Abstract
The cellular response to DNA double-strand breaks involves direct activation of ataxia telangiectasia mutated (ATM) and indirect activation of ataxia telangiectasia and Rad3 related (ATR) in an ATM/Mre11/cell-cycle-dependent manner. Here, we report that the crucial checkpoint signalling proteins—p53, structural maintainance of chromosomes 1 (SMC1), p53 binding protein 1 (53BP1), checkpoint kinase (Chk)1 and Chk2—are phosphorylated rapidly by ATR in an ATM/Mre11/cell-cycle-independent manner, albeit at low levels. We observed the sequential recruitment of replication protein A (RPA) and ATR to the sites of DNA damage in ATM-deficient cells, which provides a mechanistic basis for the observed phosphorylations. The recruitment of ATR and consequent phosphorylations do not require Mre11 but are dependent on Exo1. We show that these low levels of phosphorylation are biologically important, as ATM-deficient cells enforce an early G2/M checkpoint that is ATR-dependent. ATR is also essential for the late G2 accumulation that is peculiar to irradiated ATM-deficient cells. Interestingly, phosphorylation of KRAB associated protein 1 (KAP-1), a protein involved in chromatin remodelling, is mediated by DNA-dependent protein kinase catalytic subunit (DNA-PKcs) in a spatio-temporal manner in addition to ATM. We posit that ATM substrates involved in cell-cycle checkpoint signalling can be minimally phosphorylated independently by ATR, while a small subset of proteins involved in chromatin remodelling are phosphorylated by DNA-PKcs in addition to ATM.
Keywords: ATM, ATR, DNA-PKcs, DNA double-strand break (DSB), DNA repair
Introduction
The mammalian DNA damage response (DDR) consists of a vast network of signal transduction cascades that amplify and relay the signal from DNA double-strand breaks (DSBs) to DNA repair, cell-cycle arrest and programmed cell death machineries (Harper & Elledge, 2007). The response is driven by a multitude of serine/threonine phosphorylation events and involves sensors, proximal kinases, distal kinases, mediators and effector proteins (Kurz & Lees-Miller, 2004). Although some of the proteins phosphorylated during this response enforce cell-cycle checkpoints directly (for example, p53 and structural maintainance of chromosomes 1 (SMC1)), some reinforce checkpoints by amplifying the original damage signal (for example, p53 binding protein 1 (53BP1)), whereas others are second-tier kinases (checkpoint kinase (Chk)1 and Chk2) that relay the damage signal triggered by proximal signalling kinases (ataxia telangiectasia mutated (ATM) and ataxia telangiectasia and Rad3 related (ATR)). In addition, a small subset of these proteins (such as histone H2AX and KRAB associated protein 1 (KAP-1)) induce chromatin changes in response to DNA breaks, perhaps to facilitate DNA repair (Ziv et al, 2006; Goodarzi et al, 2008; Pandita & Richardson, 2009). At the hub of this complex network of signalling events lie the ATM and ATR kinases, which are primary enforcers of checkpoints in response to ionizing radiation (IR) and replication stress, respectively.
ATM and ATR belong to the phosphatidylinositol 3-kinase-like kinase (PIKK) family of protein kinases; a third member of this family, DNA-dependent protein kinase catalytic subunit (DNA-PKcs), is an important enzyme in the non-homologous end joining pathway of DSB repair (Burma et al, 2006) but does not have a major role in cell-cycle checkpoint signalling (Burma et al, 1999; Burma & Chen, 2004). According to the canonical model of ATM and ATR activation, ATM is activated directly by DNA breaks and relays/amplifies the damage signal by phosphorylating Chk2 kinase and many additional DDR proteins (Shiloh, 2006). ATR responds primarily to stalled replication forks and relays/amplifies the signal by phosphorylating Chk1 kinase and a large subset of ATM substrates (Cimprich & Cortez, 2008). This paradigm of ATM and ATR signalling through two independent and alternate pathways was recently challenged and refined by several reports showing that ATR can be activated directly in response to DSBs specifically in S/G2 phases of the cell cycle (Adams et al, 2006; Jazayeri et al, 2006; Myers & Cortez, 2006; Cuadrado et al, 2006a). In these cells, DSBs are resected to generate single-stranded DNA (ssDNA) in an ATM/Mre11-dependent manner. The binding of replication protein A (RPA) to ssDNA, in turn, facilitates recruitment of the ATR interacting protein (ATRIP)–ATR complex to the break sites. ATR, thus activated, specifically phosphorylates Chk1 thereby implementing a G2/M checkpoint. So far, the direct activation of ATR by DSBs has been shown to be restricted to S/G2, absolutely dependent on ATM/Mre11, with consequent phosphorylation of only Chk1. Our current understanding of these pathways, therefore, essentially places the immediate checkpoint response to DSBs under the complete control of ATM (Cuadrado et al, 2006b). However, here we report that crucial checkpoint signalling proteins are rapidly phosphorylated independently by ATR in ATM-deficient cells, resulting in the implementation of cell-cycle checkpoints, while proteins involved in chromatin remodelling are also phosphorylated by DNA-PKcs. Our results help to redefine the contributions of ATM, ATR and DNA-PKcs to DDR.
Results
DDR proteins are phosphorylated in AT cells
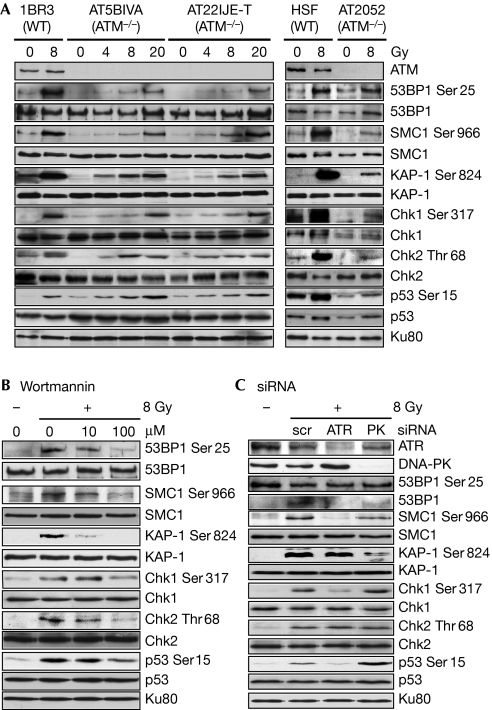
We wanted to determine whether the early phosphorylation of DDR proteins in response to IR is completely dependent on ATM. We irradiated immortalized ATM-proficient (1BR3) or ATM-deficient (AT5BIVA and AT22IJE-T) fibroblasts (Ziv et al, 1997; Mukherjee et al, 2006), primary human skin fibroblasts or primary ataxia telangiectasia (AT) fibroblasts (AT2052; Mukherjee et al, 2008), and assessed the phosphorylation of the following DDR proteins at 1 h post-irradiation: 53BP1 (Ser 25), SMC1 (Ser 966), KAP-1 (Ser 824), Chk1 (Ser 317), Chk2 (Thr 68) and p53 (Ser 15; Shiloh, 2006; Fig 1A). Interestingly, we observed radiation-inducible phosphorylation of these proteins in ATM-deficient cells, albeit at low levels. Although the actual levels of phosphorylation varied between the primary and immortalized ATM-deficient cell lines, these ATM-independent responses were seen in all three cell lines and for all six proteins. The ATM-independent phosphorylations observed were largely independent of the cell-cycle stage, as assayed by irradiation of synchronized AT5BIVA or AT22IJE-T cells (supplementary Fig S1a online). These results indicate the presence of a backup kinase that is able to phosphorylate ATM substrates, even in the absence of ATM, at early times post-IR.
Figure 1.
Phosphorylation of crucial DNA damage response proteins by ATR in ATM-deficient cells. (A) ATM-proficient (1BR3) or ATM-deficient (AT5BIVA and AT22IJE-T) immortalized fibroblasts or primary human skin fibroblasts (HSFs), or primary AT fibroblasts (AT2052) were irradiated with γ-rays and the phosphorylation of DDR proteins was assayed after 1 h by Western blotting with phospho-specific antibodies as indicated. (B) The phosphorylation of DDR proteins in AT5BIVA cells irradiated with 8 Gy of γ-rays was assayed after pretreatment with increasing doses of wortmannin or (C) after short interfering RNA (siRNA)-mediated knockdown of ATR or DNA-PKcs. AT, ataxia telangiectasia; ATM, ataxia telangiectasia mutated; ATR, ataxia telangiectasia and Rad3 related; Chk, checkpoint kinase; DDR, DNA damage response; DNA-PKcs, DNA-dependent protein kinase catalytic subunit; KAP-1, KRAB associated protein 1; scr, scrambled; SMC1, structural maintainance of chromosomes 1; WT, wild type.
ATR phosphorylates checkpoint proteins in AT cells
To identify the kinase involved in the phosphorylation of ATM substrates in ATM-deficient cells, AT cells were pre-treated with wortmannin before irradiation. Phosphorylation of these substrates (except for KAP-1) was largely unaffected by 10 μM wortmannin, which inhibits DNA-PKcs at these concentrations, but was attenuated by 100 μM wortmannin, which inhibits ATR at these concentrations (Sarkaria et al, 1998; Fig 1B and supplementary Fig S1b online). These phosphorylations were also attenuated by short interfering RNA (siRNA) knockdown of ATR, but not by knockdown of DNA-PKcs (except for KAP-1 and Chk2; Fig 1C and supplementary Fig S1c online). These results indicate that phosphorylation of cell-cycle checkpoint proteins in ATM-deficient cells is largely mediated by ATR. Interestingly, phosphorylation of Chk2 was abolished by 100 μM wortmannin—that is, when both DNA-PKcs and ATR were inhibited—but was not affected by knockdown of either ATR or DNA-PKcs alone. This indicates that both ATR and DNA-PKcs might redundantly phosphorylate Chk2 in the absence of ATM; this inference is consistent with a previous report showing that DNA-PKcs phosphorylates Chk2 in both wild-type and ATM-deficient cells (Li & Stern, 2005). KAP-1 is the only protein for which phosphorylation was inhibited by DNA-PKcs knockdown. We show later that KAP-1 is phosphorylated by both ATM and DNA-PKcs in a spatio-temporal manner.
ATR is recruited to DSBs in the absence of ATM
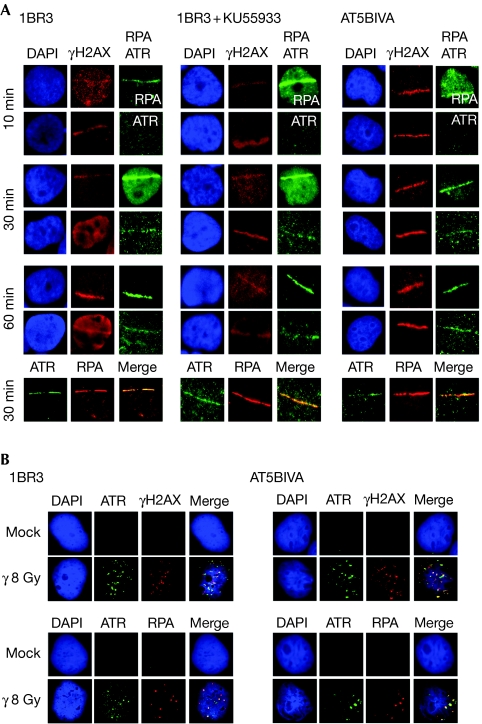
The activation of ATR directly by IR is reported to require the resection of DSBs in an ATM/Mre11-dependent manner (Jazayeri et al, 2006). However, on laser micro-irradiation of AT5BIVA (Fig 2A) and AT22IJE-T cells (supplementary Fig S2a online), primary AT2052 cells (supplementary Fig S2a online) or wild-type cells treated with the ATM inhibitor KU55933 (Fig 2A and supplementary Fig S2a online), we observed the accumulation of RPA at the sites of DNA damage within 10 min, followed by the recruitment of ATR within 30 min in most of the irradiated cells. On irradiation of AT cells with γ-rays, we observed ATR foci that largely co-localized with γH2AX in most of the irradiated cells, indicating that ATR recruitment is not peculiar to laser-induced DSBs (Fig 2B and supplementary Fig S2b online). Co-localization of RPA and ATR at the sites of DNA damage was confirmed by co-immunostaining. It is important to point out here that visualization of ATR foci after γ-irradiation requires an in situ extraction step before immunofluorescence staining (Cuadrado et al, 2006a); the less-than-expected number of γH2AX/RPA/ATR foci observed for a radiation dose of 8 Gy and the incomplete co-localization of some of these foci are possibly due to the extraction step. Therefore, we also examined ATR localization kinetics by using laser micro-irradiation, as this does not require in situ extraction and, thus, allows for more realistic visualization of ATR recruitment to DSBs. The stepwise localization of RPA and ATR at laser-irradiated sites in ATM-deficient cells indicates the generation of ssDNA even in the absence of ATM, and provides a mechanistic basis for the ATR-mediated phosphorylations observed.
Figure 2.
Sequential recruitment of RPA and ATR to double-strand breaks in ATM-deficient cells. (A) 1BR3, 1BR3 pre-treated with KU55933 or AT5BIVA cells were laser micro-irradiated and co-immunofluorescence stained with γH2AX antibodies and either RPA antibodies (top panel) or ATR antibodies (bottom panel) at the indicated times. These cells were also co-immunofluorescence stained with RPA antibodies and ATR antibodies at 30 min. (B) 1BR3 or AT5BIVA cells were immunofluorescence stained as indicated 1 h after irradiation with 8 Gy of γ-rays. ATM, ataxia telangiectasia mutated; ATR, ataxia telangiectasia and Rad3 related; DAP1, 4′,6-diamino-2-phenylindole; γH2AX, phosphorylated H2AX; RPA, replication protein A.
Recruitment of ATR to DSBs requires Exo1
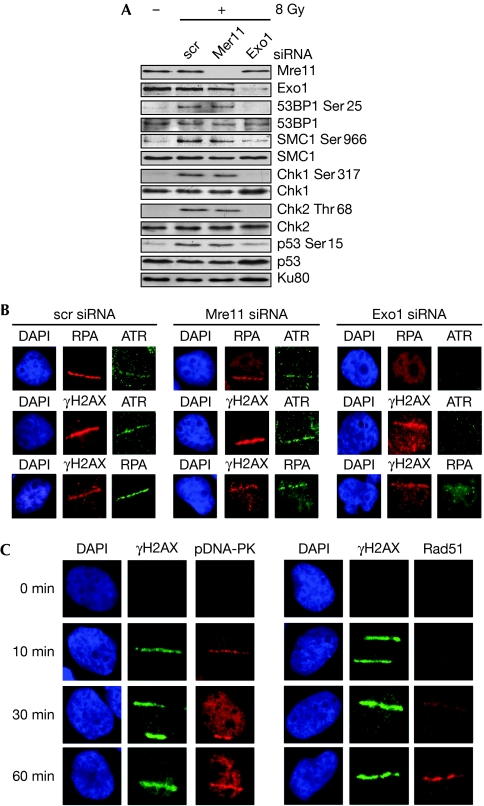
Although the existence of an ATM/Mre11-dependent pathway for the generation of ssDNA is now an established concept, three recent studies indicate the presence of additional, partly redundant pathways involving other nucleases such as Exo1 (Gravel et al, 2008; Mimitou & Symington, 2008; Zhu et al, 2008). The siRNA-mediated knockdown of Mre11 in AT cells did not substantially affect the phosphorylation of ATM substrates (Fig 3A and supplementary Fig S3a online) or the recruitment of RPA/ATR to the sites of laser-induced DNA damage (Fig 3B). Importantly, siRNA knockdown of Exo1 attenuated substrate phosphorylations and the recruitment of RPA/ATR to the sites of DNA damage. These results indicate that additional nucleases such as Exo1 are able to generate ssDNA in the absence of ATM/Mre11, thereby facilitating at least minimal levels of ATR activation. Interestingly, we observed the recruitment of DNA-PKcs to the sites of DSBs in AT cells soon after laser irradiation (10 min) and the recruitment of the homologous recombination (HR) repair protein Rad51 (Raynard et al, 2008) later (60 min; Fig 3C and supplementary Fig S3b online). This suggests that DSBs in AT cells might be amenable to repair by the non-homologous end joining pathway (Burma et al, 2006) early in the DDR. At later times, some of these breaks might be converted to ssDNA tracts and repaired, in S/G2 cells, by HR.
Figure 3.
Exo1-dependent recruitment of ATR and activation of DNA damage response signalling. (A) The phosphorylation of DDR proteins in AT5BIVA cells irradiated with 8 Gy of γ-rays was assayed by Western blotting after short interfering RNA (siRNA)-mediated knockdown of Mre11 or Exo1. (B) AT5BIVA cells with knockdown of Mre11 or Exo1 were laser micro-irradiated and co-immunofluorescence stained with the following combinations of antibodies after 30 min as indicated: RPA/ATR, γH2AX/ATR or γH2AX/RPA. (C) AT5BIVA cells were laser micro-irradiated and co-immunofluorescence stained with γH2AX antibodies and either pDNA-PKcs(Ser 2056) or Rad51 antibodies at the indicated times. ATR, ataxia telangiectasia and Rad3 related; Chk, checkpoint kinase; DAP1, 4′,6-diamino-2-phenylindole; DDR, DNA damage response; DNA-PKcs, DNA-dependent protein kinase catalytic subunit; γH2AX, phosphorylated H2AX; RPA, replication protein A; scr, scrambled; SMC1, structural maintainance of chromosomes 1.
ATR implements both early and late G2 checkpoints
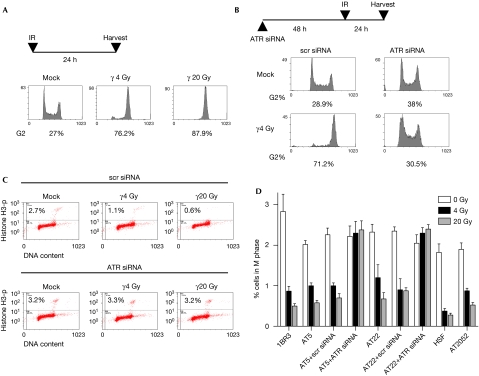
Next, we investigated whether the low levels of ATR-mediated phosphorylations are biologically relevant. ATM-deficient cells lack G1/S, intra-S and G2/M checkpoints (Shiloh, 2006), but undergo a late G2 accumulation that is peculiar to these cells (Xu et al, 2002). First we examined the late G2 accumulation in AT5BIVA and AT22IJE-T cells by using single-parameter flow cytometry. Irradiated AT cells accumulated in G2 by 24 h post-irradiation with doses as low as 4 Gy of γ-rays (Fig 4A and supplementary Fig S4a online). This accumulation could be reversed by siRNA knockdown of ATR (Fig 4B and supplementary Fig S4b online), clearly showing that the late G2 checkpoint in ATM-deficient cells is enforced by ATR. Next, we used multi-parameter flow cytometry to examine the early G2/M checkpoint in these cells using histone H3 phosphorylation to distinguish between mitotic and G2 cells (Xu et al, 2002; Fig 4C). Interestingly, AT5BIVA and AT22IJE-T cells irradiated with doses as low as 4 Gy showed a G2/M block that was, however, less robust than that shown by wild-type cells; such a block was also observed in the primary AT2052 cells (Fig 4D). Significantly, the G2/M block in AT cells could be completely reversed by siRNA knockdown of ATR. These results clearly and unequivocally show that the low levels of substrate phosphorylations by ATR do result in G2/M checkpoints even in ATM-deficient cells, thereby validating the biological significance of ATR-dependent phosphorylation events.
Figure 4.
Implementation of G2/M checkpoints in ATM-deficient cells by ATR. (A) Late G2 accumulation of AT5BIVA cells (24 h after irradiation) was quantified by single-parameter flow cytometry after PI staining for DNA content (PI; x axis). The percentage of cells in G2 are indicated at the bottom of each plot. (B) Lack of late G2 accumulation in AT5BIVA cells with short interfering RNA (siRNA)-mediated knockdown of ATR. (C) Early G2/M checkpoint in wild-type and AT cells was assayed by dual-parameter flow cytometry; representative distributions are shown for AT5BIVA cells with or without siRNA-mediated knockdown of ATR. Staining for DNA content (x axis) and for histone H3 phosphorylation (Histone H3-p; y axis) is shown (the percentage of cells in M phase—top compartment—are indicated). The G2/M checkpoint manifests as a decrease in mitotic cells at 2 h post-irradiation. (D) The percentage of cells in M-phase are plotted against the respective cell type, irradiation dose and treatment condition; error bars represent standard error of the mean. AT, ataxia telangiectasia; ATM, ataxia telangiectasia mutated; ATR, ataxia telangiectasia and Rad3 related; HSF, human skin fibroblast; IR, ionizing radiation; PI, propidium iodide; scr, scrambled; siRNA, short interfering RNA.
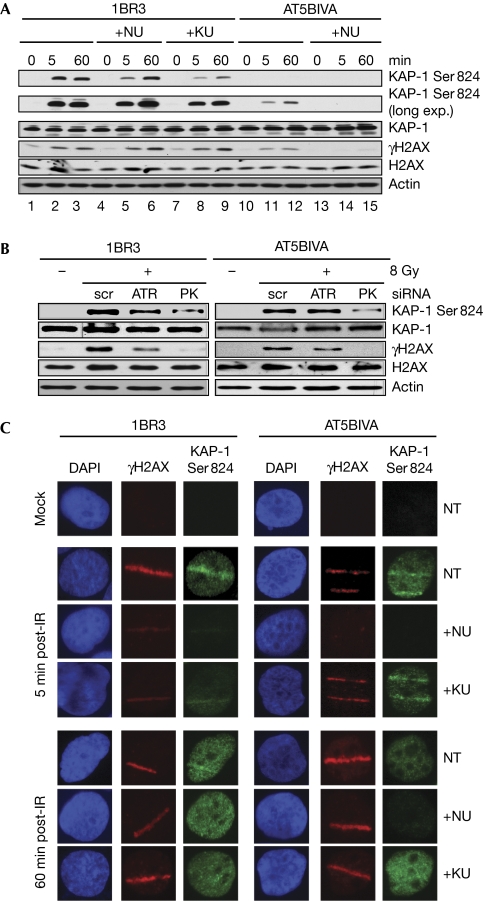
KAP-1 is phosphorylated by DNA-PKcs
We were intrigued by the fact that KAP-1 phosphorylation in AT cells was attenuated by low concentrations of wortmannin, suggesting the involvement of DNA-PKcs (Fig 1B). KAP-1 is different from the other DDR proteins examined as it is not required for the implementation of cell-cycle checkpoints but for chromatin relaxation and DSB repair on irradiation (Ziv et al, 2006; Goodarzi et al, 2008). Early phosphorylation of KAP-1 in wild-type cells was reduced by the DNA-PKcs inhibitor NU7026 (Fig 5A and supplementary Fig S5a online, compare lanes 2 and 5; top panel), whereas KAP-1 phosphorylation in AT cells was completely abrogated by this compound (compare lanes 11 and 12 with 14 and 15). Pre-treatment of wild-type cells with KU55933 reduced KAP-1 phosphorylation but did not abolish it (Fig 5A and supplementary Fig S5a online, compare lanes 2 and 3 with lanes 8 and 9). Furthermore, early KAP-1 phosphorylation in AT5BIVA cells was attenuated by siRNA knockdown of DNA-PKcs but not by knockdown of ATR (Fig 5B). Flow-cytometric analyses of 1BR3 cells revealed that KAP-1 is phosphorylated in all three phases of the cell cycle and that NU7026 had a maximum impact on phosphorylation at early times (supplementary Fig S5b and S5c online). KAP-1 is phosphorylated in the vicinity of the damaged DNA immediately on irradiation, following which it is phosphorylated in a pan-nuclear manner (Ziv et al, 2006). On micro-irradiation of 1BR3 cells with a laser beam, we observed KAP-1 phosphorylation along the laser path within 5 min, and pan-nuclear KAP-1 phosphorylation at 60 min (Fig 5C). Significantly, NU7026 largely abolished the phospho-KAP-1 tracks but did not affect the pan-nuclear KAP-1 phosphorylation. Conversely, KU55933 did not significantly affect early KAP-1 phosphorylation, but reduced pan-nuclear phosphorylation in wild-type cells and had no effect in AT cells. Taken together, these results clearly indicate that DNA-PKcs is essential for KAP-1 phosphorylation in ATM-deficient cells and is also required for KAP-1 phosphorylation at early times in wild-type cells at the sites of DNA damage. Interestingly, the requirement of both ATM and DNA-PKcs for KAP-1 phosphorylation is similar to that reported for H2AX phosphorylation (Burma et al, 2001; Stiff et al, 2004; Mukherjee et al, 2006) and observed by us here (Fig 5 and supplementary Fig S5 online). Thus, in addition to ATM, DNA-PKcs phosphorylates proteins involved in chromatin remodelling in a spatio-temporal manner, whereas ATR phosphorylates proteins involved in cell-cycle regulation.
Figure 5.
Phosphorylation of KAP-1 by both ATM and DNA-PKcs. (A) 1BR3 or AT5BIVA cells were irradiated with 8 Gy of γ-rays in the presence or absence of NU7026 (NU) or KU55933 (KU) and assayed by Western blotting with pKAP-1 or γH2AX antibodies. Longer exposures were necessary for visualization of KAP-1 phosphorylation in ATM-deficient cells (long exp.). (B) 1BR3 or AT5BIVA cells were irradiated with γ-rays after short interfering RNA (siRNA)-mediated knockdown of DNA-PKcs or ATR and assayed by Western blotting at 15 min post-ionizing radiation (IR). (C) 1BR3 or AT5BIVA cells were micro-irradiated with a laser beam and co-immunofluorescence stained with γH2AX antibodies (red) and phospho-KAP-1 antibodies (green) at the indicated times. Cells were untreated or pre-treated with NU7026 or KU55933 as indicated. AT, ataxia telangiectasia; ATM, ataxia telangiectasia mutated; ATR, ataxia telangiectasia and Rad3 related; DAP1, 4′,6-diamino-2-phenylindole; DNA-PKcs, DNA-dependent protein kinase catalytic subunit; γH2AX, phosphorylated H2AX; KAP-1, KRAB associated protein 1.
Discussion
On the basis of current models of ATM and ATR activation, one can envisage that the immediate response to DSBs would involve the direct activation of ATM, as well as the activation of ATR in an ATM/Mre11/cell-cycle-dependent manner. To this we add a third possibility, namely, the phosphorylation of cell-cycle checkpoint proteins by ATR in an ATM/Mre11/cell-cycle-independent manner. In contrast to previous reports (Jazayeri et al, 2006; Cuadrado et al, 2006a), we find that RPA accumulates at the sites of DSBs in ATM/Mre11-deficient cells with the subsequent recruitment of ATR. This is probably due to the resection of a fraction of DSBs into ssDNA by nucleases other than Mre11. Indeed, it is becoming clear that although Mre11 is responsible for limited resection at the sites of DSBs, at least two other pathways exist in yeast and humans that facilitate extensive resections, one involving the exonuclease Exo1, and the other involving the helicase and endonuclease activities of BLM and Dna2, respectively (Gravel et al, 2008; Mimitou & Symington, 2008; Raynard et al, 2008; Zhu et al, 2008). The existence of additional means of DSB resection is also suggested by our experiments showing that the ablation of Exo1 in AT cells attenuates the recruitment of RPA/ATR to DSBs and subsequent phosphorylation events. Although the contribution of ATR towards the activation of DDR proteins in AT cells is minor compared with ATM-dependent events in wild-type cells, it is, nonetheless, sufficient to enforce both early and late G2/M checkpoint responses. Our results support the concept that ATR could be, independently of ATM, important in the immediate response to DSBs. Interestingly, although the phosphorylation of cell-cycle regulatory proteins is mediated by ATR in addition to ATM, the phosphorylation of a small subset of DDR proteins involved in chromatin changes—KAP-1 and H2AX (Pandita & Richardson, 2009)—is also mediated by the repair enzyme, DNA-PKcs. ATM was previously reported to be the main kinase that phosphorylates KAP-1 (Ziv et al, 2006), while another report implicated all three PIKKs in the process without clearly demarcating the roles of individual kinases (White et al, 2006). We find that although ATM is the main KAP-1 kinase, DNA-PKcs also phosphorylates KAP-1 in a spatio-temporal manner. As DNA-PKcs has an important role in DSB repair, phosphorylation of proteins facilitating chromatin remodelling makes logical sense as these changes act to promote DSB repair. Our observations emphasize the tremendous therapeutic potential of these two kinases as they could, perhaps, be activated ectopically to compensate for the loss of ATM.
Methods
Cell culture. Cells were maintained in a humidified atmosphere with 5% CO2 in α-MEM medium supplemented with 10% fetal calf serum and penicillin/streptomycin.
Irradiation of cells. Cells were irradiated with γ-rays from a caesium source (JL Shepherd and Associates, San Fernando, CA, USA) with a total dose of 8 Gy unless otherwise indicated. For laser irradiation, cells were micro-irradiated with a pulsed nitrogen laser (Spectra-Physics, Mountain View, CA, USA; 365 nm, 10 Hz) with output set at 80% of the maximum. Cells were processed 1 h after irradiation unless otherwise indicated.
Antibodies. The following primary antibodies were used: 53BP1, p53BP1(Ser 25), SMC1, pSMC1(Ser 966), KAP-1, pKAP-1(Ser 824), H2AX (Bethyl Laboratories, Montgomery, TX, USA), p53, Exo1, Rad51 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), Chk1, pChk1(Ser 317), Chk2, pChk2(Thr 68), p53(Ser 15), ATR (Cell Signaling Technologies, Danvers, MA, USA), γH2AX, pHistone-H3(Ser 10; Upstate Biotechnology, Billerica, MA, USA), DNA-PKcs (Neomarkers, Fremont, CA, USA), ATM, Mre11 (Genetex, Irvine, CA, USA) and actin (Sigma, St Louis, MO, USA), RPA (Calbiochem, Gibbstown, NJ, USA), pDNA-PKcs(Ser 2056; Abcam, Cambridge, MA, USA), and Ku80 (a kind gift from Dr B.P. Chen). Horseradish peroxidase-conjugated secondary antibodies were from Bio-Rad (Hercules, CA, USA) and Alexa488/568-conjugated secondary antibodies were from Molecular Probes (Carlsbad, CA, USA).
Immunofluorescence staining. For laser micro-irradiation, cells were seeded onto Premium coverglass (Fisher Scientific, Rochester, NY, USA) and irradiated 24 h later. For γ-irradiation, cells were seeded onto chamber slides (Lab-Tek, Rochester, NY, USA) and irradiated 24 h later; at 1 h after irradiation, cells were subjected to in situ extraction (Cuadrado et al, 2006a) before immunofluorescence staining.
Inhibition of PIKKs. Cells were incubated with 10 or 100 μM wortmannin (Sigma), 20 μM NU7026 (Calbiochem) or 10 μM KU55933 (a kind gift from Dr D.J. Chen) for 1 h before irradiation.
Small interfering RNA (siRNA) transfections. Cells were transfected with siRNAs (Dharmacon, Lafayette, CO, USA), first by electroporation (Amaxa, Walkersville, MD, USA) and, 24 h later, by lipofection (Invitrogen, Carlsbad, CA, USA). Cells were analysed 48 h after the second transfection.
Cell-cycle synchronization and flow cytometry. Cells were synchronized in G1 by treatment with aphidicolin (2 μg/ml) and released into normal media for either 4 h (S phase) or 8 h (G2 phase). Cell-cycle stage was ascertained by flow cytometry using a BD CYTOMICS FC500 Flow Cytometer (Beckton Dickinson, Franklin Lakes, NJ, USA).
Supplementary information is available at EMBO Reports online (http://www.emboreports.org)
Supplementary Material
Supplementary Information
Acknowledgments
We are grateful to W. Wright, J. Wang and M. Cobb for their critical comments and suggestions. We thank B. McEllin for carefully reading the manuscript and C. Camacho for helping with microscopy. We gratefully acknowledge D. Chen and S.-Y. Wang for facilitating the laser micro-irradiation experiments. This work was supported by grant NNA05CS97G from the National Aeronautics and Space Administration to S.B.
Footnotes
The authors declare that they have no conflict of interest.
References
- Adams KE, Medhurst AL, Dart DA, Lakin ND (2006) Recruitment of ATR to sites of ionising radiation-induced DNA damage requires ATM and components of the MRN protein complex. Oncogene 25: 3894–3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burma S, Chen DJ (2004) Role of DNA-PK in the cellular response to DNA double-strand breaks. DNA Repair 3: 909–918 [DOI] [PubMed] [Google Scholar]
- Burma S, Kurimasa A, Xie G, Taya Y, Araki R, Abe M, Crissman HA, Ouyang H, Li GC, Chen DJ (1999) DNA-dependent protein kinase-independent activation of p53 in response to DNA damage. J Biol Chem 274: 17139–17143 [DOI] [PubMed] [Google Scholar]
- Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ (2001) ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem 276: 42462–42467 [DOI] [PubMed] [Google Scholar]
- Burma S, Chen BP, Chen DJ (2006) Role of non-homologous end joining (NHEJ) in maintaining genomic integrity. DNA Repair 5: 1042–1048 [DOI] [PubMed] [Google Scholar]
- Cimprich KA, Cortez D (2008) ATR: an essential regulator of genome integrity. Nat Rev Mol Cell Biol 9: 616–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadrado M, Martinez-Pastor B, Murga M, Toledo LI, Gutierrez-Martinez P, Lopez E, Fernandez-Capetillo O (2006a) ATM regulates ATR chromatin loading in response to DNA double-strand breaks. J Exp Med 203: 297–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadrado M, Martinez-Pastor B, Fernandez-Capetillo O (2006b) ATR activation in response to ionizing radiation: still ATM territory. Cell Div 1: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodarzi AA, Noon AT, Deckbar D, Ziv Y, Shiloh Y, Lobrich M, Jeggo PA (2008) ATM signaling facilitates repair of DNA double-strand breaks associated with heterochromatin. Mol Cell 31: 167–177 [DOI] [PubMed] [Google Scholar]
- Gravel S, Chapman JR, Magill C, Jackson SP (2008) DNA helicases Sgs1 and BLM promote DNA double-strand break resection. Genes Dev 22: 2767–2772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JW, Elledge SJ (2007) The DNA damage response: ten years after. Mol Cell 28: 739–745 [DOI] [PubMed] [Google Scholar]
- Jazayeri A, Falck J, Lukas C, Bartek J, Smith GC, Lukas J, Jackson SP (2006) ATM- and cell cycle-dependent regulation of ATR in response to DNA double-strand breaks. Nat Cell Biol 8: 37–45 [DOI] [PubMed] [Google Scholar]
- Kurz EU, Lees-Miller SP (2004) DNA damage-induced activation of ATM and ATM-dependent signaling pathways. DNA Repair 3: 889–900 [DOI] [PubMed] [Google Scholar]
- Li J, Stern DF (2005) Regulation of CHK2 by DNA-dependent protein kinase. J Biol Chem 280: 12041–12050 [DOI] [PubMed] [Google Scholar]
- Mimitou EP, Symington LS (2008) Sae2, Exo1 and Sgs1 collaborate in DNA double-strand break processing. Nature 455: 770–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee B, Kessinger C, Kobayashi J, Chen BP, Chen DJ, Chatterjee A, Burma S (2006) DNA-PK phosphorylates histone H2AX during apoptotic DNA fragmentation in mammalian cells. DNA Repair 5: 575–590 [DOI] [PubMed] [Google Scholar]
- Mukherjee B, Camacho CV, Tomimatsu N, Miller J, Burma S (2008) Modulation of the DNA-damage response to HZE particles by shielding. DNA Repair 7: 1717–1730 [DOI] [PubMed] [Google Scholar]
- Myers JS, Cortez D (2006) Rapid activation of ATR by ionizing radiation requires ATM and Mre11. J Biol Chem 281: 9346–9350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandita TK, Richardson C (2009) Chromatin remodeling finds its place in the DNA double-strand break response. Nucleic Acids Res 37: 1363–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynard S, Niu H, Sung P (2008) DNA double-strand break processing: the beginning of the end. Genes Dev 22: 2903–2907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkaria JN, Tibbetts RS, Busby EC, Kennedy AP, Hill DE, Abraham RT (1998) Inhibition of phosphoinositide 3-kinase related kinases by the radiosensitizing agent wortmannin. Cancer Res 58: 4375–4382 [PubMed] [Google Scholar]
- Shiloh Y (2006) The ATM-mediated DNA-damage response: taking shape. Trends Biochem Sci 31: 402–410 [DOI] [PubMed] [Google Scholar]
- Stiff T, O'Driscoll M, Rief N, Iwabuchi K, Lobrich M, Jeggo PA (2004) ATM and DNA-PK function redundantly to phosphorylate H2AX after exposure to ionizing radiation. Cancer Res 64: 2390–2396 [DOI] [PubMed] [Google Scholar]
- White DE, Negorev D, Peng H, Ivanov AV, Maul GG, Rauscher FJ III (2006) KAP1, a novel substrate for PIKK family members, colocalizes with numerous damage response factors at DNA lesions. Cancer Res 66: 11594–11599 [DOI] [PubMed] [Google Scholar]
- Xu B, Kim ST, Lim DS, Kastan MB (2002) Two molecularly distinct G(2)/M checkpoints are induced by ionizing irradiation. Mol Cell Biol 22: 1049–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Chung WH, Shim EY, Lee SE, Ira G (2008) Sgs1 helicase and two nucleases Dna2 and Exo1 resect DNA double-strand break ends. Cell 134: 981–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv Y, Bar-Shira A, Pecker I, Russell P, Jorgensen TJ, Tsarfati I, Shiloh Y (1997) Recombinant ATM protein complements the cellular A-T phenotype. Oncogene 15: 159–167 [DOI] [PubMed] [Google Scholar]
- Ziv Y, Bielopolski D, Galanty Y, Lukas C, Taya Y, Schultz DC, Lukas J, Bekker-Jensen S, Bartek J, Shiloh Y (2006) Chromatin relaxation in response to DNA double-strand breaks is modulated by a novel ATM- and KAP-1 dependent pathway. Nat Cell Biol 8: 870–876 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information