Abstract
Aryl hydrocarbon receptor (AhR) is a transcription factor that works as a dioxin receptor and is also involved in various physiological phenomena, including development and cell proliferation. Here, we show that the Gα13 signal destabilizes AhR by promoting the ubiquitination of AhR. Gα13 interacts directly with AhR-interacting protein (AIP) and inhibits the interaction between AhR and AIP, a crucial interacting protein of AhR. Strikingly, a reporter gene assay and a quantitative reverse transcription–PCR analysis indicate that the Gα13 signal shows a potent inhibitory effect on the ligand-induced transcriptional activation of AhR. Gα13 results in the nuclear translocation of AhR in a ligand-independent manner. However, in the presence of active Gα13, AhR fails to form the active transcriptional complex. Taken together, we propose a new negative regulation of dioxin signalling by the G protein.
Keywords: dioxin receptor, G protein, ubiquitin
Introduction
Aryl hydrocarbon receptor (AhR) is a ligand-activated transcription factor that belongs to the bHLH-PAS (basic helix–loop–helix PER/Arnt/Sim) superfamily (Schmidt & Bradfield, 1996; Mimura & Fujii-Kuriyama, 2003). AhR is broadly expressed in various tissues and is involved both in diverse responses to dioxin and in female reproduction by regulating the expression of aromatase in ovarian cells (Baba et al, 2005).
In the absence of stimulation, AhR is localized in the cytoplasm and associates with two molecules of the molecular chaperone heat-shock protein 90 (HSP90), the co-chaperone p23 and the immunophilin-like protein AIP (AhR-interacting protein, also known as XAP2 or Ara9; Mimura & Fujii-Kuriyama, 2003). On ligand binding, AhR undergoes a conformational change and translocates into the nucleus. In the nucleus, AhR binds to Arnt and forms a transcriptionally active complex. AhR in the active complex binds to xenobiotic response element (XRE) in the promoter region of the target genes, which include many drug-metabolizing enzymes, such as P450/CYP1A (Fujii-Kuriyama & Mimura, 2005). At present, the endogenous ligand for AhR remains unknown; however, the transcriptional activity of AhR can be stimulated by various xenobiotic compounds, including 2,3,7,8-TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin) and 3-MC (3-methyl cholanthrene).
AIP facilitates the activation of AhR and contributes to the maintenance of AhR in the cytoplasm. The amino-terminal part of AIP contains regions that have homology with FK506-binding protein 12 (FKBP12) and FKBP52, but AIP does not bind to FK506 (Fig 1A). The carboxy-terminal part of AIP contains three TPR (tetratricopeptide repeat) motifs, which are involved in protein–protein interactions. AIP protects AhR from ubiquitination, resulting in stabilization (Kazlauskas et al, 2000; LaPres et al, 2000). AIP also regulates the subcellular localization of AhR, indicating that AIP is crucial in AhR signalling. It has been reported that AIP binds to other nuclear receptors, including peroxisome proliferator-activated receptor-α and thyroid hormone receptor-β1, and affects their transcriptional activity (Sumanasekera et al, 2003; Froidevaux et al, 2006). These reports indicate that AIP takes part in various nuclear receptor signalling pathways.
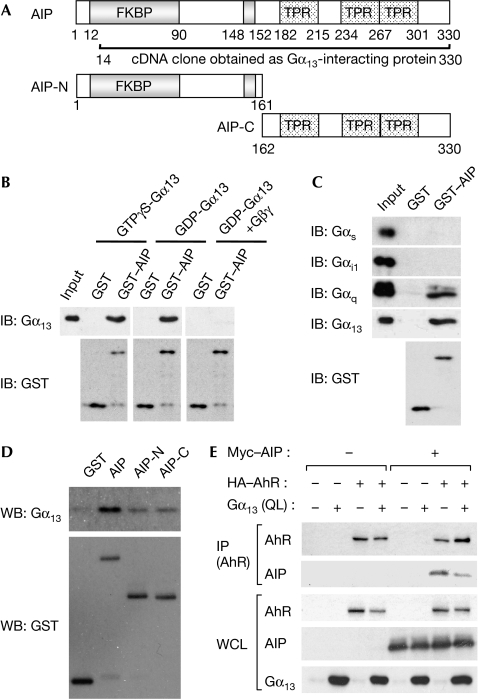
Figure 1.
AIP is identified as a Gα13-interacting protein. (A) AIP contains the FKBP domain and three TPR motifs. The AIP fragment shown was isolated by a yeast two-hybrid screen. The amino- and carboxy-terminal fragments of AIP used in subsequent experiments are also shown. (B) Recombinant Gα13 was treated with 10 μM GTPγS, 100 μM GDP or GDP plus Gβγ, and then mixed with GST or GST–AIP. The interaction between Gα13 and AIP was detected by immunoblot (IB). (C,D) The interaction between a series of Gα subunits and GST–AIP or its mutants (illustrated in (A)) was analysed by the same procedures as in (B). (E) The whole-cell lysate (WCL) of HEK293T cells transfected as indicated was used for immunoprecipitation with the HA antibody. Experiments were performed three times, and similar results were observed. AIP, AhR-interacting protein; FKBP, FK506-binding protein; GST, glutathione S-transferase; HA, haemagglutinin; HEK, human embryonic kidney; TPR, tetratricopeptide repeat.
Heterotrimeric G proteins are composed of three subunits, α, β and γ (Gα, Gβ and Gγ), and function as molecular switches that turn on intracellular signalling cascades in response to the activation of G-protein-coupled receptors (GPCRs) by extracellular stimulation including sensory signals, hormones, neurotransmitters and chemokines in mammalian cells (Gilman, 1987; Kaziro et al, 1991). G proteins are typically characterized into four main classes on the basis of the primary sequence similarity of the Gα-subunits: Gs, Gi, Gq and G12. Among them, the two members of the G12 family, G12 and G13, have been reported to interact directly with p115RhoGEF, Na+-H+ exchanger, radixin, cadherin and protein phosphatase 5 (Kurose, 2003). The α-subunits of G12 and G13 (Gα12 and Gα13) are ubiquitously expressed and coupled to the receptors, which respond to lysophosphatidic acid (LPA), sphingosine 1-phosphate (S1P) and thrombin. Although the primary structure of Gα12 and Gα13 shows 67% similarity, the physiological roles of Gα12 and Gα13 seem to be different, as only Gα13-deficient mice show the embryonic lethal phenotype and Gα12-deficient mice do not.
In the course of our study to identify the new downstream effectors of Gα13, we found that Gα13 signalling represses AhR-mediated transcription by affecting the localization and stability of AhR. We propose the new concept that AhR is negatively regulated by G-protein signalling.
Results And Discussion
Gα13 interacts with AIP
To identify new Gα13-interacting proteins, we performed a yeast two-hybrid screen by using Gα13Q226L, a mutant lacking GTPase activity, as bait. From the mouse fetal brain cDNA library, we obtained two clones, both of which encoded AIP (Fig 1A). To confirm the interaction between Gα13 and AIP, we prepared recombinant proteins and then performed in vitro pull-down analysis. As shown in Fig 1B, we observed that both the GDP and GTP forms of Gα13 interacted comparably with AIP, suggesting that the interaction between Gα13 and AIP is independent of GDP/GTP-binding status. Next, we tested the effect of the Gβγ-subunit on the association between Gα13 and AIP. The addition of Gβγ effectively abolished the interaction between Gα13 and AIP, suggesting that the dissociation of Gα13 from Gβγ seems to be required for the formation of the Gα13–AIP complex. Also, we tested the ability of other Gα-subunits—Gαs, Gαi1 and Gαq—to interact with AIP. As shown in Fig 1C, Gαq showed less binding than did Gα13; however, Gαs and Gαi1 failed to bind to AIP. As shown in Fig 1A, the C-terminus of AIP contains three TPR motifs, which are involved in protein–protein interactions (Blatch & Lässle, 1999). Some proteins containing TPR motifs, such as protein phosphatase 5 and TPR1, interact with heterotrimeric G protein through their TPR motifs (Yamaguchi et al, 2002; Marty et al, 2003). To determine the region of AIP that binds to Gα13, we prepared deletion mutants of AIP and used them for an in vitro binding assay. As shown in Fig 1D, Gα13 could interact with both the N- and C-terminal portions of AIP. It has been reported that the TPR motifs of AIP are involved in the association of AIP and AhR (Meyer et al, 2000), and that Gα13 also showed the ability to bind to the C terminus of AIP, suggesting that Gα13 might physically disturb the interaction of AhR with AIP by competition of the TPR motifs of AIP. We tested whether Gα13 counteracts the complex formation of AIP with AhR. Using human embryonic kidney 293T (HEK293T) cells expressing AIP, AhR and/or Gα13Q226L, immunoprecipitation analysis was performed. As reported previously (Carver & Bradfield, 1997), AIP interacted with AhR. However, the coexpression of Gα13Q226L suppressed the interaction between AIP and AhR (Fig 1E). AIP has been reported to form a complex with AhR and HSP90 in the cytoplasm, and this complex formation is necessary for the ligand-mediated activation of AhR (Meyer et al, 1998). Our data raise the interesting possibility that Gα13 might affect the ligand-mediated activation of AhR.
Activation of Gα13 inhibits AhR-mediated transcription
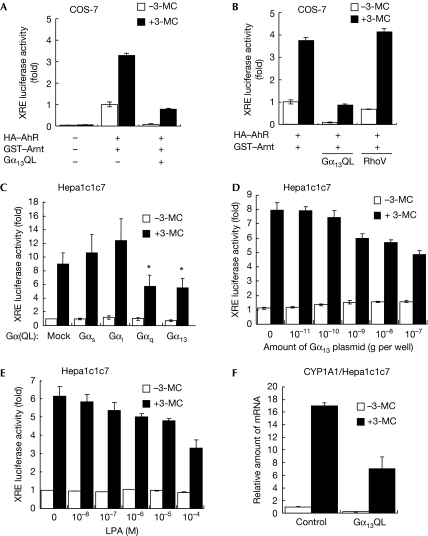
To examine whether Gα13 affects the ligand-dependent activation of AhR, we performed an XRE-driven luciferase reporter gene assay. When AhR and AIP were exogenously expressed in COS-7 cells, 3-MC induced the AhR-dependent activation of XRE, as shown in Fig 2A. Interestingly, the expression of Gα13Q226L effectively suppressed the 3-MC-induced XRE activation. Next, we examined whether a small GTP-binding protein, Rho, is involved in the inhibition of AhR by Gα13, as Gα13 induces the activation of Rho through p115RhoGEF. An active mutant of RhoA, RhoA(G14V), failed to affect the 3-MC-induced luciferase activity (Fig 2B). These data suggest that the active Gα13 inhibits the activation of AhR in a RhoA-independent manner. Next, we tested the effect of various Gα-subunits on endogenous activation of AhR using Hepa1c1c7 cells, which express highly the endogenous AhR and AIP. As shown in Fig 2C, Gα13Q226L and GαqQ209L inhibited the XRE-driven luciferase activity and Gα13Q226L showed the dose-dependent inhibition of AhR (Fig 2D). The Gα specificity of suppression of luciferase activity was correlated with the ability of Gα to bind to AIP (Figs 1C, 2C), implying that the interaction of Gα with AIP could trigger the suppression of AhR. LPA is known to activate Gα13 through its receptor. Stimulation by LPA also suppressed 3-MC-induced activation of AhR in a dose-dependent manner (Fig 2E), indicating that the physiological activation of the Gα13 signal suppresses the activation of AhR. Next, we investigated the effect of Gα13 on AhR-induced CYP1A1 expression using quantitative reverse transcription–PCR (RT–PCR) analysis. In Hepa1c1c7 cells, 3-MC induced the expression of CYP1A1, and this induction was markedly decreased when Gα13Q226L was expressed (Fig 2F). These data suggest that the activation of Gα13 inhibits the AhR-mediated transcriptional activity.
Figure 2.
Activated Gα13 inhibits AhR-mediated transcriptional activity. (A,B) COS-7 cells transfected as indicated were stimulated with 1 μM 3-MC and then used for the luciferase reporter gene analysis. (C,D) Hepa1c1c7 cells expressing constitutively active mutants of Gα were stimulated with or without 1 μM 3-MC for 24 h, and then the luciferase reporter gene analysis was performed. In (D), Hepa1c1c7 cells were transfected with various amounts of Gα13Q226L. (E) Hepa1c1c7 cells harbouring a reporter gene were pretreated with various concentrations of LPA, and were then stimulated with 3-MC and used for reporter gene analysis. (F) Hepa1c1c7 cells transfected with or without Gα13Q226L were treated with 1 μM 3-MC for 12 h. The expression of CYP1A1 was analysed by the quantitative RT–PCR method. Error bar means s.e. (n=3, *P<0.05). 3-MC, 3-methyl cholanthrene; AhR, aryl hydrocarbon receptor; GST, glutathione S-transferase; HA, haemagglutinin; LPA, lysophosphatidic acid; RT–PCR, reverse transcription–PCR.
The localization of AhR is altered by Gα13
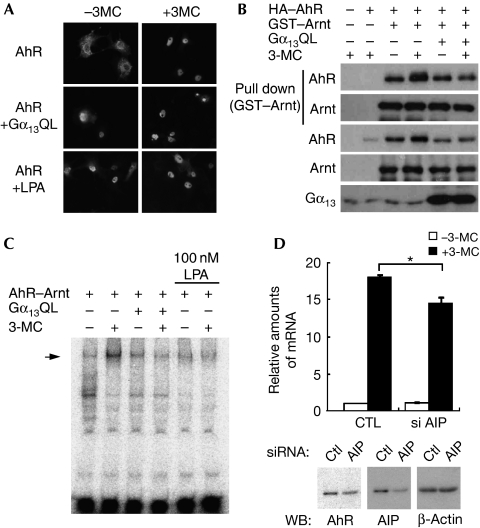
AhR is a nucleocytoplasmic shuttling protein, and AIP is crucial in the cytoplasmic localization of AhR (Petrulis et al, 2003). To examine the effect of Gα13 on the localization of AhR, we examined by using immunofluorescence analysis where AhR localizes under the activation of Gα13. Haemagglutinin-tagged AhR expressed in COS-7 cells was observed in the cytoplasm in quiescent cells. Once cells were stimulated by 3-MC, AhR translocated to the nucleus (Fig 3A). Interestingly, the expression of Gα13Q226L and LPA stimulation resulted in the nuclear translocation of AhR despite the absence of 3-MC (Fig 3A). It is known that an interaction between AhR and AIP is required for the localization of AhR in the cytoplasm, as well as for the ligand-mediated transcriptional activation of AhR (Meyer et al, 1998). As Gα13 inhibited the association of AhR with AIP (Fig 1E), the Gα13-induced dissociation of AhR from AIP might lead to the translocation of AhR to the nucleus. Although 3-MC induced the translocation of AhR to the nucleus followed by the transactivation of AhR, the nuclear localization of AhR induced by Gα13 failed to induce the transactivation of AhR (Fig 2). To investigate whether nuclear-accumulated AhR by Gα13 is not in an ‘active state', we examined the effect of Gα13 on the ability of AhR to interact with Arnt. We introduced GST–Arnt and AhR with or without Gα13Q226L into COS-7 cells, and then GST pull-down analysis was performed to detect the AhR/GST–Arnt protein complex. As shown in Fig 3B, the stimulation with 3-MC enhanced the complex formation of Arnt and AhR (Fig 3B, lanes 3 and 4). Interestingly, the expression of Gα13Q226L prevented the 3-MC-induced complex formation of Arnt and AhR (Fig 3B, lanes 5 and 6). This result suggests that AhR accumulated in the nucleus by Gα13 is not in an active complex. To verify this hypothesis, we next tested by using an electrophoretic mobility shift assay (EMSA) whether the Gα13 signal affects the binding activity of AhR to XRE. When AhR and Arnt were ectopically expressed in COS-7 cells, the nuclear extract from the cells treated with 3-MC showed binding activity to a DNA probe including XRE (supplementary Fig 1 online). The expression of Gα13Q226L prevented the 3-MC-induced DNA-binding activity of AhR (Fig 3C). LPA also inhibited the formation of the AhR–DNA complex. It is well established that the transcriptional activation of AhR requires a ligand-induced conformational change that confers the ability of nuclear translocation and heterodimer formation of AhR with Arnt. This heterodimer formation leads to their binding to an XRE element. By contrast, our results indicated that Gα13 induces the nuclear accumulation of AhR without ligand stimulation. However, in the presence of the Gα13 signal, AhR showed no ability to form the complex with Arnt and failed to bind to XRE, because of lacking the conformational change of AhR. Next, to test whether sequestering AIP from the AhR complex is enough for the Gα13-induced suppressive effect on the activation of AhR, we used RNA interference to knock down AIP. The activation of AhR was partly reduced by small interfering RNA (siRNA) of AIP but could still be activated by 3-MC stimulation (Fig 3D). In addition, we fractionated the cytoplasm and nucleus from Hepa1c1c7 cells and analysed the localization of AhR. As shown in supplementary Fig 2 online, knockdown of AIP showed a fairly weak effect on AhR localization in the nucleus without 3-MC. The knockdown of AIP did not fully mimic the effect of Gα13 on the AhR signal, indicating that Gα13 not only disturbed the association between AhR and AIP but also affected other signals to suppress the activation of AhR. Lees & Whitelaw (2002) reported that antisense oligonucleotide for AIP decreased the activation of AhR in HEK293T cells. Conversely, it has also been reported that knockdown of AIP failed to shut down ligand-induced activation of AhR (Pollenz & Dougherty, 2005). Our current result seems to fall somewhere inbetween these two reports, and we have no additional information to explain this discrepancy. However, a study using an animal experiment showed that AIP is essential for AhR signalling (Lin et al, 2008). Clarification of the physiological role of AIP is a problem that needs to be explored.
Figure 3.
The subcellular localization, dimerization with Arnt and DNA-binding activity of AhR are altered by the activation of Gα13. (A) COS-7 cells expressing HA–AhR, GST–Arnt or Gα13Q226L were stimulated with 3-MC and/or LPA for 24 h. The localization of AhR was visualized by immunostaining with the HA antibody. (B) COS-7 cells transfected as indicated were stimulated with 1 μM 3-MC for 6 h. The protein complexes were precipitated and analysed by immunoblot analysis. (C) COS-7 cells transfected with the indicated combinations of plasmids were stimulated with 1 μM 3-MC for 6 h. Nuclear extracts were analysed by EMSA with the radioactively labelled AhR-binding element DNA probe. An arrow indicates the AhR–Arnt complex binding to the probe. (D) Hepa1c1c7 cells were transfected with the indicated siRNA mixture, and stimulated with 1 μM 3-MC for 12 h. The expression of CYP1A1 was analysed by the quantitative RT-PCR method. Expression of AhR, AIP and β-actin were analysed by immunoblot. 3-MC, 3-methyl cholanthrene; AhR, aryl hydrocarbon receptor; AIP, AhR-interacting protein; Ctl, control; EMSA, electrophoretic mobility shift assay; GST, glutathione S-transferase; HA, haemagglutinin; LPA, lysophosphatidic acid; RT–PCR, reverse transcription–PCR; siRNA, short interfering RNA.
Gα13 promotes the destabilization of AhR
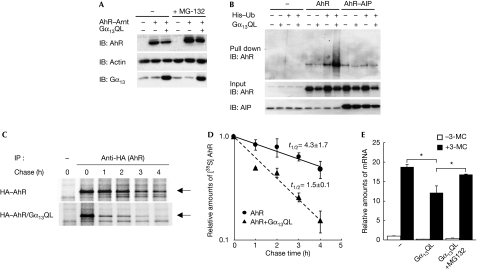
As described in the Introduction, in quiescent cells, AIP forms a stable complex with AhR and protects it from degradation by the ubiquitin–proteasome pathway. We observed that Gα13 overexpression disturbed the association of AIP with AhR, suggesting that Gα13 might impede the stabilization of AhR by AIP. We confirmed that the overexpression of Gα13Q226L resulted in the downregulation of the AhR protein (Fig 4A, lanes 2 and 3) and that the proteasome inhibitor, MG-132, restored the downregulation of AhR (Fig 4A, lanes 5 and 6). Then, we tested the effect of Gα13 on the ubiquitination of AhR. HEK293T cells were transfected with AhR and histidine-tagged ubiquitin. Ubiquitinated AhR was precipitated by Ni-agarose and detected by immunoblot. As expected, the ubiquitination of AhR was promoted by Gα13Q226L (Fig 4B, lanes 7 and 8). Conversely, the ubiquitination of AhR was suppressed by the overexpression of AIP (Fig 4B, lanes 9–12). Next, to examine whether Gα13 affects the protein stability of AhR, pulse-chase analysis was performed. The half-life of AhR was about 4.3 h in the absence of Gα13Q226L. Conversely, when Gα13Q226L was coexpressed, AhR was degraded with a half-life of 1.5 h (Fig 4C,D). These data suggest that Gα13 suppresses the activation of AhR through the destabilization of AhR by the ubiquitin–proteasome pathway. This possibility was also supported by the evidence that the expression of AhR was reduced by Gα13 expression (Figs 1E, 3B, 4B). Interestingly, as shown in Fig 1E, overexpression of AIP effectively cancelled the Gα13-induced reduction of AhR in whole-cell lysate (compare lanes 3 and 4, with lanes 7 and 8), and also in Fig 4B, coexpressed AIP diminished the ubiquitination of AhR accelerated by Gα13. These results supported our hypothesis that Gα13 competitively sequesters AIP from the AhR complex and this would be the trigger for Gα13-induced suppression of activation of AhR. Also, we tested whether inhibition of proteasome is able to cancel the Gα13-induced suppression of activation of AhR. As shown in Fig 4E, a proteasome inhibitor MG-132 showed the cancellation of the inhibitory effect of Gα13, suggesting that promoting the proteasome-mediated degradation of AhR is one of the molecular mechanisms by which Gα13 diminishes ligand-dependent activation of AhR. As shown in Fig 3, Gα13 induced the ligand-independent nuclear localization of AhR, but did not allow AhR to interact with Arnt. It has been reported that the nuclear-localized AhR is unstable and degraded more rapidly in the absence of Arnt (Roberts & Whitelaw, 1999). This report supports our model that the Gα13-induced nuclear localization of AhR might be the trigger for the degradation of AhR.
Figure 4.
Gα13 induces the ubiquitination and degradation of AhR. (A) HEK293T cells transfected as indicated were treated with or without 10 μM MG-132 for 4 h. Then, cell lysates were analysed by immunoblot (IB) analysis with the indicated antibodies. (B) HEK293T cells expressing [His]6–ubiquitin (Ub), HA–AhR and Myc-AIP with or without Gα13Q226L were lysed, and the ubiquitinated proteins were precipitated by Ni-NTA agarose. The ubiquitinated AhR, AhR and AIP in the total lysate were analysed by immunoblot. (C,D) HEK293T cells transfected as indicated were tagged metabolically with [35S]-labelled methionine and cysteine for 1 h. Then, the cells were used for pulse-chase analysis. The values of t1/2 were obtained from three independent experiments. Arrows indicate immunoprecipitated AhR. (E) Hepa1c1c7 cells were transfected as indicated and stimulated with 1 μM 3-MC for 8 h in the presence or absence of 10 μM MG-132. The expression of CYP1A1 was analysed by the quantitative RT-PCR method. 3-MC, 3-methyl cholanthrene; AhR, aryl hydrocarbon receptor; AIP, AhR-interacting protein; HA, haemagglutinin; HEK, human embryonic kidney.
Through our current research, we have also shown that Gq has a potent inhibitory effect on AhR in spite of its weak interacting activity. Several signalling molecules have been reported to regulate the AhR signal. Some of them are activated by Gαq signalling, including the transcription factor NF-κB (Harper et al, 2006). Gαq might affect AhR through these downstream molecules.
The C terminus of HSC70-interacting protein (CHIP) is known as E3 ligase, which contains three TPR motifs and a U-box domain (McDonough & Patterson, 2003), and has been reported to be involved in the ubiquitination of AhR (Lees et al, 2003). CHIP might be E3 ubiquitin ligase in the Gα13-induced degradation of AhR. Here, we propose a new model to explain how the activation of AhR is attenuated by extracellular signals. The determination of which E3 ubiquitin ligase is involved in the Gα13-induced suppression of AhR activity should be the focus of a future study.
Methods
Cell culture and transfection. HEK293T, COS-7 and Hepa1c1c7 cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% FBS and 100 μg/ml kanamycin at 37°C and 5% CO2. Transfection into HEK293T and COS-7 cells was performed using the calcium phosphate method. Hepa1c1c7 cells were transfected using Lipofectamine2000 (Invitrogen; Carlsbad, CA, USA).
RNA interference. Annealed siRNA complexes for mouse AIP and firefly luciferase were purchased from Thermo Fisher Scientific Inc. (Waltham, MA, USA). The mixture of siRNA (final concentration, 50 nM) was transfected by using Lipofectamine2000 into 35-mm dishes containing 1 × 105 Hepa1c1c7 cells. At 48 h after transfection, cells were analysed as described in the text. The sequences of siRNA were shown in supplementary Fig 2A online.
Reporter gene analysis. For reporter gene analysis, cells were plated onto a 48-well plate. COS-7 or Hepa1c1c7 cells transfected with the indicated combinations of plasmids, including haemagglutinin–AhR, GST–Arnt, FLAG–Gα13Q226L, pXRE–luciferase and pEF–RL, were stimulated with 1 μM 3-MC or 10 μM LPA (Sigma-Aldrich; St Louis, MO, USA) for 24 h (reporter gene analysis) or 12 h (RT–PCR). The reporter gene analysis was performed with the Dual Luciferase Assay kit (Promega).
EMSA. The nuclear extracts were mixed with 3 μg of poly (dI-dC; GE Healthcare; Buckinghamshire, England) and a radioactively labelled probe (2 × 104 c.p.m.) in a final volume of 25 μl of EMSA-binding buffer (10 mM HEPES-KOH (pH 7.8), 1 mM EDTA, 5 mM MgCl2, 10% glycerol and 50 mM KCl) and incubated for 20 min at 25°C. The protein–DNA complex was separated by 4.5% polyacrylamide gel using 0.5 × TGE (12.5 mM Tris, 95 mM glycine and 0.5 mM EDTA) as a running buffer and detected by autoradiography. In several experiments, the AhR or Arnt (H-172; Santa Cruz Biotechnology; Santa Cruz, CA, USA) antibody was added into the reactive mixture. The annealed oligo probe 5′-GATCCGGCTCTTGTCACGCAACTCCGAGCTCA-3′ includes the XRE sequence (shown here underlined). The oligo probe was radioactively labelled by T4-polynucleotide kinase (TOYOBO; Osaka, Japan) with [γ-32P]ATP.
Pulse-chase analysis. For pulse-chase analysis, the transfected HEK293T cells were cultured in DMEM including [35S]methionine and [35S]cysteine for 60 min. Cells were then cultured in DMEM containing 2 mM non-radioactive methionine and cysteine for the indicated periods and collected in 500 μl of RIPA buffer (10 mM Tris–HCl (pH 7.5), 150 mM NaCl, 5 mM EDTA, 1% Triton X-100 and 1% deoxycholate). [35S]-labelled AhR was precipitated with the haemagglutinin antibody, separated by SDS–polyacrylamide gel electrophoresis, and detected by autoradiography.
Supplementary information is available at EMBO reports online (http://www.emboreports.org)
Supplementary Material
Supplementary Information
Acknowledgments
We thank Dr Dirk Bohmann for providing pMT107-[His]6–ubiquitin. This study was supported by grants-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (17079006).
Footnotes
The authors declare that they have no conflict of interest.
References
- Baba T, Mimura J, Nakamura N, Harada N, Yamamoto M, Morohashi K, Fujii-Kuriyama Y (2005) Intrinsic function of the aryl hydrocarbon (dioxin) receptor as a key factor in female reproduction. Mol Cell Biol 25: 10040–10051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatch GL, Lässle M (1999) The tetratricopeptide repeat: a structural motif mediating protein–protein interactions. Bioessays 21: 932–939 [DOI] [PubMed] [Google Scholar]
- Carver LA, Bradfield CA (1997) Ligand-dependent interaction of the aryl hydrocarbon receptor with a novel immunophilin homolog in vivo. J Biol Chem 272: 11452–11456 [DOI] [PubMed] [Google Scholar]
- Froidevaux MS, Berg P, Seugnet I, Decherf S, Becker N, Sachs LM, Bilesimo P, Nygard M, Pongratz I, Demeneix BA (2006) The co-chaperone XAP2 is required for activation of hypothalamic thyrotropin-releasing hormone transcription in vivo. EMBO Rep 7: 1035–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii-Kuriyama Y, Mimura J (2005) Molecular mechanisms of AhR functions in the regulation of cytochrome P450 genes. Biochem Biophys Res Commun 338: 311–317 [DOI] [PubMed] [Google Scholar]
- Gilman AG (1987) G proteins: transducers of receptor-generated signals. Annu Rev Biochem 56: 615–649 [DOI] [PubMed] [Google Scholar]
- Harper PA, Riddick DS, Okey AB (2006) Regulating the regulator: factors that control levels and activity of the aryl hydrocarbon receptor. Biochem Pharmacol 72: 267–279 [DOI] [PubMed] [Google Scholar]
- Kaziro Y, Itoh H, Kozasa T, Nakafuku M, Satoh T (1991) Structure and function of signal-transducing GTP-binding proteins. Annu Rev Biochem 60: 349–400 [DOI] [PubMed] [Google Scholar]
- Kazlauskas A, Poellinger L, Pongratz I (2000) The immunophilin-like protein XAP2 regulates ubiquitination and subcellular localization of the dioxin receptor. J Biol Chem 275: 41317–41324 [DOI] [PubMed] [Google Scholar]
- Kurose H (2003) Gα12 and Gα13 as key regulatory mediator in signal transduction. Life Sci 74: 155–161 [DOI] [PubMed] [Google Scholar]
- LaPres JJ, Glover E, Dunham EE, Bunger MK, Bradfield CA (2000) ARA9 modifies agonist signaling through an increase in cytosolic aryl hydrocarbon receptor. J Biol Chem 275: 6153–6159 [DOI] [PubMed] [Google Scholar]
- Lees MJ, Whitelaw ML (2002) Effect of ARA9 on dioxin receptor mediated transcription. Toxicology 181–182: 143–146 [DOI] [PubMed] [Google Scholar]
- Lees MJ, Peet DJ, Whitelaw ML (2003) Defining the role for XAP2 in stabilization of the dioxin receptor. J Biol Chem 278: 35878–35888 [DOI] [PubMed] [Google Scholar]
- Lin BC, Nguyen LP, Walisser JA, Bradfield CA (2008) A hypomorphic allele of aryl hydrocarbon receptor-associated protein-9 produces a phenocopy of the AHR-null mouse. Mol Pharmacol 74: 1367–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty C, Browning DD, Ye RD (2003) Identification of tetratricopeptide repeat 1 as an adaptor protein that interacts with heterotrimeric G proteins and the small GTPase Ras. Mol Cell Biol 23: 3847–3858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough H, Patterson C (2003) CHIP: a link between the chaperone and proteasome systems. Cell Stress Chaperones 8: 303–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer BK, Petrulis JR, Perdew GH (2000) Aryl hydrocarbon (Ah) receptor levels are selectively modulated by Hsp90-associated immunophilin homolog XAP2. Cell Stress Chaperones 5: 243–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer BK, Pray-Grant MG, Vanden Heuvel JP, Perdew GH (1998) Hepatitis B virus X-associated protein 2 is a subunit of the unliganded aryl hydrocarbon receptor core complex and exhibits transcriptional enhancer activity. Mol Cell Biol 18: 978–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura J, Fujii-Kuriyama Y (2003) Functional role of AhR in the expression of toxic effects by TCDD. Biochim Biophys Acta 1619: 263–268 [DOI] [PubMed] [Google Scholar]
- Petrulis JR, Kusnadi A, Ramadoss P, Hollingshead B, Perdew GH (2003) The hsp90 co-chaperone XAP2 alters importin β recognition of the bipartite nuclear localization signal of the Ah receptor and represses transcriptional activity. J Biol Chem 278: 2677–2685 [DOI] [PubMed] [Google Scholar]
- Pollenz RS, Dougherty EJ (2005) Redefining the role of the endogenous XAP2 and C-terminal hsp70-interacting protein on the endogenous Ah receptors expressed in mouse and rat cell lines. J Biol Chem 280: 33346–33356 [DOI] [PubMed] [Google Scholar]
- Roberts BJ, Whitelaw ML (1999) Degradation of the basic helix–loop–helix/Per–ARNT–Sim hoomology domain dioxin receptor via the ubiquitin/proteasome pathway. J Biol Chem 274: 36351–36356 [DOI] [PubMed] [Google Scholar]
- Schmidt JV, Bradfield CA (1996) Ah receptor signaling pathways. Annu Rev Cell Dev Biol 12: 55–89 [DOI] [PubMed] [Google Scholar]
- Sumanasekera WK, Tien ES, Turpey R, Vanden Heuvel JP, Perdew GH (2003) Evidence that peroxisome proliferator-activated receptor α is complexed with the 90-kDa heat shock protein and the hepatitis virus B X-associated protein 2. J Biol Chem 278: 4467–4473 [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Katoh H, Mori K, Negishi M (2002) Gα12 and Gα13 interact with Ser/Thr protein phosphatase type 5 and stimulate its phosphatase activity. Curr Biol 12: 1353–1358 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information