Abstract
Axin is known to have an important role in the degradation of β-catenin in the Wnt pathway. Here, we reveal a new function of Axin at the centrosome. Axin was localized to the centrosome in various cell lines and formed a complex with γ-tubulin. Knockdown of Axin reduced the localization of γ-tubulin and γ-tubulin complex protein 2—components of the γ-tubulin ring complex—to the centrosome and the centrosomal microtubule nucleation activity after treatment with nocodazole. These phenotypes could not be rescued by the reduction in the levels of β-catenin. Although the expression of Axin rescued these phenotypes in Axin-knockdown cells, overexpression of Axin2, which is highly homologous to Axin, could not. Axin2 was also localized to the centrosome, but it did not form a complex with γ-tubulin. These results suggest that Axin, but not Axin2, is involved in microtubule nucleation by forming a complex with γ-tubulin at the centrosome.
Keywords: Axin, DIX domain, microtubule, centrosome
Introduction
Axin was originally identified as the product of the mouse gene fused and has since been shown to have a crucial role in controlling axis formation during embryonic development (Zeng et al, 1997). Mutation of the mouse fused gene was found to cause axis duplication in homozygous mouse embryos, and overexpression of Axin in Xenopus embryos inhibited the formation of the dorsal axis. As Wnt signalling activity determines dorsal–ventral duplication in vertebrates, these results suggest that Axin negatively regulates this signalling pathway. It has been found that Axin is a scaffold protein that mediates the phosphorylation of β-catenin by glycogen synthase kinase-3β (GSK-3β) in cooperation with adenomatous polyposis coli (APC) protein and Dishevelled (Dvl) (Kikuchi, 1999). These proteins form a large protein assembly that regulates the subsequent degradation of β-catenin by the ubiquitin–proteasome system.
As Axin is a scaffold protein for the Wnt pathway, Axin-binding proteins could have their own functions in the cell. The functions of some Axin-binding proteins in a microtubule organization have been studied and seem to be independent of Axin (Akhmanova & Steinmetz, 2008). For example, GSK-3, which is inactivated at the plus-ends of microtubules, mediates Par6-protein kinase Cζ-dependent promotion of cell polarization through microtubules. APC is localized to the microtubule plus-end. The binding of APC to microtubules increases the stability of microtubules, and their interaction is decreased by the phosphorylation of APC by GSK-3β. In addition, β-catenin localizes to the mitotic spindle poles and its knockdown increases the frequency of monoastral mitotic spindles (Kaplan et al, 2004). β-Catenin is also reported to localize to the centrosome and to be involved in centrosome separation (Bahmanyar et al, 2008). However, the functional relationship between Axin itself and the microtubules is not well understood. It has been reported that overexpressed Axin forms puncta throughout the cytosol in transfected cells (Kishida et al, 1999; Smalley et al, 1999; Cong et al, 2004; Schwarz-Romond et al, 2007). Here, we found that endogenous Axin is colocalized with γ-tubulin at the centrosome and show that Axin is involved in the efficient nucleation of microtubules.
Results And Discussion
Localization of Axin to the centrosome
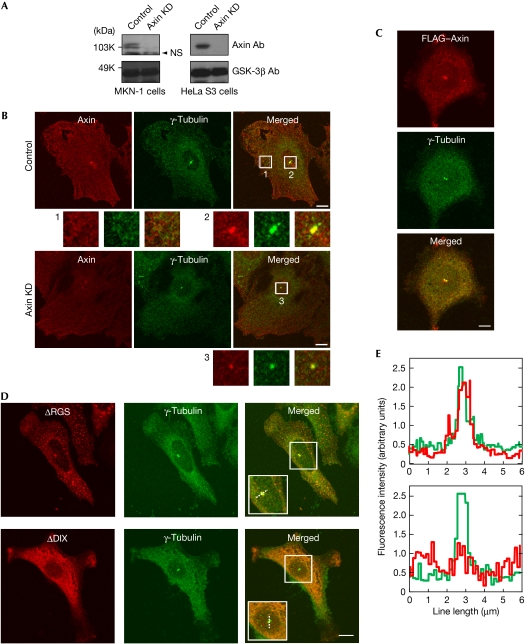
To examine the subcellular localization of Axin at an endogenous level, five types of Axin antibodies were tested to detect the levels of endogenous Axin. One antibody detected Axin proteins clearly in MKN-1, HeLa S3, human embryonic kidney 293T (HEK293T), NIH3T3 and L cells (supplementary Fig 1A online). Small interference RNA (siRNA) for Axin reduced the levels of the protein bands recognized by this Axin antibody (Fig 1A). The subcellular localization of endogenous Axin in MKN-1 cells fixed with methanol was observed as fine punctate cytoplasmic structures with a filamentous component. In addition, a clear staining of Axin was detected with that of γ-tubulin in the centre of the cell, suggesting that Axin localized to the centrosome (Fig 1B). Supporting this observation, Axin colocalized with outer dense fibre of sperm tails 2 (Odf2), a centriole marker (supplementary Fig 2 online). When Axin was knocked down, the area of Axin staining at the centrosome was reduced (Fig 1B; supplementary Fig 2 online). HeLa S3, NIH3T3 and L cells also showed the colocalization of Axin and γ-tubulin at the centrosome (supplementary Fig 1B,C online). Ectopically expressed FLAG–Axin showed various sizes of punctate structures throughout the cytoplasm of MKN-1 cells when it was expressed at a 8.3-fold higher level compared with endogenous Axin (supplementary Fig 3A,B online). When the expression level of Axin was less than 1.8-fold compared with the levels of endogenous Axin, cytoplasmic FLAG–Axin puncta became smaller, but the Axin punctate structure that colocalized with γ-tubulin and centrin 3 at the centrosome remained clear (Fig 1C; supplementary Fig 3C,D online). The centrosomal localization of FLAG–Axin was also observed in NIH3T3 cells (supplementary Fig 4 online).
Figure 1.
Localization of Axin to the centrosome. (A) The lysates of MKN-1 and HeLa S3 cells transfected with control or Axin siRNA were probed with the indicated antibodies. GSK-3β was used as a loading control. (B) Control or Axin-knockdown MKN-1 cells were stained with Axin (red) and γ-tubulin (green) antibodies. Cytoplasmic (1) and centrosomal regions (2 and 3) are enlarged. (C) MKN-1 cells expressing FLAG–Axin were stained with FLAG (red) and γ-tubulin (green) antibodies. (D) MKN-1 cells expressing FLAG–Axin deletion mutants were stained with FLAG (red) and γ-tubulin (green) antibodies. (E) The distribution of fluorescence intensity was measured along the dotted line across the centrosome shown in the boxed area of (D). Scale bars, 10 μm. Ab, antibody; DIX, Dishevelled–Axin; GSK-3β, glycogen synthase kinase-3β; KD, knockdown; NS, nonspecific band; RGS, regulator of G-protein signalling; siRNA, small interference RNA.
Axin has four unique domains (Kikuchi, 1999): regulator of G-protein signalling (RGS; APC-binding), GSK-3β-binding, β-catenin-binding and Dishevelled–Axin (DIX) domains (see Fig 3B). Among the Axin deletion mutants in which these domains were removed, FLAG–Axin–(ΔRGS), FLAG–Axin–(ΔGSK-3β) and FLAG–Axin–(Δβ-catenin) were localized to the centrosome with γ-tubulin (Fig 1D; supplementary Fig 5A online). FLAG–Axin–(ΔDIX) was distributed diffusely and was not colocalized with γ-tubulin (Fig 1D). The immunofluorescence signal intensity from γ-tubulin was completely overlaid with those from Axin–(ΔRGS), Axin–(ΔGSK-3β) and Axin–(Δβ-catenin) but not with the signal from Axin–(ΔDIX) (Fig 1E; supplementary Fig 5B online). Taken together, these results suggest that the DIX domain is necessary for the localization of Axin to the centrosome.
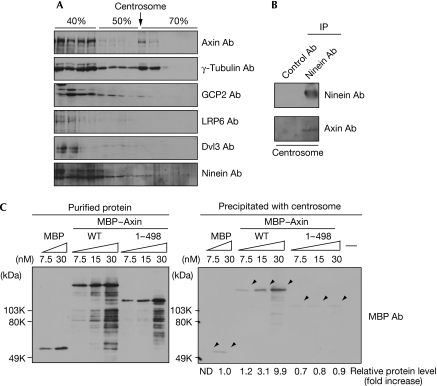
The centrosome was isolated from HeLa S3 cells by discontinuous sucrose density gradient centrifugation (Mitchison & Kirschner, 1986). The interface fraction between 50% and 70% sucrose has been shown to contain the centrosomes, as assessed by a microtubule nucleation assay, and this fraction indeed showed the presence of γ-tubulin and γ-tubulin complex protein 2 (GCP2), markers of the γ-tubulin ring complex (γ-TuRC), and ninein, a marker for the centriole (Fig 2A). Axin, but not low-density lipoprotein receptor-related protein 6 (LRP6) and Dvl3—other Wnt signal components—was observed in this fraction and coimmunoprecipitated with ninein (Fig 2A,B). To confirm the association of Axin with the centrosome, the isolated centrosome fractions were incubated with recombinant maltose-binding protein (MBP) fused to Axin (MBP–Axin). MBP–Axin, but not MBP–Axin–(1–498) and MBP, was coprecipitated with the centrosome (Fig 2C), and was not precipitated without the centrosome (data not shown). These biochemical results also support the centrosomal localization of Axin through its carboxy-terminal region.
Figure 2.
Association of Axin with the centrosome. (A) The lysates from HeLa S3 cells were fractionated by discontinuous sucrose density gradient centrifugation and probed with the indicated antibodies. The arrow indicates the centrosome-enriched fraction. (B) The centrosome fraction was immunoprecipitated (IP) with ninein antibody, and the immunoprecipitates were probed with the indicated antibodies. (C) Left panel, the indicated concentrations of recombinant MBP, MBP–Axin (WT) and MBP–Axin–(1–498) purified from Escherichia coli were probed with MBP antibodies. Right panel, MBP, MBP–Axin (WT) and MBP–Axin–(1–498) used in the left panel were incubated with the centrosome fraction isolated from HeLa S3 cells. After incubation of the mixture, the samples were separated by centrifugation and then the precipitate was probed with MBP antibodies. The levels of precipitated MBP–Axin were expressed as the fold increase compared with that of precipitated MBP. Ab, antibody; Dvl3, Dishevelled 3; GCP2, γ-tubulin complex protein 2; LRP6, low-density lipoprotein receptor-related protein 6; MBP, maltose-binding protein; ND, not determined; WT, wild type.
Complex formation between Axin and γ-tubulin
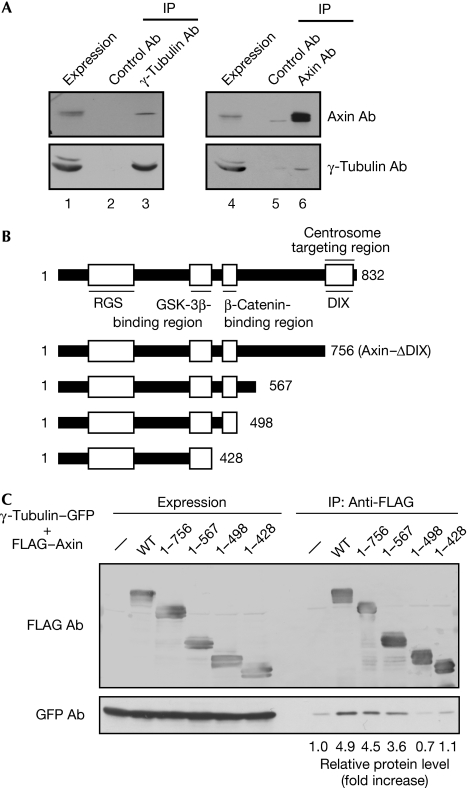
Axin and γ-tubulin were coprecipitated with each other reciprocally in HeLa S3 cell lysates (Fig 3A). To identify the region of Axin that is required for the formation of the complex with γ-tubulin, various FLAG–Axin deletion mutants were expressed in HEK293T cells. γ-Tubulin was immunoprecipitated with FLAG–Axin–(1–756), which lacks the DIX domain, to a similar efficiency as wild-type Axin (Fig 3B,C). FLAG–Axin–(1–567), but not FLAG–Axin–(1–498) or FLAG–Axin–(1–428), formed a complex with γ-tubulin (Fig 3B,C). These results suggest that Axin–(499–756) is important for the interaction between Axin and γ-tubulin, and that the region is different from the centrosome target region. Knockdown of γ-tubulin did not affect the centrosomal localization of Axin (supplementary Fig 6 online), suggesting that the presence of Axin at the centrosome is not due to binding to γ-tubulin.
Figure 3.
Complex formation of Axin with γ-tubulin. (A) The lysates of HeLa S3 cells (lanes 1 and 4) were immunoprecipitated (IP) with control (lanes 2 and 5), γ-tubulin (lane 3) or Axin (lane 6) antibodies, and the immunoprecipitates were probed with the indicated antibodies. (B) Schematic representation of the deletion mutants of Axin. (C) The lysates of HEK293T cells expressing wild-type or deletion mutants of FLAG–Axin with γ-tubulin–GFP were immunoprecipitated (IP) with FLAG antibodies and probed with the indicated antibodies. The levels of precipitated GFP–γ-tubulin were quantified and expressed as the fold increase compared with that in control cells. Ab, antibody; DIX, Dishevelled–Axin; GFP, green fluorescent protein; GSK-3β, glycogen synthase kinase-3β; HEK, human embryonic kidney; RGS, regulator of G-protein signalling; WT, wild type.
Axin and centrosomal γ-TuRC
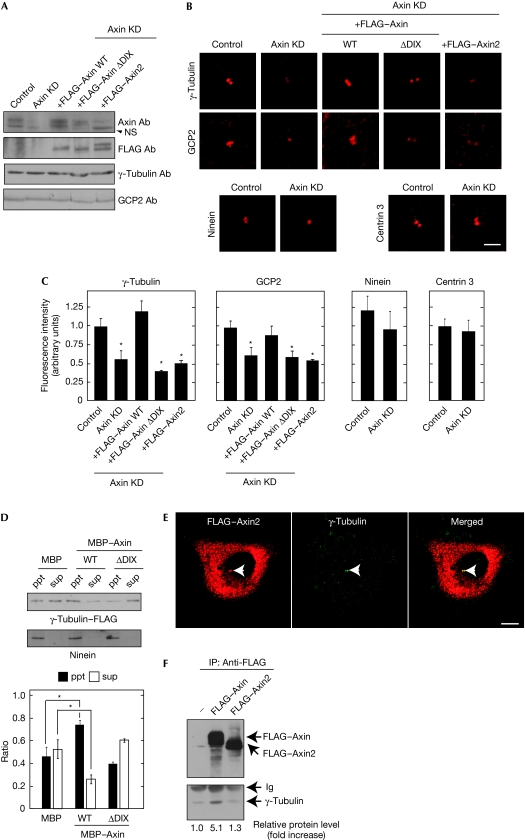
To gain insights into the function of Axin at the centrosome, Axin was depleted in MKN-1 cells by siRNA (Fig 4A). The appearance of the centrosomal and non-centrosomal microtubule arrays was not changed by the reduction of Axin protein levels (data not shown). The staining areas of γ-tubulin and GCP2 at the centrosome were reduced significantly, but those of ninein, centrin 3 and Odf2 were not affected (Figs 1B, 4B,C; supplementary Fig 2 online). As the total amounts of γ-tubulin and GCP2 were not changed by the depletion of Axin (Fig 4A), Axin seemed to be involved in the localization of γ-TuRC to the centrosome. Similar results were also observed in HeLa S3 cells (supplementary Fig 7A online). Therefore, Axin might be involved in the association of γ-tubulin to the centrosome.
Figure 4.
Role of Axin in the localization of γ-tubulin ring complex to the centrosome. (A) Control MKN-1 or MKN-1 cells stably expressing FLAG–Axin, FLAG–Axin–(ΔDIX) or FLAG–Axin2 were transfected with Axin siRNA and the lysates were probed with the indicated antibodies. (B) The same cells as prepared in (A) were stained with the indicated antibodies. Scale bar, 5 μm. (C) Fluorescence intensities from centrosomal proteins γ-tubulin, GCP2, ninein and centrin 3 in the cells used in (B) were quantified; *P<0.01. (D) Upper panel, γ-tubulin–FLAG/γ-TuRC purified from U2OS cells stably expressing γ-tubulin–FLAG were incubated with centrosomes isolated from HeLa S3 cells in the presence of MBP, MBP–Axin (WT) or MBP–Axin–(1–498). After incubation of the mixture, the samples were separated by centrifugation and then the precipitate (ppt) and supernatant (sup) fractions were probed with FLAG and ninein antibodies. Lower panel, the band intensity in the precipitate and supernatant fractions and the sum of the intensity in both fractions were measured using Image J software, and the former value was divided by the latter. The ratio of the amount of γ-tubulin–FLAG in the precipitate and supernatant fractions was expressed as arbitrary units; *P<0.01. (E) MKN-1 cells expressing FLAG–Axin2 were stained with FLAG (red) and γ-tubulin (green) antibodies. Arrowheads indicate the centrosome. Scale bar, 10 μm. (F) The lysates of HEK293T cells expressing FLAG–Axin or FLAG–Axin2 were immunoprecipitated (IP) with FLAG antibodies and probed with the FLAG and γ-tubulin antibodies. The levels of γ-tubulin coprecipitated with FLAG–Axin were quantified and expressed as the fold increase compared with that of FLAG–Axin in control cells. Ab, antibody; DIX, Dishevelled–Axin; GCP2, γ-tubulin complex protein 2; HEK, human embryonic kidney; Ig, immunoglobulin; KD, knockdown; MBP, maltose binding protein; NS, nonspecific band; siRNA, small interference RNA; γ-TuRC, γ-tubulin ring complex; WT, wild type.
The reduction in the localization of γ-tubulin and GCP2 to the centrosome by the knockdown of Axin was rescued in cells stably expressing wild-type Axin and Axin–(Δβ-catenin) that were siRNA resistant, but not in cells stably expressing Axin–(ΔDIX) (Fig 4A–C; supplementary Fig 8A–C online). These results suggest that the binding of Axin to the centrosome, but not to β-catenin, is required for this function of Axin at the centrosome. The increase in the expression levels of FLAG–Axin at the centrosome in Axin-knockdown cells showed a tendency to enhance the localization of γ-tubulin to the centrosome, although it was not statistically significant, suggesting that the expression of a certain amount of Axin at the centrosome is sufficient for the localization of γ-tubulin to the centrosome (supplementary Fig 9 online). As the levels of APC and γ-tubulin were reduced to similar degrees by siRNA in various MKN-1 cells stably expressing different proteins (supplementary Fig 10 online), the effects of Axin knockdown qre not due to the different efficiencies of siRNA-mediated knockdown.
γ-TuRC was partly purified from U2OS cells expressing γ-tubulin–FLAG and incubated with centrosomes isolated from HeLa S3 cells with or without MBP–axin in vitro (Izumi et al, 2008). γ-TuRC was precipitated with isolated centrosomes by centrifugation (ppt fraction; Fig 4D). By adding MBP–Axin, the amounts of γ-TuRC coprecipitated with the centrosomes increased, but MBP–Axin–(ΔDIX) did not affect the coprecipitation of γ-TuRC (Fig 4D). Therefore, Axin could have a role in the enhancement of γ-TuRC localization to the centrosome.
Human Axin2, which is identical to rat axil and mouse conductin, is a homologue of Axin that has similar biochemical activities in the degradation of β-catenin (Behrens et al, 1998; Yamamoto et al, 1998). In addition, Axin2 is known to be one of the target gene products induced by β-catenin and T-cell factor/lymphoid-enhancer factor (Tcf/Lef) (Lustig et al, 2002). Axin depletion induced the accumulation of β-catenin in the nucleus and markedly increased the levels of Axin2 messenger RNA in HeLa S3 cells, whereas β-catenin stabilized in Axin-knockdown MKN-1 cells did not induce an increase in the levels of Axin2 messenger RNA (supplementary Fig 11 online). The finding that the localization of γ-tubulin to the centrosome was decreased in Axin-knockdown HeLa S3 cells to a similar level to Axin-knockdown MKN-1 cells irrespective of Axin2 expression (Fig 4C; supplementary Fig 7A online) prompted an investigation into whether Axin2 shares similar abilities to Axin at the centrosome. FLAG–Axin2 was localized to the centrosome and to FLAG–Axin; however, FLAG–Axin was not associated with γ-tubulin (Fig 4E,F). Furthermore, Axin2 could not rescue the decrease in the localization of γ-tubulin and GCP2 to the centrosome in Axin-knockdown cells (Fig 4A–C). These results suggest that Axin and Axin2 have different roles in the centrosome.
Centrosomal function of Axin
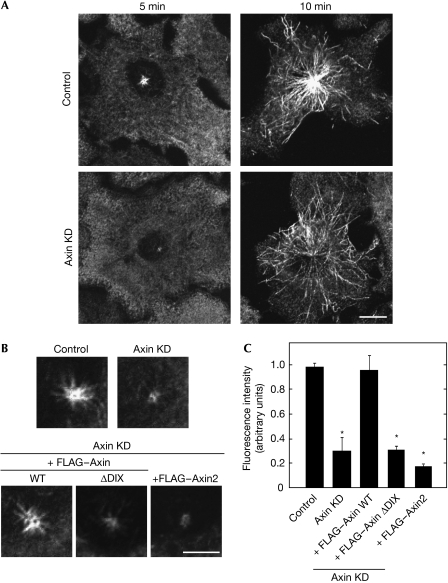
As γ-TuRC has important roles in microtubule nucleation from the centrosome (Luders & Stearns, 2007), the function of Axin in the nucleation of microtubules was tested. When microtubule aster formation was measured at 5 and 10 min after treatment with nocodazole, regrowth of centrosomal microtubules was suppressed in Axin-knockdown MKN-1 and HeLa S3 cells (Fig 5A; supplementary Fig 7B online). It is known that sub-populations of microtubules are released from the centrosome, and it appeared as if they were spread throughout the cell (Fig 5A). The density of microtubules around the centrosome in control cells was high, whereas it was low in Axin-knockdown cells, suggesting that the numbers of microtubules are reduced by the knockdown of Axin. In addition, there was no difference in aster formation between control and Axin-knockdown cells at 20 min after regrowth (data not shown). Therefore, it is likely that Axin is involved in the regulation of the initial polymerization of microtubules.
Figure 5.
The function of Axin in microtubule nucleation at the centrosome. (A) Control or Axin-knockdown (KD) MKN-1 cells were subjected to the microtubule regrowth assay and stained with β-tubulin antibodies at 5 or 10 min after regrowth. Scale bar, 10 μm. (B) Control MKN-1 or MKN-1 cells stably expressing FLAG–Axin (WT), FLAG–Axin–(ΔDIX) or FLAG–Axin2 transfected with control or Axin siRNA were subjected to the microtubule regrowth assay and stained with β-tubulin antibody at 5 min after regrowth. Scale bar, 5 μm. (C) The fluorescence intensity of microtubule asters in MKN-1 cells used in (B) was quantified and the relative intensity was expressed as arbitrary units; *P<0.01. DIX, Dishevelled–Axin; siRNA, small interference RNA; WT, wild type.
The phenotype induced by the knockdown of Axin in MKN-1 cells was rescued by expressing wild-type Axin and Axin–(Δβ-catenin), but not Axin–(ΔDIX) or Axin2 (Fig 5B,C; supplementary Fig 8D,E online). Axin–(ΔDIX) was not localized to the centrosome, and Axin2 did not form a complex with γ-tubulin. Therefore, Axin could have different regions that bind to the centrosome and γ-tubulin, and both regions are necessary for the function of Axin at the centrosome. It is also possible that Axin2 is not involved in microtubule nucleation owing to its failure to bind to γ-tubulin. It is also possible that the phenotypes observed in Axin-knockdown cells were due to the accumulation of β-catenin. However, this possibility is unlikely because neither the expression of dominant-negative Tcf-4 nor the knockdown of β-catenin could rescue the reduction in the centrosomal localization of γ-tubulin and microtubule regrowth activity in Axin-knockdown cells (supplementary Fig 12 online). These findings are consistent with the results that Axin2 overexpression did not rescue the phenotypes.
The association of γ-tubulin with the centrosome is dynamic and changes during the cell cycle. As GSK-3β is involved in the recruitment of γ-tubulin to the spindle poles in the mitotic phase (Izumi et al, 2008), the function of Axin at the centrosome in interphase might be due to GSK-3β associating with Axin. However, this possibility is unlikely because GSK-3β is involved in anchoring the microtubule minus-end to the centrosome, but not the centrosomal localization of γ-tubulin and microtubule nucleation in interphase (Fumoto et al, 2006).
Axin–(499–756), which is involved in the association with γ-tubulin, shows a low amino-acid similarity to Axin2–(468–763); therefore, the ability to interact with γ-tubulin is specific to Axin. It has been shown that Axin2 (conductin) localizes to the mitotic spindles and to the centrosome during the mitotic phase in colon cancer cells, and that Axin2 overexpression results in the generation of chromosomal instability, whereas Axin neither localizes to the mitotic spindles nor induces chromatin instability (Hadjihannas et al, 2006). Our results show that Axin is observed in the spindles but is not localized to the spindle poles in mitotic cells (supplementary Fig 13A online), and that the knockdown of Axin does not affect the distribution of each phase in mitosis (data not shown). Taken together, these results suggest that Axin and Axin2 have specific functions in the dynamics of microtubules in a cell cycle-dependent manner.
Here, a new function of Axin at the centrosome was identified. In addition, it was found that the knockdown of Axin perturbs the structure of the Golgi apparatus in interphase (supplementary Fig 13B online). As microtubules are required for determining the localization and organization of the Golgi apparatus (Rios & Bornens, 2003), the function of Axin at the centrosome might be involved indirectly in the regulation of this organelle. Clarifying the roles of Axin in these phenomena might lead to an improved understanding of the new functions of Axin in microtubule regulation.
Methods
Immunocytochemistry. The immunocytochemical analyses of the cultured cells were performed as described previously (Yamamoto et al, 2006), except that the cultured cells were fixed with 100% methanol for 10 min at −20 °C, or were simultaneously fixed and permeabilized with PBS containing 3.7% paraformaldehyde and 0.05% Triton X-100. All experiments were carried out using interphase cells. For the quantitative measurement of centrosomal proteins or microtubule asters, the background of all images was subtracted and the integrated intensity of the region of interest was measured. At least 100 cells were evaluated for each experimental group. All processing and measurements were carried out using LSM510 (Carl Zeiss, Tokyo, Japan) with Metamorph (Molecular Devices, Downingtown, PA, USA) and Image J software.
Association of γ-tubulin–FLAG/γ-TuRC, Axin and centrosomes. In vitro sedimentation assays were performed by mixing equal volumes (10 μl each) of centrosome fractions (40 ng) and purified γ-tubulin–FLAG/γ-TuRC with MBP, MBP–Axin or MBP–Axin–(ΔDIX) (Izumi et al, 2008). After incubation for 30 min at 30 °C, the mixture was separated into pellet and supernatant fractions by centrifugation at 15 000g for 10 min at 4 °C. When the association of Axin with the centrosome was examined in vitro, MBP–Axin and its deletion mutants were incubated with the centrosome fractions. The pellet was washed once with Nonidet P-40 buffer (20 mM Tris–HCl, pH 7.5, 137 mM NaCl, 1% Nonidet P-40 and 10% glycerol), twice with LiCl buffer (0.1 M Tris–HCl (pH 7.5) and 0.5 M LiCl), and once with 10 mM Tris–HCl (pH 7.5) before being resuspended in Laemmli's buffer. The samples were subjected to SDS–polyacrylamide gel electrophoresis followed by immunoblotting with the indicated antibodies.
Microtubule regrowth assay. The microtubule regrowth assay was carried out as reported previously (Delgehyr et al, 2005). Briefly, siRNA-transfected cells were treated with 20 μM nocodazole for 45 min at 37 °C. Nocodazole was then removed by washing with DMEM and the cells were incubated at 37 °C. At 5 or 10 min after regrowth, the cells were fixed with 100% methanol and stained with β-tubulin antibodies. At least 300 cells were evaluated for each experimental group.
Supplementary information is available at EMBO reports online (http://www.emboreports.org)
Supplementary Material
Supplementary Information
Acknowledgments
We thank W. Yasui, K. Matsumoto, H. Saya, Y. Ono, M. Bornens and S. Tsukita for donating cells, plasmids and antibodies. This study was supported by Grants-in-Aid for Scientific Research and for Scientific Research on Priority Areas from the Ministry of Education, Science, and Culture of Japan (2007, 2008) and by The Uehara Memorial Foundation (2007).
Footnotes
The authors declare that they have no conflict of interest.
References
- Akhmanova A, Steinmetz MO (2008) Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat Rev Mol Cell Biol 9: 309–322 [DOI] [PubMed] [Google Scholar]
- Bahmanyar S, Kaplan DD, Deluca JG, Giddings TH Jr, O'Toole ET, Winey M, Salmon ED, Casey PJ, Nelson WJ, Barth AI (2008) Beta-catenin is a Nek2 substrate involved in centrosome separation. Genes Dev 22: 91–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J, Jerchow B-A, Wurtele M, Grimm J, Asbrand C, Wirtz R, Kuhl M, Wedlich D, Birchmeier W (1998) Functional interaction of an Axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science 280: 596–599 [DOI] [PubMed] [Google Scholar]
- Cong F, Schweizer L, Varmus H (2004) Wnt signals across the plasma membrane to activate the beta-catenin pathway by forming oligomers containing its receptors, Frizzled and LRP. Development 131: 5103–5115 [DOI] [PubMed] [Google Scholar]
- Delgehyr N, Sillibourne J, Bornens M (2005) Microtubule nucleation and anchoring at the centrosome are independent processes linked by ninein function. J Cell Sci 118: 1565–1575 [DOI] [PubMed] [Google Scholar]
- Fumoto K, Hoogenraad CC, Kikuchi A (2006) GSK-3beta-regulated interaction of BICD with dynein is involved in microtubule anchorage at centrosome. EMBO J 25: 5670–5682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjihannas MV, Bruckner M, Jerchow B, Birchmeier W, Dietmaier W, Behrens J (2006) Aberrant Wnt/beta-catenin signaling can induce chromosomal instability in colon cancer. Proc Natl Acad Sci USA 103: 10747–10752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi N, Fumoto K, Izumi S, Kikuchi A (2008) GSK-3beta regulates proper mitotic spindle formation in cooperation with a component of the gamma-tubulin ring complex, GCP5. J Biol Chem 283: 12981–12991 [DOI] [PubMed] [Google Scholar]
- Kaplan DD, Meigs TE, Kelly P, Casey PJ (2004) Identification of a role for beta-catenin in the establishment of a bipolar mitotic spindle. J Biol Chem 279: 10829–10832 [DOI] [PubMed] [Google Scholar]
- Kikuchi A (1999) Roles of Axin in the Wnt signalling pathway. Cell Signal 11: 777–788 [DOI] [PubMed] [Google Scholar]
- Kishida S, Yamamoto H, Hino S-I, Ikeda S, Kishida M, Kikuchi A (1999) DIX domains of Dvl and Axin are necessary for protein interactions and their ability to regulate beta-catenin stability. Mol Cell Biol 19: 4414–4422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders J, Stearns T (2007) Microtubule-organizing centres: a re-evaluation. Nat Rev Mol Cell Biol 8: 161–167 [DOI] [PubMed] [Google Scholar]
- Lustig B et al. (2002) Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol 22: 1184–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison TJ, Kirschner MW (1986) Isolation of mammalian centrosomes. Methods Enzymol 134: 261–268 [DOI] [PubMed] [Google Scholar]
- Rios RM, Bornens M (2003) The Golgi apparatus at the cell centre. Curr Opin Cell Biol 15: 60–66 [DOI] [PubMed] [Google Scholar]
- Schwarz-Romond T, Metcalfe C, Bienz M (2007) Dynamic recruitment of axin by Dishevelled protein assemblies. J Cell Sci 120: 2402–2412 [DOI] [PubMed] [Google Scholar]
- Smalley MJ, Sara E, Paterson H, Naylor S, Cook D, Jayatilake H, Fryer LG, Hutchinson L, Fry MJ, Dale TC (1999) Interaction of Axin and Dvl-2 proteins regulates Dvl-2-stimulated TCF-dependent transcription. EMBO J 18: 2823–2835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Kishida S, Uochi T, Ikeda S, Koyama S, Asashima M, Kikuchi A (1998) Axil, a member of the Axin family, interacts with both glycogen synthase kinase-3beta and beta-catenin and inhibits axis formation of Xenopus embryos. Mol Cell Biol 18: 2867–2875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Komekado H, Kikuchi A (2006) Caveolin is necessary for Wnt-3a-dependent internalization of LRP6 and accumulation of beta-catenin. Dev Cell 11: 213–223 [DOI] [PubMed] [Google Scholar]
- Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek TJ, Perry WL III, Lee JJ, Tilghman SM, Gumbiner BM, Costantini F (1997) The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell 90: 181–192 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information