Abstract
The influence of circadian rhythms on memory has long been studied; however, the molecular prerequisites for their interaction remain elusive. The hippocampus, which is a region of the brain important for long-term memory formation and temporary maintenance, shows circadian rhythmicity in pathways central to the memory-consolidation process. As neuronal plasticity is the translation of numerous inputs, illuminating the direct molecular links between circadian rhythms and memory consolidation remains a daunting task. However, the elucidation of how clock genes contribute to synaptic plasticity could provide such a link. Furthermore, the idea that memory training could actually function as a zeitgeber for hippocampal neurons is worth consideration, based on our knowledge of the entrainment of the circadian clock system. The integration of many inputs in the hippocampus affects memory consolidation at both the cellular and the systems level, leaving the molecular connections between circadian rhythmicity and memory relatively obscure but ripe for investigation.
Keywords: circadian, hippocampus, long-term memory, oscillation, sleep
Glossary
Bdnf brain-derived neurotrophic factor
bHLH basic helix–loop–helix
BMAL1 brain and muscle Arnt-like protein 1
CA1 cornu ammonis 1 (group of hippocampal pyramidal cells)
cAMP cyclic AMP
CREB cyclic AMP-response element-binding protein
Cry cryptochrome
GABA γ-aminobutyric acid
MAPK mitogen-activated protein kinase
MSK1 mitogen- and stress-activated protein kinase
NMDA N-methyl-D-aspartic acid
Npas2 neuronal PAS domain protein 2
PAS period–aryl hydrocarbon receptor nuclear translocator–single minded
Per period
Introduction
Do you wonder why you cannot remember being asked to bring home the dry cleaning but can list the dog breeds serving as runners up to the new Portuguese water dog at the White House? The answers to these types of question are probably complicated; however, why some memories are consolidated and maintained at the expense of others remains a central question in the field of learning and memory. Some studies indicate that circadian rhythms might be among the cast of neuronal players that regulate memory formation and consolidation. A progressive understanding of how neural circuits cooperate has resulted in new discoveries about the role of circadian rhythms in the brain. Circadian rhythms have long been acknowledged to synchronize the phases of activity and inactivity, and only recently have they been shown to exist in most regions of the brain as well as the periphery, controlling complex physiology that ranges from thermoregulation and olfactory sensitivity to hormone secretion, glucose metabolism and even memory processes (Granados-Fuentes et al, 2006; Lamia et al, 2008; Schibler & Sassone-Corsi, 2002; Stratmann & Schibler, 2006; Walker & Stickgold, 2006). The hippocampus, which is a region of the brain important for long-term declarative memory formation, is not immune to the presence of the circadian clock, hosting 24 4-h oscillations in both gene expression and enzyme activity (Cirelli & Tononi, 2000; Dolci et al, 2003; Eckel-Mahan et al, 2008). As the hippocampus has a crucial role in long-term memory (LTM) formation and temporary storage, these cyclical events raise new questions about how it integrates and temporarily sustains new memories amid a backdrop of circadian and homeostatically regulated events (Fig 1; Sidebar A). How cellular oscillations contribute to the encoding and consolidation of memory needs to be investigated in greater detail, both within individual cells and at the molecular level.
Figure 1.
Is memory training a zeitgeber for hippocampal neurons? In view of the molecular similarities between the processes of light-induced phase shifting in suprachiasmatic nucleus neurons and hippocampal memory consolidation, memory training could induce a distinct phase-response curve in memory-specific neuronal ensembles. The initial spike drawn in the phase-response curve after a training event reflects the early increase in mitogen-activated protein kinase activity and gene expression that occurs in many hippocampal neurons after a training event. Depending on when training occurs relative to existing circadian oscillations, the phase response might be advanced, delayed or unchanged. If unchanged, there could be increased phase coupling between neurons or an increase in the amplitude of oscillations in neurons that are already keeping time. One of these outcomes might be preferable for the hippocampal–cortical communication that occurs during slow-wave sleep. Phase-adjusted or amplitude-adjusted neurons could be marked and selected for the subsequent transfer and storage of information in the cortex.
Sidebar A | Basic concepts in circadian rhythmicity and memory.
Circadian
Occurring with around 24-h periodicity. Derived from the Latin words ‘circa' meaning ‘about' and ‘diem' meaning ‘day'.
Zeitgebers
External cues such as light or food that entrain endogenous circadian rhythms.
Long-term memory (LTM)
Memory that depends on de novo transcription and translation. Many forms of declarative LTM require a hippocampus-dependent cellular-consolidation period. Over time (usually weeks in rodents), LTMs are redistributed to the neocortex, which is a state of memory sometimes referred to as ‘remote'.
Long-term potentiation (LTP)
The increase in postsynaptic potential induced in response to repeated presynaptic stimuli. LTP can persist for hours or longer and is generally thought to be a molecular correlate of LTM formation.
Memory acquisition
The initial encoding of information that relies on short-term modifications of existing synapses. It is typically gauged by memory tests administered shortly after training.
Slow-wave sleep (SWS)
Part of non-rapid eye movement (non-REM) sleep that is distinguishable from REM and other non-REM stages of sleep by the presence of slow (0.5–2 Hz) delta waves of large amplitude, as measured by an electroencephalogram. Sometimes referred to as ‘deep sleep', it is thought to contribute to the consolidation of declarative memory.
Homeostasis
The physiological processes that maintain internal equilibrium despite variations in the external environment. An example of sleep homeostasis is the ability of the brain to respond to sleep deprivation by an increase in subsequent sleep (particularly SWS) duration and intensity, whereas an excess of sleep results in a decrease in subsequent sleep time and intensity.
Circadian cycles take place over the course of approximately 24 h. A small region of the brain known as the suprachiasmatic nucleus (SCN) is the anatomical centre that generates these rhythms and provides the necessary synchronization of other central nervous system and peripheral tissues (Hastings et al, 2008, and references therein). The organs that rely on the SCN for rhythmicity are considered to be ‘slave' oscillators. In mammals, the entrainment of the SCN by a zeitgeber, light, is initiated by neurons that project from the eye through the retinohypothalamic tract. At the individual cell level, the circadian clock uses the CLOCK and BMAL1 proteins, which are two bHLH–PAS transcription activators that heterodimerize and activate clock genes. These proteins bind to E-box elements—the sequence of which is CACGTG—that are present in clock gene promoters (Bell-Pedersen et al, 2005; Reppert & Weaver, 2002). The clock genes Per and Cry encode additional elements of the core clock machinery, and function as heterodimeric complexes that translocate to the nucleus and inhibit CLOCK:BMAL1-mediated transcription through direct protein–protein interactions (Okamura et al, 2002, and references therein). Therefore, at their core, circadian rhythms are supported by intracellular transcriptional and translational feedback loops that perpetuate oscillations in gene expression. Timekeeping in this system is complex: auxiliary enzymes impinge on the circadian proteins by mechanisms that include methylation, acetylation, phosphorylation and ubiquitination, which keep the core clock strictly timed, yet surprisingly plastic and able to adapt to changes in its environment (Belden & Dunlap, 2008; Lee et al, 2004; Naidoo et al, 1999; Nakahata et al, 2008; Toh et al, 2001). Although SCN neurons are responsive to zeitgebers, such as light, their molecular rhythms are self-sustaining and continue to oscillate in a circadian manner even in ‘free running' (constant dark) conditions.
Cells rhythmically synthesize as much as 10% of their transcripts (Duffield et al, 2002; Lowrey & Takahashi, 2004; Panda et al, 2002). As these transcripts include those involved in neuronal signalling and synaptic plasticity, it is conceivable that many of the functions of the brain occur within a changing, circadian phase-dependent sensitivity to stimuli. With this in mind, determining how memory formation and maintenance is affected by circadian systems at the molecular level is crucial for explaining the behavioural link between the two. The existence of interplay between memory and circadian rhythmicity is not a new observation; however, numerous experiments from the past few decades underscore the complexity of the interaction. One question that emerges from these studies is whether memory training functions as a type of zeitgeber for the hippocampus. Considering the similarities with which light entrains the central oscillator, it is possible that training actually functions as a zeitgeber of sorts in regions of the brain that are responsible for temporary encoding and storage of memory. Moreover, the contribution of clock genes to memory formation remains largely unexplored. Although some clock-gene-knockout organisms have been tested for memory deficits, more spatially and temporally confined gene-targeting strategies would be useful for determining the precise role—if any—that these genes have in synaptic plasticity and memory formation. Finally, the molecular consequences of sleep—which is controlled by both circadian and homeostatic processes—in memory circuits remain an enigma. A more detailed look at memory-activated neuron ensembles within the context of sleep and circadian time might improve our understanding of how these processes cooperate at the molecular level and how they might be coordinated to maximize information storage.
Does memory training function as a zeitgeber?
There is considerable interest in the molecular mechanisms that underlie LTM (Sidebar B). LTM formation differs from short-term memory (STM) formation in that the latter occurs rapidly and involves changes in the activity of existing proteins to mediate synaptic responses, whereas the former depends on de novo transcription and translation, and therefore requires considerably more time. Much of the progress made in the field of memory is based on discoveries about long-term potentiation (LTP), which is the enduring increase in synaptic response that occurs after strong, repetitive stimulation. Although LTP can be NMDA-receptor-dependent or NMDA-receptor-independent (Grover & Teyler, 1990; Nicoll & Malenka, 1999), it also requires new protein synthesis for consolidation (Abraham & Williams, 2008). The time-dependent process of memory formation is often referred to as cellular consolidation and occurs over several hours. The subsequent transfer of memories from the hippocampus to the cortex seems to take much longer—probably weeks to months—and might rely on sleep-dependent communication between the two structures (Ji & Wilson, 2007). Once a memory has been redistributed to the neocortex, it is commonly referred to as ‘remote' (Frankland & Bontempi, 2005).
Sidebar B | In need of answers.
Does the hippocampus function as a ‘slave' oscillator or a self-autonomous one?
How do clock genes contribute to synaptic plasticity?
Does memory training function as a zeitgeber for hippocampal neurons?
How does circadian rhythmicity in the hippocampus promote temporary memory maintenance?
Is circadian rhythmicity necessary for the redistribution of hippocampus-dependent memory to the cortex?
The contributions of the MAPK and cAMP signalling pathways to memory consolidation are well established (Sweatt, 2004, and references therein). Therefore, recent evidence that cAMP and MAPK activity oscillate in the hippocampus allows speculation as to whether they might provide a point of intersection between the circadian clock and memory. Interestingly, several studies support the idea that the signalling pathways that mediate hippocampal long-term plasticity must be reactivated to consolidate memory, perhaps even for its maintenance as a ‘remote', cortically dependent entity (Bekinschtein et al, 2007; Cui et al, 2004; Schulz et al, 1999; Trifilieff et al, 2006).
The idea of reactivation, perhaps accentuated by circadian rhythmicity, could make sense considering the persistent nature of LTP and LTM. LTP, which is thought to be a prerequisite for LTM formation, occurs together with circadian rhythmicity in mice and a similar circadian dependence has been observed in other species (Chaudhury et al, 2005). Interestingly, in vivo cortical LTP in rodents is partly occluded after hours of wakefulness, when the net synaptic facilitation is high; however, it is again inducible after several hours of sleep, during which cortical and hippocampal synaptic strength undergoes a net depression (Vyazovskiy et al, 2008). This time-dependent variation in cortical plasticity is paralleled in the hippocampus. The observed changes in net plasticity seem to be predominantly dependent on sleep, although signals from the SCN and surrounding hypothalamic circuits might also contribute.
Circadian effects on memory formation and maintenance have also been observed. For example, SCN lesions impair hippocampus-dependent LTM in rodents (Stephan & Kovacevic, 1978). Similarly, circadian disruptions imposed by other means—such as light-phase shifting—can also produce retrograde amnesia (Devan et al, 2001; Tapp & Holloway, 1981), indicating that circadian rhythmicity could have a distinct role in memory storage. The crosstalk between circadian rhythms and memory has been shown in many species, ranging from Aplysia to humans; however, our understanding of the molecular events connecting these two physiological processes is limited (Chaudhury & Colwell, 2002; Devan et al, 2001; Hauber & Bareiss, 2001; Holloway & Wansley, 1973; Leirer et al, 1994; Lyons et al, 2006; Tapp & Holloway, 1981; Valentinuzzi et al, 2004). One recent study that used a hippocampus-dependent novel object-recognition task brings GABA signalling to the forefront of the processes (Ruby et al, 2008). In this study of the nocturnal Siberian hamster (Phodopus sungorus), the normal circadian sensitivity for learning a new object-recognition task was absent when animals were made arrhythmic by a single 2-h exposure to light during the night (zeitgeber time 17) followed by a 3-h phase delay the next day. Interestingly, GABA antagonists restore the circadian sensitivity of learning the task. Similarly to GABA, circulating levels of melatonin might also affect memory encoding. Melatonin is released by the pineal gland and its levels oscillate notably over the circadian cycle in zebrafish, as they do in humans. Recent studies in zebrafish indicate that high levels of melatonin at night impair nocturnal memory acquisition, which is an effect that can be rescued by treatment with a melatonin receptor antagonist or by pinealectomy (Rawashdeh et al, 2007). Melatonin might also affect memory consolidation, as pinealectomized and sham-operated animals were both able to acquire active-avoidance conditioning during the subjective night, whereas pinealectomized animals showed better long-term retention than their sham-operated controls. In addition to its role in memory encoding, additional studies implicate the clock in memory consolidation. For example, circadian phase shifting seems to be particularly detrimental for the consolidation and maintenance of spatial memory. Rats that are phase-shifted before and throughout place-acquisition training for the Morris water maze—which is a predominantly hippocampus-dependent task—show normal acquisition but have impaired retention when tested for LTM days later, even if normal circadian rhythmicity is fully restored (Devan et al, 2001). Interestingly, tests performed using C-3H mice and the melatonin-deficient C-57/6J mice show circadian sensitivity in hippocampus-dependent memory encoding and recall, as well as in its extinction (Chaudhury & Colwell, 2002). Several recent studies indicate that wakefulness supports encoding and sleep promotes consolidation (Donlea et al, 2009; Rasch et al, 2007). Admittedly, it is difficult to separate the effects of circadian rhythmicity on memory maintenance from those of sleep, as most manipulations of the circadian cycle also involve sleep disruption. Nevertheless, these studies are beginning to bridge a gap in our understanding of how the biological clock affects different memory processes, although the cellular consequences of time-keeping in neurons remain vague. Why does the circadian clock sensitize the hippocampus to memory formation and maintenance, and how does LTM consolidation intersect with circadian rhythmicity on a cellular level?
One possibility is that memory training actually functions as a zeitgeber that is able to induce an immediate response that reflects the timing of input relative to existing intracellular oscillations, while altering the phase or amplitude of subsequent cycles. The similarities between memory-training-induced signalling and zeitgeber-induced molecular events in the SCN are remarkably homogeneous. This is the case in SCN neurons, for example, which individually keep time. When activated by light, the SCN responds to glutamate released from retinohypothalamic neurons, which is an event that precipitates signalling cascades that are notably similar to those induced in the hippocampus after memory training. Both inputs activate NMDA receptors, resulting in calcium-induced increases in cAMP production (O‘Neill et al, 2008; Shimizu et al, 2000; Vindlacheruvu et al, 1992; Wong et al, 1999). The activation of the MAPK pathway is necessary both for light-induced phase shifting in the SCN and for memory consolidation, and is a prerequisite for MSK1 activation and for CREB phosphorylation and activity in both systems (Butcher et al, 2005; Obrietan et al, 1999; Sindreu et al, 2007; Tischkau et al, 2003; Travnickova-Bendova et al, 2002). Chromatin remodelling also contributes to light-induced phase shifting and the consolidation of hippocampal memories (Naruse et al, 2004; Sweatt, 2009). In the light of these similarities, is it possible that memory training actually entrains memory-specific neuronal ensembles in the hippocampus? This question could be addressed by tracking individual neurons after training using a bioluminescent reporter that is specific for training-activated neurons. Other studies using such tools provide precedents for this approach (Han et al, 2009; Impey et al, 1998). Using such a reporter, it might be possible to determine whether circadian oscillations in training-activated neurons are amplified or phase shifted. Such detailed analysis would be challenging, but would shed light on the temporal organization of memory encoding and temporary maintenance in the presence of already oscillating pathways. Using light-induced alterations in circadian clock gene expression as a model, Fig 1 depicts possible ways in which memory training might affect signalling within individual neurons or neuron ensembles. Memory training is known to induce at least temporary increases in cAMP, MAPK activation and CREB-mediated gene transcription; therefore, phase-response curves are drawn to model what initially occurs after a training event—that is, an immediate spike in cAMP and MAPK activity—followed by phase responses observed in the circadian system after the administration of a zeitgeber at various points in the circadian cycle. Although speculative, it is possible that training either amplifies or phase shifts circadian rhythms in neuron ensembles and, by doing so, marks them for subsequent replay or sleep-dependent communication. Alternatively, phase coupling might also occur, meaning that cells with oscillators functioning out of phase to one another might adjust so as to relay information collectively for further consolidation.
Do clock genes contribute to memory formation?
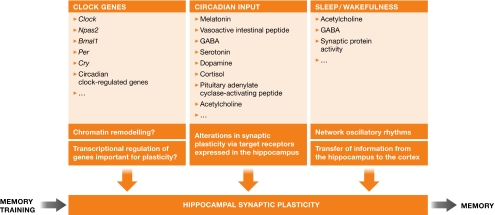
The signalling required for memory consolidation mirrors, in part, that which takes place in the SCN in response to light. So, where do clock genes fit into memory encoding (Fig 2)? The expression of some clock genes in the hippocampus is relatively robust, although whether they have an essential role in synaptic plasticity is unclear. The CREB-driven Per1 and Per2 genes are expressed and reported to oscillate in both the dentate gyrus and CA1 regions of the hippocampus (Feillet et al, 2008), and Clock and the Clock homologue Npas2 are strongly expressed in the forebrain. The oscillation of the Per2 gene in the dentate gyrus has been reported to be abolished by lesion of the SCN, indicating that the hippocampus might function as a ‘slave' oscillator (Lamont et al, 2005). To understand better the role of the clock genes in memory formation, several organisms that are deficient in clock gene expression have been analysed for memory defects. Rodents that lack functional PER1 and PER2 show no deficits in spatial memory (Feillet et al, 2008; Zueger et al, 2006), whereas mice that lack Npas2 in the forebrain have impaired hippocampus-dependent context and cued fear memory (Garcia et al, 2000). Unlike in rodents, the PER protein seems to be necessary for memory in Drosophila melanogaster, in which per mutants show deficits in experience-dependent courtship and the overexpression of PER improves LTM (Sakai et al, 2004). The role of PER in consolidation is now thought to be mediated, at least in part, by generating the need to sleep that precedes LTM training (Donlea et al, 2009). Bmal1—the only circadian gene essential for circadian rhythmicity—is thought to be negatively regulated by MAPK (Sanada et al, 2002), indicating that MAPK oscillations in the hippocampus might impinge on CLOCK:BMAL1-driven or NPAS2:BMAL1-driven gene expression. However, it is unknown whether Bmal1-deficient animals have specific memory impairments.
Figure 2.
Memory training: one hippocampal input among many. Memory consolidation occurs within the context of fluctuating states of synaptic plasticity in the hippocampus. The regulation of hippocampal neurons by the sleep-wake cycle, circadian cues and, possibly, the expression of circadian clock genes dynamically alters the state of plasticity on which a training response can be generated. A few of the molecules and proteins that could link the circadian clock and sleep to hippocampal plasticity are listed, as well as how they might affect memory consolidation mechanistically. The integration of these inputs in the hippocampus might determine the functional output, memory, at the level of formation and maintenance. Bmal1, brain and muscle aryl hydrocarbon receptor nuclear translocator (Arnt)-like protein-1; Cry, cryptochrome; GABA, γ-aminobutyric acid; Npas2, neuronal period–aryl hydrocarbon receptor nuclear translocator–single-minded domain protein 2; Per, period.
An important consideration when interpreting data from circadian mutant animals is that circadian genes contribute to other neuronal processes and might, therefore, indirectly affect memory formation. For example, the arrhythmic Cry1−/− and Cry2−/− mice show aberrant sleep patterns, and therefore abnormal sleep could affect memory in these animals (Wisor et al, 2002; Van der Zee et al, 2008). Furthermore, the Bmal1−/− mice age prematurely and their ageing is accompanied by increased oxidative stress in numerous tissues (Kondratov et al, 2006). Memory studies in rodents with tissue-specific alterations in the clock machinery would be useful to determine the exact role—if any—that circadian clock genes have in hippocampus-dependent memory. For example, ablating genes such as Bmal1 in a spatially restricted manner would allow one to determine whether circadian disruptions—such as those elicited by SCN lesions—affect behaviour because the hippocampus functions as a ‘slave' oscillator or because an oscillation intrinsic to the hippocampus has been abolished. Such spatially restricted interference with clock gene expression could be addressed by the administration of one of several viruses known to be effective at gene delivery in the hippocampus. In addition, transgenic mouse models such as the one engineered for Per1-driven luciferase expression might be used to monitor PER1 expression after training. This would allow determination of whether individual cells or groups of cells that have circadian rhythmicity in clock gene expression are activated or undergo a phase response after training.
The abundant expression of CLOCK in hippocampal neurons raises additional interesting questions. For example, the recent discovery that CLOCK functions not only as a transcriptional activator but also as a histone acetyltransferase (Doi et al, 2006; Nader et al, 2009; Nakahata et al, 2007) indicates that CLOCK could induce chromatin remodelling in the hippocampus as well. The intrinsic acetyltransferase activity of the CLOCK protein is necessary for circadian rhythmicity and mediates sufficient chromatin remodelling to enable rhythmic clock gene expression, as well as for direct acetylation of its binding partner BMAL1 (Hirayama et al, 2007). Chromatin remodelling at relevant gene promoters is reported to be an important component of circadian rhythms in the SCN as well as of memory persistence in the hippocampus (Borrelli et al, 2008; Naruse et al, 2004; Sweatt, 2009). Specifically, memory consolidation requires chromatin remodelling at memory-activated gene target sites, such as at the Bdnf promoter (Lubin et al, 2008). CLOCK or NPAS2 might also contribute transcriptionally to the generation of E-box-driven promoter transcripts in the hippocampus, which could be determined easily by established biochemical and histological methods. However, the presence of circadian clock machinery in hippocampal neurons does not make clock proteins essential for memory formation or maintenance; until the study of clock gene expression is limited to individual hippocampal neurons, it is premature to propose the mechanism by which they could contribute to memory formation.
The enigmatic role of sleep in memory
Perhaps one of the more interesting and controversial topics associated with circadian rhythms and memory is that of sleep. It is evident that both circadian and homeostatic processes contribute to sleep (Dijk & von Schantz, 2005). The neuronal replay of experiences during sleep is supported by in vivo electrophysiology and remains one of the most convincing pieces of evidence that sleep intersects with memory by reactivating already assimilated information (Ji & Wilson, 2007; Shank & Margoliash, 2009). Evidence is growing that non-rapid eye movement, particularly slow-wave sleep (SWS), might assist in the consolidation and strengthening of hippocampus-dependent, declarative forms of memory (Gais & Born, 2004). This idea is supported by sleep-deprivation studies (Walker & Stickgold, 2006, and references therein), although it is underscored by the fact that learning also alters the architecture of post-training SWS. Some studies indicate that wakefulness can generate a cellular, homeostatic need to sleep (Ganguly-Fitzgerald et al, 2006; Huber et al, 2004). These studies show that slow-wave activity—which is a marker of need for sleep—can be induced locally by learning and correlates with increased performance of memory tasks after a period of sleep. The increase in need for sleep rises throughout wakefulness and correlates with specific but widespread alterations in synaptic makers that result in a net synaptic potentiation (Gilestro et al, 2009; Vyazovskiy et al, 2008). Conversely, sleep is associated with a net synaptic depression in the cortex and hippocampus. Increasing the amount of SWS through electrical stimulation or by the presentation of training-associated cues—such as odorants—during SWS produces enhanced hippocampus-dependent memory performance (Rasch et al, 2007). Transcranial magnetic stimulation, which effectively mimics the patterns of synchronized neuronal activity—such as spindles and slow waves—that occur during SWS (Massimini et al, 2007) also increases LTM performance for some associative learning tasks in humans (Marshall et al, 2006). A recent study has provided evidence that the increased sleep need acquired through learning is controlled directly by clock neurons in Drosophila (Donlea et al, 2009), and that it depends on cAMP signalling within these circuits. In humans, the homeostatic drive to sleep also seems to be related directly to the clock, and activity in the suprachiasmatic area is inversely correlated with homeostatic sleep pressure (Schmidt et al, 2009). Finally, direct in vivo electrophysiological measurements in rodents, primates and birds have shown that SWS might provide the replay necessary for refinement or consolidation of memory at activated synapses (Kudrimoti et al, 1999; Lee & Wilson, 2002; Nadasdy et al, 1999; Pavlides & Winson, 1989; Wilson & McNaughton, 1994), although such replay involves substantial time compression.
It is clear that waking experiences are replayed during sleep. Does this depend on the circadian rhythmicity that exists within memory circuits or the rhythmicity acting on them? Again, current imaging technology places the answers to questions such as these within reach. For example, using bioluminescence to track neurons activated by a memory task, one could follow changes in gene expression that are specific to consolidation during or after SWS manipulation—such as by transcranial stimulation—while monitoring the expression of circadian-regulated genes within memory circuits. SCN lesions impair consolidated sleep sessions; however, would replay be impaired by a hippocampus-specific deletion of Bmal1 or Npas2? As fibre-optic fluorescence technology in the brain develops, the monitoring of freely behaving rodents will be useful in addressing these questions experimentally.
The discovery that most cells in the body have a cell-autonomous circadian oscillator (Nagoshi et al, 2004; Yoo et al, 2004) gives a new perspective on how signalling in individual cells and groups of cells might contribute to a physiological response. In the case of memory formation, the presence of circadian variations in cortical and hippocampal cells (Cirelli & Tononi, 2000; Prolo et al, 2005; Wakamatsu et al, 2001), as well as the vast number of reports implicating circadian rhythmicity in memory formation and maintenance, indicate that there are molecular links yet to be explored. As discussed, the details of the interaction between circadian rhythmicity and memory formation need to be demonstrated at the molecular and cellular level. However, with new imaging tools and more sophisticated conditional gene-targeting schemes these studies can and should be performed in vivo. In the end, memory formation and circadian rhythms probably interact in a similar manner to cogs and gears; however, the elucidation of their spatial and temporal connectivity awaits further experimentation.

Kristin L. Eckel-Mahan

Daniel R. Storm
References
- Abraham WC, Williams JM (2008) LTP maintenance and its protein synthesis-dependence. Neurobiol Learn Mem 89: 260–268 [DOI] [PubMed] [Google Scholar]
- Bekinschtein P, Cammarota M, Igaz LM, Bevilaqua LR, Izquierdo I, Medina JH (2007) Persistence of long-term memory storage requires a late protein synthesis- and BDNF-dependent phase in the hippocampus. Neuron 53: 261–277 [DOI] [PubMed] [Google Scholar]
- Belden WJ, Dunlap JC (2008) SIRT1 is a circadian deacetylase for core clock components. Cell 134: 212–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell-Pedersen D, Cassone VM, Earnest DJ, Golden SS, Hardin PE, Thomas TL, Zoran MJ (2005) Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat Rev Genet 6: 544–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrelli E, Nestler EJ, Allis CD, Sassone-Corsi P (2008) Decoding the epigenetic language of neuronal plasticity. Neuron 60: 961–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher GQ, Lee B, Cheng HY, Obrietan K (2005) Light stimulates MSK1 activation in the suprachiasmatic nucleus via a PACAP-ERK/MAP kinase-dependent mechanism. J Neurosci 25: 5305–5313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury D, Colwell CS (2002) Circadian modulation of learning and memory in fear-conditioned mice. Behav Brain Res 133: 95–108 [DOI] [PubMed] [Google Scholar]
- Chaudhury D, Wang LM, Colwell CS (2005) Circadian regulation of hippocampal long-term potentiation. J Biol Rhythms 20: 225–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirelli C, Tononi G (2000) Differential expression of plasticity-related genes in waking and sleep and their regulation by the noradrenergic system. J Neurosci 20: 9187–9194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Z, Wang H, Tan Y, Zaia KA, Zhang S, Tsien JZ (2004) Inducible and reversible NR1 knockout reveals crucial role of the NMDA receptor in preserving remote memories in the brain. Neuron 41: 781–793 [DOI] [PubMed] [Google Scholar]
- Devan BD et al. (2001) Circadian phase-shifted rats show normal acquisition but impaired long-term retention of place information in the water task. Neurobiol Learn Mem 75: 51–62 [DOI] [PubMed] [Google Scholar]
- Dijk DJ, von Schantz M (2005) Timing and consolidation of human sleep, wakefulness, and performance by a symphony of oscillators. J Biol Rhythms 20: 279–290 [DOI] [PubMed] [Google Scholar]
- Doi M, Hirayama J, Sassone-Corsi P (2006) Circadian regulator CLOCK is a histone acetyltransferase. Cell 125: 497–508 [DOI] [PubMed] [Google Scholar]
- Dolci C, Montaruli A, Roveda E, Barajon I, Vizzotto L, Grassi Zucconi G, Carandente F (2003) Circadian variations in expression of the trkB receptor in adult rat hippocampus. Brain Res 994: 67–72 [DOI] [PubMed] [Google Scholar]
- Donlea JM, Ramanan N, Shaw PJ (2009) Use-dependent plasticity in clock neurons regulates sleep need in Drosophila. Science 324: 105–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffield GE, Best JD, Meurers BH, Bittner A, Loros JJ, Dunlap JC (2002) Circadian programs of transcriptional activation, signaling, and protein turnover revealed by microarray analysis of mammalian cells. Curr Biol 12: 551–557 [DOI] [PubMed] [Google Scholar]
- Eckel-Mahan KL, Phan T, Han S, Wang H, Chan GC, Scheiner ZS, Storm DR (2008) Circadian oscillation of hippocampal MAPK activity and cAMP: implications for memory persistence. Nat Neurosci 11: 1074–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feillet CA, Mendoza J, Albrecht U, Pevet P, Challet E (2008) Forebrain oscillators ticking with different clock hands. Mol Cell Neurosci 37: 209–221 [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B (2005) The organization of recent and remote memories. Nat Rev Neurosci 6: 119–130 [DOI] [PubMed] [Google Scholar]
- Gais S, Born J (2004) Low acetylcholine during slow-wave sleep is critical for declarative memory consolidation. Proc Natl Acad Sci USA 101: 2140–2144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly-Fitzgerald I, Donlea J, Shaw PJ (2006) Waking experience affects sleep need in Drosophila. Science 313: 1775–1781 [DOI] [PubMed] [Google Scholar]
- Garcia JA, Zhang D, Estill SJ, Michnoff C, Rutter J, Reick M, Scott K, Diaz-Arrastia R, McKnight SL (2000) Impaired cued and contextual memory in NPAS2-deficient mice. Science 288: 2226–2230 [DOI] [PubMed] [Google Scholar]
- Gilestro GF, Tononi G, Cirelli C (2009) Widespread changes in synaptic markers as a function of sleep and wakefulness in Drosophila. Science 324: 109–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granados-Fuentes D, Tseng A, Herzog ED (2006) A circadian clock in the olfactory bulb controls olfactory responsivity. J Neurosci 26: 12219–12225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover LM, Teyler TJ (1990) Two components of long-term potentiation induced by different patterns of afferent activation. Nature 347: 477–479 [DOI] [PubMed] [Google Scholar]
- Han JH et al. (2009) Selective erasure of a fear memory. Science 323: 1492–1496 [DOI] [PubMed] [Google Scholar]
- Hastings MH, Maywood ES, Reddy AB (2008) Two decades of circadian time. J Neuroendocrinol 20: 812–819 [DOI] [PubMed] [Google Scholar]
- Hauber W, Bareiss A (2001) Facilitative effects of an adenosine A1/A2 receptor blockade on spatial memory performance of rats: selective enhancement of reference memory retention during the light period. Behav Brain Res 118: 43–52 [DOI] [PubMed] [Google Scholar]
- Hirayama J, Sahar S, Grimaldi B, Tamaru T, Takamatsu K, Nakahata Y, Sassone-Corsi P (2007) CLOCK-mediated acetylation of BMAL1 controls circadian function. Nature 450: 1086–1090 [DOI] [PubMed] [Google Scholar]
- Holloway FA, Wansley RA (1973) Multiple retention deficits at periodic intervals after active and passive avoidance learning. Behav Biol 9: 1–14 [DOI] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Tononi G (2004) Local sleep and learning. Nature 430: 78–81 [DOI] [PubMed] [Google Scholar]
- Impey S, Smith DM, Obrietan K, Donahue R, Wade C, Storm DR (1998) Stimulation of cAMP response element (CRE)-mediated transcription during contextual learning. Nat Neurosci 1: 595–601 [DOI] [PubMed] [Google Scholar]
- Ji D, Wilson MA (2007) Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci 10: 100–107 [DOI] [PubMed] [Google Scholar]
- Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP (2006) Early aging and age-related pathologies in mice deficient in BMAL1, the core component of the circadian clock. Genes Dev 20: 1868–1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudrimoti HS, Barnes CA, McNaughton BL (1999) Reactivation of hippocampal cell assemblies: effects of behavioral state, experience, and EEG dynamics. J Neurosci 19: 4090–4101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamia KA, Storch KF, Weitz CJ (2008) Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci USA 105: 15172–15177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont EW, Robinson B, Stewart J, Amir S (2005) The central and basolateral nuclei of the amygdala exhibit opposite diurnal rhythms of expression of the clock protein Period2. Proc Natl Acad Sci USA 102: 4180–4184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AK, Wilson MA (2002) Memory of sequential experience in the hippocampus during slow wave sleep. Neuron 36: 1183–1194 [DOI] [PubMed] [Google Scholar]
- Lee C, Weaver DR, Reppert SM (2004) Direct association between mouse PERIOD and CKIepsilon is critical for a functioning circadian clock. Mol Cell Biol 24: 584–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leirer VO, Tanke ED, Morrow DG (1994) Time of day and naturalistic prospective memory. Exp Aging Res 20: 127–134 [DOI] [PubMed] [Google Scholar]
- Lowrey PL, Takahashi JS (2004) Mammalian circadian biology: elucidating genome-wide levels of temporal organization. Annu Rev Genomics Hum Genet 5: 407–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin FD, Roth TL, Sweatt JD (2008) Epigenetic regulation of BDNF gene transcription in the consolidation of fear memory. J Neurosci 28: 10576–10586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons LC, Collado MS, Khabour O, Green CL, Eskin A (2006) The circadian clock modulates core steps in long-term memory formation in Aplysia. J Neurosci 26: 8662–8671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L, Helgadottir H, Molle M, Born J (2006) Boosting slow oscillations during sleep potentiates memory. Nature 444: 610–613 [DOI] [PubMed] [Google Scholar]
- Massimini M, Ferrarelli F, Esser SK, Riedner BA, Huber R, Murphy M, Peterson MJ, Tononi G (2007) Triggering sleep slow waves by transcranial magnetic stimulation. Proc Natl Acad Sci USA 104: 8496–8501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadasdy Z, Hirase H, Czurko A, Csicsvari J, Buzsaki G (1999) Replay and time compression of recurring spike sequences in the hippocampus. J Neurosci 19: 9497–9507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader N, Chrousos GP, Kino T (2009) Circadian rhythm transcription factor CLOCK regulates the transcriptional activity of the glucocorticoid receptor by acetylating its hinge region lysine cluster: potential physiological implications. FASEB J 23: 1572–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagoshi E, Saini C, Bauer C, Laroche T, Naef F, Schibler U (2004) Circadian gene expression in individual fibroblasts: cell-autonomous and self-sustained oscillators pass time to daughter cells. Cell 119: 693–705 [DOI] [PubMed] [Google Scholar]
- Naidoo N, Song W, Hunter-Ensor M, Sehgal A (1999) A role for the proteasome in the light response of the timeless clock protein. Science 285: 1737–1741 [DOI] [PubMed] [Google Scholar]
- Nakahata Y, Grimaldi B, Sahar S, Hirayama J, Sassone-Corsi P (2007) Signaling to the circadian clock: plasticity by chromatin remodeling. Curr Opin Cell Biol 19: 230–237 [DOI] [PubMed] [Google Scholar]
- Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente LP, Sassone-Corsi P (2008) The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell 134: 329–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naruse Y, Oh-hashi K, Iijima N, Naruse M, Yoshioka H, Tanaka M (2004) Circadian and light-induced transcription of clock gene Per1 depends on histone acetylation and deacetylation. Mol Cell Biol 24: 6278–6287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA, Malenka RC (1999) Expression mechanisms underlying NMDA receptor-dependent long-term potentiation. Ann NY Acad Sci 868: 515–525 [DOI] [PubMed] [Google Scholar]
- O‘Neill JS, Maywood ES, Chesham JE, Takahashi JS, Hastings MH (2008) cAMP-dependent signaling as a core component of the mammalian circadian pacemaker. Science 320: 949–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrietan K, Impey S, Smith D, Athos J, Storm DR (1999) Circadian regulation of cAMP response element-mediated gene expression in the suprachiasmatic nuclei. J Biol Chem 274: 17748–17756 [DOI] [PubMed] [Google Scholar]
- Okamura H, Yamaguchi S, Yagita K (2002) Molecular machinery of the circadian clock in mammals. Cell Tissue Res 309: 47–56 [DOI] [PubMed] [Google Scholar]
- Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB (2002) Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 109: 307–320 [DOI] [PubMed] [Google Scholar]
- Pavlides C, Winson J (1989) Influences of hippocampal place cell firing in the awake state on the activity of these cells during subsequent sleep episodes. J Neurosci 9: 2907–2918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prolo LM, Takahashi JS, Herzog ED (2005) Circadian rhythm generation and entrainment in astrocytes. J Neurosci 25: 404–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasch B, Buchel C, Gais S, Born J (2007) Odor cues during slow-wave sleep prompt declarative memory consolidation. Science 315: 1426–1429 [DOI] [PubMed] [Google Scholar]
- Rawashdeh O, de Borsetti NH, Roman G, Cahill GM (2007) Melatonin suppresses nighttime memory formation in zebrafish. Science 318: 1144–1146 [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR (2002) Coordination of circadian timing in mammals. Nature 418: 935–941 [DOI] [PubMed] [Google Scholar]
- Ruby NF, Hwang CE, Wessells C, Fernandez F, Zhang P, Sapolsky R, Heller HC (2008) Hippocampal-dependent learning requires a functional circadian system. Proc Natl Acad Sci USA 105: 15593–15598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T, Tamura T, Kitamoto T, Kidokoro Y (2004) A clock gene, period, plays a key role in long-term memory formation in Drosophila. Proc Natl Acad Sci USA 101: 16058–16063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanada K, Okano T, Fukada Y (2002) Mitogen-activated protein kinase phosphorylates and negatively regulates basic helix–loop–helix–PAS transcription factor BMAL1. J Biol Chem 277: 267–271 [DOI] [PubMed] [Google Scholar]
- Schibler U, Sassone-Corsi P (2002) A web of circadian pacemakers. Cell 111: 919–922 [DOI] [PubMed] [Google Scholar]
- Schmidt C et al. (2009) Homeostatic sleep pressure and responses to sustained attention in the suprachiasmatic area. Science 324: 516–519 [DOI] [PubMed] [Google Scholar]
- Schulz S, Siemer H, Krug M, Hollt V (1999) Direct evidence for biphasic cAMP responsive element-binding protein phosphorylation during long-term potentiation in the rat dentate gyrus in vivo. J Neurosci 19: 5683–5692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank SS, Margoliash D (2009) Sleep and sensorimotor integration during early vocal learning in a songbird. Nature 458: 73–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu E, Tang YP, Rampon C, Tsien JZ (2000) NMDA receptor-dependent synaptic reinforcement as a crucial process for memory consolidation. Science 290: 1170–1174 [DOI] [PubMed] [Google Scholar]
- Sindreu CB, Scheiner ZS, Storm DR (2007) Ca2+-stimulated adenylyl cyclases regulate ERK-dependent activation of MSK1 during fear conditioning. Neuron 53: 79–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan FK, Kovacevic NS (1978) Multiple retention deficit in passive avoidance in rats is eliminated by suprachiasmatic lesions. Behav Biol 22: 456–462 [DOI] [PubMed] [Google Scholar]
- Stratmann M, Schibler U (2006) Properties, entrainment, and physiological functions of mammalian peripheral oscillators. J Biol Rhythms 21: 494–506 [DOI] [PubMed] [Google Scholar]
- Sweatt JD (2004) Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol 14: 311–317 [DOI] [PubMed] [Google Scholar]
- Sweatt JD (2009) Experience-dependent epigenetic modifications in the central nervous system. Biol Psychiatry 65: 191–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapp WN, Holloway FA (1981) Phase shifting circadian rhythms produces retrograde amnesia. Science 211: 1056–1058 [DOI] [PubMed] [Google Scholar]
- Tischkau SA, Mitchell JW, Tyan SH, Buchanan GF, Gillette MU (2003) Ca2+/cAMP response element-binding protein (CREB)-dependent activation of Per1 is required for light-induced signaling in the suprachiasmatic nucleus circadian clock. J Biol Chem 278: 718–723 [DOI] [PubMed] [Google Scholar]
- Toh KL, Jones CR, He Y, Eide EJ, Hinz WA, Virshup DM, Ptacek LJ, Fu YH (2001) An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science 291: 1040–1043 [DOI] [PubMed] [Google Scholar]
- Travnickova-Bendova Z, Cermakian N, Reppert SM, Sassone-Corsi P (2002) Bimodal regulation of mPeriod promoters by CREB-dependent signaling and CLOCK/BMAL1 activity. Proc Natl Acad Sci USA 99: 7728–7733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifilieff P, Herry C, Vanhoutte P, Caboche J, Desmedt A, Riedel G, Mons N, Micheau J (2006) Foreground contextual fear memory consolidation requires two independent phases of hippocampal ERK/CREB activation. Learn Mem 13: 349–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentinuzzi VS, Menna-Barreto L, Xavier GF (2004) Effect of circadian phase on performance of rats in the Morris water maze task. J Biol Rhythms 19: 312–324 [DOI] [PubMed] [Google Scholar]
- Van der Zee EA, Havekes R, Barf RP, Hut RA, Nijholt IM, Jacobs EH, Gerkema MP (2008) Circadian time-place learning in mice depends on Cry genes. Curr Biol 18: 844–848 [DOI] [PubMed] [Google Scholar]
- Vindlacheruvu RR, Ebling FJ, Maywood ES, Hastings MH (1992) Blockade of glutamatergic neurotransmission in the suprachiasmatic nucleus prevents cellular and behavioural responses of the circadian system to light. Eur J Neurosci 4: 673–679 [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G (2008) Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci 11: 200–208 [DOI] [PubMed] [Google Scholar]
- Wakamatsu H, Yoshinobu Y, Aida R, Moriya T, Akiyama M, Shibata S (2001) Restricted-feeding-induced anticipatory activity rhythm is associated with a phase-shift of the expression of mPer1 and mPer2 mRNA in the cerebral cortex and hippocampus but not in the suprachiasmatic nucleus of mice. Eur J Neurosci 13: 1190–1196 [DOI] [PubMed] [Google Scholar]
- Walker MP, Stickgold R (2006) Sleep, memory, and plasticity. Annu Rev Psychol 57: 139–166 [DOI] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL (1994) Reactivation of hippocampal ensemble memories during sleep. Science 265: 676–679 [DOI] [PubMed] [Google Scholar]
- Wisor JP, O‘Hara BF, Terao A, Selby CP, Kilduff TS, Sancar A, Edgar DM, Franken P (2002) A role for cryptochromes in sleep regulation. BMC Neurosci 3: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong ST, Athos J, Figueroa XA, Pineda VV, Schaefer ML, Chavkin CC, Muglia LJ, Storm DR (1999) Calcium-stimulated adenylyl cyclase activity is critical for hippocampus-dependent long-term memory and late phase LTP. Neuron 23: 787–798 [DOI] [PubMed] [Google Scholar]
- Yoo SH et al. (2004) PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA 101: 5339–5346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zueger M, Urani A, Chourbaji S, Zacher C, Lipp HP, Albrecht U, Spanagel R, Wolfer DP, Gass P (2006) mPer1 and mPer2 mutant mice show regular spatial and contextual learning in standardized tests for hippocampus-dependent learning. J Neural Transm 113: 347–356 [DOI] [PubMed] [Google Scholar]