway morphology, and cyst size (2, 21, 49, 54, 55). Many reports show that the histology and microscopic findings of BPS and CCAM often coexist within the same lesions suggesting related embryologic origins for these congenital airway anomalies (3, 9, 15, 21, 35, 41). Features that are similar in both anomalies suggest that their aberrant airway development results from an imbalance of proper mesenchymal-epithelial interactions in early lung morphogenesis at a time when dynamic and orchestrated changes in cell adhesion are known to alter cell migration and cell proliferation to coordinate airway branching. However, the potential contribution of altered cell adhesion in the development of BPS and CCAM has not been studied.
Other studies have shown that integrins and E-cadherin are in part regulated by the master regulatory Hox gene transcription factor family. Interestingly, some integrin genes are localized in clusters in close genetic vicinity with Hox genes on chromosomes 2, 12, and 17 in humans. This suggests that Hox genes that help determine embryonic and organ-specific patterning evolved along with integrins that help recognize cell-cell and cell-matrix interactions coordinating cell positioning and cellular communication in developing and mature organs and tissues (13, 28, 63). In mice, we have reported that regulated spatial and temporal expression of the Hox protein, Hoxb5, helps regulate airway branching patterns partially through regulation of cell matrix molecules (57, 60, 61). We have also shown that HoxB5 (nomenclature for human Hoxb5) is expressed in human lung development in a similar pattern to that seen in mouse and that human BPS and CCAM tissue has abnormally high levels of HoxB5 protein, analogous to the canalicular stage of lung development and significantly increased over age and lung development stage-matched controls (59, 64). The persistent overexpression of HoxB5 in BPS and CCAM tissues may have deleterious effects on downstream genes governing mesenchymal-epithelial cell adhesion and cellular interactions, but whether Hox gene regulation and altered cell adhesion coexist in BPS and CCAM has not been studied.
The objectives of the current study were 1) to investigate integrin α2, α3, their receptor partner β1, and E-cadherin expression in normal human lung development and in BPS and CCAM, where we have previously identified abnormal HoxB5 expression patterns, and 2) to study the relationship of cell adhesion molecule expression to HoxB5 regulation. We hypothesized that integrin and E-cadherin expression would be altered in BPS and CCAM tissue that we have previously shown to have increased levels of HoxB5 protein (59).
MATERIALS AND METHODS
Reagents.
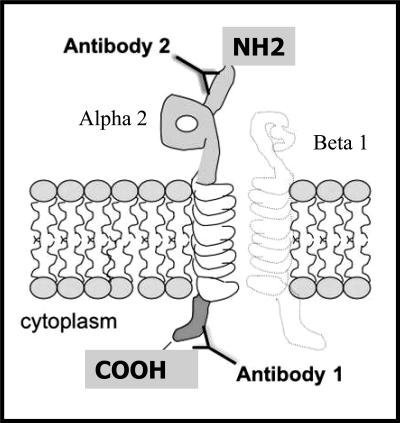
Two different antibodies were used for detection of α2-integrin in normal human lung, BPS, and CCAM tissue (Fig. 1). Antibody 1 (Chemicon, Temecula, CA) is a rabbit polyclonal antibody that detects essential peptide sequences in the cytoplasmic domain of human and mouse α2-integrin including two important sites necessary for negative regulation of FAK (31, 34, 38). Antibody 2 (Santa Cruz Biotechnology, Santa Cruz, CA) is a goat polyclonal antibody that only detects peptide sequences in the extracellular domain of human α2-integrin but does not cross-react with α2-integrin of mouse origin. Goat polyclonal antibodies to α3-integrin and E-cadherin and rabbit polyclonal antibody to β1-integrin were purchased from Santa Cruz Biotechnology. Mouse monoclonal antibody to GAPDH was purchased from Ambion (Austin, TX). Western blot reagents were from BioRad (Hercules, CA). Immunostaining reagents were from Vector (Burlingame, CA). siRNA molecules were purchased from Ambion as previously described (60). All other reagents were purchased from Fisher (Pittsburgh, PA) unless otherwise specified.
Fig. 1.
Schematic of primary antibody binding sites to α2-integrin transmembrane protein. Antibody 1 (Chemicon, Temecula, CA) detects essential regulatory sequences in the cytoplasmic domain of α2-integrin of mouse and human origin. Antibody 2 (Santa Cruz Biotechnology, Santa Cruz, CA) detects sequences in the extracellular domain of α2-integrin of human origin.
Human lung, BPS, and CCAM tissue specimens.
The protocol for this study was approved by the Tufts Medical Center Human Investigation Review Committee. As previously described, normal human lung tissue from 17 wk of gestation to 1.5 years of age was obtained from therapeutic abortuses and autopsies for non-pulmonary disease. BPS and CCAM tissue from children up to 3 years of age was obtained at surgical resection. CCAM tissue specimens used in this study were pathologically defined by our institution's Department of Pathology as type II CCAM (1, 54).Tissue from each specimen was separated for Western blot and immunostaining studies as previously described (59).
Mouse lung fibroblasts cultures.
To compliment the studies in human tissue, we evaluated α2-integrin expression in E13.5 (E0.5 identification of vaginal plug, term is 19 days) mouse lung fibroblast cultures in which we have previously reported Hoxb5 downregulation by Hoxb5-specific siRNA molecules (60). The animal protocols were approved by Tufts University School of Medicine IACUC. As we previously described, timed dated pregnant mice were killed at E13.5. Fetal mouse lungs were isolated, and fetal mouse lung fibroblasts were cultured with no treatment, TKO transfection vehicle (Mirus, Madison, WI), scrambled siRNA control, or Hoxb5-specific siRNA. This siRNA protocol and the sequences for Hoxb5 siRNA and scrambled siRNA control have been published (60). After 72 h of treatment, mouse fetal lung fibroblast cultures were harvested and prepared for Western blot analysis as described below. Using the same protein aliquots that previously showed decreased Hoxb5 protein levels, α2-integrin Western blots were performed using the rabbit anti-human/anti-mouse antibody that detects important cytoplasmic regulatory regions in the α2-integrin protein of human and mouse origin (Chemicon, Temecula, CA) (34) Therefore, with this antibody, we can only detect the 150-kDa isoform and cannot determine the presence or absence of the 130-kDa isoform in these samples. This antibody that reacts against the extracellular domain of α2-integrin does not cross-react with mouse tissue. α2-integrin protein levels in mouse fetal lung fibroblasts with Hoxb5-specific siRNA downregulation of Hoxb5 protein were compared with α2-integrin levels in scramble siRNA-treated cells as previously described (60).
Western blot analysis.
Normal lung, BPS, and CCAM tissue samples in which we have previously studied HoxB5 protein expression were used for this study (59). Western blot analysis using methodology we have previously described was used to identify temporal and quantitative changes in α2-, α3-, and β1-integrins and E-cadherin protein levels in human lung tissue from the canalicular period (17- to 22-wk gestation, n = 7) and the alveolar period (term gestation to 1.5 years, n = 3) and in BPS (1–3 yr of age, n = 4) and CCAM tissue (1–3 yr of age, n = 7) (58). Briefly, proteins were first homogenized in the presence of proteinase inhibitors with protein concentration determined by the Lowry method. Twenty micrograms of total lung, BPS, or CCAM protein were loaded onto a 7.5% polyacrylamide gel, and proteins were separated by SDS-PAGE. After SDS-PAGE, proteins were transferred to nitrocellulose membranes by wet transfer. Individual membranes from the same protein samples were probed for each of the cell adhesion molecules under study. The top of each membrane was used to evaluate α2-integrin, α3-integrin, β1-integrin, or E-cadherin with the bottom portion of each membrane used for GAPDH protein determination as the internal control. Normal human lung, including canalicular and alveolar stage lung tissue, was loaded on each blot along with the BPS and CCAM tissue samples to allow comparisons of cell adhesion protein levels between normal canalicular and normal alveolar stage lung and BPS and CCAM tissue. BPS and CCAM tissues were obtained from patients whose lung development by their chronological age would correspond to the alveolar stage of lung development. Therefore, we focused our comparisons mainly on the relationship of BPS and CCAM tissue findings to normal human lung tissue findings from patients whose normal lungs would also be in the alveolar stage of lung development. This was considered the best possible comparison as we cannot obtain isolated normal lung tissue from the patients with BPS and CCAM. These comparisons were also made to help us determine if BPS and CCAM tissues with aberrant airway branching show integrin and E-cadherin expression that is different from the stage of lung development of the person from which the lung lesion was removed and more similar to earlier stages of fetal lung development, suggesting that in these congenital lung lesions, there is an arrest of lung development and lung cellular regulation at an earlier stage of development. This same comparison was also used in our previous study (58). The nitrocellulose membranes were blocked with appropriate blocking solutions (5% blotto or 5% donkey serum) and then subjected to sequential reactions with primary antibody, HRP-labeled secondary antibody followed by chemiluminescence detection of the protein-antibody reactions (Perkin Elmer, Waltham, MA). Quantitative changes in the levels of each of the cell adhesion proteins at each gestational age and in the BPS and CCAM tissues were determined by densitometry with normalization to GAPDH as internal control. Specificity of each antibody was confirmed by absence of specific protein bands in absence of primary antibody.
Immunohistochemistry.
Sequential lung cryosections (6 μm) from normal human lung and BPS and CCAM tissue in which we have previously seen abnormal HoxB5 protein expression were incubated overnight (4°C) with either α2 antibody 1 (1/800), α2 antibody 2 (1/200), or α3 (1/400) primary antibodies, followed by room temperature incubation with corresponding secondary antibody (1/200), avidin-biotin complex conjugated to alkaline phosphatase or horseradish peroxidase. Blue alkaline phosphatase chromagen plus levamisole (endogenous alkaline phosphatase blocker) was used for alkaline phosphatase detection followed by Fast Red counterstaining. Brown diaminobenzadine (DAB) or purple VIP was used to detect HRP followed by methyl green counterstaining. Lung sections from normal human lung and BPS and CCAM were incubated for the same period of time in the alkaline phosphatase or HRP reaction solutions. Colabeling of HoxB5 and some of the cell adhesion molecules was performed as we have previously described with modifications as appropriate for the antibodies being used in these reactions (58). Each experiment (n = 3 for each antibody) included controls of adjacent sections with omission of respective primary antibody.
Data analysis.
Computer densitometry (Alpha Innotech, San Leandro, CA) results of individual blots for α2-, α3-, β1-integrins, and E-cadherin with normalization to corresponding GAPDH internal control were statistically analyzed for comparison of levels of these proteins in BPS and CCAM to that seen in canalicular and alveolar stage lung tissue on each Western blot by nonparametric ANOVA or t-test with Welch correction where appropriate (Instat; Graphpad Software, San Diego, CA). For fetal mouse lung fibroblasts, Western blot α2-integrin protein levels were also normalized to GAPDH values as an internal control with statistical analysis (t-test with Welch correction) performed between Hoxb5-siRNA and scramble-siRNA control-treated mouse fetal lung fibroblasts loaded on each blot. The level of significance was defined at P < 0.05. For immunostaining, lung sections from normal human lung and BPS and CCAM tissue were visually assessed and compared by light microscopy.
RESULTS
Altered α2 protein isoforms and protein levels exist in BPS and CCAM tissue.
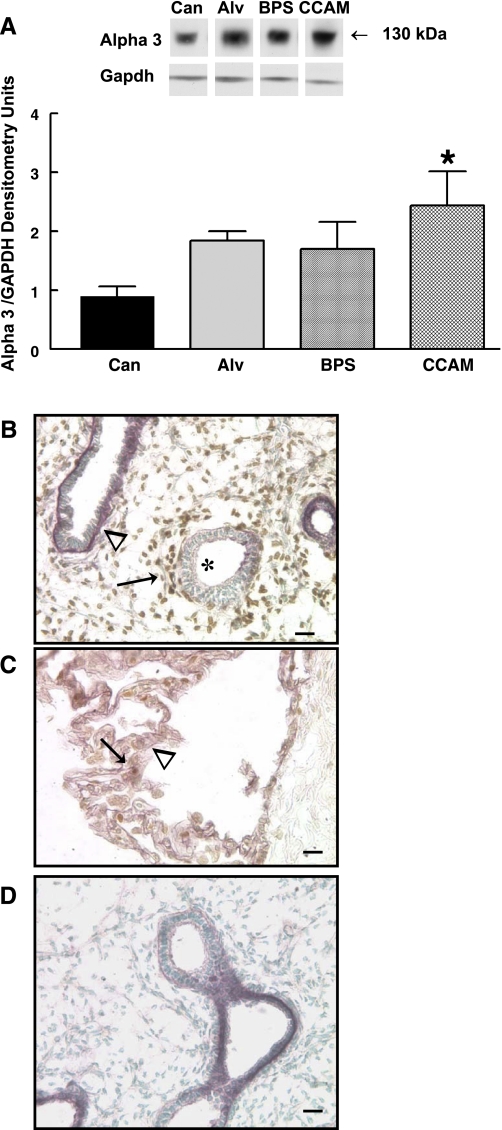
Western blot analysis was used to determine if protein levels of α2-integrin, a key cell adhesion molecule expressed during normal airway branching morphogenesis, would be altered in BPS and CCAM. Using antibody 1 (binds to peptide sequences in cytoplasmic region of α2-integrin) (Fig. 1) (37), no differences were detected between the 150-kDa protein isoform of α2-integrin protein in BPS or CCAM tissue compared with canalicular or alveolar stage normal human lung tissue (Fig. 2A). However, when these same tissue samples were evaluated using antibody 2 (Fig. 1) that binds peptide sequences in the extracellular domain of α2-integrin, an additional protein isoform of 130 kDa was detected and significantly increased by at least sixfold in BPS and CCAM compared with alveolar period normal lung tissue (lung development stage matched to BPS and CCAM tissue) and compared with canalicular period normal lung tissue. The levels of the 150-kDa isoform remained relatively unchanged (Fig. 2B). To confirm the identity of the 130- and 150-kDa isoforms detected with antibody 2, we performed Western blots on a subset of samples using an aliquot of antibody 2 prereacted with the peptide sequence used to produce antibody 2 (blocking peptide) (Fig. 2C). Using antibody 2 alone, Western blots reproduced the 150- and 130-kDa bands. However, when Western blot analysis was performed using aliquots of antibody 2 prereacted with its blocking peptide, the 150- and 130-kDa bands were not detected, confirming that both these bands as seen in BPS and CCAM specimens were specific for α2-integrin protein extracellular domains detected by this antibody.
Fig. 2.
α2-integrin representative Western blots and summary densitometry. A: Western blots performed with antibody 1 (detects cytoplasmic sequences of α2-integrin) did not demonstrate any difference in protein levels of the 150-kDa protein isoform of α2-integrin between normal human lung from the canalicular (Can) and alveolar (Alv) stage of lung development and bronchopulmonary sequestration (BPS) and congenital cystic adenomatoid malformation (CCAM) tissue. B: Western blots using antibody 2 (detects extracellular sequences of α2-integrin) detected a significantly upregulated 130-kDa protein isoform of α2-integrin in BPS and CCAM compared with normal alveolar or canalicular stage lung tissue, but there were no differences in the 150-kDa α2-integrin isoform (*P = 0.005, means ± SE; BPS and CCAM vs. Alv or Can). A and B: n = 6, Can; n = 3, Alv; n = 4, BPS; n = 7, CCAM. C: representative Western blot of one CCAM sample in absence (−) or presence (+) of blocking peptide for antibody 2 showed that when antibody 2 activity was blocked with α2-specific blocking peptide against antibody 2, neither the 150- nor the 130-kDa protein isoform was detected. D: mouse fetal lung fibroblasts treated with Hoxb5-specific siRNA to downregulate Hoxb5 protein had significantly decreased α2-integrin protein levels compared with scramble-treated cells or control (no treatment) (*P = 0.04, means ± SE, n = 5).
α2-integrin protein levels are decreased in fetal mouse lung fibroblasts with in vitro downregulation of Hoxb5 protein.
We evaluated α2-integrin protein levels in mouse fetal lung fibroblasts in which we have previously reported siRNA-specific downregulation of Hoxb5 protein. This was done to further understand the potential relationship between altered α2-integrin protein expression in BPS and CCAM and the aberrant expression of HoxB5 protein that we have previously reported in these same tissue samples (59, 60). Compared with mouse fetal lung fibroblasts treated with scramble siRNA (50 nM) (negative control), the 150-kDa protein isoform of α2-integrin was significantly decreased in fetal mouse lung fibroblasts treated with Hoxb5 siRNA (50 nM) in association with the previously described decrease in Hoxb5 protein levels (Fig. 2D) (60).
BPS and CCAM tissue has more intense α2-integrin immunostaining.
With the same α2-integrin antibodies used in Western blot analysis, we detected interesting differences in the pattern of α2-integrin immunohistochemistry in consecutive tissue sections from BPS and CCAM specimens. Using antibody 1 (Fig. 3, A–C), cytoplasmic localization and intensity of α2-integrin protein around CCAM cysts (Fig. 3, A and B) and in adjacent compressed lung tissue (Fig. 3, A and C) appeared similar. However, adjacent tissue sections from this same CCAM tissue sample immunostained simultaneously using antibody 2 (detects extracellular domain of α2 and 130-kDa protein isoform on Western blots) showed that the intensity of α2-integrin immunostaining was greater in cells in and around the CCAM cysts (Fig. 3, D–F) than in adjacent compressed lung tissue. BPS tissue immunostained using antibody 2 (Fig. 3G) also showed intense purple HRP staining for α2-integrin in epithelial and mesenchymal cells that was more intense than that seen in canalicular stage human lung (17-wk human fetus) (Fig. 3G). Compared with CCAM tissue, canalicular stage (17-wk gestation human fetus) (Fig. 3H) immunostained with α2-integrin (using antibody 2, extracellular domain) had similar epithelial α2-integrin cellular localization but less intense mesenchymal staining. These immunostaining results along with our Western blot results suggest that the increased intensity of staining seen in CCAM cysts and BPS tissue may be secondary to the localization of the 130-kDa α2-integrin protein isoform detected in BPS and CCAM. Colabeling of canalicular stage human fetal lung for HoxB5 protein and α2-integrin (using antibody 2) (Fig. 3H) showed intense brown nuclear staining for HoxB5 protein in mesenchymal cells that were also positive for α2-integrin and immediately adjacent to purple α2-integrin positive epithelial cells at branching airway tips. Specificity of the individual antibody reactions was confirmed by absence of specific staining in absence of primary antibody, as shown in Fig. 3I, where absence of primary antibody for α2-integrin demonstrates only the presence of brown mesenchymal nuclear staining for HoxB5 and no α2-integrin purple staining.
Fig. 3.
α2-integrin immunostaining in CCAM and BPS. A–F: representative CCAM tissue sections stained for α2-integrin with blue alkaline phosphatase immunohistochemistry and nuclear Fast Red counterstain. G–I: α2-integrin stained with purple VIP-HRP immunolocalization and Hoxb5 protein (H, I) immunolocalized with brown DAB-HRP immunolocalization and counterstained with methyl green. Bar = 100 μm in D, 50 μm in A and E, and 25 μm in B, C, F, G, H, and I. A: α2-integrin antibody 1, immunolocalized α2-integrin protein to mesenchymal and epithelial cells of CCAM cysts (* in A and B) and adjacent compressed lung (arrow in A and C) with similar intensity. D, E, F: in contrast, α2-integrin antibody 2 immunostaining of adjacent section of CCAM shown in A–C revealed different regional localization of α2-integrin. Compared with adjacent compressed lung regions (# in D, E, F), increased α2-integrin staining in mesenchyme and epithelial cells was seen around CCAM cyst (* in D, E, F). Using antibody 2, BPS tissue (G) showed intense purple epithelial (arrow in G) and mesenchymal localization (* in G) of α2-integrin compared with human fetal lung (17-wk gestation) (H), which had strong but less intense purple α2-integrin localization in epithelial cells of branching airway tips (long arrows in H). These α2-positive purple epithelial cells were surrounded by closely adjacent subepithelial fibroblasts with intense brown nuclear staining for HoxB5 protein (arrowheads in H). Regions of airways with less purple α2-integrin staining (< in H) are not surrounded by closely adjacent HoxB5-positive brown cells. α2-integrin was also localized diffusely in mesenchyme, especially in fibroblasts with brown nuclear Hoxb5 staining (short arrow in H). In absence of α2-integrin antibody, there is no purple α2-integrin immunostaining (I) but continued Hoxb5 brown nuclear staining in presence of Hoxb5 antibody.
α3-integrin protein levels are dysregulated in CCAM but not in BPS tissues.
Whereas both BPS and CCAM tissue demonstrated altered α2-integrin expression, Western blot of α3-integrin in these same tissue samples showed that CCAM but not BPS had significantly increased levels of α3-integrin protein compared with canalicular stage lung tissue, but neither CCAM nor BPS tissue had altered α3-integrin compared with alveolar stage lung tissue (Fig. 4A). Canalicular stage (17-wk human fetus) lung tissue (Fig. 4B) colabeled for HoxB5 and α3-integrin showed that purple α3-positive cells are not regionally associated with closely adjacent brown nuclear-stained HoxB5-positive cells. Rather, branching airway tips that had weak α3-integrin staining had closely adjacent brown HoxB5-positive cells. In CCAM (Fig. 4C), α3-integrin purple staining was more intense than in canalicular stage normal human lung and sometimes seen in cells that were also positive for HoxB5 protein (brown nuclei). The specificity of HoxB5 immunostaining was confirmed by absence of HoxB5 brown nuclear staining in absence of HoxB5 primary antibody with detection of only purple α3-integrin staining with presence of α3-integrin antibody (Fig. 4D).
Fig. 4.
α3-integrin Western blots and immunohistochemistry. A: representative α3-integrin Western blot and summary densitometry showed similar levels of α3-integrin in BPS and CCAM tissue compared with alveolar stage lung tissue. CCAM tissue, however, had significantly increased α3-integrin protein levels compared with canalicular period lung tissue samples (*P = 0.03, CCAM vs. Can, means ± SE; n = 6, Can; n = 3, Alv; n = 4, BPS; n = 7, CCAM). B: human fetal lung tissue (17-wk human gestation) showed α3-integrin purple VIP staining at basal surface of columnar epithelial cells that had less intense subepithelial brown nuclear staining for Hoxb5 protein in mesenchymal cells (arrowhead). In contrast, airways (*) with less intense purple epithelial α2-integrin staining had more intense brown Hoxb5 staining in adjacent subepithelial fibroblasts (arrow). α3-integrin (purple) was also seen diffusely in mesenchyme but to a lesser degree than in airway epithelial cells and subepithelial fibroblasts. C: CCAM tissue had diffuse purple staining for α3-integrin (arrowhead) and scattered brown nuclear staining for Hoxb5 protein (arrow). D: immunostaining control showed that in absence of Hoxb5 primary antibody, no brown DAB staining was seen with only purple VIP staining for α3-integrin in presence of α3-integrin primary antibody.
β1-integrin protein levels are not altered in BPS and CCAM.
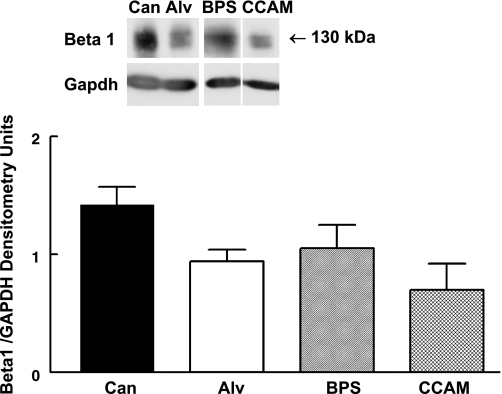
β1-integrin is the major partner for α2- and α3-integrin in lung with formation of activated α2β1- or α3β1-integrin receptors leading to changes in inside-out and outside-in integrin signaling partly through recruitment of signaling molecules to sites in the cytoplasmic tail of β1-integrin (16, 17, 53). Although we detected differential changes in α2- and α3-integrin in BPS and CCAM, no differences in β1-integrin were detected between canalicular and alveolar stage normal human lung tissue and BPS and CCAM tissue samples (Fig. 5).
Fig. 5.
β1-integrin representative Western blot and summary densitometry. β1-integrin protein levels were not altered in BPS and CCAM compared with alveolar or canalicular stage human lung tissue. N = 6, Can; n = 3, Alv; n = 4, BPS; n = 7, CCAM.
E-cadherin protein levels are increased in CCAM.
We evaluated E-cadherin protein levels in these same tissue samples to determine if abnormal E-cadherin regulation was associated with abnormal airway branching and altered α2-integrin expression patterns in BPS and CCAM. Compared with alveolar stage normal human lung tissue, E-cadherin protein levels were significantly upregulated 20-fold in CCAM but only 10-fold in BPS. No significant difference was seen between BPS and CCAM tissue compared with canalicular stage human fetal lung tissue (Fig. 6).
Fig. 6.
E-cadherin representative Western Blot and summary densitometry. Compared with alveolar stage human lung, E-cadherin protein levels were increased significantly in CCAM and showed a trend towards increased levels in BPS. Levels of E-cadherin in CCAM and BPS were more similar to canalicular stage lung tissue. *P = 0.04, CCAM vs. Alv, means ± SE, n = 6, Can; n = 3, Alv; n = 4, BPS; n = 7, CCAM.
DISCUSSION
In this study, we have demonstrated that in BPS and CCAM tissue from infants age 1–3 years, there is the presence and increased protein levels of a previously undetected 130-kDa protein isoform of α2-integrin. Splice variants lacking specific cytoplasmic residues have been described for α3- and α6-integrin, but to our knowledge this is the first description of such a finding for α2-integrin (56). Similar to our study, others using antibodies only to the intracellular cytoplasmic domain of α2-integrin have detected a single isoform for α2-integrin (66). Using two different antibodies, one to the intracellular and one to the extracellular domain of α2, we detected this apparent additional isoform of α2 in BPS and CCAM. The specificity of both the 130- and 150-kDa isoforms was confirmed with a peptide generated to match the extracellular domain of α2-integrin detected by antibody 2 showing that this peptide competes for binding of antibody 2 to the 150- and 130-kDa integrin α2 protein isoforms detected in our study. In this current study, the derivation of the 130-kDa α2-integrin protein isoform could not be determined, but similar findings for other proteins with multiple isoforms suggest the possibility of chromosomal, transcriptional, posttranscriptional, and posttranslational modifications as potential mechanisms to be investigated (23, 45).
The antibody designated antibody 1 was made against and detects peptide sequences in the intracellular cytoplasmic tail of α2-integrin including the GFFKR and KYEKMTK regions (Kevin Long, Chemicon, personal communication) known to interact with FAK (31, 34). We deduce that the short α2-integrin isoform (130 kDa) was not detected by antibody 1 due to its lacking the cytoplasmic peptide sequences against which antibody 1 was made. The number of amino acids calculated to be absent from the 130-kDa α2-integrin isoform to cause this apparent size difference (up to 166 amino acids, Swiss Prot Data Base) compared with the 150-kDa isoform is also consistent with these important peptides being absent from the cytoplasmic tail of the 130-kDa isoform, resulting in the inability of antibody 1 to detect the 130-kDa isoform of α2-integrin in the BPS and CCAM tissues. We could only detect this 130-kDa α2-integrin isoform with antibody 2 as this antibody is made against peptide sequences in the extracellular domain of α2. The cytoplasmic regions of α2-integrin detected by antibody 1 are nearly 100% homologous in humans and mice consistent with antibody 1 in our study, only recognizing the 150-kDa band and not being able to detect any presence of the 130-kDa isoform in Western blots from mouse fetal lung fibroblast cultures. Antibody 2, which detects peptide sequences in the extracellular domain of α2-integrin of human origin, does not cross-react with α2 of mouse origin and therefore could not be used in Western blots of the mouse fetal lung fibroblast cultures.
These FAK-interacting cytoplasmic peptide regions (GFFKR and KYEKMTK) of α2-integrin influence specific signaling pathways, including EGFR signaling, that are important for changes in cell migration and cell cycle progression (12, 34, 38). We have not studied EGFR signaling in BPS and CCAM, but previous studies show that in vitro EGF-mediated cell migration and entry into the S-phase of cell proliferation required a full length α2-integrin cytoplasmic domain including the specific cytoplasmic domains that are likely absent from the 130-kDa α2-integrin isoform detected in our BPS and CCAM tissue samples (8, 14, 19, 34). On the other hand, other studies have shown that deletion of these same regions in the α2 cytoplasmic tail alters binding of cytoplasmic mediators to the β1-integrin tail of α2β1-integrin receptors, causing altered inside-out integrin signaling, altered adhesiveness to extracellular matrix molecules, as well as altered growth factor-stimulated chemotaxis (12, 31). Outside-in signaling might also be altered by aberrant α2β1-integrin receptors formed with this altered 130-kDa α2-integrin isoform. Furthermore, in developing lung, β1-integrin signaling is important for migration of epithelial cells (17). These altered molecular signaling mechanisms may be partially responsible for the aberrant airway branching seen in BPS and CCAM lesions through altered α2β1-integrin signaling causing altered cell-cell adhesion and subsequent changes in epithelial cell migration and cell proliferation (10, 39, 52, 64).
Consistent with other studies in mouse and human lung, our results show that α2-integrin is expressed in epithelia and adjacent mesenchyme of branching airway tips, whereas α3-integrin is expressed in established airway branches (15, 34, 62). α2β1-integrin receptors are thought to promote mesenchymal-epithelial cell contacts as epithelial cells migrate into surrounding mesenchyme and associate with extracellular matrix binding partners and other cells (16, 67). In CCAM tissue, the increased intensity of α2-integrin localization with antibody 2 compared with lung tissue adjacent to CCAM cysts suggests that part of the intensity of α2-integrin immunostaining in the CCAM cysts was due to the 130-kDa α2-isoform detected with Western blotting. BPS tissue also stained intensely with antibody 2 compared with less intense staining for α2-integrin in human fetal lung. We speculate that this increased α2-integrin expression contributed to increased and aberrant airway branching in these lung lesions. Experiments designed to isolate and then block or increase this specific α2-integrin isoform will be needed to confirm this speculation. Furthermore, aberrant α2 signaling in BPS in the presence of modestly changed α3-integrin expression may have led to established new branches in these lung lesions, whereas significantly increased α3-integrin levels and cellular expression in CCAM may have further modified other distinct cell-cell interactions that exist in CCAM but not in BPS. Synergistic activity between increased α2- and α3-integrin expression in CCAM compared with BPS in the pathogenesis of these congenital anomalies is also possible and has been shown for other integrin family members (16, 20, 27, 36, 50, 53, 66). Differences in cell adhesion between BPS and CCAM may also contribute to differences in their malignant potential (4, 30, 64).
The importance of E-cadherin for maintenance of epithelial cell polarity and stability as well as the necessity of integrin-E-cadherin clustering to control cell signaling and epithelial cell stabilization suggests that increased E-cadherin along with increased and aberrant expression of α2-integrin may have altered the formation of integrin-E-cadherin heterodimers, thereby contributing to dysregulated cell-adhesion and cellular migration in BPS and CCAM while controlling or preventing any invasive potential of the abnormal airways in these lung lesions (24, 47, 69). E-cadherin expression is also linked to β-catenin signaling with β-catenin being bound to its cytoplasmic domain. β-catenin binding to E-cadherin assists with linkage of E-cadherin to the actin cytoskeleton decreasing β-catenin regulation of Wnt gene expression and subsequent altered cellular behavior, cell fate, and differentiation (46, 51). We are not aware of any information addressing β-catenin and Wnt signaling in BPS and CCAM, but the increased E-cadherin levels that we observed in these lung anomalies may be one of the mechanisms causing altered cell fate and cell differentiation, possibly through altered Wnt downstream signaling (10, 39, 51).
In these same BPS and CCAM tissues evaluated in this study, we have previously shown upregulation and abnormal cellular localization of HoxB5 protein (59). In the current study, we have now shown that fetal mouse lung fibroblasts with in vitro downregulation of Hoxb5 (60) have decreased α2-integrin protein levels. In both development and in certain diseases, integrins have been shown to be downstream targets of Hox genes, as well as being clustered in close chromosomal locations, both indicating a close association between Hox gene regulation and integrin-mediated signaling (18, 28, 63). Additionally, in lung cancer and in melanoma cell lines, specific Hox gene expression is associated with certain changes in integrin expression patterns (13). Our study along with this information from the literature suggests that α2-integrin is a potential downstream target of HoxB5 and that increased HoxB5 in BPS and CCAM could directly or indirectly contribute to the presence of the altered α2-integrin splice variant. Lack of available and appropriate human tissue and lack of appropriate animal models of BPS and CCAM currently limits the ability to more directly study this phenomenon. Our immunostaining findings show a potential relationship between HoxB5 and α2-integrin. As in normal human fetal lung, α2-integrin colocalizes with HoxB5 around branching airways. However, any potential interaction between HoxB5 and α3-integrin is less likely in normal fetal lung, but is possible in CCAM with upregulated α3-integrin in the same cellular location as HoxB5 protein. A direct interaction between HoxB5 and E-cadherin is also less likely as E-cadherin is exclusively localized to epithelial cells (24, 25). However, we and others have shown that certain Hox genes and divergent homeobox genes are localized to lung epithelial cells (22, 32, 42, 43, 61, 62). We have not yet investigated the expression pattern of other Hox genes in BPS and CCAM, but it is possible that other Hox genes are aberrantly expressed in these lung lesions either independently of HoxB5 or through Hox gene auto- and cross-regulation, that might contribute to more direct Hox regulation of epithelial-expressed cell adhesion molecules in BPS and CCAM (6, 26, 44, 60, 68).
In summary, we have shown for the first time that specific cell adhesion molecules important to lung development and airway morphogenesis are altered in BPS and CCAM, lung anomalies characterized by altered airway formation. Furthermore, this study also demonstrates that BPS and CCAM pathogenesis is associated with potentially altered integrin cytoplasmic signaling. This work along with our previous work showing aberrant HoxB5 expression in BPS and CCAM suggests that aberrant Hox protein and cell adhesion are part of the mechanisms involved in the development of these lung lesions (59).
GRANTS
This work was supported by National Institutes of Health Grants HD-038419, HD-044784, and HL-37930 and the Peabody Foundation.
Acknowledgments
We thank Dr. Sandy Murray and Lucia Pham for helpful comments and assistance and Erdene Haltiwanger for secretarial support.
REFERENCES
- 1.Achiron R, Hegesh J, Yagel S. Fetal lung lesions: a spectrum of disease. New classification based on pathogenesis, two dimensional and color doppler ultrasound. Ultrasound Obstet Gynecol 24: 107–114, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Achiron R, Zalel Y, Lipitz S, Hegesh J, Mazkereth R, Kuint J, Jacobson J, Yagel S. Fetal lung dysplasia: clinical outcome based on a new classification system. Ultrasound Obstet Gynecol 24: 127–133, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Aulicino MR, Reis ED, Dolgin SE, Unger PD, Shah KD. Intra-abdominal pulmonary sequestration exhibiting congenital cystic adenomatoid malformation: report of a case and review of literature. Arch Pathol Lab Med 118: 1034–1037, 1994. [PubMed] [Google Scholar]
- 4.Barr LF, Cambell SE, Bochner BS, Dang CV. Association of the decreased expression of alpha3 beta 1 integrin with the altered cell: environmental interactions and enhanced soft agar cloning ability of c-myc overexpressing small cell cancer cells. Cancer Res 58: 5537–5545, 1998. [PubMed] [Google Scholar]
- 5.Blystone SD Integrating an integrin: a direct route to actin. Biochim Biophys Acta 1692: 47–54, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Bondos S Variations on a theme: Hox and Wnt combinatorial regulation during animal development. Sci STKE 38: 1–4, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Brunton VG, MacPherson IRJ, Frame MC. Cell adhesion receptors, tyrosine kinases and actin modulators. Biochem Biophys Acta 1692: 121–144, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Cabodi S, Moro L, Bergatto EB, DiStefano P, Turco E, Tarone G, Defilippi P. Integrin regulation of epidermal growth factor (EGF) receptor and of EGF-dependent responses. Biochem Soc Trans 32: 438–442, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Cass DL, Crombleholme TM, Howell LJ, Stafford PW, Ruchelli ED, Adzick NS. Cystic lung lesions with systemic arterial blood supply: a hybrid of congenital cystic adenomatoid malformation and bronchopulmonary sequestration. J Pediatr Surg 32: 986–990, 1997. [DOI] [PubMed] [Google Scholar]
- 10.Cass DL, Quinn TM, Yang EY, Liechty KW, Crombleholme TM, Flake AW, Adzick NS. Increased cell proliferation and decreased apoptosis characterize congenital cystic adenomatoid malformation of the lung. J Pediatr Surg 33: 1043–1046, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Chen J, Diacovo TG, Grenache DG, Santoro SA, Zutter MM. The alpha 2 integrin subunit deficient mouse: a multiphaceted phenotype including defects of branching morphogenesis and hemostasis. Am J Pathol 161: 337–344, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen YP, O'Toole E, Shipley T, Forsyth J, LaFlamme SE, Yamada KM, Shattil SJ, Ginsberg MH. Inside-out signal transduction inhibited by isolated integrin cytoplasmic domains. J Biol Chem 269: 18307–18310, 1994. [PubMed] [Google Scholar]
- 13.Cillo C, Cantile M, Mortarini R, Barba P, Parmiani G, Anichini A. Differential patterns of Hox gene expression are associated with specific integrin and ICAM profiles in clonal populations isolated from a single human melanoma metastasis. Int J Cancer 66: 692–697, 1996. [DOI] [PubMed] [Google Scholar]
- 14.Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science 268: 233–238, 1995. [DOI] [PubMed] [Google Scholar]
- 15.Conran RM, Stocker JT. Extralobar sequestration with frequently associated congenital cystic adenomatoid malformation, type 2: a report of 50 cases. Pediatr Dev Pathol 2: 454–463, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Coraux C, Delplanque A, Hinnrasky J, Peault B, Puchelle E, Gaillard D. Distribution of integrins during human fetal lung development. J Histochem Cytochem 46: 803–810, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Coraux C, Zahm JM, Puchelle E, Gaillard D. β1-integrins are involved in migration of human fetal tracheal epithelial cells and tubular morphogenesis. Am J Physiol Lung Cell Mol Physiol 279: L224–L234, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Daftary GS, Troy PJ, Bagot CN, Young SL, Taylor HS. Direct regulation of beta3-integrin subunit gene expression by HoxA10 in endometrial cells. Mol Endocrinol 16: 571–579, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Damsky CH, Ilic D. Integrin signaling: it's where the action is. Curr Opin Cell Biol 14: 594–602, 2002. [DOI] [PubMed] [Google Scholar]
- 20.DeArcangelis A, Mark M, Kreidberg J, Sorokin L, Georges-Labouesse E. Synergestic activities of (alpha)3 and (alpha)6 integrins are required during apical ectodermal ridge formation and organogenesis in the mouse. Development 126: 3957–3968, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Freedom RM, Yoo S, Goo HW, Mikailian H, Anderson RH. The bronchopulmonary foregut malformation complex. Cardiol Young 16: 229–251, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Golpon HA, Gerace MW, Moore MD, Miller HL, Tuder RM, Voelkel NF. Hox genes in human lung: altered expression in primary pulmonary hypertension and emphysema. Am J Pathol 158: 955–966, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Good WV, Gendron RL. Genomics and proteomics of retinopathy of prematurity: DNA-based prevention and treatment. Br J Ophthalmol 91: 1577, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halbleib JM, Nelson WJ. Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev 20: 3199–3214, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Hirai Y, Nose A, Kobayashi S, Takeichi M. Expression and role of E- and P-cadherin adhesion molecules in embryonic histogenesis. I. Lung epithelial morphogenesis. Development 105: 263–270, 1989. [DOI] [PubMed] [Google Scholar]
- 26.Hooiveld MH, Morgan R, In Der Rieden P, Houtzager E, Pannese M, Boncinelli E, Durston AJ. Novel interactions between vertebrate Hox genes. Int J Dev Biol 43: 665–674, 1999. [PubMed] [Google Scholar]
- 27.Hynes RO Integrins: versatility, modulation, and signaling in cell adhesion. Cell 69: 11–25, 1992. [DOI] [PubMed] [Google Scholar]
- 28.Jones FS, Prediger EA, Bittner DA, DeRobertis EM, Edelman GM. Cell adhesion molecules as targets for Hox genes. Proc Natl Acad Sci USA 89: 2086–2090, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Juliano RL, Reddig P, Alahari S, Edin M, Howe A, Aplin A. Integrin regulation of cell signaling and motility. Biochem Soc Trans 32: 443–446, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Kavlovsky RA, Purdy S, Dangman BC, McKenna BJ, Brien T, Ilves R. Bronchioloalveolar carcinoma in a child with congenital cystic adenomatoid malformation. Chest 112: 548–551, 1997. [DOI] [PubMed] [Google Scholar]
- 31.Kawaguchi S, Bergelson JM, Finberg RW, Hemler ME. Integrin alpha 2 cytoplasmic domain deletion effects: loss of adhesive activity parallels ligand-independent recruitment into focal adhesions. Mol Biol Cell 5: 977–988, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim C, Nielsen HC. Hoxa-5 in mouse developing lung: cell-specific expression and retinoic acid regulation. Am J Physiol Lung Cell Mol Physiol 279: L863–L871, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Kirchhofer D, Languino LR, Ruoslahti E, Pierschbacher MD. alpha 2 beta 1 integrins from different cell types show different binding specificities. J Biol Chem 265: 615–618, 1990. [PubMed] [Google Scholar]
- 34.Klekotka PA, Santoro SA, Wang H, Zutter MM. Specific residues within the alpha 2 integrin subunit cytoplasmic domain regulate migration and cell cycle progression via distinct MAPK Pathways. J Biol Chem 276: 32353–32361, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Kousseff BG, Gilbert-Barness E, Debich-Spicer D. Bronchopulmonary-foregut malformations: a continuum of paracrine hamartomas? Am J Med Genet 68: 12–17, 1997. [DOI] [PubMed] [Google Scholar]
- 36.Kreidberg JA, Donovan MJ, Goldstein SI, Rennke H, Shepherd K, Jones RC, Jaenisch R. Alpha 3 Beta 1 integrin has a crucial role in kidney and lung organogenesis. Development 122: 3537–3547, 1996. [DOI] [PubMed] [Google Scholar]
- 37.Lai JF, Kao SC, Jiang ST, Tang MJ, Chan PC, Chen HC. Involvement of focal adhesion kinase in hepatocyte growth factor-induced scatter of madin-darby canine kidney cells. J Biol Chem 275: 7474–7480, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Liang CC, Chen HC. Sustained activation of extracellular signal-regulated kinase stimulated by hepatocyte growth factor leads to integrin alpha 2 expression that is involved in cell scattering. J Biol Chem 276: 21146–21152, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Liechty KW, Crombleholme TM, Quinn TM, Cass DL, Flake AW, Adzick NS. Elevated platelet-derived growth factor-B in congenital cystic adenomatoid malformations requiring fetal resection. J Pediatr Surg 34: 805–809, 1999. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y, Martinez L, Ebine K, Abe MK. Role of mitogen-activated protein kinase p38alpha in lung epithelial branching morphogenesis. Dev Biol 314: 224–235, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McLean SE, Pfeifer JD, Siegel MJ, Jensen ER, Schuler PM, Hirsch R, Mychaliska GB. Congenital cystic adenomatoid malformation connected to an extralobar sequestration in the contralateral chest: common origin? J Pediatr Surg 39: e13–e17, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Minoo P, Hamdan H, Bu D, Warburton D, Stepanik P, deLemos R. TTF-1 regulates lung epithelial morphogenesis. Dev Biol 172: 694–698, 1995. [DOI] [PubMed] [Google Scholar]
- 43.Morotti RA, Gutierrez MC, Askin F, Profitt SA, Wert SE, Whitsett JA, Greco MA. Expression of thyroid transcription factor-1 in congenital cystic adenomatoid malformation of the lung. Pediatr Dev Pathol 3: 455–461, 2000. [DOI] [PubMed] [Google Scholar]
- 44.Newman SA Sticky fingers: Hox genes and cell adhesion in vertebrate limb development. Bioessays 18: 171–174, 1996. [DOI] [PubMed] [Google Scholar]
- 45.Peng Q, Pevzner PA, Tesler G. The fragile breakage versus random breakage models of chromosome evolution. PLoS Comp Biol 2: 100–111, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Piedra J, Martinez D, Castano J, Miravet S, Dunach M, Garcia de Herreros A. Regulation of beta-catenin structure and activity by tyrosine phosphorylation. J Biol Chem 276: 20436–20443, 2001. [DOI] [PubMed] [Google Scholar]
- 47.Runswick SK, O'Hare MJ, Jones L, Streuli CH, Garrod DR. Desmosomal adhesion regulates epithelial morphogenesis and cell positioning. Nature Cell Biol 3: 823–830, 2001. [DOI] [PubMed] [Google Scholar]
- 48.Ruoslahti E Control of cell motility and tumour invasion by extracellular matrix interactions. Br J Cancer 66: 239–242, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sade RM, Clouse M, Ellis FH. The spectrum of pulmonary sequestration. Ann Thoracic Surg 18: 644–658, 1974. [DOI] [PubMed] [Google Scholar]
- 50.Sanchez-Esteban J, Wang Y, Filardo EF, Rubin LP, Ingber DE. Integrins β1, α6, and α3 contribute to mechanical strain-induced differentiation of fetal lung type II epithelial cells via distinct mechanisms. Am J Physiol Lung Cell Mol Physiol 290: L343–L350, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Schambony A, Kunz M, Gradl D. Cross-regulation of Wnt signaling and cell adhesion. Differentiation 72: 307–318, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Schlaepfer DD, Mitra SK, Ilic D. Control of motile and invasive phenotypes by focal adhesion kinase. Biochem Biophys Acta 1692: 77–102, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Sheppard D Functions of pulmonary epithelial integrins from development to disease. Physiol Rev 83: 673–686, 2003. [DOI] [PubMed] [Google Scholar]
- 54.Stocker JT Congenital pulmonary airway malformation: a new name for an expanded classification of congenital cystic adenomatoid malformation of the lung. Histopathology 41, Suppl 2: 424–431, 2002. [Google Scholar]
- 55.Stocker JT, Madewell JE, Drake RM. Congenital cystic adenomatoid malformation of the lung: classification and morphologic spectrum. Hum Pathol 8: 155–171, 1977. [DOI] [PubMed] [Google Scholar]
- 56.Tamura RN, Cooper HM, Collo G, Quaranta V. Cell type-specific integrin variants with alternative alpha chain cytoplasmic domains. Proc Natl Acad Sci USA 88: 10183–10187, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Volpe MV, Martin A, Vosatka RJ, Mazzoni CL, Nielsen HC. Hoxb-5 expression in the developing mouse lung suggests a role in branching morphogenesis and epithelial cell fate. Histochem Cell Biol 108: 495–504, 1997. [DOI] [PubMed] [Google Scholar]
- 58.Volpe MV, Nielsen HC, Archavachotikul K, Ciccone TJ, Chinoy MR. Thyroid hormone affects airway morphogenesis and epithelial cell fate during the late pseudoglandular period of mouse lung development. Mol Genet Metab 80: 242–254, 2003. [DOI] [PubMed] [Google Scholar]
- 59.Volpe MV, Pham L, Lessin M, Ralston SJ, Bhan I, Cutz E, Nielsen HC. Expression of Hoxb-5 during human lung development and in congenital lung malformations. Birth Defects Res A Clin Mol Teratol 67: 550–556, 2003. [DOI] [PubMed] [Google Scholar]
- 60.Volpe MV, Ramadurai SM, Pham LD, Nielsen HC. Hoxb-5 downregulation alters tenascin-C, FGF10 and Hoxb gene expression patterns in pseudoglandular period fetal mouse lung. Front Biosci 12: 860–873, 2007. [DOI] [PubMed] [Google Scholar]
- 61.Volpe MV, Vosatka RJ, Nielsen HC. Hoxb-5 control of early airway formation during branching morphogenesis in the developing mouse lung. Biochim Biophys Acta 1475: 337–345, 2000. [DOI] [PubMed] [Google Scholar]
- 62.Volpe MV, Wang KT, Nielsen HC, Chinoy MR. Unique spatial and cellular expression patterns of Hoxa5, Hoxb4 and Hoxb6 proteins in normal developing mouse lung are modified in pulmonary hypoplasia. Birth Defects Res A Clin Mol Teratol 82: 571–584, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang W, Wu W, Desai T, Ward DC, Kaufman SJ. Localization of the alpha 7 integrin gene (ITGA7) on human chromosome 12q13: clustering of integrin and hox genes implies parallel evolution of these gene families. Genomics 26: 563–570, 1995. [DOI] [PubMed] [Google Scholar]
- 64.Wilson RD, Hedrick HL, Liechty KW, Flake AW, Johnson MP, Bebbington M, Adzick NS. Cystic adenomatoid malformation of the lung: review of genetics, prenatal diagnosis and in utero treatment. Am J Med Genet 140A: 151–155, 2006. [DOI] [PubMed] [Google Scholar]
- 65.Wozniak MA, Modzelewska K, Kwong L, Keely PJ. Focal adhesion regulation of cell behavior. Biochem Biophys Acta 1692: 103–119, 2004. [DOI] [PubMed] [Google Scholar]
- 66.Wu JE, Santoro SA. Complex patterns of expression suggest extensive roles for the alpha 2 beta 1 integrin in murine development. Dev Dyn 199: 292–314, 1994. [DOI] [PubMed] [Google Scholar]
- 67.Wu JE, Santoro SA. Differential expression of integrin subunits supports distinct roles during lung branching morphogenesis. Dev Dyn 206: 169–181, 1996. [DOI] [PubMed] [Google Scholar]
- 68.Yokouchi Y, Nakazato S, Yamamoto M, Goto Y, Kameda T. Misexpression of Hoxa13 induces cartilage homeotic transformation and changes cell adhesiveness in chick limb buds. Genes Dev 9: 2509–2522, 1995. [DOI] [PubMed] [Google Scholar]
- 69.Zhang F, Tom CC, Kugler MC, Ching TT, Kreidberg JA, Wei Y, Chapman HA. Distinct ligand binding sites in integrin alpha 3 beta 1 regulate matrix adhesion and cell-cell contact. J Biol Chem 163: 177–188, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]