Abstract
Plexins are receptors for axonal guidance molecules known as semaphorins. We recently reported that the semaphorin 4D (Sema4D) receptor, Plexin-B1, induces axonal growth cone collapse by functioning as an R-Ras GTPase activating protein (GAP). Here, we report that Plexin-B1 shows GAP activity for M-Ras, another member of the Ras family of GTPases. In cortical neurons, the expression of M-Ras was upregulated during dendritic development. Knockdown of endogenous M-Ras—but not R-Ras—reduced dendritic outgrowth and branching, whereas overexpression of constitutively active M-Ras, M-Ras(Q71L), enhanced dendritic outgrowth and branching. Sema4D suppressed M-Ras activity and reduced dendritic outgrowth and branching, but this reduction was blocked by M-Ras(Q71L). M-Ras(Q71L) stimulated extracellular signal-regulated kinase (ERK) activation, inducing dendrite growth, whereas Sema4D suppressed ERK activity and down-regulation of ERK was required for a Sema4D-induced reduction of dendrite growth. Thus, we conclude that Plexin-B1 is a dual functional GAP for R-Ras and M-Ras, remodelling axon and dendrite morphology, respectively.
Keywords: plexin, semaphorin, M-Ras, R-Ras, dendrite
Introduction
Neurons are highly polarized cells with two distinct components, a single long axon and multiple dendrites, which establish synaptic contacts. Dendrites extend from the cell body and often form a highly branched structure that receives inputs from other neurons, and the pattern of dendritic outgrowth and branching determines neuronal functions (Jan & Jan, 2003). Semaphorins are a large family of secreted and transmembrane molecules, which function as axon guidance factors (Kolodkin et al, 1993; Tamagnone et al, 1999). The function of semaphorins is mediated by plexins, which are classified into four subfamilies: Plexin-A, -B, -C and -D (Tamagnone et al, 1999). Plexin-B1 has been identified as a receptor for semaphorin 4D (Sema4D; Tamagnone et al, 1999). Semaphorins were originally identified as repulsive and attractive axonal guidance molecules, and Sema4D/Plexin-B1 induces axonal growth cone collapse in hippocampal neurons (Swiercz et al, 2002; Oinuma et al, 2004a). Plexin-B1 has been shown to activate RhoA through the interaction of its carboxy-terminal PSD-95/Dlg/ZO-1 (PDZ) domain-binding motif with PDZ–Rho guanine nucleotide exchange factors (Aurandt et al, 2002; Swiercz et al, 2002); however, the PDZ-domain-binding motif is restricted to the Plexin-B1 subfamily. Conversely, the cytoplasmic regions are highly conserved among all plexin subfamilies. We recently revealed that the cytoplasmic region of Plexin-B1 directly encodes a GTPase activating protein (GAP) for R-Ras, and that Plexin-B1 specifically down-regulates R-Ras activity in response to Sema4D, inducing axonal growth cone collapse in cultured hippocampal neurons (Oinuma et al, 2004a). Furthermore, R-Ras-GAP activity of Plexin-A1 has been shown to be required for the Sema3A-induced repulsive response, suggesting that R-Ras GAP activity is a common signalling activity of the plexin family for the axonal repulsive response (Oinuma et al, 2004a; Toyofuku et al, 2005). R-Ras is known to promote cell adhesion and axon outgrowth (Zhang et al, 1996; Ivins et al, 2000), and its activity has been shown to increase during axon formation and elongation in hippocampal neurons (Oinuma et al, 2007).
The Ras family comprises a huge number of small GTPases and among them R-Ras, TC21 and M-Ras show relatively similar homology (Matsumoto et al, 1997). However, in spite of their structural similarity, R-Ras and M-Ras have different functions owing to distinct effector coupling. R-Ras activates phosphoinositide-3-kinase (PI(3)K) but not the extracellular signal-regulated kinase (ERK) pathway, promoting cell adhesion and migration through integrin activation (Kinbara et al, 2003), whereas M-Ras stimulates the ERK pathway by activating B-Raf, thereby promoting neurite outgrowth in PC12 cells (Kimmelman et al, 2002; Sun et al, 2006). M-Ras is predominantly expressed in the central nervous system and in situ hybridization revealed that it is highly expressed in the cortex and hippocampus (Kimmelman et al, 2002; Rodriguez et al, 2006). However, the functional roles of M-Ras in neurons are not well understood. Here, we show that M-Ras is highly expressed during dendrite development in cortical neurons and promotes dendritic outgrowth and branching, and that Plexin-B1 suppresses M-Ras activity, reducing dendritic outgrowth and branching through its GAP activity. Thus, Plexin-B1 functions as a GAP for both R-Ras and M-Ras, inducing axonal growth cone collapse and remodelling dendrite morphology, respectively.
Results
Sema4D shows M-Ras GAP activity
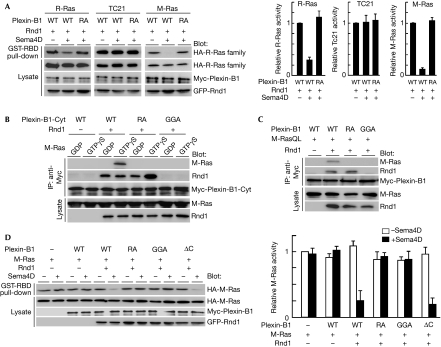
We have previously shown that Plexin-B1 functions as an R-Ras GAP but that the expression of R-Ras GAP activity requires the binding of Rnd1—a member of the Rho family and a constitutively active GTPase—to the cytoplasmic domain of Plexin-B1 (Oinuma et al, 2004a). First, we examined the GAP activity of Plexin-B1 for three R-Ras-related Ras members—R-Ras, TC21 and M-Ras—by using a pull-down assay with glutathione S-transferase-fused Ras-binding domain (GST-RBD) of c-Raf1 in COS-7 cells expressing Plexin-B1, Rnd1 and Ras GTPases. Sema4D stimulation decreased guanosine 5'-triphosphate (GTP)-bound R-Ras and M-Ras, but not TC21, indicating that Plexin-B1 shows GAP activity for R-Ras and M-Ras but not for TC21 (Fig 1A). To determine the direct interaction between Plexin-B1 and M-Ras, we prepared the recombinant Rnd1, M-Ras and cytoplasmic domain of Plexin-B1 or its two mutants; RA, which has mutations at arginine motifs of GAP domains essential for GAP activity, and GGA, which is unable to interact with Rnd1 (Oinuma et al, 2004a). The cytoplasmic domain of Plexin-B1 interacted with GTPγS-bound M-Ras in the presence of Rnd1, but this interaction was not observed with the RA and GGA mutants (Fig 1B). The Rnd1-dependent interaction between M-Ras and Plexin-B1 also occurred in transfected COS-7 cells expressing full-length Plexin-B1, a constitutively active form of M-Ras, M-Ras(Q71L), and Rnd1 (Fig 1C). To characterize the GAP activity of Plexin-B1 in cells, we determined the amount of GTP-bound M-Ras in transfected COS-7 cells expressing Rnd1, M-Ras and Plexin-B1 or its three mutants, RA, GGA and ΔC, by using a pull-down assay with GST-RBD; ΔC is a mutant lacking the C-terminal PDZ-domain-binding motif, which cannot activate RhoA (Swiercz et al, 2002). Sema4D stimulation of the wild-type Plexin-B1 decreased GTP-bound M-Ras in the presence of Rnd1; this decrease was also observed with the ΔC mutant, but not with the RA or GGA mutants (Fig 1D). These results indicate that Plexin-B1 acts as an M-Ras GAP through its GAP domains and that the interaction with Rnd1 is essential for the GAP activity, whereas RhoA activation is not required. Rnd3, but not Rnd2, supported the M-Ras GAP activity of Plexin-B1 (supplementary Fig 1 online). The extracellular-domain-deleted Plexin-A1, a constitutively active receptor, also showed M-Ras GAP activity in the presence of Rnd1 (supplementary Fig 2 online).
Figure 1.
Plexin-B1 shows GTPase activating protein activity for M-Ras. (A) COS-7 cells expressing Myc-Plexin-B1 (WT or RA mutant), green fluorescent protein (GFP)-Rnd1 and indicated HA-tagged Ras GTPases were stimulated with Sema4D. The cell lysates were incubated with glutathione S-transferase-fused Ras-binding domain (GST-RBD) and bound Ras GTPases; total cell lysates were analysed by immunoblotting. Relative Ras activities were determined by the amounts of Ras bound to GST-RBD normalized to the amount of Ras in cell lysates analysed by National Institutes of Health Image software. (B) Recombinant Myc-tagged cytoplasmic domain of Plexin-B1 (WT, RA or GGA mutant) and GTPγS-loaded Rnd1 were incubated with M-Ras preloaded with GDP or GTPγS, and then immunoprecipitated (IP) with an antibody against Myc. Bound and total proteins were analysed by immunoblotting with the indicated antibodies. (C) Lysates from transfected COS-7 cells expressing the indicated proteins were immunoprecipitated with an antibody against Myc, and bound and total proteins were analysed by immunoblotting with the indicated antibodies. (D) COS-7 cells, transfected with the indicated plasmids, were stimulated with Sema4D. The cell lysates were incubtated with GST-RBD and bound M-Ras; total cell lysates were analysed by immunoblotting. Results are the means±s.e.m. of three independent experiments. GAP, GTPase activating protein; HA, haemagglutinin; WT, wild type.
M-Ras regulates dendritic development
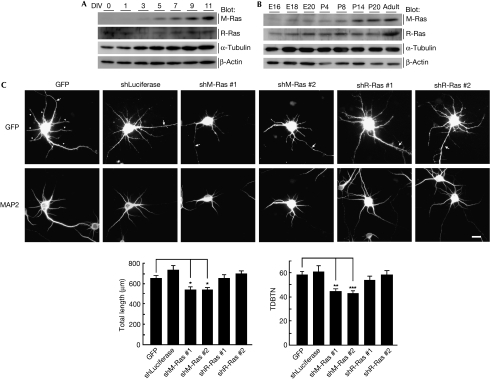
M-Ras is predominantly expressed in the cortex (Kimmelman et al, 2002); therefore, we examined its expression in primary cultured cortical neurons. The expression of M-Ras protein was up-regulated after 5 days in vitro (DIV), when dendrites begin to grow (Fig 2A). We also examined M-Ras expression in the developing rat brain. M-Ras expression was poor in the embryonic stage but gradually increased in the postnatal days (Fig 2B). To assess the role of M-Ras in dendrite morphogenesis, we generated two short hairpin RNA (shRNA) expression vectors designed to target two different regions of the M-Ras transcript; these shRNAs effectively reduced the amount of endogenous M-Ras (Fig 3A). At 5 DIV, dissociated cultured cortical neurons were transfected with green fluorescent protein (GFP) and shRNAs, and their dendrite morphology was observed at 7 DIV. Expression of the control shRNA had little effect on the dendrite development of cortical neurons, but the expression of two M-Ras-specific shRNAs impaired dendritic outgrowth and branching (Fig 2C). Conversely, the expression of R-Ras-specific shRNAs, which showed similar efficiency to M-Ras-specific shRNAs in silencing (Fig 3B), failed to affect dendrite morphology (Fig 2C). These results indicate that M-Ras, but not R-Ras, is required for normal dendrite development in cortical neurons.
Figure 2.
Knockdown of M-Ras suppresses dendritic outgrowth and branching in cortical neurons. (A) Lysates from primary cultured cortical neurons after 0, 1, 3, 5, 7, 9 and 11 days in vitro (DIV) were analysed by immunoblotting with the antibodies against M-Ras, R-Ras, α-tubulin and β-actin. (B) Lysates from whole brain at different developmental stages, from E16 to adult were analysed by immunoblotting with the indicated antibodies. (C) Cortical neurons were transfected with green florescent protein (GFP) and control shRNA (shLuciferase), M-Ras shRNA (#1 or #2) or R-Ras shRNA (#1 or #2) at 5 DIV and fixed at 7 DIV. GFP and microtubule associated protein 2 (MAP2) labelling are shown in the upper and lower panels, respectively. Arrows and arrowheads indicate axons and dendrites, respectively. Scale bar, 20 μm. Total length of dendrites and total dendritic branch tip number (TDBTN) of transfected neurons were measured. The results are the means±s.e.m. of three independent experiments (n=40, *P<0.05, **P<0.01 and ***P<0.005). E, embryonic day; P, postnatal day; shRNA, short hairpin RNA.
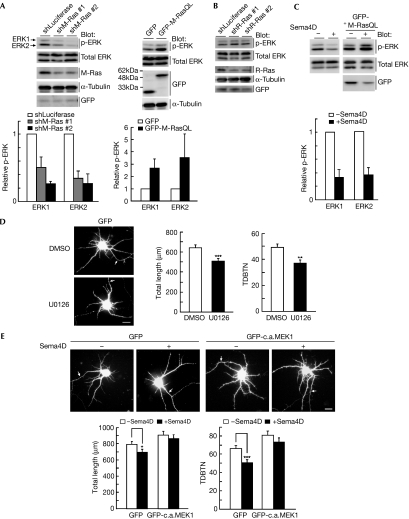
Figure 3.
Sema4D reduces dendrite growth through the down-regulation of extracellular signal-regulated kinase activity. (A) Cortical neurons were transfected with control shRNA (shLuciferase), M-Ras shRNA (#1 or #2), green fluorescent protein (GFP) or GFP-fused M-Ras(Q71L), before plating, using nucleofection technology; cell lysates at 7 days in vitro (DIV) were analysed by immunoblotting with antibodies against phospho-ERK, ERK, M-Ras, α-tubulin and GFP. (B) Cortical neurons were transfected with control shRNA or R-Ras shRNA (#1 or #2) with GFP, before plating, using nucleofection technology; cell lysates at 7 DIV were analysed by immunoblotting with the indicated antibodies. (C) Cortical neurons transfected with or without M-Ras(Q71L) were treated at 7 DIV with or without Sema4D for 1.5 h; cell lysates were analysed by immunoblotting with antibodies against phospho-ERK and ERK. (D) Cortical neurons transfected with GFP at 5 DIV were treated with or without 20 μM MAPK/extracellular signal-regulated kinase kinase (MEK) inhibitor U0126 for 2 d and fixed at 7 DIV. (E) Cortical neurons were transfected with GFP or GFP-fused constitutively active MEK1 (GFP-c.a.MEK1) at 5 DIV. Neurons at 7 DIV were stimulated with Sema4D for 1.5 h and fixed. Transfected cells are shown by GFP fluorescence. Arrows indicate axons; scale bar, 20 μm. Total length of dendrites and total dendritic branch tip number (TDBTN) were measured. The results are the means±s.e.m. of three independent experiments (n=40, *P<0.05, **P<0.01 and ***P<0.005). ERK, extracellular signal-regulated kinase; shRNA, short hairpin RNA.
Sema4D/Plexin-B1 reduces dendritic growth
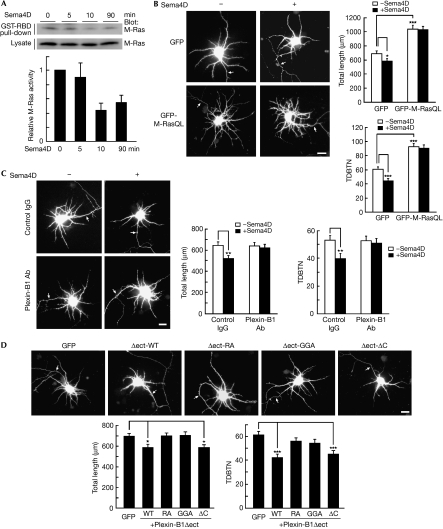
Next, we examined whether Sema4D/Plexin-B1 regulates dendrite morphology. First, we examined the effect of Sema4D on endogenous M-Ras activity in cultured cortical neurons by using a pull-down assay with GST-RBD. M-Ras activity was suppressed by Sema4D stimulation within 10 min (Fig 4A). We then examined the effect of Sema4D on dendrite morphology. Sema4D stimulation significantly reduced dendritic outgrowth and branching (Fig 4B). Expression of M-Ras(Q71L) not only promoted dendritic outgrowth and branching, but also blocked the Sema4D-reduced dendritic outgrowth and branching, suggesting that down-regulation of M-Ras activity by Plexin-B1 correlates to Sema4D-induced dendrite remodelling. The Plexin-B1-specific antibody also blocked the effects of Sema4D, indicating that these effects are mediated by Plexin-B1 (Fig 4C). Inhibition of M-Ras activity and dendrite growth by Sema4D was also observed in primary cultured hippocampal neurons (supplementary Fig 3 online). We have recently reported that the extracellular-domain-deleted Plexin-B1, Plexin-B1Δect, shows constitutive, ligand-independent GAP activity (Oinuma et al, 2004b). Thus, we examined the effect of Plexin-B1Δect on dendrite morphology (Fig 4D). Expression of Plexin-B1Δect reduced dendritic outgrowth and branching, but the two mutants, Plexin-B1Δect-RA and Plexin-B1Δect-GGA, had little effect on morphology. Dendrite development was also suppressed by Plexin-B1Δect-ΔC; similar results were observed with full-length Plexin-B1 (supplementary Fig 4 online). These results indicate that Plexin-B1-reduced dendrite growth requires Rnd1 binding and is mediated by its GAP activity but not by RhoA activation. In vivo electroporation of a dominant negative Plexin-B1 into a mouse embryo at embryonic day (E)15 promoted the morphological complexity of cortical neurons at postnatal day (P)3 and P7 (supplementary Fig 5 online).
Figure 4.
Sema4D/Plexin-B1 downregulates M-Ras activity and suppreses dendritic growth through its GTPase activating protein domain. (A) Cortical neurons at 7 days in vitro (DIV) were treated with Sema4D for the indicated times. Guanosine 5'-triphosphate (GTP)-bound M-Ras isolated with glutathione S-transferase-fused Ras-binding domain (GST-RBD) was detected with an M-Ras antibody. Relative M-Ras activity was determined by the amount of M-Ras bound to GST-RBD normalized to the amount of M-Ras in cell lysates. (B) Cortical neurons at 5 DIV were transfected with green fluorescent protein (GFP) or GFP-M-Ras(Q71L) and were fixed at 7 DIV after stimulation with Sema4D for 1.5 h. (C) Cortical neurons at 7 DIV were pretreated with control IgG or an antibody (Ab) against Plexin-B1 and were fixed after stimulation with Sema4D for 1.5 h. (D) Cortical neurons at 5 DIV were co-transfected with GFP and indicated Plexin-B1 constructs and fixed at 7 DIV. Transfected cells are shown by GFP fluorescence. Arrows indicate axons; scale bar, 20 μm. Total length of dendrites and total dendritic branch tip number (TDBTN) were measured. The results are the means±s.e.m. of three independent experiments (n=40, *P<0.05, **P<0.01 and ***P<0.005). WT, wild type.
Sema4D suppresses ERK activity
M-Ras is known to stimulate the ERK pathway, inducing neurite outgrowth in PC12 cells (Kimmelman et al, 2002; Sun et al, 2006). We examined whether M-Ras regulates ERK activity in cortical neurons. Knockdown of endogenous M-Ras with M-Ras-specific shRNAs decreased ERK phosphorylation, whereas overexpression of M-Ras(Q71L) promoted phosphorylation, indicating that M-Ras activation induces ERK activation (Fig 3A). By contrast, R-Ras knockdown with R-Ras-specific shRNAs did not affect ERK phosphorylation (Fig 3B). To evaluate the involvement of the ERK pathway in dendrite growth, we examined the effect of a MAPK/extracellular signal-regulated kinase kinase (MEK) inhibitor, U0126, on dendrite morphology. U0126 treatment significantly suppressed dendritic outgrowth and branching (Fig 3D). Next, we examined the effect of Sema4D on ERK activity. Sema4D stimulation decreased ERK phosphorylation and M-Ras(Q71L) blocked the decrease (Fig 3C). Sema4D-reduced dendritic outgrowth and branching were suppressed by the overexpression of the constitutively active form of MEK1, an upstream kinase of ERK (Fig 3E), indicating that down-regulation of ERK signalling is required for the Sema4D-reduced dendrite growth.
Discussion
We recently reported that the Sema4D receptor, Plexin-B1, functions as a GAP for R-Ras, inducing axonal growth cone collapse (Oinuma et al, 2004a). We have shown here that Plexin-B1 acts as a GAP for M-Ras, remodelling dendrite morphology. Previously, we have shown that Plexin-B1 does not show GAP activity for classical Ras such as H-Ras (Oinuma et al, 2004a). Thus, the GAP of Plexin-B1 shows substrate selectivity, and R-Ras and M-Ras are preferential substrates among the Ras family of GTPases. The biochemical properties of M-Ras differ from those of R-Ras but are similar to those of classical Ras (Ohba et al, 2000). M-Ras strongly stimulates the ERK pathway through the binding and activation of B-Raf (Sun et al, 2006). Conversely, R-Ras preferentially activates PI(3)K and integrin (Marte et al, 1997; Suire et al, 2002). Therefore, Plexin-B1 regulates functionally different Ras family GTPases.
We have shown that M-Ras is up-regulated after 5 DIV in cultured cortical neurons, when dendrites begin to grow, suggesting that M-Ras has a role in dendrite development. By contrast, R-Ras activity increased during the second and third stages of neuron development (2–3 DIV) when axons are specified and elongated, and that the knockdown of R-Ras impaired axon formation (Oinuma et al, 2007). We have shown that the knockdown of M-Ras, but not of R-Ras, impaired dendrite growth. In addition, overexpression of a constitutively active form of M-Ras promoted dendrite growth. Taken together, these results suggest that R-Ras and M-Ras have differential roles in neuronal development, axon formation and dendrite growth.
We examined the downstream signalling pathway of M-Ras for the regulation of dendrite development and have shown the involvement of the ERK pathway. M-Ras is known to activate the ERK pathway through binding to B-Raf, inducing neurite outgrowth in PC12 cells (Sun et al, 2006). Thus, M-Ras promotes dendrite growth, in part, through ERK activation. We have also shown that Sema4D suppressed ERK activity and active MEK1 blocked Sema4D-suppressed dendrite growth, suggesting that the inhibition of M-Ras-meditated ERK activation through M-Ras GAP activity correlates with Sema4D/Plexin-B1-induced dendrite remodelling. By contrast, we have shown previously that Sema4D/Plexin-B1 induces axonal growth cone collapse by inhibiting R-Ras-mediated PI(3)K-Akt activation through R-Ras GAP activity (Ito et al, 2006). Thus, Plexin-B1 regulates axon and dendrite morphology through distinct signalling pathways.
Semaphorins were originally identified as axon guidance factors (Kolodkin et al, 1993); however, Sema3A has been shown to regulate dendritic branching and spine maturation (Morita et al, 2006). We have shown that Sema4D induces remodelling of dendrite morphology in cortical neurons. Regulation of the morphological structures of dendrites is a crucial part of the neuronal architecture that underlies synaptic contacts. Here, we propose that in addition to its role as an axon guidance cue, Sema4D has a role in regulating dendrite morphology through distinct signalling pathways in the neurons. Dual regulation by Sema4D/Plexin-B1 of axon and dendrite morphology might lead to fine-tuning of the complex formation of the neuronal network.
Methods
DNA constructs and reagents. Human Plexin-B1 and mouse Plexin-A1 complementary DNAs were from L. Tamagnone (Torino University, Torino, Italy) and H. Fujisawa (Nagoya University, Nagoya, Japan). A soluble form of Sema4D fused to human IgG1-Fc was from H. Kikutani (Osaka University, Osaka, Japan), and Sema4D stimulation was carried out by replacing the culture medium with a Sema4D-containing conditioned medium, which contains approximately 1.3 nM Sema4D-Fc. Details of other DNA constructs, antibodies and reagents used in this study are in the supplementary information online.
Cell culture and transfection. COS-7 cells were cultured and transfected as described previously (Oinuma et al, 2004a). Primary cortical neurons and hippocampal neurons were dissociated from E18.5 rats, and were plated onto the poly-L-lysine and laminin (Sigma, St Louis, MO, USA)-coated cover slips (circular, 13 mm in diameter) or plastic dishes (60 mm in diameter), at a density of 0.7 × 104 cells/cm2. After 5 days in culture, the medium was changed to OPTI-MEM (Invitrogen, Carlsbad, CA, USA), and the neurons were transfected using Lipofactamine 2000 (Invitrogen) for immunofluorescence microscopy or the nucleofector kit (Amaxa Biosystems, Cologne, Germany) for immunoblotting. Co-transfection efficiency of each plasmid and GFP was greater than 90% as revealed by immunostaining (data not shown).
Measurement of activity of Ras family GTPases. Measurement of the activity of the R-Ras family of GTPases using COS-7 cells was carried out as described previously (Oinuma et al, 2004a). For the measurement of the M-Ras activity in the neurons, cortical neurons (4 × 106 cells) at 7 DIV were washed with ice-cold PBS and lysed directly on dishes with an ice-cold cell lysis buffer (25 mM HEPES-NaOH, pH 7.5, 150 mM NaCl, 0.5% Nonidet P-40, 0.5% sodium deoxycholate, 10% glycerol, 20 mM MgCl2, 1 mM ethylene glycol tetraacetic acid (EGTA), 25 mM NaF, 0.1 mM orthvanadate, 1 mM dithiothreitol (DTT), 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mM phenylmethylsulphonyl fluoride (PMSF)) containing 60 μg of cRaf-1-GST-RBD.
Immunofluorescence microscopy. Primary cultured cortical and hippocampal neurons on coverslips were fixed with 4% paraformaldehyde in phosphate buffered saline (PBS). After residual paraformaldehyde had been quenched with 50 mM NH4Cl in PBS, the cells were permeabilized with 0.2% Triton X-100 in PBS and incubated with 10% foetal bovine serum in PBS. Cells on coverslips were mounted on 90% glycerol containing 0.1% p-phenylenediamine dihydrochloride in PBS. The cells were photographed with a Leica DC350F digital camera system (Leica Microsystems, Nussloch, Germany) equipped with a Nikon Eclipse E800 microscope (Tokyo, Japan). For quantification of dendrite morphology, total dendritic length and total dendritic branch tip number (TDBTN) were measured as described previously (Ishikawa et al, 2006), using ImageJ software (Wayne Rasband, National Institutes of Health). At least 40 neurons were collected per construct from three independent experiments and statistical significance was determined by using the analysis of variance (ANOVA) test (Dunnett) or Student's t test. Dendritic tips were scored when they were longer than 3 μm as described previously (Ishikawa et al, 2006).
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
supplementary information
Acknowledgments
We thank L. Tamagnone, H. Kikutani and H. Fujisawa for providing plasmids for Plexin-B1, Sema4D and Plexin-A1, respectively. This work was in part supported by the Ministry of Education, Science, Sports and Culture of Japan.
Footnotes
The authors declare that they have no conflict of interest.
References
- Aurandt J, Vikis HG, Gutkind JS, Ahn N, Guan KL (2002) The semaphorin receptor plexin-B1 signals through a direct interaction with the Rho-specific nucleotide exchange factor, LARG. Proc Natl Acad Sci USA 99: 12085–12090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa Y, Katoh H, Negishi M (2006) Small GTPase Rnd1 is involved in neuronal activity-dependent dendritic development in hippocampal neurons. Neurosci Lett 400: 218–223 [DOI] [PubMed] [Google Scholar]
- Ito Y, Oinuma I, Katoh H, Kaibuchi K, Negishi M (2006) Sema4D/plexin-B1 activates GSK-3β through R-Ras GAP activity, inducing growth cone collapse. EMBO Rep 7: 704–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivins JK, Yurchenco PD, Lander AD (2000) Regulation of neurite outgrowth by integrin activation. J Neurosci 20: 6551–6560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan YN, Jan LY (2003) The control of dendrite development. Neuron 40: 229–242 [DOI] [PubMed] [Google Scholar]
- Kimmelman AC, Rodriguez NN, Chan AML (2002) R-Ras3/M-Ras induces neuronal differentiation of PC12 cells through cell-type-specific activation of the mitogen-activated protein kinase cascade. Mol Cell Biol 22: 5946–5961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinbara K, Goldfinger LE, Hansen M, Chou FL, Ginsberg MH (2003) Ras GTPases: integrins friends or foes. Nat Rev Mol Cell Biol 4: 767–776 [DOI] [PubMed] [Google Scholar]
- Kolodkin AL, Matthes DJ, Goodman CS (1993) The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell 75: 1389–1399 [DOI] [PubMed] [Google Scholar]
- Marte BM, Rodriguez-Viciana P, Wennstrom S, Warne PH, Downward J (1997) R-Ras can activate the phosphoinositide 3-kinase but not the MAP kinase arm of the Ras effector pathways. Curr Biol 7: 63–70 [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Asano T, Endo T (1997) Novel small GTPase M-Ras participates in reorganization of actin cytoskeleton. Oncogene 15: 2409–2417 [DOI] [PubMed] [Google Scholar]
- Morita A et al. (2006) Regulation of dendritic branching and spine maturation by semaphorin3A-Fyn signaling. J Neurosci 26: 2971–2980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohba Y, Mochizuki N, Yamashita S, Chan AM, Schrader JW, Hattori S, Nagashima K, Matsuda M (2000) Regulatory proteins of R-Ras, TC21/R-Ras2, and M-Ras/R-Ras3. J Biol Chem 275: 20020–20026 [DOI] [PubMed] [Google Scholar]
- Oinuma I, Ishikawa Y, Katoh H, Negishi M (2004a) The semaphorin 4D receptor Plexin-B1 is a GTPase activating protein for R-Ras. Science 305: 862–865 [DOI] [PubMed] [Google Scholar]
- Oinuma I, Katoh H, Negishi M (2004b) Molecular dissection of the semaphorin 4D receptor Plexin-B1-stimulated R-Ras GTPase-activating protein activity and neurite remodeling in hippocampal neurons. J Neurosci 24: 11473–11480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oinuma I, Katoh H, Negishi M (2007) R-Ras controls axon specification upstream of glycogen synthase kinase-3β through integrin-linked kinase. J Biol Chem 282: 303–318 [DOI] [PubMed] [Google Scholar]
- Rodriguez NN, Lee INL, Banno A, Qiao HF, Yao Z, Hoang T, Kimmelman AC, Chan AML (2006) Characterization of R-Ras3/M-Ras null mice reveals a potential role in trophic factor signaling. Mol Cell Biol 26: 7145–7154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suire S, Hawkins P, Stephens L (2002) Activation of phosphoinositide 3-kinase γ by Ras. Curr Biol 12: 1068–1075 [DOI] [PubMed] [Google Scholar]
- Sun P, Watanabe H, Takano K, Yokoyama T, Fujisawa J, Endo T (2006) Sustained activation of M-Ras induced by nerve growth factor is essential for neuronal differentiation of PC12 cells. Genes Cells 11: 1097–1113 [DOI] [PubMed] [Google Scholar]
- Swiercz JM, Kuner R, Behrens J, Offermanns S (2002) Plexin-B1 directly interacts with PDZ-RhoGEF/LARG to regulate RhoA and growth cone morphology. Neuron 35: 51–63 [DOI] [PubMed] [Google Scholar]
- Tamagnone L et al. (1999) Plexins are large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell 99: 71–80 [DOI] [PubMed] [Google Scholar]
- Toyofuku T, Yoshida J, Sugimoto T, Zhang H, Kumanogoh A, Hori M, Kikutani H (2005) FARP2 triggers signals for Sema3A-mediated axonal repulsion. Nat Neurosci 12: 1712–1719 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Vuori K, Wang H, Reed JC, Ruoslahti E (1996) Integrin activation by R-Ras. Cell 85: 61–69 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary information