Abstract
The transcription factor p53 protects neurons from transformation and DNA damage through the induction of cell-cycle arrest, DNA repair and apoptosis in a range of in vitro and in vivo conditions. Indeed, p53 has a crucial role in eliciting neuronal cell death during development and in adult organisms after exposure to a range of stressors and/or DNA damage. Nevertheless, accumulating evidence challenges this one-sided view of the role of p53 in the nervous system. Here, we discuss how—unexpectedly—p53 can regulate the proliferation and differentiation of neural progenitor cells independently of its role in apoptosis, and p53 post-translational modifications might promote neuronal maturation, as well as axon outgrowth and regeneration, following neuronal injury. We hope to encourage a more comprehensive view of the non-apoptotic functions of p53 during neural development, and to warn against oversimplifications regarding its role in neurons. In addition, we discuss how further insight into the p53-dependent modulation of these mechanisms is necessary to elucidate the decision-making processes between neuronal cell death and differentiation during development, and between neuronal degeneration and axonal regeneration after injury.
Keywords: axon outgrowth and regeneration, brain tumours, cell fate, neuron differentiation, p53
Glossary
See Glossary for abbreviations used in this article.
APAF1 apoptotic protease-activating factor 1
BAX BCl-2-associated X protein
Brn-3a brain-specific homeobox/POU domain protein 3a
C/EBP CCAAT/enhancer binding protein
CBP CREB binding protein
CHD8 chromodomain helicase DNA-binding protein 8
ChIP chromatin immunoprecipitation
Cip1 cdk-interacting protein 1
CREB cyclic-AMP-response-element-binding protein
EphA2 EPH receptor A2
GADD45 growth arrest and DNA damage
GAP43 growth associated protein 43
grhl3 grainyhead-like 3
MAPK mitogen-activated protein kinase
MSK1 mitogen-activated and stress-activated protein kinase 1
P/CAF CBP-associated factor
p300 E1A-binding protein p300
p53AIP1 p53-regulated apoptosis-inducing protein 1
PC12 pheochromocytoma
PI(3)K phosphoinositide 3-kinase
Pten phosphatase and tensin homologue
PUMA p53-upregulated modulator of apoptosis
Rab13 Ras-associated protein 13
Rsk2 p90 ribosomal S6 protein kinase 2
SET9 SUV39 enhancer of zeste and trithorax
STAT3 signal transducer and activator of transcription
TrkA tyrosine kinase receptor A
Unc-5B uncoordinated 5B
WAF1 wild-type p53-activated fragment 1
WNT7b Wingless-type MMTV integration site family member 7b
XRCC4 X-ray-repair cross-complementing protein 4 gene
Introduction
During development of the nervous system, a delicate and dynamic balance involving cell death and survival, proliferation and differentiation is continuously adjusted in order to generate a correctly structured and functional mature tissue. The decision-making process that takes place, leading to either neuronal death or survival during development, is analogous to the one that decides between axonal retraction and outgrowth after injury in the adult nervous system. In both scenarios—development and injury—regulation of the cell cycle and regulation of transcription are instrumental in shifting the balance between the two possible outcomes (Becker & Bonni, 2004; Galderisi et al, 2003; Herrup & Yang, 2007; Hughes et al, 1999; Ohnuma & Harris, 2003; Teng & Tang, 2006). Such complex processes require the coordinated action of a range of extracellular signals and intracellular pathways that are highly dependent on the specific cellular environment. The transcription factor and tumour suppressor p53 has a leading role in these molecular events, probably as the master regulator that contributes to directing neurons towards a specific phenotype in critical conditions, such as during development and following cellular damage (Helton & Chen, 2007; Jacobs et al, 2006). In fact, p53 should be viewed as a crucial decision-maker, rather than as an inducer of cell-cycle arrest, a pro-apoptotic factor or a tumour suppressor.
p53 functions by binding to specific DNA sequences on its target genes to regulate transcription, thereby triggering cell-cycle arrest, promoting apoptosis, regulating differentiation or altering cellular lifespan (Harms et al, 2004; Riley et al, 2008; Xu, 2003). When cells are subjected to DNA damage and/or oxidative stress, the transcription and post-translational modifications (PTMs) of p53 are rapidly activated, leading to the expression of typical p53 target genes, such as the pro-death Bax, Noxa, Puma and APAF1, the cell-cycle arrest and DNA-repair gene p21, and GADD45 (Harms et al, 2004). When cellular stressors—such as DNA damage, oxidative stress and nerve growth factor (NGF) withdrawal in developing or mature cortical and sympathetic neurons (Aloyz et al, 1998; Anderson & Tolkovsky, 1999; Cregan et al, 2004; Song et al, 2007; Vaughn & Deshmukh, 2007)—lead to irreversible damage, or when the elimination of cells is required—as occurs during development—the activation of p53 leads to apoptotic cell death. In several models of cellular stress, MAPK–p38 signalling cascades phosphorylate p53 at specific sites and enhance its pro-apoptotic effects. However, p53 is subject to a myriad of PTMs on its highly variable amino and carboxyl termini, which are not limited to phosphorylation, but also include acetylation, sumoylation, neddylation and ubiquitination (Lavin & Gueven, 2006). These modifications could allow p53 to mediate a wide range of different functional outcomes, which are controlled by specific transcriptional-dependent effects.
As previously stated, the importance of p53 in neuronal apoptosis is well established and supported by a rich and convincing literature, and has been covered by several comprehensive reviews (Culmsee & Mattson, 2005; Jacobs et al, 2004, 2005, 2006; Miller & Kaplan, 2007). Here, we focus on our current knowledge—gathered from both older and more recent experiments—regarding other processes in which p53 probably has a role, such as the regulation of neuronal terminal differentiation during development, the control of the proliferation and differentiation of neural progenitor cells towards the neuronal phenotype, and the promotion of axonal outgrowth and regeneration after nerve injury.
In vitro experiments in models of neuronal maturation, and in vivo analyses of axonal injury and regeneration suggest that some 'atypical' p53-dependent cellular functions could depend on specific patterns of p53 PTMs, such as acetylation in its C-terminus. These modifications affect directly the transcriptional activity of p53 and regulate its affinity to diverse cofactors, which, in turn, regulate the occupancy of p53 in specific promoters (Sims & Reinberg, 2008). However, the effects that p53 PTMs have during proliferation and differentiation of neural stem cells (NSCs) remain unknown. Our current knowledge is limited to some studies in embryonic stem cells and in neuronal cell lines undergoing differentiation. These issues are discussed in this Concept paper.
p53 in mouse nervous system development
In vivo experimental evidence has suggested a role for p53 in cell differentiation, as well as in the cell-fate decisions that occur during development of the nervous system. Early in situ hybridization studies during mouse embryonic development showed that p53 messenger RNA levels reach a maximum during the differentiation of several tissues, including early neuronal precursor cells of the brain. However, the levels of p53 decline strongly in cells undergoing terminal differentiation, and weaker hybridization signals were observed in post-mitotic cells (Louis et al, 1988; Rogel et al, 1985; Schmid et al, 1991). In addition, an analysis of p53-dependent transcriptional activation during normal development in vivo—performed using a lacZ reporter gene under the control of a p53-responsive promoter—indicated that p53 activity is at a maximum during neuronal differentiation and is clustered in areas that are not correlated with apoptosis (Gottlieb et al, 1997; Komarova et al, 1997). Studies performed in p53-null animals also support the importance of p53 for normal neural development. Some p53-null embryos die during development with a female versus male bias, and up to 16% of the surviving mice develop exencephaly owing to either cellular overgrowth or reduced apoptosis (Armstrong et al, 1995; Choi & Donehower, 1999; Sah et al, 1995). Interestingly, there are also signs of increased neuronal apoptosis in the spinal cord of p53-null mice (Armstrong et al, 1995), implying that p53 can protect against neuronal apoptosis under certain conditions, although the molecular mechanisms underlying this unusual observation were not elucidated in this study. Nonetheless, approximately 80% of the p53-null mice that survive to term develop normally, but have an increased incidence of cancer, predominantly lymphomas (Sansom & Clarke, 2000). This suggests an ancillary or dispensable role for p53 in neural development and neural differentiation in these mice, probably owing to the compensatory role of the two other p53 family members, p63 and, in particular, p73. In fact, the truncated isoforms of p73, known as ΔN, have an important role in neuronal survival during nervous system development, as shown by the reduced survival of cortical, olfactory, brain-stem and sympathetic neurons in p73-null mice (Pozniak et al, 2000). ΔN p73—particularly the δ-isoform—protects cortical neurons from cell death after various apoptotic stimuli, in part by inhibiting the pro-apoptotic activation of p53 (Pozniak et al, 2002), which is a function that is also conserved in mature cortical neurons.
p53 seems to have a role in the death of developing and differentiating cells, in addition to that of mature neurons. In this respect, a lack of p53 has been shown to rescue the embryonic lethality, cellular proliferation and neuronal apoptosis that occur in mice lacking the DNA double-strand break-repair gene XRCC4 (Gao et al, 2000). In addition, p53 is required for the death of sympathetic neurons after NGF withdrawal during development and in response to p75-receptor signalling (Aloyz et al, 1998). However, a p63 and p53 mouse deletion study has shown that p63 is essential to ensure the complete p53-mediated phenotype. In fact, full-length p63 is required and sufficient to induce the death of sympathetic neurons after NGF withdrawal, and the absence of p53 does not affect p63-induced apoptosis (Jacobs et al, 2005), whereas p63 is an obligate partner required for p53-mediated apoptosis. The γ-isoform of p63, which is expressed most strongly during development, seems to be crucial to mediate this effect. Surprisingly, the possible role of p63 in mature neuronal death has not been addressed.
These data suggest the existence of a specific hierarchical organization in the capacity of the p53 family members to induce neuronal apoptosis, which might reflect the relative expression of each transcriptional or splice variant of p53, p63 and p73 in a specific cellular context. In addition, each member undergoes specific PTMs and protein–protein interactions that determine its affinity for various promoters, such as the phosphorylation of p53 at Ser 46, which specifically enhances its ability to interact with the promoter of p53AIP1, but not with that of p21 (Oda et al, 2000). Although the three family members can influence and partly rescue the function of one another, this is not always the case. For example, the absence of p63 and p73 does not allow p53 to activate the Bax promoter and induce cell death in response to DNA damage in mouse embryonic fibroblasts (Flores et al, 2002). These complex influences must be taken into consideration when critically evaluating the biological effects of each family member, especially in fields—such as the non-apoptotic function of p53 in the nervous system—in which their relationship has not been addressed.
p53: a decision-maker in neural progenitor cells
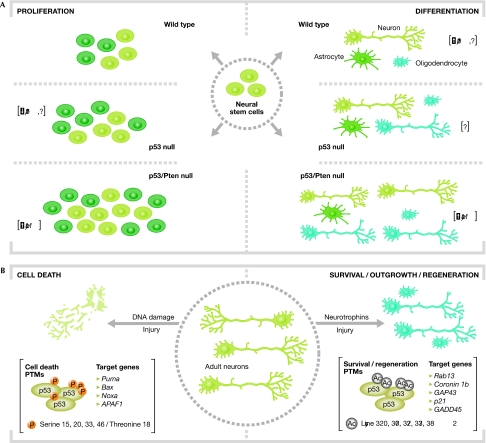
The demonstration that p53 is expressed in proliferating and newly formed neurons of the embryonic and postnatal rat brain—and that its expression is associated with that of cell-cycle regulators and p53 target genes such as p21 (van Lookeren Campagne & Gill, 1998)—was the first indication that p53-dependent molecular pathways may affect neural progenitor cell proliferation and differentiation. It has subsequently been shown that p21—an established mediator of p53-dependent cell-cycle arrest—can negatively regulate the self-renewal of adult NSCs (Mori et al, 2001), thereby revealing a possible link between p53, cell-cycle regulation and the differentiation of NSCs. Later work has supported this hypothesis by showing a direct involvement of p53 in the control of the proliferation and differentiation of NSCs, both in vitro and in vivo (Fig 1). One report showed that p53 negatively regulates the proliferation and survival of adult NSCs, without affecting their differentiation potential (Meletis et al, 2006). By comparing the transcriptome of adult NSCs from p53-null and wild-type mice, the authors identified an alteration in the expression of several cell-cycle regulators—including the downregulation of p21 expression—in the absence of p53, although the expression of all well-known neuronal differentiation markers was not affected. However, recent experiments in embryonic NSCs extracted from the olfactory bulb of wild-type and p53-null mice showed that p53 is required for both physiological NSC proliferation and differentiation, which seems to be dependent on the maintenance of chromosomal stability. In fact, p53-null NSCs have an increased proliferation and a bias towards the neuronal phenotype, which is correlated with chromosomal abnormalities (Armesilla-Diaz et al, 2008). The role of p53 on adult NSCs resident in the subventricular zone (SVZ) has also been studied in p53-null mice with respect to cell differentiation and transformation; loss of p53 was not sufficient for tumour formation, but led to increased cell proliferation and altered differentiation, which was associated with increased p53-independent apoptosis (Gil-Perotin et al, 2006). Importantly, transformation in the SVZ occurred only when the loss of p53 was accompanied by a mutagenic stimulus, whereas impaired neuronal differentiation—with a bias towards the neuronal phenotype—was found in the SVZ of p53-null mice under physiological conditions. Notably, p53 was not essential to promote cell death in any of these studies, which supports the idea that p53 is required to maintain the physiological proliferation rate in NSCs. However, it is currently unclear whether p53 is required for correct progenitor cell differentiation.
Figure 1.
p53-mediated decision-making processes in neural progenitor cells and neurons. (A) The self-renewal and differentiation of NSCs is dependent on p53. In its absence (the p53-null condition), NSC self-renewal and the number of differentiated neurons increases. Furthermore, in the absence of both p53 and Pten (the p53/Pten-null condition), NSCs show a higher renewal and neuronal differentiation potential. The genes with altered expression in p53-null and p53/Pten-null mice are indicated in brackets. (B) p53 PTMs and related target genes involved in cell death or survival/outgrowth/regeneration after lesion in the adult nervous system. APAF1, apoptotic protease-activating factor 1; Bax, BCl-2-associated X protein; GADD45, growth arrest and DNA damage; GAP43, growth associated protein 43; NSC, neural stem cell; Pten, phosphatase and tensin homologue; PTM, post-translation modification; Puma, p53-upregulated modulator of apoptosis; Rab13, Ras-associated protein 13.
Accumulating data show that at least some tumours contain a subpopulation of cells that have stem-cell characteristics. Therefore, understanding the molecular pathways that control the self-renewal of NSCs might be relevant to clarifying the mechanisms of cancer development. As a tumour suppressor and regulator of the cell cycle, p53 is a logical candidate to contribute to the control of stem-cell subpopulations in tumours. Perhaps the most insightful contribution regarding the role of p53 in NSC differentiation and cancer was made recently by Zheng and colleagues, who showed that p53 regulates the cell differentiation, self-renewal and tumorigenic potential of normal and malignant stem/progenitor cells (Zheng et al, 2008). By using integrated transcriptome profiling, in silico-promoter analysis and functional studies of murine NSCs, the authors showed that the dual inactivation of p53 and Pten promotes an undifferentiated state with high renewal potential and increased Myc protein levels (Table 1; Fig 1). Functional studies demonstrated that Myc activity is required for the differentiation and enhanced renewal of NSCs lacking p53 and Pten, as well as for the maintenance of tumour neurospheres with tumorigenic potential (Zheng et al, 2008). Incidentally, approximately 70% of patients with giant-cell glioblastoma have pathogenic mutations in p53, and approximately 30% have them in Pten (Kleihues & Ohgaki, 1999), and their tumour cells show a similar transcriptional profile to that found in the double-null murine model. Therefore, the data obtained in mice have potential therapeutic relevance for the treatment of human glioblastoma.
Table 1.
Non-apoptosis-related p53 target genes involved in neuronal differentiation, axon outgrowth or guidance identified by ChIP, luciferase and gene-expression analysis
| Cell types | Target genes | References |
|---|---|---|
| Neural progenitor cells | p21, Myc | Mori et al, 2001; Zheng et al, 2008 |
| Neurons | Coronin 1b,Rab13, GAP43 | Di Giovanni et al, 2006; Tedeschi et al, 2008 |
| PC12 cells | Wnt7b, TrkA | Brynczka et al, 2007; Zhang et al, 2006 |
| Non-neuronal cells | EphA2, semaphorin 3B, semaphorin 3F, Unc-5B | Dohn et al, 2001; Ochi et al, 2002; Futamura et al, 2007; Tanikawa et al, 2003 |
ChIP, chromatin immunoprecipitation; EphA2, EPH receptor A2; GAP43, growth associated protein 43 gene; PC12, pheochromocytoma; Rab13, Ras-associated protein 13; TrkA, tyrosine kinase receptor A; Unc-5B, uncoordinated 5B; Wnt7b, Wingless-type MMTV integration site family member 7B.
In summary, although the role of p53 in NSC differentiation is still controversial, these data clearly implicate it as a suppressor of tissue and cancer stem-cell self-renewal in the nervous system. However, many uncertainties remain regarding the specific signalling context in which p53 modulates neuronal stem-cell proliferation and differentiation in vivo (Sidebar A). In addition, most of the relevant p53 transcriptional targets have yet to be determined, with the exception of those related to cell-cycle arrest, apoptosis and DNA repair. Experiments aimed at addressing these questions should include high-throughput proteomics analysis of the pattern of p53 PTMs and ChIP-on-chip assays to determine the p53-dependent transcriptome during NSC proliferation and differentiation.
Sidebar A | In need of answers.
Do post-translational modifications contribute to the specific role of p53 in neural stem cell proliferation and differentiation?
Which are the key p53 binding partners in the p53-dependent pro-axon outgrowth pathways? How do they cross-talk with p53 post-translational modifications?
Which are the non-apoptotic p53 transcriptional targets in developing and in mature neurons following injury?
Do histone modifications have a role in p53-dependent decision-making in neurons?
p53 signalling and PTMs
The p53 family functions in a stimulus-dependent and cell-type-dependent manner. This is made possible by the multiple PTMs that target p53, p63 and p73 on their N-and C-termini, which lead to conformational changes that affect protein–protein interactions with transcriptional cofactors. Such interactions ultimately dictate the promoter occupancy and, therefore, the regulation of a specific set of promoters. The consequences of specific patterns of p53 PTMs are also highly context dependent, meaning that specific p53 codes might lead to different biological outcomes depending on the transcriptional context in a given cell or tissue (Murray-Zmijewski et al, 2008).
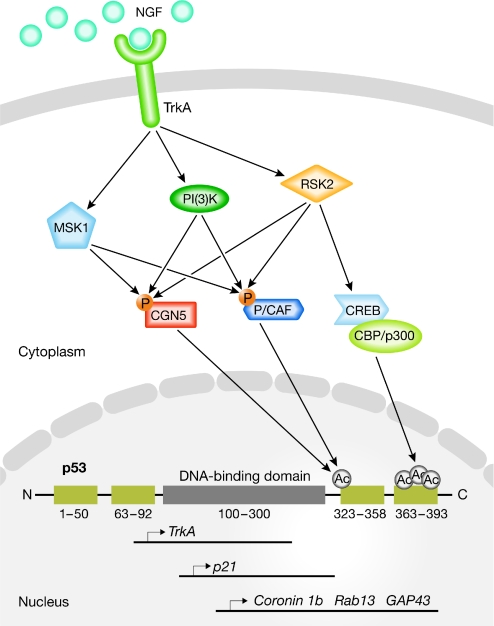
The best characterized p53 PTMs are phosphorylation and acetylation, which are induced by a complex kinase cascade and by acetyltransferases, respectively (Xu, 2003). Kinases phosphorylate several serine or threonine residues, mainly in the p53 N-terminal region. Among these, Ser 15, Ser 20, Ser 33, Ser 46 and Thr 18 are the best studied, and their phosphorylation promotes the stabilization of p53 by preventing its nuclear export or by favouring its recruitment to specific sets of promoters. These PTMs are often associated with increased neuronal death following genotoxic stress (Culmsee & Mattson, 2005). Acetylation targets mainly two distinct regions of the p53 C-terminus and, in neurons, involves the activity of at least two histone acetyltransferases (HATs): CBP/p300 and P/CAF. CBP/p300 acetylates Lys 370, Lys 372, Lys 373 and Lys 382, whereas P/CAF has been linked to the acetylation of a single residue—Lys 320—located within a flexible linker domain near the oligomerization domain, which also contains a nuclear localization signal (Brooks & Gu, 2003). Importantly, these kinase and acetyltransferase pathways are activated downstream from NGF and brain-derived nerve growth factor (BDNF) signalling during neuronal differentiation and axon outgrowth. Interestingly, the acetylation of p53 at Lys 320 by P/CAF and the highly homologous acetyltransferase CGN5 leads to increased transcriptional activation of the p21 promoter, which triggers G1/S arrest and promotes neuronal differentiation in PC12 cells (Wong et al, 2004). P/CAF and CGN5 are activated upon the phosphorylation of specific serine and threonine residues within their HAT domains—which is mediated by the kinases PI(3)K, Rsk2 and MSK1 (Fig 2). This observation clearly shows the presence of cross-talk between the phosphorylation and acetylation cascades. It is unclear whether these p53-dependent molecular pathways are representative of what occurs in neurons, given that the studies were performed in PC12 cells. However, they represent important background knowledge for the development of experiments aimed at clarifying the non-apoptotic p53-dependent pathways in neurons.
Figure 2.
p53-dependent signalling in neuronal differentiation and neurite-axonal outgrowth. NGF engagement of TrkA induces the activation of PI(3)K, MSK1 and RSK2, which promotes P/CAF and CGN5 phosphorylation. Phosphorylated P/CAF and CGN5 acetylate p53 at Lys 320, which increases p21 transcription, thereby triggering G1/S arrest and promoting differentiation. Activated PI(3)K and RSK2 also elicit a phosphorylation cascade that activates the CREB-binding protein CBP and p300, which acetylate several lysine residues located in the carboxy-terminal region of p53 (Lys 370, Lys 372, Lys 373 and Lys 382). The acetylation of p53 is responsible for its increased binding to the promoters of pro-outgrowth genes (see Table 1 and main text for details), which leads to increased neurite outgrowth. CBP, CREB-binding protein; CREB, cyclic-AMP-response-element-binding protein; GAP43 growth associated protein 43; MSK1, mitogen-activated and stress-activated protein kinase 1; NGF, nerve growth factor; P/CAF, CBP-associated factor; PI(3)K, phosphoinositide 3-kinase; Rab13, Ras-associated protein 13; RSK2, p90 ribosomal S6 protein kinase 2; TrkA, tyrosine kinase receptor A.
Studies in neuronal-like PC12 and neuroblastoma cells have shown that p53 gene expression is induced and required during neurotrophin-dependent neuronal differentiation and maturation (Hughes et al, 2000; Montano, 1997; Poluha et al, 1996, 1997). p53 was also reported to bind to the NGF receptor TrkA—which is required for PC12 neuronal differentiation (Browes et al, 2001)—and to activate TrkA expression (Zhang et al, 2006). Moreover, by genome-wide ChIP, Brynczka and colleagues have recently identified new putative p53 target genes during NGF-mediated PC12 neuronal differentiation, among which are wnt7b—which is involved in dendritic development—and the grhl3 factor—which is involved in pro-ectodermal differentiation (Brynczka et al, 2007).
Several years ago, p53 was proposed to elicit neuronal maturation in vitro after BDNF administration (Eizenberg et al, 1996), in addition to regulating apoptosis. However, the molecular explanation for this dual role of p53 in neuronal apoptosis and maturation was unknown. This encouraged us to investigate whether the PTMs of p53 could dictate specific non-apoptotic pathways to promote neuronal outgrowth and maturation. Indeed, by comparing cultured embryonic cortical neurons—obtained at embryonic day 18—from wild-type and p53-null mice, we observed that p53 is required for correct neurite outgrowth and neuronal maturation. In addition, the expression of acetylated p53 at Lys 373 and Lys 320 was enriched compared with that of total p53 during neuronal outgrowth and maturation in vitro (Di Giovanni et al, 2006; Tedeschi et al, 2008). Notably, the overexpression of specific p53 mutants that mimic acetylation at Lys 320 and Lys 373 was shown to promote neurite outgrowth and maturation in cultured neurons without affecting cell survival. Importantly, the acetyltransferases P/CAF and CBP/p300 formed a transcriptional complex with p53 on promoters of pro-neurite and axon-outgrowth genes such as Coronin 1b, Rab13 and GAP43 (Di Giovanni et al, 2006; Tedeschi et al, 2008). Finally, we found that such acetylated transcriptional complexes increased the affinity of p53 to specific promoters compared with the affinity shown by the total pool of p53.
Axon outgrowth and regeneration occur spontaneously—to a certain extent—in the peripheral nervous system after nerve injury. Transcription has a crucial role in driving the gene expression-dependent pro-regenerative response by affecting the remodelling of the cytoskeleton, which is required for axonal and growth-cone plasticity. The molecular mechanisms that drive neurite outgrowth and neuronal maturation in cortical neurons are partly recapitulated in the adult organism during axonal outgrowth and regeneration (Emery et al, 2003; Harel & Strittmatter, 2006). Therefore, taking advantage of a transection nerve model—such as the facial nerve axotomy model—that allows a certain degree of functional nerve regeneration in a physiologically relevant setting, we studied the requirement of p53 for peripheral nerve regeneration. p53-null mice show reduced spontaneous regeneration compared with wild-type mice, with no significant impact on cell survival, indicating that p53 is indeed required in this process. In addition, we demonstrated that C-terminal acetylation of p53 within CBP/p300-p53 transcriptional complexes increases its affinity to the promoters of pro-outgrowth genes in regenerating facial motor neurons (Di Giovanni et al, 2006; Tedeschi et al, 2008). Whether these or related p53-dependent pathways also mediate axonal regeneration within the central nervous system is now under investigation. In addition, several transcription factors, including c-jun, C/EBP, CREB and STAT3 (Lane & Bailey, 2005; Makwana & Raivich, 2005; Raivich & Behrens, 2006; Teng & Tang, 2006; Zhou & Snider, 2006), have been reported to promote axonal regeneration; however, their specific transcriptional partners, cofactors and targets in this process have not yet been elucidated.
The lack of removal of damaged cells probably influences the impairment in neuronal outgrowth and axonal regeneration that occurs in p53-null mice, given the essential role of p53 in DNA repair and the elimination of damaged cells. However, the overexpression of p53 mutants that mimic acetylation drives neuronal outgrowth in cultured neurons, and acetylated p53 occupies the promoter of pro-axonal regeneration genes and drives their expression in vivo, supporting a role for specific p53-dependent pro-neuronal outgrowth and pro-axonal regeneration pathways. For effective axonal outgrowth and regeneration to occur, appropriate axon guidance and pathfinding are also required. There is currently no evidence for a role of p53 in these neuronal processes, although p53 drives the expression of EphA2, semaphorin 3B, semaphorin 3F and the netrin-1 receptor Unc-5B in cancer cells to control tumour invasion and metastasis (Dohn et al, 2001; Futamura et al, 2007; Ochi et al, 2002; Tanikawa et al, 2003). Interestingly, these factors also regulate axon guidance (Arakawa, 2005; Barallobre et al, 2005; Harel & Strittmatter, 2006; Pasterkamp & Verhaagen, 2006); hence, studying their regulation on p53 induction in the nervous system could lead to the discovery of unknown transcriptionally mediated events in the control of axon guidance.
In addition to PTMs, protein–protein interactions also regulate the binding of transcription factors to promoters. Studies in neuronal cells have suggested that the interaction of p53 with the neuronal specific and pro-differentiation transcription factor Brn-3a facilitates a shift of p53 transcriptional activity from cell death to neuronal differentiation (Hudson et al, 2005). In fact, when forming a complex with Brn-3a, p53 is not able to activate pro-cell-death genes such as Bax, but rather shows increased affinity for the pro-differentiation gene p21. Brn-3b has also been shown to interact physically with p53 (Budhram-Mahadeo et al, 2006), although this interaction seems to increase the transactivation of the pro-apoptotic Bax promoter but not that of p21Cip1/WAF1. Consequently, the co-expression of Brn-3b with p53 enhances apoptosis, which is in contrast to the increased survival and differentiation observed when Brn-3a is co-expressed with p53. Whether specific PTMs influence the binding of p53 to either Brn-3 isoform is currently unknown and would be important to elucidate.
Conclusions, limitations and perspectives
We have presented and discussed the evidence supporting a role for p53 in the regulation of NSC proliferation and differentiation, as well as axon outgrowth and regeneration. Once again, p53 seems to be a crucial sensor for a multitude of cellular stresses and signals, which are processed through still unclearly orchestrated events that include PTMs and protein–protein interactions. Together, they lead to cell selection and phenotypic changes during development, as well as neuronal death or axon outgrowth in adulthood. The apparent 'dark side' of p53 that triggers mainly neuronal apoptosis following 'above threshold' DNA damage seems to be counterbalanced by its ability to promote axon growth in the presence of the appropriate neurotrophic signalling. However, there are limitations to our understanding of the role of p53 in these processes because our knowledge regarding the non-apoptotic p53-dependent signalling in neurons is based, in part, on studies carried out in cell lines and not in primary neurons or in vivo. Nonetheless, increasing experimental evidence has accumulated on this aspect of p53 biology in neurons, both in vitro and in vivo. Future work should aim to provide a better understanding of the signalling-dependent fine-tuning of the PTMs of p53 in neurodevelopment and post-axonal injury models, in order to elucidate the differential p53-dependent molecular mechanisms that control neuronal survival, differentiation, axonal outgrowth and regeneration (Sidebar A).
It is exciting to foresee that the ability of p53 to modulate different transcriptional responses in neurons depending on the specific signalling and transcriptional context might be linked not only to the PTMs on p53, but also to the cross-talk between p53 and a set of PTMs on histones. Histone codes contribute to the transcriptional environment and, therefore, to the generation of specific phenotypes during development, as well as to the regulation of cellular proliferation and transformation in cancer. Similar enzymes—such as the acetyltransferases CBP/p300 and P/CAF, and methyltransferases such as SET9—are responsible for generating the PTMs on both p53 and histones, establishing a parallelism between both codes. The transcriptional environment is generated by transcription factors (for example, p53), cofactors (such as CBP/p300, P/CAF and SET9) and histone modifications, which influence the chromatin state (or structure) and control gene expression. A unique chromatin environment could regulate the affinity of p53 to specific promoters in cancer cells, in which p53 could also influence chromatin structure by regulating gene expression or by forming transcriptional complexes with histone-modifying enzymes (Sims & Reinberg, 2008). Analogously, CHD8 binds to p53 during early embryogenesis, thereby promoting its association to histone H1 and forming a complex on chromatin that is required for the inhibition of p53-dependent transactivation and apoptosis (Nishiyama et al, 2009). In this setting, the recruitment of histone H1 can counteract p53-dependent apoptosis. Understanding whether such mechanisms also have a role in the regulation of the neuronal phenotype during development or following neuronal injury undoubtedly represents an exciting challenge for future investigation.

Andrea Tedeschi (left) & Simone Di Giovanni
Acknowledgments
We thank S. U. Steele and S. Vakil for their help in reading and editing this manuscript, and the members of the Laboratory for NeuroRegeneration and Repair for their support. Research in the Di Giovanni laboratory is supported by the Hertie Foundation, the Fortune grant (University of Tuebingen), the National Institutes of Health R21 NS052640 grant, the German Research Foundation (DFG) DI 1497/1-1 grant and the IRP-D-021/07 grant (all to S.D.G.).
References
- Aloyz RS, Bamji SX, Pozniak CD, Toma JG, Atwal J, Kaplan DR, Miller FD (1998) p53 is essential for developmental neuron death as regulated by the TrkA and p75 neurotrophin receptors. J Cell Biol 143: 1691–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CN, Tolkovsky AM (1999) A role for MAPK/ERK in sympathetic neuron survival: protection against a p53-dependent, JNK-independent induction of apoptosis by cytosine arabinoside. J Neurosci 19: 664–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa H (2005) p53, apoptosis and axon-guidance molecules. Cell Death Differ 12: 1057–1065 [DOI] [PubMed] [Google Scholar]
- Armesilla-Diaz A, Bragado P, Del Valle I, Cuevas E, Lazaro I, Martin C, Cigudosa JC, Silva A (2008) p53 regulates the self-renewal and differentiation of neural precursors. Neuroscience 158: 1378–1389 [DOI] [PubMed] [Google Scholar]
- Armstrong JF, Kaufman MH, Harrison DJ, Clarke AR (1995) High-frequency developmental abnormalities in p53-deficient mice. Curr Biol 5: 931–936 [DOI] [PubMed] [Google Scholar]
- Barallobre MJ, Pascual M, Del Rio JA, Soriano E (2005) The Netrin family of guidance factors: emphasis on Netrin-1 signalling. Brain Res Brain Res Rev 49: 22–47 [DOI] [PubMed] [Google Scholar]
- Becker EB, Bonni A (2004) Cell cycle regulation of neuronal apoptosis in development and disease. Prog Neurobiol 72: 1–25 [DOI] [PubMed] [Google Scholar]
- Brooks CL, Gu W (2003) Ubiquitination, phosphorylation and acetylation: the molecular basis for p53 regulation. Curr Opin Cell Biol 15: 164–171 [DOI] [PubMed] [Google Scholar]
- Browes C, Rowe J, Brown A, Montano X (2001) Analysis of trk A and p53 association. FEBS Lett 497: 20–25 [DOI] [PubMed] [Google Scholar]
- Brynczka C, Labhart P, Merrick BA (2007) NGF-mediated transcriptional targets of p53 in PC12 neuronal differentiation. BMC Genomics 8: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budhram-Mahadeo VS, Bowen S, Lee S, Perez-Sanchez C, Ensor E, Morris PJ, Latchman DS (2006) Brn-3b enhances the pro-apoptotic effects of p53 but not its induction of cell cycle arrest by cooperating in trans-activation of bax expression. Nucleic Acids Res 34: 6640–6652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Donehower LA (1999) p53 in embryonic development: maintaining a fine balance. Cell Mol Life Sci 55: 38–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cregan SP, Arbour NA, Maclaurin JG, Callaghan SM, Fortin A, Cheung EC, Guberman DS, Park DS, Slack RS (2004) p53 activation domain 1 is essential for PUMA upregulation and p53-mediated neuronal cell death. J Neurosci 24: 10003–10012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culmsee C, Mattson MP (2005) p53 in neuronal apoptosis. Biochem Biophys Res Commun 331: 761–777 [DOI] [PubMed] [Google Scholar]
- Di Giovanni S, Knights CD, Rao M, Yakovlev A, Beers J, Catania J, Avantaggiati ML, Faden AI (2006) The tumor suppressor protein p53 is required for neurite outgrowth and axon regeneration. EMBO J 25: 4084–4096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohn M, Jiang J, Chen X (2001) Receptor tyrosine kinase EphA2 is regulated by p53-family proteins and induces apoptosis. Oncogene 20: 6503–6515 [DOI] [PubMed] [Google Scholar]
- Eizenberg O, Faber-Elman A, Gottlieb E, Oren M, Rotter V, Schwartz M (1996) p53 plays a regulatory role in differentiation and apoptosis of central nervous system-associated cells. Mol Cell Biol 16: 5178–5185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery DL, Royo NC, Fischer I, Saatman KE, McIntosh TK (2003) Plasticity following injury to the adult central nervous system: is recapitulation of a developmental state worth promoting? J Neurotrauma 20: 1271–1292 [DOI] [PubMed] [Google Scholar]
- Flores ER, Tsai KY, Crowley D, Sengupta S, Yang A, McKeon F, Jacks T (2002) p63 and p73 are required for p53-dependent apoptosis in response to DNA damage. Nature 416: 560–564 [DOI] [PubMed] [Google Scholar]
- Futamura M, Kamino H, Miyamoto Y, Kitamura N, Nakamura Y, Ohnishi S, Masuda Y, Arakawa H (2007) Possible role of semaphorin 3F, a candidate tumor suppressor gene at 3p21.3, in p53-regulated tumor angiogenesis suppression. Cancer Res 67: 1451–1460 [DOI] [PubMed] [Google Scholar]
- Galderisi U, Jori FP, Giordano A (2003) Cell cycle regulation and neural differentiation. Oncogene 22: 5208–5219 [DOI] [PubMed] [Google Scholar]
- Gao Y, Ferguson DO, Xie W, Manis JP, Sekiguchi J, Frank KM, Chaudhuri J, Horner J, DePinho RA, Alt FW (2000) Interplay of p53 and DNA-repair protein XRCC4 in tumorigenesis, genomic stability and development. Nature 404: 897–900 [DOI] [PubMed] [Google Scholar]
- Gil-Perotin S, Marin-Husstege M, Li J, Soriano-Navarro M, Zindy F, Roussel MF, Garcia-Verdugo JM, Casaccia-Bonnefil P (2006) Loss of p53 induces changes in the behavior of subventricular zone cells: implication for the genesis of glial tumors. J Neurosci 26: 1107–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb E, Haffner R, King A, Asher G, Gruss P, Lonai P, Oren M (1997) Transgenic mouse model for studying the transcriptional activity of the p53 protein: age- and tissue-dependent changes in radiation-induced activation during embryogenesis. EMBO J 16: 1381–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel NY, Strittmatter SM (2006) Can regenerating axons recapitulate developmental guidance during recovery from spinal cord injury? Nat Rev Neurosci 7: 603–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms K, Nozell S, Chen X (2004) The common and distinct target genes of the p53 family transcription factors. Cell Mol Life Sci 61: 822–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helton ES, Chen X (2007) p53 modulation of the DNA damage response. J Cell Biochem 100: 883–896 [DOI] [PubMed] [Google Scholar]
- Herrup K, Yang Y (2007) Cell cycle regulation in the postmitotic neuron: oxymoron or new biology? Nat Rev Neurosci 8: 368–378 [DOI] [PubMed] [Google Scholar]
- Hudson CD, Morris PJ, Latchman DS, Budhram-Mahadeo VS (2005) Brn-3a transcription factor blocks p53-mediated activation of proapoptotic target genes Noxa and Bax in vitro and in vivo to determine cell fate. J Biol Chem 280: 11851–11858 [DOI] [PubMed] [Google Scholar]
- Hughes AL, Gollapudi L, Sladek TL, Neet KE (2000) Mediation of nerve growth factor-driven cell cycle arrest in PC12 cells by p53. Simultaneous differentiation and proliferation subsequent to p53 functional inactivation. J Biol Chem 275: 37829–37837 [DOI] [PubMed] [Google Scholar]
- Hughes PE, Alexi T, Walton M, Williams CE, Dragunow M, Clark RG, Gluckman PD (1999) Activity and injury-dependent expression of inducible transcription factors, growth factors and apoptosis-related genes within the central nervous system. Prog Neurobiol 57: 421–450 [DOI] [PubMed] [Google Scholar]
- Jacobs WB, Walsh GS, Miller FD (2004) Neuronal survival and p73/p63/p53: a family affair. Neuroscientist 10: 443–455 [DOI] [PubMed] [Google Scholar]
- Jacobs WB, Govoni G, Ho D, Atwal JK, Barnabe-Heider F, Keyes WM, Mills AA, Miller FD, Kaplan DR (2005) p63 is an essential proapoptotic protein during neural development. Neuron 48: 743–756 [DOI] [PubMed] [Google Scholar]
- Jacobs WB, Kaplan DR, Miller FD (2006) The p53 family in nervous system development and disease. J Neurochem 97: 1571–1584 [DOI] [PubMed] [Google Scholar]
- Kleihues P, Ohgaki H (1999) Primary and secondary glioblastomas: from concept to clinical diagnosis. Neuro Oncol 1: 44–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komarova EA, Chernov MV, Franks R, Wang K, Armin G, Zelnick CR, Chin DM, Bacus SS, Stark GR, Gudkov AV (1997) Transgenic mice with p53-responsive lacZ: p53 activity varies dramatically during normal development and determines radiation and drug sensitivity in vivo. EMBO J 16: 1391–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, Bailey SJ (2005) Role of retinoid signalling in the adult brain. Prog Neurobiol 75: 275–293 [DOI] [PubMed] [Google Scholar]
- Lavin MF, Gueven N (2006) The complexity of p53 stabilization and activation. Cell Death Differ 13: 941–950 [DOI] [PubMed] [Google Scholar]
- Louis JM, McFarland VW, May P, Mora PT (1988) The phosphoprotein p53 is down-regulated post-transcriptionally during embryogenesis in vertebrates. Biochim Biophys Acta 950: 395–402 [DOI] [PubMed] [Google Scholar]
- Makwana M, Raivich G (2005) Molecular mechanisms in successful peripheral regeneration. FEBS J 272: 2628–2638 [DOI] [PubMed] [Google Scholar]
- Meletis K, Wirta V, Hede SM, Nister M, Lundeberg J, Frisen J (2006) p53 suppresses the self-renewal of adult neural stem cells. Development 133: 363–369 [DOI] [PubMed] [Google Scholar]
- Miller FD, Kaplan DR (2007) To die or not to die: neurons and p63. Cell Cycle 6: 312–317 [DOI] [PubMed] [Google Scholar]
- Montano X (1997) P53 associates with trk tyrosine kinase. Oncogene 15: 245–256 [DOI] [PubMed] [Google Scholar]
- Mori S, Matsuyama K, Mori F, Nakajima K (2001) Supraspinal sites that induce locomotion in the vertebrate central nervous system. Adv Neurol 87: 25–40 [PubMed] [Google Scholar]
- Murray-Zmijewski F, Slee EA, Lu X (2008) A complex barcode underlies the heterogeneous response of p53 to stress. Nat Rev Mol Cell Biol 9: 702–712 [DOI] [PubMed] [Google Scholar]
- Nishiyama M, Oshikawa K, Tsukada Y, Nakagawa T, Iemura S, Natsume T, Fan Y, Kikuchi A, Skoultchi AI, Nakayama KI (2009) CHD8 suppresses p53-mediated apoptosis through histone H1 recruitment during early embryogenesis. Nat Cell Biol 11: 172–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi K, Mori T, Toyama Y, Nakamura Y, Arakawa H (2002) Identification of semaphorin3B as a direct target of p53. Neoplasia 4: 82–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda K et al. (2000) p53AIP1, a potential mediator of p53-dependent apoptosis, and its regulation by Ser-46-phosphorylated p53. Cell 102: 849–862 [DOI] [PubMed] [Google Scholar]
- Ohnuma S, Harris WA (2003) Neurogenesis and the cell cycle. Neuron 40: 199–208 [DOI] [PubMed] [Google Scholar]
- Pasterkamp RJ, Verhaagen J (2006) Semaphorins in axon regeneration: developmental guidance molecules gone wrong? Philos Trans R Soc Lond B Biol Sci 361: 1499–1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poluha W, Poluha DK, Chang B, Crosbie NE, Schonhoff CM, Kilpatrick DL, Ross AH (1996) The cyclin-dependent kinase inhibitor p21 (WAF1) is required for survival of differentiating neuroblastoma cells. Mol Cell Biol 16: 1335–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poluha W, Schonhoff CM, Harrington KS, Lachyankar MB, Crosbie NE, Bulseco DA, Ross AH (1997) A novel, nerve growth factor-activated pathway involving nitric oxide, p53, and p21WAF1 regulates neuronal differentiation of PC12 cells. J Biol Chem 272: 24002–24007 [DOI] [PubMed] [Google Scholar]
- Pozniak CD, Radinovic S, Yang A, McKeon F, Kaplan DR, Miller FD (2000) An anti-apoptotic role for the p53 family member, p73, during developmental neuron death. Science 289: 304–306 [DOI] [PubMed] [Google Scholar]
- Pozniak CD, Barnabe-Heider F, Rymar VV, Lee AF, Sadikot AF, Miller FD (2002) p73 is required for survival and maintenance of CNS neurons. J Neurosci 22: 9800–9809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raivich G, Behrens A (2006) Role of the AP-1 transcription factor c-Jun in developing, adult and injured brain. Prog Neurobiol 78: 347–363 [DOI] [PubMed] [Google Scholar]
- Riley T, Sontag E, Chen P, Levine A (2008) Transcriptional control of human p53-regulated genes. Nat Rev Mol Cell Biol 9: 402–412 [DOI] [PubMed] [Google Scholar]
- Rogel A, Popliker M, Webb CG, Oren M (1985) p53 cellular tumor antigen: analysis of mRNA levels in normal adult tissues, embryos, and tumors. Mol Cell Biol 5: 2851–2855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah VP, Attardi LD, Mulligan GJ, Williams BO, Bronson RT, Jacks T (1995) A subset of p53-deficient embryos exhibit exencephaly. Nat Genet 10: 175–180 [DOI] [PubMed] [Google Scholar]
- Sansom OJ, Clarke AR (2000) P53 null mice: damaging the hypothesis? Mutat Res 452: 149–162 [DOI] [PubMed] [Google Scholar]
- Schmid P, Lorenz A, Hameister H, Montenarh M (1991) Expression of p53 during mouse embryogenesis. Development 113: 857–865 [DOI] [PubMed] [Google Scholar]
- Sims RJ 3rd, Reinberg D (2008) Is there a code embedded in proteins that is based on post-translational modifications? Nat Rev Mol Cell Biol 9: 815–820 [DOI] [PubMed] [Google Scholar]
- Song J, Chao C, Xu Y (2007) Ser18 and Ser23 phosphorylation plays synergistic roles in activating p53-dependent neuronal apoptosis. Cell Cycle 6: 1412–1414 [PubMed] [Google Scholar]
- Tanikawa C, Matsuda K, Fukuda S, Nakamura Y, Arakawa H (2003) p53RDL1 regulates p53-dependent apoptosis. Nat Cell Biol 5: 216–223 [DOI] [PubMed] [Google Scholar]
- Tedeschi A, Nguyen T, Puttagunta R, Gaub P, Di Giovanni S (2008) A p53-CBP/p300 transcription module is required for GAP-43 expression, axon outgrowth, and regeneration. Cell Death Differ 16: 543–554 [DOI] [PubMed] [Google Scholar]
- Teng FY, Tang BL (2006) Axonal regeneration in adult CNS neurons: signaling molecules and pathways. J Neurochem 96: 1501–1508 [DOI] [PubMed] [Google Scholar]
- van Lookeren Campagne M, Gill R (1998) Tumor-suppressor p53 is expressed in proliferating and newly formed neurons of the embryonic and postnatal rat brain: comparison with expression of the cell cycle regulators p21Waf1/Cip1, p27Kip1, p57Kip2, p16Ink4a, cyclin G1, and the proto-oncogene Bax. J Comp Neurol 397: 181–198 [DOI] [PubMed] [Google Scholar]
- Vaughn AE, Deshmukh M (2007) Essential postmitochondrial function of p53 uncovered in DNA damage-induced apoptosis in neurons. Cell Death Differ 14: 973–981 [DOI] [PubMed] [Google Scholar]
- Wong K, Zhang J, Awasthi S, Sharma A, Rogers L, Matlock EF, Van Lint C, Karpova T, McNally J, Harrod R (2004) Nerve growth factor receptor signaling induces histone acetyltransferase domain-dependent nuclear translocation of p300/CREB-binding protein-associated factor and hGCN5 acetyltransferases. J Biol Chem 279: 55667–55674 [DOI] [PubMed] [Google Scholar]
- Xu Y (2003) Regulation of p53 responses by post-translational modifications. Cell Death Differ 10: 400–403 [DOI] [PubMed] [Google Scholar]
- Zhang J, Yan W, Chen X (2006) p53 is required for nerve growth factor-mediated differentiation of PC12 cells via regulation of TrkA levels. Cell Death Differ 13: 2118–2128 [DOI] [PubMed] [Google Scholar]
- Zheng H et al. (2008) p53 and Pten control neural and glioma stem/progenitor cell renewal and differentiation. Nature 455: 1129–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FQ, Snider WD (2006) Intracellular control of developmental and regenerative axon growth. Philos Trans R Soc Lond B Biol Sci 361: 1575–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]