Abstract
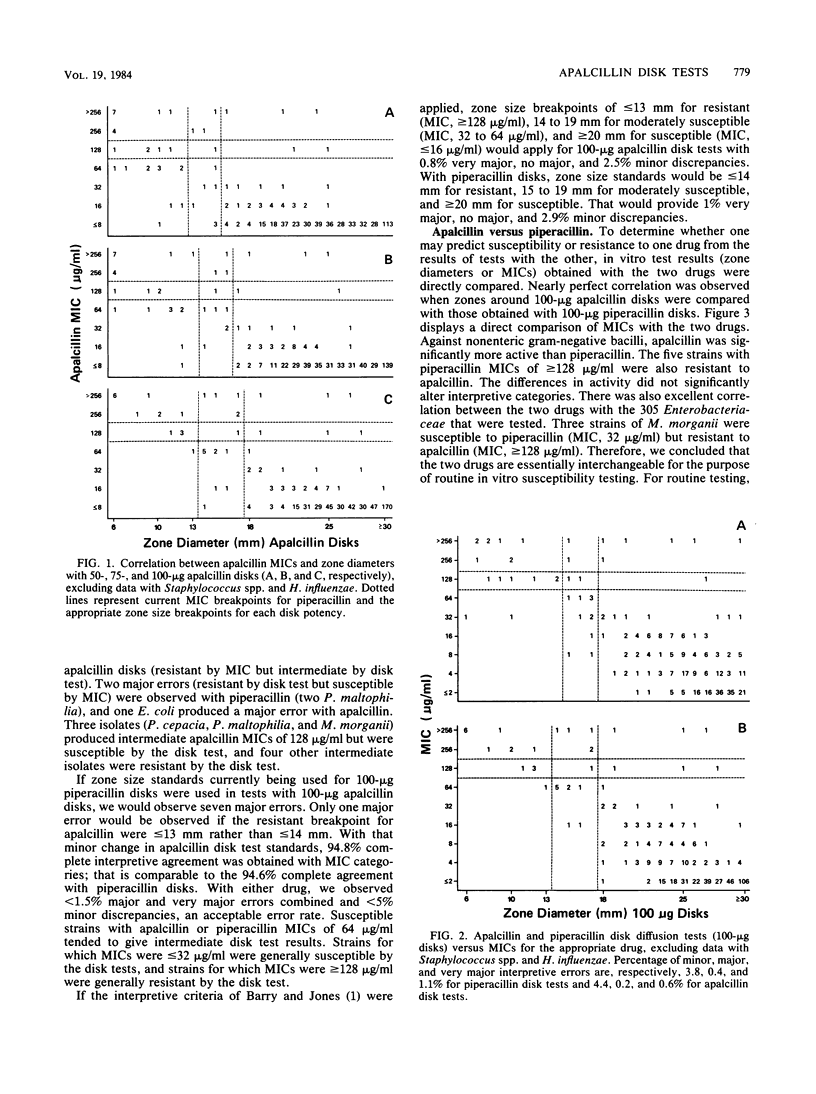
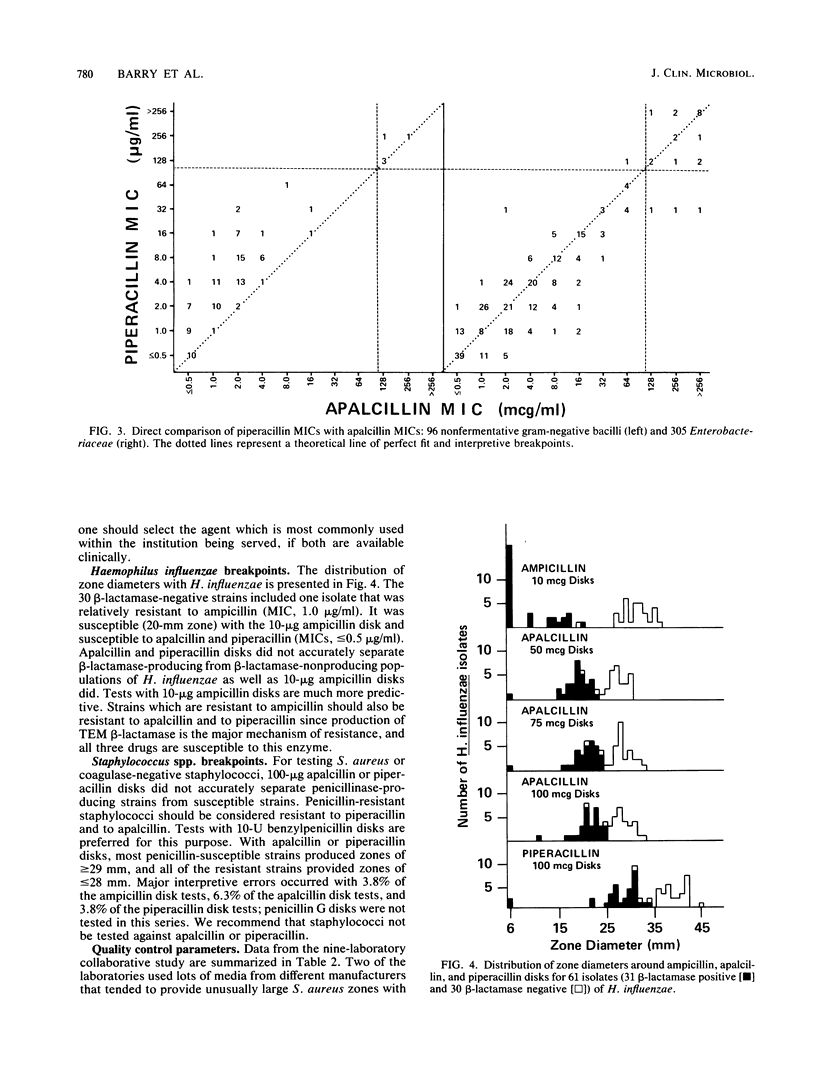
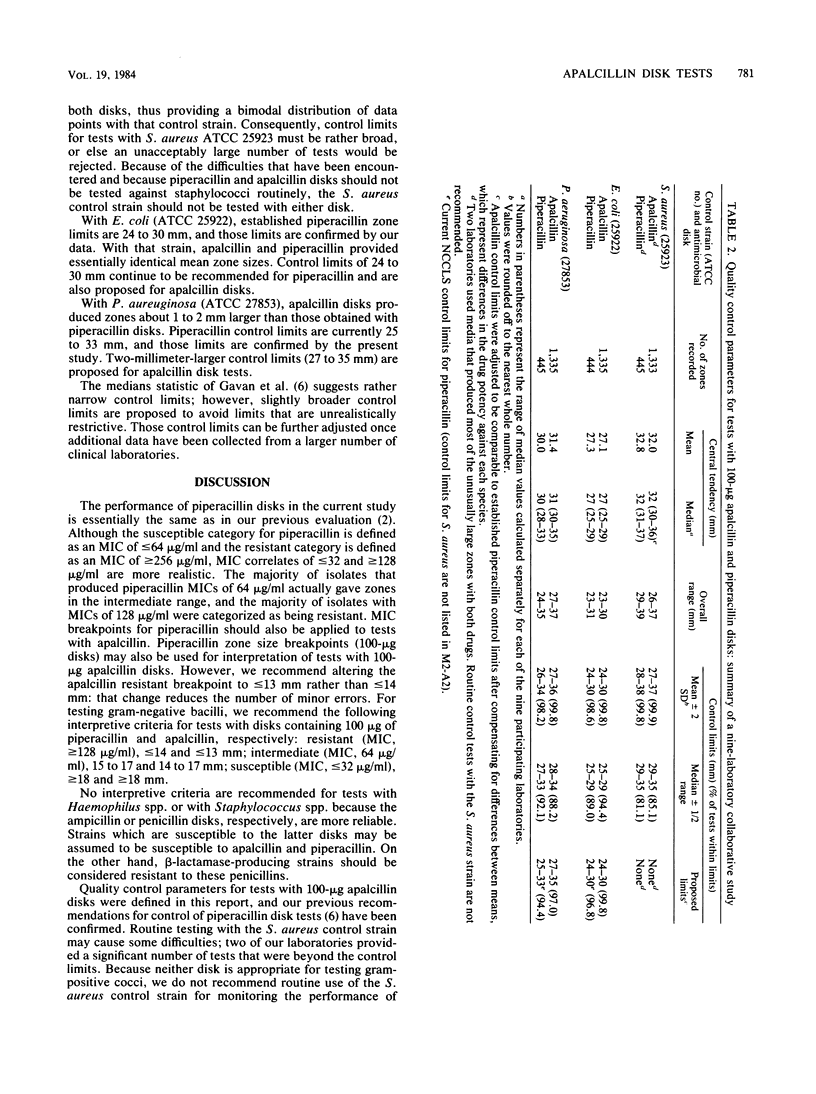
In vitro studies with 661 bacterial isolates were performed to establish interpretive criteria. In addition, a nine-laboratory study was performed to establish quality control limits for tests with 100-micrograms apalcillin disks and to confirm testing criteria for tests with 100-micrograms piperacillin disks. The two drugs were very similar, and nearly identical criteria were recommended for interpretation and for control of the two types of disks. Neither disk is recommended for testing Staphylococcus spp. or Haemophilus spp.; with other microorganisms, zone size limits of less than or equal to 13 mm (resistant) and greater than or equal to 18 mm (susceptible) are proposed for tests with 100-micrograms apalcillin disks.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry A. L., Jones R. N. A three category system for interpretation of disk tests for Pseudomonas-active penicillins and beta-lactamase hydrolysis/inhibition studies. J Antimicrob Chemother. 1982 Jan;9 (Suppl A):35–45. doi: 10.1093/jac/9.suppl_a.35. [DOI] [PubMed] [Google Scholar]
- Barry A. L., Thornsberry C., Badal R. E., Baker C. N., Jones R. N., Gerlach E. H. Piperacillin susceptibility tests by the single-disk agar diffusion technique. Antimicrob Agents Chemother. 1979 Sep;16(3):378–385. doi: 10.1128/aac.16.3.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry A. L., Thornsberry C., Jones R. N., Gavan T. L. In vitro activity of mezlocillin and azlocillin compared with that of four other penicillins and two aminoglycosides. Cleve Clin Q. 1980 Winter;47(4):311–319. doi: 10.3949/ccjm.47.4.311. [DOI] [PubMed] [Google Scholar]
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- Bodey G. P., Weaver S., Pan T. PC-904, a new semisynthetic penicillin. Antimicrob Agents Chemother. 1978 Jan;13(1):14–18. doi: 10.1128/aac.13.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavan T. L., Jones R. N., Barry A. L., Fuchs P. C., Gerlach E. H., Matsen J. M., Reller L. B., Thornsberry C., Thrupp L. D. Quality control limits for ampicillin, carbenicillin, mezlocillin, and piperacillin disk diffusion susceptibility tests: a collaborative study. J Clin Microbiol. 1981 Jul;14(1):67–72. doi: 10.1128/jcm.14.1.67-72.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Labthavikul P. In vitro activity of apalcillin compared with that of other new penicillins and anti-Pseudomonas cephalosporins. Antimicrob Agents Chemother. 1982 Jun;21(6):906–911. doi: 10.1128/aac.21.6.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi H., Eda Y., Tobiki H., Nakagome T., Komatsu T. PC-904, a novel broad-spectrum semisynthetic penicillin with marked antipseudomonal activity: microbiological evaluation. Antimicrob Agents Chemother. 1976 Feb;9(2):262–273. doi: 10.1128/aac.9.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi H., Kubo M., Kurashige S., Mitsuhashi S. Antibacterial activity of apalcillin (PC-904) against gram-negative bacilli, especially ampicillin-, carbenicillin-, and gentamicin-resistant clinical isolates. Antimicrob Agents Chemother. 1978 May;13(5):745–752. doi: 10.1128/aac.13.5.745. [DOI] [PMC free article] [PubMed] [Google Scholar]


