Abstract
Proper regulation of the cAMP-dependent protein kinase (protein kinase A, PKA) is necessary for cellular homeostasis, and dysregulation of this kinase is crucial in human disease. Mouse embryonic fibroblasts (MEFs) lacking the PKA regulatory subunit Prkar1a show altered cell morphology and enhanced migration. At the molecular level, these cells showed increased phosphorylation of cofilin, a crucial modulator of actin dynamics, and these changes could be mimicked by stimulating the activity of PKA. Previous studies of cofilin have shown that it is phosphorylated primarily by the LIM domain kinases Limk1 and Limk2, which are under the control of the Rho GTPases and their downstream effectors. In Prkar1a−/− MEFs, neither Rho nor Rac was activated; rather, we showed that PKA could directly phosphorylate Limk1 and thus enhance the phosphorylation of cofilin. These data indicate that PKA is crucial in cell morphology and migration through its ability to modulate directly the activity of LIM kinase.
Keywords: cell migration, cofilin, LIM kinase, protein kinase A
Introduction
Protein kinase A (PKA), or cAMP-dependent protein kinase, is an evolutionarily conserved, ubiquitously expressed serine–threonine kinase. The importance of proper regulation of PKA has been underscored by the observation that null mutations in the gene encoding the type 1A regulatory subunit (PRKAR1A) cause the inherited tumour syndrome Carney complex (CNC; Kirschner et al, 2000), and are also observed in sporadic endocrine tumours (Sandrini et al, 2002; Bertherat et al, 2003; Lania et al, 2004). Similar to human patients, Prkar1a+/− mice develop a spectrum of tumours in cAMP-responsive tissues (Kirschner et al, 2005). Analysis of Prkar1a−/− mouse embryonic fibroblasts (MEFs) showed that loss of Prkar1a led to constitutive PKA signalling and immortalization (Nadella & Kirschner, 2005). These cells also showed a shift towards an epithelial cell identity, a phenomenon echoed in CNC patient tumours (Nadella et al, 2008).
Structural organization is known to be crucial in cell fate, and PKA has a complex role in regulating cytoarchitecture. PKA phosphorylates many cytoskeletal proteins, including microtubules, intermediate filaments and actin (Shabb, 2001). Cytoskeletal-associated proteins might act as A-kinase-anchoring proteins to modulate the intracellular localization of PKA (Howe, 2004). Some characteristics of migration and actin cytoskeletal assembly are positively regulated by PKA (Feoktistov et al, 2000; O'Connor & Mercurio, 2001; Dormond et al, 2002), whereas others are inhibited (Howe & Juliano, 2000; Ellerbroek et al, 2003). Thus, the impact of activation of PKA as observed in Prkar1a−/− cells is difficult to predict. Here, we describe morphological and mobility changes in Prkar1a−/− MEFs, and report that PKA is able to modulate actin function by phosphorylation of LIM domain kinase 1 (Limk1), thereby enhancing its ability to phosphorylate cofilin, a protein that is crucial in actin filament turnover.
Results And Discussion
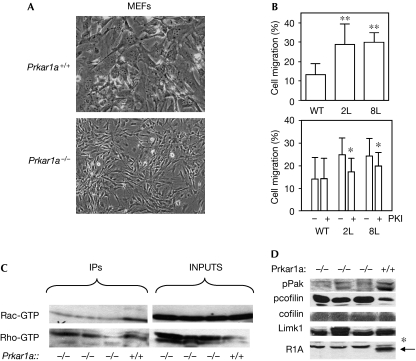
Prkar1a−/− MEFs showed altered cell shape and size, and grew in a nested pattern compared with wild-type MEFs (Fig 1A). To determine whether altered cell morphology correlated with changes in cell migration, we investigated cell mobility using a Boyden chamber assay (Fig 1B) and in a real-time wound-healing assay by electronic coupled impedance sensing (ECIS; Hadjout et al, 2001; supplementary Fig 1a online). These assays showed that Prkar1a−/− cells had enhanced migration rates compared with control MEFs. Treatment of Prkar1a−/− cells with the PKA inhibitor (PKI) peptide caused a significant reduction in migration rate, whereas control cells were unaffected. Thus, activation of PKA is associated with increased cell motility.
Figure 1.
Loss of Prkar1a causes altered cell morphology and enhanced migration independent of Rho GTPases. (A) Phase-contrast images of Prkar1a+/+ and Prkar1a−/− MEFs illustrating altered cell morphology induced by the loss of Prkar1a. (B) Top: migration of WT MEFs and two Prkar1a−/− MEF lines (2L and 8L) in a Boyden chamber assay. **P<0.01 compared with WT cells. Bottom: PKA inhibitor (PKI) reduces migration of knockout but not of WT MEFs. *P<0.05 for drug-treated versus vehicle-treated cells. (C) Measurement of total and activated (GTP-bound) Rac and Rho. (D) Immunoblot analysis of phospho-Pak (pPak), pcofilin, total cofilin, Limk1 and Prkar1a (R1A) levels in knockout (−/−) and WT (+/+) lysates. Note the presence of a nonspecific band (*) in the Prkar1a lane. IPs, immunoprecipitates (of activated proteins); Limk1, LIM domain kinase 1; MEF, mouse embryonic fibroblast; PKA, protein kinase A; WT, wild type.
Given that Rho family GTPases are thought to coordinate the cellular responses required for migration, we investigated the activation of Rac and Rho GTPases in Prkar1a−/− cells. We had shown earlier that Ras, which lies upstream from Rac and Rho, was not activated in these cells (Nadella & Kirschner, 2005). Using a pull-down assay specific for active (GTP-bound) Rac and Rho, we observed that neither of these signalling proteins is activated in response to ablation of Prkar1a; in fact, levels of GTP-loaded Rac and Rho seemed to be modestly decreased (Fig 1C). Consistent with the decrease of GTP-loaded Rac, levels of T423 phospho-Pak were also reduced (Fig 1D).
Alterations in cell motility are associated with changes in the actin cytoskeleton, and the turnover rate of actin filaments might be mediated by cofilin/ADF, a small family of 21-kDa proteins that stimulate actin filament cycling. The activity of cofilin is primarily regulated by Limk1 and Limk2, which inactivate the depolymerizing activity of cofilin by phosphorylating it on Ser 3 (Arber et al, 1998; Nishita et al, 2005; Wang et al, 2006). Analysis of phospho-cofilin (pcofilin) in Prkar1a−/− cells showed a marked increase in Ser 3-phosphorylated cofilin, whereas both total cofilin and Limk1 levels remained unchanged (Fig 1D). Taken together, the increase in pcofilin in the absence of activated Rac or Rho GTPase activities suggests that PKA might regulate migration by a different mechanism.
To verify the relevance of these observations in intact cells, we evaluated pcofilin in tumour osteoblasts derived from osteochondromyxomas arising in Prkar1a+/− mice (Pavel et al, 2008). These cells show increased activity of PKA in a manner similar to knockout MEFs. This analysis showed both increased pcofilin and increased migration in tumour cells when compared with wild-type osteoblasts (supplementary Fig 2 online), indicating that the observations made in this in vitro model were relevant to tumorigenesis in vivo.
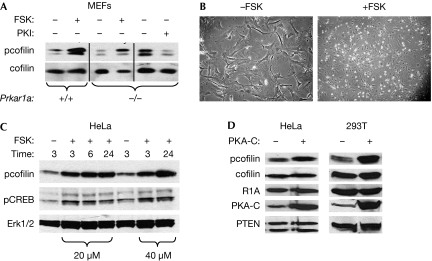
To address the role of increased activity of PKA on enhanced levels of pcofilin, we treated wild-type or Prkar1a−/− MEFs with forskolin (FSK), a stimulator of PKA activation. In both cases, treatment of the cells led to an increase in the levels of pcofilin. Conversely, treatment of Prkar1a−/− MEFs with PKI led to a reduction in pcofilin (Fig 2A). Also, treatment of wild-type MEFs with FSK caused morphological changes in the MEFs at either high (Fig 2B) or low (supplementary Fig 3 online) density, reminiscent of the observations in Prkar1a−/− cells. These data indicate that, in MEFs, the activation of PKA signalling has important consequences for cell morphology in association with modifications in actin cytoskeletal function.
Figure 2.
Activation of PKA causes enhanced pcofilin levels and morphological changes. (A) Immunoblot analysis of pcofilin and cofilin levels in WT (+/+) and knockout (−/−) MEFs treated with forskolin (20 μM) or PKI (5 μM) for 24 h. (B) Morphological and growth pattern changes induced in high-density WT MEFs treated with forskolin for 24 h. Note the similarity to Fig 1A. (C) Time–course analysis of pcofilin and pCREB levels in HeLa cells treated with 20 or 40 μm forskolin. Erk1/2 levels are used as a loading control. (D) Analysis of the levels of pcofilin in HeLa and 293T cells 48 h after transfection with PKA-C shows increased pcofilin without an increase in total cofilin. PTEN was used as a loading control. Erk, extracellular signal-regulated kinase; FSK, forskolin; MEF, mouse embryonic fibroblast; PKA, protein kinase A; PKI, PKA inhibitor; PTEN, phosphatase and tensin homologue; R1A, Prkar1a; WT, wild type.
To see whether these observations could be recapitulated in other cell systems, we examined the effects of FSK on the levels of pcofilin in HeLa cells (Fig 2C). This experiment showed that treatment with FSK produced not only the expected increase in phosphorylation of the cyclic-AMP response element-binding factor (CREB), but also produced robust increases in the levels of pcofilin. Also, transfection of the active catalytic subunit of PKA (PKA-C) into either HeLa or 293T cells also increased pcofilin (Fig 2D). Thus, in all assays tested, increases in the activity of PKA caused an increase in the levels of pcofilin.
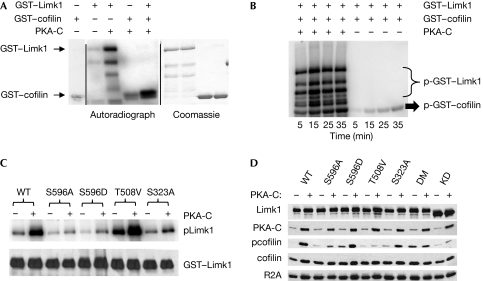
To identify the mechanism by which PKA affects the phosphorylation of cofilin, we first tested the possibility that cofilin is a substrate for PKA. Incubation of purified glutathione S-transferase (GST)–cofilin with PKA-C in the presence of radiolabelled ATP (Fig 3A) showed no phosphorylation of cofilin above background levels (supplementary Fig 4 online), indicating that the effect of PKA on its phosphorylation status is indirect.
Figure 3.
PKA phosphorylates Limk1 and increases its ability to phosphorylate cofilin. (A) GST–Limk1 and GST–cofilin were subject to an in vitro kinase assay with PKA-C. The left panel shows the autoradiograph, whereas the right panel shows total protein lysates stained with Coomassie. The panel at the far left shows GST–cofilin incubated without other proteins to assess the background level of the assay. (B) Autoradiograph of the time course of Limk1-mediated phosphorylation of cofilin with or without pre-incubation with PKA-C. (C) Autoradiograph of purified WT or phosphomutant Limk1 proteins treated with PKA-C in vitro. Note reductions in the label caused by mutation of Ser 596 or Ser 323 but not of Thr 508. (D) Immunoblot analysis of lysates from 293T cells transfected with WT or phosphomutant Limk1 with and without PKA-C. DM indicates S596A/S323A double mutant, whereas KD indicates the D460A dominant-negative (kinase-dead) mutant. Prkar2a (R2A) is shown as a loading control. GST, glutathione S-transferase; PKA, protein kinase A; WT, wild type.
As noted above, phosphorylation of cofilin is primarily performed by Limk1 and Limk2, which are classically activated by either the Rac–Pak or the Rho–Rock pathways by phosphorylation in the activation loop at Thr 508 (Limk1) or Thr 505 (Limk2; Edwards et al, 1999; Ohashi et al, 2000). As we did not observe activation of Rac (to stimulate Pak) or Rho (to stimulate Rock), we considered that Limk1 might be a substrate of PKA. To evaluate this possibility, we treated purified GST–Limk1 with PKA-C in the presence of radiolabelled ATP (Fig 3A). Autoradiography showed rapid incorporation of the label into Limk1 in the presence of PKA, whereas the amount of auto-phosphorylation by Limk1 was minimal. Pre-incubation of Limk1 with PKA-C markedly enhanced both the total amount (supplementary Fig 4 online) and the rate (Fig 3B) at which Limk1 phosphorylated cofilin. Taken together, these data indicate that PKA is able to phosphorylate Limk1 and that this modification enhances the catalytic ability of Limk1.
Analysis of the Limk1 sequence identified an evolutionarily conserved potential PKA phosphorylation site (KRPS) at Ser 596 within the kinase domain of Limk1 (supplementary Table 1 online). It is noted that this residue is not conserved in Limk2, supporting the concept of functional differences (Bernard, 2007). To assess the role of this site, we generated phosphorylation site mutants at Ser 596, as well as at the mitogen-activated protein kinase-activated protein 2 (MapkAP2) site (Ser 323; Kobayashi et al, 2006), and the Pak/Rock activation site (Thr 508; Edwards et al, 1999; Ohashi et al, 2000). Wild-type and mutant proteins were subjected to in vitro kinase assays with PKA-C (Fig 3C). Both the S596A and S323A mutants showed substantial reduction in phosphorylation of Limk1, indicating that these are both target sites for PKA phosphorylation. By contrast, mutation of Thr 508 did not affect the incorporation of radioactivity when incubated with PKA-C, indicating that this residue is not a PKA phosphorylation target in vitro.
To understand the effects of these mutations on the function of Limk1 in vivo, we introduced the Limk1 non-phosphorylatable mutants S596A, S323A, S596A/S323A (double mutant) and T508V, or the phosphomimetic S596D into 293T cells with or without PKA-C and studied the effects on the levels of pcofilin (Fig 3D; supplementary Fig 5 online). The 293T cell line was used in these assays because of its low endogenous Limk1 levels (Yoshioka et al, 2003). Also, we used a dominant-negative Limk1 construct to block the activity of Limk1. In cells co-transfected with PKA-C and wild-type Limk1, pcofilin was significantly increased compared with transfection with Limk1 or PKA-C alone (supplementary Fig 3 online), confirming our in vitro data. Introduction of the phosphosite mutants S596A or S323A showed no change in the levels of pcofilin when transfected alone; however, when these constructs were transfected with PKA-C, there was an increase in pcofilin. However, this effect was attenuated approximately 4- to 6-fold when compared with wild-type Limk1. Interestingly, transfection of the S596A/S323A double mutant showed enhanced basal pcofilin, but no enhancement with PKA. These data indicate that these two sites might be the only PKA targets within Limk1 in vivo. Use of the activation site T508V mutant or the kinase-dead D460A mutant showed no phosphorylation of cofilin, even with PKA stimulation. Conversely, cells co-expressing the phosphomimetic S596D mutant had an increased basal level of pcofilin, which increased to the same level as in wild-type Limk1 after PKA stimulation.
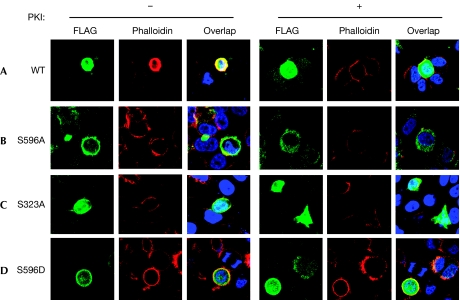
To assess the effects of the Ser 323 and Ser 596 mutations on the ability of Limk to affect the distribution of actin, we transfected wild-type or phosphomutant Limk1 into HeLa cells and studied the formation of F-actin by phalloidin immunofluorescence. When wild-type Limk1 was introduced into cells, there was a marked increase in F-actin fibres at the cell periphery (Fig 4A). The PKA dependence of this phenomenon was confirmed by incubation with PKI, which abolished actin bundling in transfected cells. In cells transfected with S596A (Fig 4B) or (Fig 4C) S323A mutants, no phalloidin-staining structures were observed, and no effects of inhibition of PKA were noted. By contrast, the phosphomimetic mutant S596D showed strong actin fibre formation, which was not reduced when PKA was inhibited (Fig 4D). These observations provide strong support for the model that PKA-mediated phosphorylation of Limk1 Ser 596 is required for Limk1 to cause the formation of actin fibres, presumably through the phosphorylation and subsequent inactivation of cofilin.
Figure 4.
Limk1 phosphorylation sites are required for actin filament stabilization. HeLa cells were transfected with (A) WT FLAG–Limk1 or (B–D) the indicated mutants and treated for 24 h without or with PKI (10 μM) to inhibit PKA-mediated phosphorylation. Cells were studied by using immunofluorescence for FLAG and F-actin (phalloidin). Note that WT Limk1 causes actin filament formation only in the absence of PKI, whereas the S596D phosphomimetic mutation causes actin bundling regardless of the activity of PKA. The serine-to-alanine mutants S323A and S596A cause minimal changes in the actin cytoskeleton. PKA, protein kinase A; PKI, PKA inhibitor; WT, wild type.
Overall, the data provided here show for the first time that PKA is able to phosphorylate Limk1, which occurs independently of Rac/Cdc42 and Rho activation. Phosphorylation of Limk1 at Ser 596 by PKA enhances the activity of Limk1, leading to phosphorylation and inactivation of cofilin, and providing a crucial link between PKA signalling and cell morphology and mobility. It was shown previously that PKA is a negative regulator of Pak during anchorage-independent growth (Howe & Juliano, 2000). Our results are consistent with these studies and suggest that PKA reduces the activity of Pak and Rock by inhibiting the Rho family GTPases that stimulate their activity. Whether PKA acts directly to inhibit Rac and Rho is at present unknown. Previous studies have indicated that PKA phosphorylates Rho and diminishes its activity by causing its extraction from the plasma membrane (Lang et al, 1996), but the fact that we do not observe increases in GTP-loaded Rho indicates that a different mechanism might be in operation in Prkar1a−/− cells.
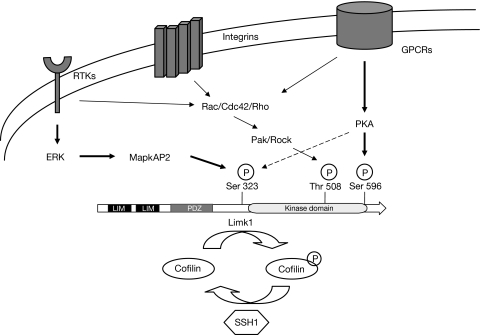
On the basis of our analysis of the phosphorylation of Limk1 site mutants, we propose a new model for its activation. In this model, phosphorylation by many kinases must occur to achieve full activation of Limk1 (Fig 5). Pak or Rock seem to be required for its activity, but this modification can only partly activate it. For Limk1 to achieve full activity, it must be phosphorylated at Thr 508 (by Pak or Rock), at Ser 596 (by PKA) and at Ser 323 (by either PKA or MapkAP2). This complexity provides for the possibility of dynamic control of Limk1 activity and its resultant modulation of cytoskeletal turnover. Indeed, the ability to control carefully the phosphorylation of cofilin by a dynamic balance of the LIM kinases and the Slingshot phosphatases (Nishita et al, 2005) supports a central role of Limk1 in the regulation of the actin cytoskeleton in response to extracellular cues. Some of these cues might be due to growth factors signalling through receptor tyrosine kinases, integrins or G-protein-coupled receptors. Although each of these pathways can signal through Rac/Cdc42 and Rho, they also signal through Erk/MapkAP and PKA to allow dynamic variation in Limk activity, phosphorylation of cofilin and ultimately actin polymerization. Although evidence so far indicates that three kinase cascades influence the activity of Limk1, further work is still needed to understand the impact of other pathways, such as bone morphogenetic protein receptor II signalling, which might affect other physiological functions of Limk1 (Foletta et al, 2003).
Figure 5.
Model for the control of Limk1/cofilin by many kinases. Limk1 acts as an integration point of cytoskeletal control by receiving inputs from receptor tyrosine kinases (RTKs), integrins and G-protein-coupled receptors (GPCRs). Each pathway can either interact with the Rac/Cdc42/Rho class proteins or it can modulate the activity of Limk1 by phosphorylation on other residues by additional protein kinases. SSH1, Slingshot homologue 1.
In summary, our results provide evidence that PKA-dependent signalling is crucial for the activation of Limk1. We have observed that PKA phosphorylation of Limk1 resulted in the aggregation of F-actin and increased the levels of pcofilin. Taken together, these results define a signal-transduction pathway by which alterations in PKA cause cytoskeletal changes. As PKA and LIM kinases are ubiquitously expressed, this new signalling pathway will further our understanding of pathways that regulate actin dynamics in disease and normal states.
Methods
Plasmids and pharmacological reagents. GST–Limk1 in pEBG1 was a gift from Dr Ora Bernard. GST–Limk1 mutants were generated by PCR-based site-directed mutagenesis. GST-tagged proteins were purified from 293T cells using a GST affinity kit (Pierce, Rockford, IL, USA). For generation of FLAG–Limk1, wild-type and mutant Limk1 were cloned from pEBG1 to pCMV-Tag2c (Stratagene, La Jolla, CA, USA) and the sequence was verified. Dominant-negative Limk1 (D460A) and haemagglutinin–PKA-C constructs were the gifts of Dr Gary Bokoch and Dr Constantine Stratakis, respectively. FSK and myristoylated PKI were purchased from Calbiochem (San Diego, CA, USA).
Cells and migration assays. Prkar1a−/− and control MEFs were generated and cultured as described (Nadella & Kirschner, 2005), as were Prkar1a+/− and control osteoblasts (Pavel et al, 2008). Cell migration was performed as described (Saji et al, 2005). PKI treatment (10 μM) included a 30-min pretreatment and fresh drug during migration. Basal migration experiments were repeated 4–6 times, and PKI studies were repeated 3–4 times. Additional details for this section are included in the supplementary information online.
Immunoblotting. Immunoblots using 50 μg of protein lysates were performed with the following antibodies: PKA-R1A, PKA-C and Limk1 (BD Biosciences, Franklin Lakes, NJ, USA); cofilin and pcofilin (Santa Cruz Biotechnology, Santa Cruz, CA, USA); phospho-CREB PTEN, Erk1/2 and phospho-Pak (Cell Signaling, Beverly MA, USA). Activation of Rac and Rho were measured using commercial kits (Upstate Biotechnology, Bedford, MA, USA).
Cell transfection and immunofluorescence. Transfections were performed with Superfect (Qiagen, Valencia, CA, USA) with efficiencies for HeLa cells around 35% and for 293T cells >70%. For immunofluorescence, cells were fixed with paraformaldehyde 48 h after transfection and permeabilized with 0.2% Triton X-100. FLAG–Limk1 was visualized with a FLAG antibody (Sigma, Rockford, IL, USA) and FITC-conjugated secondary antibodies. F-actin was imaged with Alexa 594 Phalloidin (Invitrogen, Carlsbad, CA, USA). Cover slips were mounted with VectaShield medium with 4,6-diamidino-2-phenylindole (Vector Labs, Burlingame, CA, USA) and visualized with an Axioskop 40 (Zeiss, Thornwood, NY, USA) equipped with an Axiocam HRC camera and Axiovision software.
Kinase assays. GST-tagged wild-type or mutant Limk1 (1 μg) was suspended in 30 μl of 1 × PKA reaction buffer (50 mM Tris–HCl, pH 7.5 and 10 mM MgCl2) and incubated with 200 μM ATP and 0.5 μl (250 ng) of purified PKA-C (New England BioLabs, Ipswich, NY, USA) and 5 μCi of [γ-32P] ATP at 30°C for 20 min. Reactions were terminated by boiling in sample buffer for 5 min. For the phosphorylation of cofilin, the same conditions were used except that 6 μg of cofilin was used as a substrate and Limk1 was reduced to 250 ng.
Supplementary information is available at EMBO reports online (http://www.emboreports.org)
Supplementary Material
supplementary information
Acknowledgments
We thank Dr Donna Kusewitt and Dr Heather Chandler for their help with ECIS. This study was supported in part by grants HD01323 and CA112268 (L.S.K.), CA102572 (M.D.R.) and CA16058 (The Ohio State University Comprehensive Cancer Center).
Footnotes
The authors declare that they have no conflict of interest.
References
- Arber S, Barbayannis FA, Hanser H, Schneider C, Stanyon CA, Bernard O, Caroni P (1998) Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature 393: 805–809 [DOI] [PubMed] [Google Scholar]
- Bernard O (2007) Lim kinases, regulators of actin dynamics. Int J Biochem Cell Biol 39: 1071–1076 [DOI] [PubMed] [Google Scholar]
- Bertherat J et al. (2003) Molecular and functional analysis of PRKAR1A and its locus (17q22–24) in sporadic adrenocortical tumors: 17q losses, somatic mutations, and protein kinase A expression and activity. Cancer Res 63: 5308–5319 [PubMed] [Google Scholar]
- Dormond O, Bezzi M, Mariotti A, Ruegg C (2002) Prostaglandin E2 promotes integrin α Vbeta 3-dependent endothelial cell adhesion, rac-activation, and spreading through cAMP/PKA-dependent signaling. J Biol Chem 277: 45838–45846 [DOI] [PubMed] [Google Scholar]
- Edwards DC, Sanders LC, Bokoch GM, Gill GN (1999) Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat Cell Biol 1: 253–259 [DOI] [PubMed] [Google Scholar]
- Ellerbroek SM, Wennerberg K, Burridge K (2003) Serine phosphorylation negatively regulates RhoA in vivo. J Biol Chem 278: 19023–19031 [DOI] [PubMed] [Google Scholar]
- Feoktistov I, Goldstein AE, Biaggioni I (2000) Cyclic AMP and protein kinase A stimulate Cdc42: role of A(2) adenosine receptors in human mast cells. Mol Pharmacol 58: 903–910 [DOI] [PubMed] [Google Scholar]
- Foletta VC, Lim MA, Soosairajah J, Kelly AP, Stanley EG, Shannon M, He W, Das S, Massague J, Bernard O (2003) Direct signaling by the BMP type II receptor via the cytoskeletal regulator LIMK1. J Cell Biol 162: 1089–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjout N, Laevsky G, Knecht DA, Lynes MA (2001) Automated real-time measurement of chemotactic cell motility. Biotechniques 31: 1130–1138 [DOI] [PubMed] [Google Scholar]
- Howe AK (2004) Regulation of actin-based cell migration by cAMP/PKA. Biochim Biophys Acta 1692: 159–174 [DOI] [PubMed] [Google Scholar]
- Howe AK, Juliano RL (2000) Regulation of anchorage-dependent signal transduction by protein kinase A and p21-activated kinase. Nat Cell Biol 2: 593–600 [DOI] [PubMed] [Google Scholar]
- Kirschner LS, Carney JA, Pack SD, Taymans SE, Giatzakis C, Cho YS, Cho-Chung YS, Stratakis CA (2000) Mutations of the gene encoding the protein kinase A type I-alpha regulatory subunit in patients with the Carney complex. Nat Genet 26: 89–92 [DOI] [PubMed] [Google Scholar]
- Kirschner LS, Kusewitt DF, Matyakhina L, Towns WH II, Carney JA, Westphal H, Stratakis CA (2005) A mouse model for the Carney complex tumor syndrome develops neoplasia in cyclic AMP-responsive tissues. Cancer Res 65: 4506–4514 [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Nishita M, Mishima T, Ohashi K, Mizuno K (2006) MAPKAPK-2-mediated LIM-kinase activation is critical for VEGF-induced actin remodeling and cell migration. EMBO J 25: 713–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang P, Gesbert F, Delespine-Carmagnat M, Stancou R, Pouchelet M, Bertoglio J (1996) Protein kinase A phosphorylation of RhoA mediates the morphological and functional effects of cyclic AMP in cytotoxic lymphocytes. EMBO J 15: 510–519 [PMC free article] [PubMed] [Google Scholar]
- Lania AG et al. (2004) Proliferation of transformed somatotroph cells related to low or absent expression of protein kinase a regulatory subunit 1A protein. Cancer Res 64: 9193–9198 [DOI] [PubMed] [Google Scholar]
- Nadella KS, Kirschner LS (2005) Disruption of protein kinase a regulation causes immortalization and dysregulation of D-type cyclins. Cancer Res 65: 10307–10315 [DOI] [PubMed] [Google Scholar]
- Nadella KS, Jones GN, Trimboli A, Stratakis CA, Leone G, Kirschner LS (2008) Targeted deletion of Prkar1a reveals a role for protein kinase A in mesenchymal-to-epithelial transition. Cancer Res 68: 2671–2677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishita M, Tomizawa C, Yamamoto M, Horita Y, Ohashi K, Mizuno K (2005) Spatial and temporal regulation of cofilin activity by LIM kinase and Slingshot is critical for directional cell migration. J Cell Biol 171: 349–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor KL, Mercurio AM (2001) Protein kinase A regulates Rac and is required for the growth factor-stimulated migration of carcinoma cells. J Biol Chem 276: 47895–47900 [DOI] [PubMed] [Google Scholar]
- Ohashi K, Nagata K, Maekawa M, Ishizaki T, Narumiya S, Mizuno K (2000) Rho-associated kinase ROCK activates LIM-kinase 1 by phosphorylation at threonine 508 within the activation loop. J Biol Chem 275: 3577–3582 [DOI] [PubMed] [Google Scholar]
- Pavel E, Nadella K, Towns WH II, Kirschner LS (2008) Mutation of Prkar1a causes osteoblast neoplasia driven by dysregulation of protein kinase A. Mol Endocrinol 22: 430–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saji M, Vasko V, Kada F, Allbritton EH, Burman KD, Ringel MD (2005) Akt1 contains a functional leucine-rich nuclear export sequence. Biochem Biophys Res Commun 332: 167–173 [DOI] [PubMed] [Google Scholar]
- Sandrini F, Matyakhina L, Sarlis NJ, Kirschner LS, Farmakidis C, Gimm O, Stratakis CA (2002) Regulatory subunit type I-α of protein kinase A (PRKAR1A): a tumor-suppressor gene for sporadic thyroid cancer. Genes Chromosomes Cancer 35: 182–192 [DOI] [PubMed] [Google Scholar]
- Shabb JB (2001) Physiological substrates of cAMP-dependent protein kinase. Chem Rev 101: 2381–2411 [DOI] [PubMed] [Google Scholar]
- Wang W, Mouneimne G, Sidani M, Wyckoff J, Chen X, Makris A, Goswami S, Bresnick AR, Condeelis JS (2006) The activity status of cofilin is directly related to invasion, intravasation, and metastasis of mammary tumors. J Cell Biol 173: 395–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka K, Foletta V, Bernard O, Itoh K (2003) A role for LIM kinase in cancer invasion. Proc Natl Acad Sci USA 100: 7247–7252 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary information