The Keystone Symposium on Plant Sensing, Response and Adaptation to the Environment took place between 11 and 16 January 2009, at Big Sky, Montana, USA, and was organized by S.A. Kay & J. Chory.
Glossary
Introduction
In spite of the fanfare surrounding the two-hundredth anniversary of the birth of Charles Darwin, and the one-hundred and fiftieth anniversary of his most celebrated book ‘On the Origin of Species' (Darwin, 1859), many people would still be surprised to learn that Darwin wrote a nearly 600 page book entitled ‘The Power of Movement in Plants' (Darwin, 1880). As most plants have their location fixed as soon as their roots enter the soil, non-specialists might wonder how much movement plants can make. However, movement is not limited to locomotion, and many of the movements studied by Darwin probably evolved in response to the unique challenges imposed by the rooted habit. Unable to change location, plants have developed exquisite abilities to sense, respond to and adapt to their local environment. One example that captured Darwin's attention is the navigation of the radicle (root) tip through the complex soil environment to optimize water and nutrient acquisition. He stated, “It is hardly an exaggeration to say that the tip of the radicle thus endowed, and having the power of directing the movements of the adjoining parts, acts like the brain of one of the lower animals[...].”
These root-growth movements were but one of many environmental responses discussed by plant biologists gathered at the recent Keystone Symposium on Plant Sensing, Response and Adaptation to the Environment. Root growth is controlled by gravity, water and nutrient acquisition; whereas growth of the aerial shoot is regulated primarily by the light environment. Various photoreceptors allow plants to judge light intensity, direction and competing vegetation, and responses to these cues modify plant growth to maximize photosynthetic input. In addition, plants perceive and respond to many abiotic and biotic stresses, including drought, cold, heat, salt, animal herbivory and microbial pathogens. Fundamental to plant sensing and responding to the environment is the circadian oscillator (Hotta et al, 2007)—informally known as the clock. Similar to most organisms, plants have an internal oscillator with a periodicity of approximately 24 h. This oscillator allows plants to prepare for daily changes in the environment; for example, by upregulating photosynthetic genes before dawn. The clock also regulates (gates) the sensitivity to environmental inputs; for example, cold-responsive genes are most inducible at dusk. In addition, clocks allow photoperiod measurement, and therefore regulate seasonal responses such as flowering and bud set.
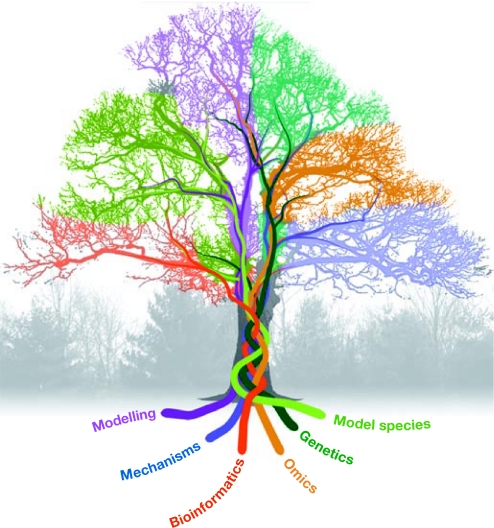
The symposium gathered scientists with expertize in various aspects of plant environmental responses at levels ranging from biochemical mechanisms to ecology and genomics. The extent of cross-disciplinary studies described at the meeting was particularly rewarding. A true understanding of biochemical mechanisms must be informed by evolutionary and ecological knowledge, and vice versa. The meeting illustrated that we have entered an age in which information flow—fuelled by genomics and informatics—has markedly increased among researchers, disciplines and organisms, thereby benefiting all fields (Fig 1). We have structured this report around the different aspects of information flow: foundational studies, acquisition, transfer and modelling.
Figure 1.
The flow of plant research information. Information at many levels is being gathered, interleaved and dispersed, transforming our understanding of plant perception and response to the environment.
Foundation of information: mechanisms
The information highway depends on detailed mechanistic studies that define protein function and signalling mechanisms. Such studies lay an essential foundation for more comparative and high-throughput methods.
Several talks focused on the mechanisms of light perception. An important part of any environmental response pathway is the attenuation of signalling after stimulation; this topic was discussed by W. Briggs (Palo Alto, CA, USA), who described the association of PP2A with the PHOT2 photoreceptor. Activated PHOT2 usually reverts to an inactive form within minutes of transfer to darkness, which is a process associated with PHOT2 dephosphorylation. Briggs showed that dark reversion requires PP2A: mutants that reduced PP2A activity had reduced PHOT2 reversion, accompanied by enhanced phototropism and stomatal opening. He reported a different attenuation mechanism for PHOT1: PHOT1–GFP is localized to the plasma membrane in plants grown in darkness but rapidly translocates to cytoplasmic speckles after exposure to blue light. Briggs found that red-light pretreatment—sensed by the photoreceptor phytochrome (PHY) A—prevented PHOT1 translocation, allowing it to stay in an active signalling location for longer (Han et al, 2008), thereby explaining the long-standing observation that red-light pretreatment enhances phototropism. C. Fankhauser (Lausanne, Switzerland) investigated the attenuation of phytochrome signalling. In the dark, phytochromes are cytoplasmic and light induces their translocation to the nucleus where they bind to and promote the degradation of bHLH transcription factors known as PIFs. Previous work from the Fankhauser group has shown that PIF4 and PIF5 function downstream of PHYB to activate transcription transiently in response to a low ratio of red-to-far-red light, which occurs when plants are shaded by a chlorophyll-containing leaf (Lorrain et al, 2007). At the symposium, Fankhauser reported that the activation is transient because an atypical bHLH protein, HFR1, is induced by leaf shade and subsequently heterodimerizes with PIF4 or PIF5 to prevent the binding of PIF to target gene promoters. Relatively little is known about the earliest events that occur in phytochrome signalling, before translocation to the nucleus where it localizes to nuclear bodies and interacts with PIFs. J. Chory (La Jolla, CA, USA) described a screen for mutants that are defective in the localization of PHYB–GFP to nuclear bodies. One such mutant, hmr, is defective in all aspects of phytochrome signalling; this phenotype is seen only in seedlings with mutations that disrupt chromophore photosynthesis, indicating that HMR acts close to phytochrome activation.
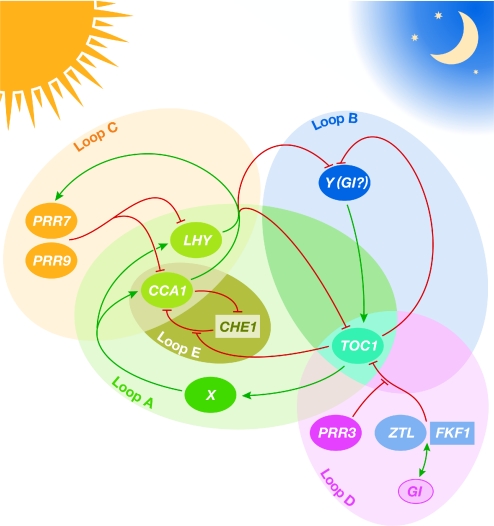
The mechanisms of circadian clock function were also a popular subject. T. Kondo (Nagoya, Japan) presented the latest on the pioneering in vitro oscillator that his laboratory has reconstituted from three cyanobacterial Kai proteins (Nakajima et al, 2005). One characteristic of circadian oscillators is that they are entrainable by external stimuli; remarkably, Kondo showed that the simplified in vitro Kai system is temperature entrainable (Yoshida et al, 2009). He also demonstrated that the intrinsic periodicity of the oscillator is determined by the rate of KaiC ATPase activity (Kitayama et al, 2008). Moving on to the Arabidopsis clock (Fig 2), T. Imaizumi (Seattle, WA, USA) presented work on the photoreceptor FKF1, which is important for the photoperiodic control of flowering (Imaizumi et al, 2005). Although overexpression or loss-of-function mutations of FKF1 have little effect on clock function, double mutants of fkf1 and its homologue ztl were found to have a clock period that was significantly longer than either single mutant, establishing a function for FKF1 in the regulation of the oscillator itself. E. Tobin (Los Angeles, CA, USA) discussed the development of alcohol-inducible constructs to test whether the pulsed expression of clock components can shift the circadian phase. Pulses of CCA1 and LHY caused phase shifts, whereas TOC1 did not, possibly owing to its post-transcriptional regulation (Knowles et al, 2008). The regulation of processes that are downstream of the clock was discussed by S. Harmer (Davis, CA, USA). The gene RVE1 encodes a transcription factor with homology to the core clock component CCA1; however, unlike CCA1, RVE1 functions downstream of the core oscillator. Both CCA1 and RVE1 promote cell elongation, although CCA1 does so through the bHLH transcription factors PIF4 and PIF5, whereas RVE1 promotes growth through the regulation of auxin availability.
Figure 2.
Model of the Arabidopsis circadian clock. The circadian oscillator consists of a series of interlocking feedback loops (A–E). Genes newly reported to function in the clock are shown within rectangles. Loop A represents the transcriptional feedback loop that was identified initially and contains the morning-phased transcription factors CCA1 and LHY, which negatively regulate TOC1. Component X, the existence of which has been inferred from mathematical modelling, induces the transcription of CCA1 and LHY. CHE1 could provide some of the functionality represented by X. Two evening-phased genes, TOC1 and the yet-unidentified component Y, make up loop B. Morning-phased PRR7, PRR9, CCA1 and LHY make up loop C. As reported at the meeting, FKF1 works with ZTL to regulate TOC1 negatively, and this process is, in turn, regulated by GI and PRR3, thereby constituting loop D. The existence of loop E, which provides a link between TOC1 and CCA1, was proposed for the first time at the meeting. Some genes implicated in clock function have been omitted for clarity. Adapted, with permission, from Harmer (2009). CCA1, CIRCADIAN CLOCK-ASSOCIATED 1; CHE1, CCA1 HIKING EXPEDITION 1; FKF1, FLAVIN-BINDING KELCH REPEAT, F-BOX 1; GI, GIGANTEA; LHY, LATE ELONGATED HYPOCOTYL; PRR, PSEUDORESPONSE REGULATOR; TOC1, TIMING OF CAB EXPRESSION 1; ZTL, ZEITLUPE.
Cargo for the highway: information gathering
Recent advances in technology are transforming the study of plant genetics. Researchers now have the ability to obtain data using methods that are orders of magnitude faster, more accurate and less expensive than those available even 5 years ago. Whole-genome sequencing and analysis techniques are allowing researchers to untangle obscure regulatory cascades that underlie the interactions between plants and their environment. S. Kay (La Jolla, CA, USA) and collaborators are developing a collection of vectors that contain most of the known Arabidopsis transcription factors. By performing yeast one-hybrid screens using this tool, Kay identified CHE, which is a TCP transcription factor that binds specifically to the promoter of CCA1 and interacts with TOC1 to regulate circadian rhythms (Fig 2; Pruneda-Paz et al, 2009).
Two important resources being developed are the genome sequences of 1,001 A. thaliana strains and those of the close relative A. lyrata, as presented by D. Weigel (Tübingen, Germany). By using short-read sequencing, 80 Arabidopsis strains have already been sequenced with enough depth to provide an average of 6 to 12 reads for each nucleotide in the genome, and promising results have been obtained by identifying both induced and spontaneous mutations. The previous elucidation of the partial sequence of 20 Arabidopsis accessions by the Weigel group allowed the construction of a microarray chip that queries 250,000 single-nucleotide polymorphisms (Clark et al, 2007). This chip is being used by M. Nordborg (Los Angeles, CA, USA) to genotype 1,300 natural accessions, emphasizing the importance of this Arabidopsis sequencing project. The association mapping of 96 genotyped accessions and 101 different phenotypes has allowed an unprecedented resolution for some traits; the full 1,300 should increase mapping power and help to overcome complications from population structure. This microarray slide also opens many more opportunities; J. Borevitz (Chicago, IL, USA) proposed its use in assaying allele-specific expression levels, determining methylation patterns, alternative splicing analyses and expression quantitative trait locus (QTL) studies.
The symposium also illustrated that information gathering in the field—such as micro-meteorological observations—and the study of plant genetic diversity, fitness and performance, are crucial to understanding the mechanisms of adaptation to different environments. Contrary to other genetic systems, the lack of mobility in plants allows hypotheses on adaptation to be more easily tested, which—in combination with the current advances in genomics and the ease of genetic manipulation in Arabidopsis and other plants—provides a fantastic opportunity for studying the molecular nature of adaptation, and interactions between genotype and environment. Some presentations at the symposium focused on this emerging field of ecological genetics by analysing the responses of specific genotypes in diverse changing and natural environments, in contrast to the constant conditions used by most researchers. For example, Borevitz programmed growth chambers to mimic the photoperiod, temperature, light intensity and humidity in Sweden and Spain, and phenotyped Arabidopsis segregating populations and accessions, finding QTLs and associations exclusive to particular (simulated) locales (Li et al, 2006). E. Holub (Warwick, UK) is studying long-term recurrent populations of Arabidopsis. Focusing on pathogen response, he is characterizing the diversity and distribution of candidate disease-resistance genes across geographic and genomic contexts (Holub, 2007). J. Schmitt and A. Wilczek (Providence, RI, USA) coordinated an exciting project in which Arabidopsis populations and mutants with known laboratory phenotypes were planted across a matrix of sowing times and locations (Wilczek et al, 2009). An analysis of plants in the field instead of the laboratory revealed unexpected environmental responses from many known mutants, highlighting the importance of field studies.
Information transfer
There was particular excitement at the symposium about the transfer of information from model organisms to other species. J. Irwin (Norwich, UK) is characterizing variation in flowering time and vernalization requirements in Brassica oleracea, a species in which flowering time is an important determinant of harvestable tissue. Crosses and field trials are underway to use the Arabidopsis vernalization pathway to understand Brassica variants and to increase yield. Crop improvement could also potentially be achieved by the optimization of circadian pathways to elevate energy conversion rates. With this idea in mind, C.R. McClung (Hanover, NH, USA) showed promising preliminary results using video recordings of leaf movement to determine the variation of circadian rhythms in B. rapa accessions and recombinant inbred-line populations. To perform solid research in these new systems, successful high-throughput techniques must also be transferred from model systems to field crops. McClung has developed transformation methods for B. rapa calli that allow the monitoring of gene expression using a luciferase-based reporter, and has shown that shoots regenerated from B. rapa calli that overexpress the Arabidopsis circadian clock genes TOC1 and ZTL have altered circadian rhythms.
One could say that the level of mechanistic understanding achieved in model species raises more questions that it answers. Are the crucial components implicated in environmental sensing and responses conserved across all plants? Is their function similar? The diversity of strategies and phenotypes is certainly enormous; however, in many cases the individual proteins are shared. Those attending the symposium realized this during the keynote address by W. Briggs, who reported on the notable diversity of LOV domain proteins in nature. Briggs and his collaborators G. Paris (Buenos Aires, Argentina), F. Goldbaum (Buenos Aires, Argentina) and R. Bogomolni (Santa Cruz, CA, USA) are studying LOV proteins in the pathogenic bacterium Brucella, and have found that infectivity is enhanced by blue light and LOV function (Swartz et al, 2007), emphasizing the influence that research in plant species can have in opening new research fields in different domains of life. More examples of diversity in well-known genes come from the study of flowering time in perennial plants. In the annual Arabidopsis, ‘winter-annual' ecotypes need to be exposed to long periods of cold before the longer days of spring can induce flowering. The repression of flowering is largely due to FLC, the expression of which is reduced during cold treatment. In addition, epigenetic modifications are acquired in the FLC locus that maintain low levels of expression after cold exposure, thereby allowing photoperiodic induction (Amasino, 2005). Perennials such as Arabis alpina—a close relative of Arabidopsis—flower repeatedly year after year, and are being studied by G. Coupland (Cologne, Germany). Coupland presented a genetic screen for A. alpina mutants that flower without vernalization, which led to the identification of PEP1 as the A. alpina orthologue of FLC (Wang et al, 2009). The characterization of PEP1 showed that, unlike for Arabidopsis FLC, epigenetic downregulation is not maintained after vernalization, ensuring vegetative growth at some apices and promoting the perennial life cycle.
Finding homologous genes that have evolved new functions to promote different environmental responses across species was a recurrent feature discussed at the meeting. An example can be found in the Populus genus, which contains poplar, aspen and cottonwood trees. The relatively close phylogenetic relationship of this genus to Arabidopsis, coupled with the release of the whole genome sequence of the black cottonwood Populus trichocarpa (Djerbi et al, 2005), aid this type of research. O. Nilsson (Umeå, Sweden) has taken advantage of these tools to explore, in aspen trees, the role of photoperiodic flowering genes originally discovered in Arabidopsis. The Arabidopsis flowering-time gene FT acts as a florigen, moving from leaves to the meristems to promote flowering after photoperiodic induction (Corbesier et al, 2007). In aspen trees, bud set in preparation for winter is also photoperiodic and FT has a role in its regulation (Bohlenius et al, 2006). Surprisingly, FT is also involved in the temperature regulation of spring bud flush. In Arabidopsis, FT expression is induced by the CO protein, which only accumulates during long days when high levels of CO messenger RNA—regulated by the clock through GI—coincide with the perception of light (Sawa et al, 2007; Yanovsky & Kay, 2003). Nilsson assigned further evolutionary importance to the circadian regulation of CO by GI in aspen, by showing a latitudinal cline in the phase of oscillation of CO, which is at least partly due to the differential regulation of GI in these trees. The modulation of the CO phase allows FT expression to peak at different times of the year in trees from different latitudes, promoting flowering and bud set under the optimal environmental conditions at each location.
Mathematical modelling
The use of new technologies is spurring the development of faster and more-efficient algorithms to process and interpret data. The development of new methods that combine the various types of information available is also crucial to building new hypotheses. J.M. Maloof (Davis, CA, USA) illustrated how novel data mining can help to determine the gene underlying a QTL—typically a labour-intensive process. In this case, network analysis was used to combine publicly available genome resequencing, microarray expression and gene-annotation databases to identify a gene responsible for a QTL that affects the acceleration of flowering in response to foliar shade.
An exciting field is the development of mathematical models that integrate the information on well-studied processes gathered by molecular biologists, which is possible in organisms for which abundant data can be readily obtained or compiled. These models not only reproduce what has been observed but also predict how pathways and organisms will respond to future perturbations. In this regard, A. Millar (Edinburgh, Scotland) presented the latest analysis of an Arabidopsis circadian clock model that yields quantitative and dynamic predictions that are difficult to obtain from experimental data (Locke et al, 2006). Millar's model is able to reproduce the changes in clock phase that occur in response to the changing times of dawn and dusk through the year. This model predicted that changes in the entrainment of the clock must be accompanied by quantitative changes in the timing of clock gene expression, which were subsequently confirmed experimentally. In addition, according to the model, the phase of TOC1 expression is controlled by light input into the evening loop of the clock. New mathematical analysis aimed to test how such timing changes could be manipulated; counter-intuitively, changing light input into the morning loop of the clock was most effective in altering the photoperiod response of the evening loop. Planned and spontaneous presentations at the meeting illustrated the importance of the interactions between ‘wet bench' and computational approaches. Millar found that the clock he modelled was resistant to resetting by pulses of TOC1, as Tobin had previously found for the real clock. CHE1, which was described by Kay, could represent part of the component ‘X' predicted by modelling. Finally, the modification of the parameters of the Millar clock model allowed H. Nimmo (Glasgow, UK) to validate his recent observation of a modified root clock, which is a version of the clock that has no light input, and in which the morning loop proteins CCA1 and LHY do not bind to the promoters of the evening loop genes (James et al, 2008).
Also remarkable is the model put forward by J. Schmitt (Manhattan, KS, USA), which was developed in collaboration with S. Welch (Manhattan, KS, USA) using the field-collected data on Arabidopsis flowering-time mutants discussed above (Wilczek et al, 2009). This model calculated the timing of bolting as a function of accumulated temperature, photoperiod and vernalization exposure, reproduced the observed data and, importantly, predicted unexpectedly sharp transitions from rapid cycling to winter annual life histories depending on germination date—a prediction that was subsequently verified experimentally.
Summary
This is a truly remarkable time to be studying plant environmental sensing and response. Understanding and manipulating these responses will be crucial for maintaining agricultural productivity and conserving biodiversity in the face of increasing climate change. The symposium showed that research in these areas is moving at a rapid pace, fuelled by fundamental discoveries in model organisms, and the increasing availability of high-throughput technology in model and non-model systems alike.
bHLH basic helix–loop–helix motif
CCA1 CIRCADIAN CLOCK-ASSOCIATED 1
CHE CCA1 HIKING EXPEDITION
CO CONSTANS
FKF1 FLAVIN-BINDING KELCH REPEAT, F-BOX 1
FLC FLOWERING LOCUS C
FT FLOWERING LOCUS T
GFP green fluorescent protein
GI GIGANTEA
HFR1 LONG HYPOCOTYL IN FAR RED 1
HMR HEMERA
LHY LATE ELONGATED HYPOCOTYL
LOV light oxygen voltage
PEP1 PERPETUAL FLOWERING 1
PHOT PHOTOTROPIN
PIF PHYTOCHROME-INTERACTING FACTORS
PP2A PROTEIN PHOSPHATASE 2A
RVE1 REVEILLE 1
TCP TEOSINTE BRANCHED 1, CYCLOIDEA AND PCNA FACTORS
TOC1 TIMING OF CAB EXPRESSION 1
ZTL ZEITLUPE

José M. Jiménez-Gómez

Julin N. Maloof
Acknowledgments
We thank S. Harmer for helpful discussion and for providing the template for Fig 2. Work in the Maloof laboratory is funded by National Science Foundation grant DBI-0820854.
References
- Amasino RM (2005) Vernalization and flowering time. Curr Opin Biotechnol 16: 154–158 [DOI] [PubMed] [Google Scholar]
- Bohlenius H, Huang T, Charbonnel-Campaa L, Brunner AM, Jansson S, Strauss H, Nilsson O (2006) CO/FT regulatory module controls timing of flowering and seasonal growth cessation in trees. Science 312: 1040–1043 [DOI] [PubMed] [Google Scholar]
- Clark RM et al. (2007) Common sequence polymorphisms shaping genetic diversity in Arabidopsis thaliana. Science 317: 338–342 [DOI] [PubMed] [Google Scholar]
- Corbesier L et al. (2007) FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 316: 1030–1033 [DOI] [PubMed] [Google Scholar]
- Darwin C (1859) On the Origin of Species. London, UK: John Murray [Google Scholar]
- Darwin C (1880) The Power of Movement in Plants. London, UK: John Murray [Google Scholar]
- Djerbi S, Lindskog M, Arvestad L, Sterky F, Teeri TT (2005) The genome sequence of black cottonwood (Populus trichocarpa) reveals 18 conserved cellulose synthase (CesA) genes. Planta 22: 739–746 [DOI] [PubMed] [Google Scholar]
- Han IS, Tseng TS, Eisinger W, Briggs WR (2008) Phytochrome A regulates the intracellular distribution of phototropin 1-green fluorescent protein in Arabidopsis thaliana. Plant Cell 20: 2835–2847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer SL (2009) The circadian system in higher plants. Annual Rev Plant Biol 60: 357–377 [DOI] [PubMed] [Google Scholar]
- Holub EB (2007) Natural variation in innate immunity of a pioneer species. Curr Opin Plant Biol 10: 415–424 [DOI] [PubMed] [Google Scholar]
- Hotta CT, Gardner MJ, Hubbard KE, Baek SJ, Dalchau N, Suhita D, Dodd AN, Webb AAR (2007) Modulation of environmental responses of plants by circadian clocks. Plant Cell Environ 30: 333–349 [DOI] [PubMed] [Google Scholar]
- Imaizumi T, Schultz TF, Harmon FG, Ho LA, Kay SA (2005) FKF1 F-box protein mediates cyclic degradation of a repressor of CONSTANS in Arabidopsis. Science 309: 293–297 [DOI] [PubMed] [Google Scholar]
- James AB, Monreal JA, Nimmo GA, Kelly CL, Herzyk P, Jenkins GI, Nimmo HG (2008) The circadian clock in Arabidopsis roots is a simplified slave version of the clock in shoots. Science 322: 1832–1835 [DOI] [PubMed] [Google Scholar]
- Kitayama Y, Nishiwaki T, Terauchi K, Kondo T (2008) Dual KaiC-based oscillations constitute the circadian system of cyanobacteria. Genes Dev 22: 1513–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles SM, Lu SX, Tobin EM (2008) Testing time: can ethanol-induced pulses of proposed oscillator components phase shift rhythms in Arabidopsis? J Biol Rhythms 23: 463–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Roycewicz P, Smith E, Borevitz JO (2006) Genetics of local adaptation in the laboratory: flowering time quantitative trait loci under geographic and seasonal conditions in Arabidopsis. PLoS ONE 1: e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke JCW, Kozma-Bognár L, Gould PD, Fehér B, Kevei E, Nagy F, Turner MS, Hall A, Millar AJ (2006) Experimental validation of a predicted feedback loop in the multi-oscillator clock of Arabidopsis thaliana. Mol Syst Biol 2: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorrain S, Allen T, Duek PD, Whitelam GC, Fankhauser C (2007) Phytochrome-mediated inhibition of shade avoidance involves degradation of growth-promoting bHLH transcription factors. Plant J 53: 312–323 [DOI] [PubMed] [Google Scholar]
- Nakajima M, Imai K, Ito H, Nishiwaki T, Murayama Y, Iwasaki H, Oyama T, Kondo T (2005) Reconstitution of circadian oscillation of cyanobacterial KaiC phosphorylation in vitro. Science 308: 414–415 [DOI] [PubMed] [Google Scholar]
- Pruneda-Paz JL, Breton G, Para A, Kay SA (2009) A functional genomics approach reveals CHE as a component of the Arabidopsis circadian clock. Science 323: 1481–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa M, Nusinow DA, Kay SA, Imaizumi T (2007) FKF1 and GIGANTEA complex formation is required for day-length measurement in Arabidopsis. Science 318: 261–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz TE et al. (2007) Blue-light-activated histidine kinases: two-component sensors in bacteria. Science 317: 1090–1093 [DOI] [PubMed] [Google Scholar]
- Wang R, Farrona S, Vincent C, Joecker A, Schoof H, Turck F, Alonso-Blanco C, Coupland G, Albani MC (2009) PEP1 regulates perennial flowering in Arabis alpina. Nature 459: 423–427 [DOI] [PubMed] [Google Scholar]
- Wilczek AM et al. (2009) Effects of genetic perturbation on seasonal life history plasticity. Science 323: 930–934 [DOI] [PubMed] [Google Scholar]
- Yanovsky MJ, Kay SA (2003) Living by the calendar: how plants know when to flower. Nat Rev Mol Cell Biol 4: 265–275 [DOI] [PubMed] [Google Scholar]
- Yoshida T, Murayama Y, Ito H, Kageyama H, Kondo T (2009) Nonparametric entrainment of the in vitro circadian phosphorylation rhythm of cyanobacterial KaiC by temperature cycle. Proc Natl Acad Sci USA 106: 1648–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]