Abstract
The maintenance and preservation of distinct phases during the cell cycle is a highly complex and coordinated process. It is regulated by phosphorylation — through the activity of cyclin-dependent kinases (CDKs) — and protein degradation, which occurs through ubiquitin ligases such as SCF (SKP1–CUL1–F-box protein) complexes and APC/C (anaphase-promoting complex/cyclosome). Here, we explore the functionality and biology of the F-box proteins, SKP2 (S-phase kinase-associated protein 2) and β-TrCP (β-transducin repeat-containing protein), which are emerging as important players in cancer biogenesis owing to the deregulated proteolysis of their substrates.
Cellular transformation results from aberrant responses to otherwise normal cues that regulate processes involved in proliferation, differentiation and apoptosis. These processes are regulated by transcription, translation, post-translational modifications and degradation of key regulatory proteins, and, as such, the ubiquitin–proteasome system (UPS) has a crucial role in maintaining and regulating cellular homeostasis. In order for target proteins to be recognized and degraded by the proteasome, the small protein ubiquitin is transferred and covalently attached to substrates by the sequential action of three enzymes, namely E1 (ubiquitin-activating enzyme), one of many E2s (ubiquitin-conjugating enzymes (UBCs)) and one of many E3s (ubiquitin ligases). The specifics of how these three enzymes work in concert are reviewed elsewhere1. Important for this Review, however, is the understanding that the specificity of ubiquitylation is provided by ubiquitin ligases, which physically interact with target substrates. With the number of ubiquitin ligases extending to several hundred, these complex molecular machines provide specificity and adaptability to regulated protein degradation.
Ubiquitin ligases are classified into two main classes on the basis of structural similarities: the RING-finger proteins and the HECT-domain proteins. Many multi-subunit, RING-finger type ubiquitin ligases contain a cullin (Cul) protein subunit, a name derived from the ability of cullin RING ubiquitin ligases (CRLs) to ‘cull’ or sort substrates for degradation2. In mammals there are six different CRLs, including the SKP1–CUL1–F-box protein (SCF) complex. In addition, there are CRL-like ligases such as the anaphase-promoting complex/cyclosome (APC/C).
The SCF ubiquitin ligases are the most characterized mammalian CRLs3. The SCF complex, together with the UBC component, forms a ‘super-enzyme’ (BOX 1). Most SCF substrates are recognized and bound by the F-box protein subunit only when they are post-translationally modified, usually through phosphorylation at specific sites. This is in contrast to other ligases, such as APC/C, which are only activated when needed and recognize substrates on the basis of a degradation motif (degron) in the primary sequence of their targets.
At a glance
Targeted protein proteolysis of key regulatory proteins by the ubiquitin–proteasome system (UPS) has a central role in maintaining and regulating growth. As such, components of the UPS can promote or prevent cellular transformation, which results from an aberrant response to otherwise normal cues that regulate processes involved in proliferation, differentiation and apoptosis.
The SCF (SKP1–CUL1–F-box protein) ubiquitin ligases are the best characterized mammalian cullin RING ubiquitin ligases, and the F-box protein provides the substrate targeting specificity of the complex.
Out of sixty-nine F-box proteins that have been identified in humans, only nine have been matched with their respective substrates. The F-box proteins SKP2 (S-phase kinase-associated protein 2) and β-TrCP (β-transducin repeat-containing protein) have emerged as key regulatory molecules with roles in cellular processes that are intimately related to tumorigenesis.
SKP2 is an oncogenic protein that targets tumour suppressor proteins for degradation. As a positive regulator of cell cycle progression, a major target of SKP2 is the cyclin-dependent kinase (CDK) inhibitor p27, as has been shown in vivo and in vitro. Increased levels of SKP2 and reduced levels of p27 are observed in many types of cancer, and these levels are in several cases used as independent prognostic markers.
Whereas β-TrCP has been previously suggested to possess both oncogenic and tumour suppressive characteristics — mainly owing to the diversity in β-TrCP substrates — recent evidence indicates β-TrCP is mainly oncogenic.
Previous attempts at targeting components of the degradation machinery have been successful for laboratory and clinical use, as observed in the effectiveness of the proteasome inhibitor bortezomib (Velcade) in multiple myeloma. The development of pharmaceutical compounds targeting specific SCF ubiquitin ligases is timely and is complemented by structural and basic biochemical studies that have identified substrates for important cellular regulators such as SKP2 and β-TrCP.
Sixty-nine F-box proteins have been identified in humans4–6, and they have been classified into three categories: those with WD40 domains (FBXWs), those with leucine-rich repeats (FBXLs) and those with other diverse domains (FBXOs). Notably, only nine of the sixty-nine SCF ubiquitin ligases have well-established or proposed substrates; these include SCFβ-TrCP1, SCFβ-TrCP2, SCFSKP2, SCFFBXL3, SCFFBXL20, SCFFBXO4, SCFFBXW7, SCFFBXO7 and SCFFBXW8. Substrates of these SCF complexes can be sub-divided into two main groups: direct regulators of cyclin-dependent kinases (CDKs) and regulators of gene transcription (or both). Numerous studies have described the roles of SCF ubiquitin ligases in controlling cell size, proliferation and survival, and, given the diverse and important roles of SCF ligases, their deregulation has been implicated in aberrant cellular growth and tumorigenesis. Such is the case for FBXW7, which has a central role in cell cycle progression, cellular growth and differentiation by targeting oncogenic proteins for degradation, and several cancer-associated mutations have been shown to exist in FBXW7 (REF. 7). In this Review, we will focus on SKP2 (S-phase kinase-associated protein 2, also known as FBXL1) and β-TrCP (β-transducin repeat-containing protein), the two other prototypical and best characterized mammalian F-box proteins. Notably, mammals express two distinct paralogues of β-TrCP with biochemical properties that are indistinguishable: β-TrCP1 (also known as FBXW1, FBW1A and FWD1) and β-TrCP2 (also known as FBXW11, FBW11, FBXW1B, FBX1B and HOS). We will therefore use the term β-TrCP to refer to both, unless specified. SKP2 and β-TrCP are key players in regulating many cellular processes that are related to cancer through targeted degradation of substrates. We will highlight their respective substrates and discuss the role of these molecules in cancer biogenesis and tumour progression.
SKP2 and β-TrCP control the activity of CDKs
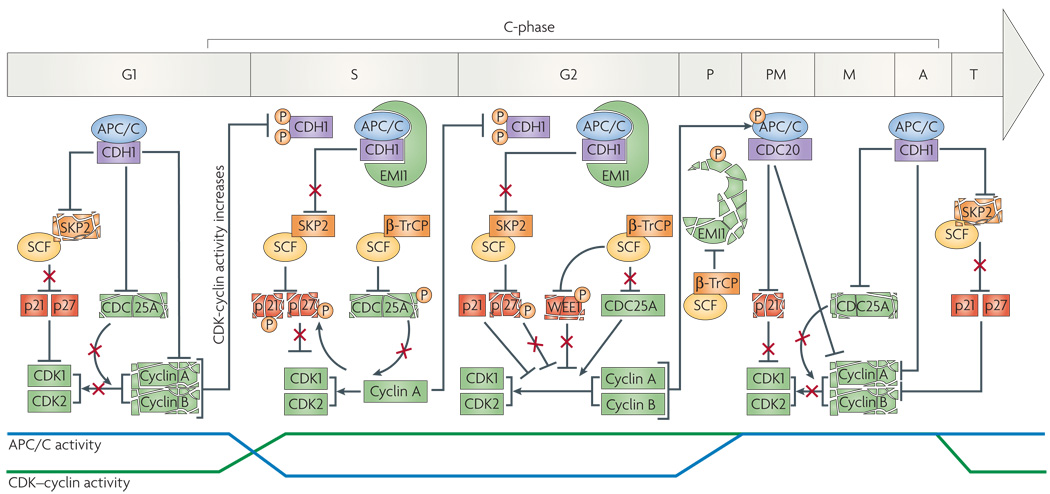
Tight regulation of the cell division cycle through proper activation and inactivation of CDKs is crucial to preventing cellular transformation8. In addition to the catalytic subunit of the CDK, each CDK complex contains one of many activating subunits, called cyclins, the levels of which oscillate during the cell cycle. Although CDK1 (also known as cell division cycle 2) is the only CDK in mammals that is essential for viability, several distinct cyclin–CDK complexes finely regulate progression through distinct phases of the cell cycle. CKIs (CDK inhibitors), such as p21 and p27, function by inhibiting CDK activity and promoting cell cycle arrest and/or delaying the response to anti-mitogenic stimuli. CDK1 and CDK2 are active from late G1 to anaphase, and for this reason, this temporal interval is defined as CDK phase (or C phase)9. Whereas CDK2 activity peaks in S and G2 phases, CDK1 activity is relatively low in S, increases in G2, and peaks in early mitosis, only to disappear abruptly during anaphase. SCF complexes and APC/C provide specific, rapid and timely proteolysis of cell cycle regulators (which ultimately control CDKs), thereby finely modulating their activities during cell cycle progression. Whereas SKP2 activates CDK1 and CDK2 by directing the degradation of p21 and p27, β-TrCP has a dual role in controlling CDK1 activity: turning it off by inducing the degradation of CDC25A (cell division cycle 25A) and EMI1 (also known as F-box protein 5), in S phase and mitosis, respectively, and turning it on by inducing the degradation of claspin and WEE1 at G2–M (FIG. 1).
Figure 1. The UPS controls the cell cycle.
The cell division cycle is regulated primarily by the activity of cyclin-dependent kinases (CDKs) and protein degradation by the ubiquitin–proteasome system (UPS). Each CDK complex contains one of many activating subunits, termed cyclins, the levels of which oscillate during the cell cycle. CKIs (CDK inhibitors), such as p27 and p21, inhibit CDK activity and promote cell cycle arrest and/or delay. SCF complexes and the APC/C (anaphase-promoting complex/cyclosome) provide the specific, rapid and timely proteolysis of cell cycle regulators, which ultimately controls CDK1 and CDK2 to finely modulate their activities during cell cycle progression. The best characterized cell cycle ubiquitin ligases are SCFSKP2, SCFFBXW7 (not shown), SCFβ-TrCP, APC/CCDH1 and APC/CCDC20. SCFSKP2 is a positive regulator of cell cycle progression (by promoting the degradation of p21 and p27), whereas SCFβ-TrCP is both a positive and negative regulator of the cell cycle (by targeting CDC25A (cell division cycle 25A), claspin, WEE1 and EMI1 (also known as F-box protein 5)). APC/CCDH1 and APC/CCDC20 always attenuate CDK1 activity (by directing the degradation of cyclins A and B), except in early mitosis, when APC/CCDC20 targets p21 for degradation. Finally, SCFFBXW7 attenuates CDK1 and CDK2 by inducing the degradation of cyclin E. SCF complexes and the APC/C control each other, with SKP2 being ubiquitylated by APC/CCDH1 in G1 and SCFβ-TrCP targeting EMI1, which is an inhibitor of APC/CCDH1, for proteolysis in early mitosis. Additionally, SCF complexes and the APC/C share common substrates that are targeted by their respective ubiquitin ligase(s) only at particular times during the cell cycle. For example, SCFSKP2 targets p21 for degradation at G1–S, whereas APC/CCDC20 targets p21 during prometaphase. This scenario is also true for the targeted degradation of CDC25A by APC/CCDH1 in G1 phase, which is followed by SCFβ-TrCP-mediated degradation during S phase. Moreover, phosphorylation by CDKs modulates the activity of SCF complexes and the APC/C. CDK activity inhibits binding of CDH1 to the APC/C while promoting the activation of APC/CCDC20, and phosphorylation of certain SCF substrates by CDKs allows recognition by the F-box protein subunit. β-TrCP, β-transducin repeat-containing protein; CDH1, also known as FZR1 (fizzy/cell division cycle 20 related 1); FBXW7, F-box protein with WD domain 7; SKP2, S-phase kinase-associated protein 2.
Notably, β-TrCP has also emerged as a key player in the S and G2 DNA-damage response checkpoint10, the main function of which is to mediate cell cycle arrest to allow time to repair DNA lesions. This is mainly accomplished by limiting the activity of CDKs to prevent premature hyperactivation of CDK1 and entry into mitosis before the completion of DNA repair. Upon DNA damage, activation of CHK1 (checkpoint kinase-1) and CHK2 through phosphorylation by ATR (ataxia-telangiectasia mutated and RAD3-related) and ATM (ataxia-telangiectasia mutated), respectively, results in hyperphosphorylation of CDC25A, leading to increased proteolysis of CDC25A (mediated by β-TrCP) and attenuation of CDK1 activity (FIG. 1). Importantly, upon recovery from DNA damage, β-TrCP additionally functions to restore CDK1 activity by targeting claspin and WEE1 for degradation. Therefore, given the crucial function of the cell cycle machinery in regulating cell cycle progression, the altered proteolysis of cell cycle regulators is clearly a contributing factor in the unrestrained proliferation that is typical in cancer cells.
SKP2 targets tumour suppressor proteins
SKP2 was first identified as a component of the cyclin-A– CDK2 complex and was subsequently shown to promote entry into S phase11. Since then, SKP2 has been extensively characterized as an SCF ubiquitin ligase that is crucial for cell cycle progression (see above and FIG. 1) and cell proliferation.
Biochemical studies identified SKP2 as the rate-limiting component of the SCF machinery that ubiquitylates the tumour suppressor p27 in vitro and in vivo12–14. In particular, SKP2 recognizes p27 only when p27 is phosphorylated on Thr187 by one of many CDK complexes. Moreover, the ubiquitylation of p27 requires CKS1 (CDK subunit 1), which binds to SKP2 and increases the affinity of phosphorylated p27 for SKP2 (REFS 15,16). Importantly, this result was the first demonstration of a requirement of an accessory protein for SCF function.
Mouse models of p27 function have provided genetic evidence that the loss of Cdkn1b (which encodes p27) promotes cell proliferation, which leads to endocrine dysfunction and the development of cancer17. Mice lacking p27 have been shown to be larger than wild-type, control littermates and exhibit generalized organomegaly. Similar to mice lacking Rb1 (retinoblastoma 1), p27−/− mice spontaneously develop pituitary tumours, confirming that p27 and RB1 function in similar regulatory pathways. Interestingly, all p27-null 129/Sv mice develop pituitary adenomas by the age of ten weeks and die with massively enlarged pituitary tumours that result in compression of the brain. Thymic enlargement in p27−/− mice was associated with an increase in T-lymphocyte proliferation, and, in the spleen, the absence of p27 enhanced proliferation of haematopoietic progenitor cells.
Box 1 | SCF complexes are super-enzymes
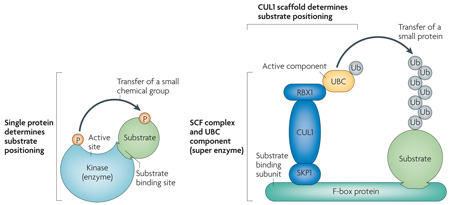
The SCF (SKP1–CUL1–F-box protein) ubiquitin ligase complex is the best characterized mammalian cullin RING ubiquitin ligase (CRL). The cullin subunit CUL1 functions as a molecular scaffold that interacts at the amino terminus with the adaptor subunit SKP1 (S-phase kinase-associated protein 1) and at the carboxyl terminus with a RING-finger protein RBX1 (also known as ROC1), RBX2 (also known as ROC2) or Ro52 and a specific E2 enzyme or ubiquitin-conjugating enzyme (UBC), such as UBC3, UBC4 or UBC5 (REF. 3). The F-box protein functions as the variable component that binds SKP1, through the F-box domain, and the substrate, through different protein–protein interaction motifs, which in most cases are localized C-terminally of the F-box. There is a debate as to whether SCF ligases are true enzymes. In many ways, the molecular composition and functionality of SCF ligases, together with the UBC component, can be considered a super-enzyme. When compared to classical enzymes, such as kinases, many fundamental characteristics support this view. In the classical enzymatic reaction, the kinase transfers a small chemical group (that is, a phosphate) by way of an active catalytic site to targeted substrates. These substrates are selected on the basis of their ability to bind the specific kinase through a substrate-binding domain. Lastly, the orientation of the substrate and its positioning towards the active site is determined within a single protein chain. In comparable mechanical fashion, SCF ubiquitin ligases transfer a small protein (that is, the ubiquitin moiety) by way of an activated UBC component to specific substrates that are selected through a particular substrate-binding protein (that is, the F-box protein). Here, the cullin and F-box protein dictate the orientation of the substrate and its presentation to the RING-finger protein–UBC pair. Finally, SCF complexes have been shown to contain intrinsic ubiquitin ligase activity in vitro, as purified, recombinant CUL1–RBX1 complexes (in the absence of SKP1 and the F-box protein subunits) can catalyse UBC-dependent, substrate-independent ubiquitylation through the formation of free ubiquitin chains. Therefore, based on the action, specificity, and multi-subunit composition of the SCF, the notion of the super-enzyme can be used to characterize these ubiquitin ligases.
In agreement with biochemical data acquired in cultured cells, the absence of SKP2 in mice results in the accumulation of p27, and Skp2−/− mice were shown to be smaller than wild-type littermates18. Skp2−/− cellular phenotypes included nuclear enlargement and polyploidy in cells of the liver, lung, kidney and testis, and an increased number of centrosomes in mouse embryonic fibroblasts (MEFs)18. Importantly, all these phenotypes disappear in Skp2−/− ; p27−/− double-mutant mice, indicating that p27 is a key target of SKP2 (REFs 19,20). Cks1−/− mice are also smaller than wild-type animals, and cells derived from these mutant mice were shown to proliferate poorly, probably owing to elevated levels of p27 (REF. 15).
Other SKP2 substrates are also tumour suppressor proteins, such as the CKIs, p21 (REFs 21,22) and p57 (encoded by CDKN1C)23; TOB1 (transducer of ERBB2)24; RASSF1 (Ras association domain family 1)25; and RBL2 (retinoblastoma-like 2; also known as p130)26. FOXO1, a member of the forkhead box-containing transcription factors that are involved in various cellular processes including cell cycle regulation, differentiation, stress responses and apoptosis, is also targeted for degradation by SKP2 as a consequence of phosphorylation by Akt, which is a pro-survival kinase27.
Several other proteins have been reported to be targeted by SKP2 (TABLE 1). Importantly, SKP2 recognizes substrates for ubiquitylation through phosphorylation of consensus sequence(s) rather than recognizing a degron in the primary sequence. Therefore, although some of these substrates, such as USP18 (ubiquitin-specific peptidase 18)28 and cyclin D1 (REF. 18), have been observed to accumulate in Skp2−/− MEFs, they have never been shown to be ubiquitylated in vitro via SKP2, implying that they might be indirectly upregulated in the absence of SKP2. Another group of proposed substrates (such as CDK9, which is the catalytic subunit of the positive-transcription elongation factor B29,30) have not been validated by follow-up studies or cannot be confirmed by other groups. As Skp2−/− ; p27−/− mice and MEFs overcome phenotypes that are associated with SKP2 deficiency, it is possible that p27 is the crucial substrate of SKP2 in vivo. Alternatively, SKP2 substrates other than p27 might be efficiently targeted by additional ubiquitin ligases, reducing the role of SKP2 in their regulation. For example, human CDT1 (chromatin licensing and DNA replication factor 1) is also degraded through CRL4; p21 is also degraded through APC/CCDC20; and cyclin E is also degraded through SCFFBXW7. Future studies will be required to validate all SKP2 substrates, identify the biological relevance of their degradation and characterize their potential roles in promoting or preventing cancer.
Table 1.
Reported substrates of SKP2
| Reported substrates | Function | Upregulated in Skp2−/− MeFs | Ubiquitylation reconstituted in vitro | Refs |
|---|---|---|---|---|
| p27* | Cell cycle control | Yes | Yes | 12, 13, 14 |
| p21* | Cell cycle control | Yes | Yes | 21, 22, 126 |
| p57 | Cell cycle control | Yes | Yes | 23 |
| Cyclin A | Cell cycle control | Yes | No | 18 |
| Cyclin E‡ | Cell cycle control | Yes | Yes | 18 |
| Cyclin D1 | Cell cycle control | Yes | No | 22 |
| CDT1 | DNA replication | No | Yes | 127 |
| ORC1 | DNA replication | ND | No | 128 |
| BRCA2 | DNA repair | ND | No | 129 |
| RAG2 | DNA repair | ND | Yes | 130 |
| TOB1 | Gene transcription | Yes | Yes | 24 |
| RBL2* | Gene transcription | ND | Yes | 26 |
| FOXO1 | Gene transcription | ND | Yes | 27 |
| MEF/ETS | Gene transcription | ND | Yes | 131 |
| MLL | Gene transcription | ND | Yes | 132 |
| MYB | Gene transcription | ND | No | 133 |
| MYC§ | Gene transcription | ND | No | 134–135 |
| E2F1 | Gene transcription | No | No | 136 |
| HPV-E7 | Viral oncogenesis | Yes | Yes | 137 |
| USP18 | Interferon signaling | Yes | No | 28 |
| MKP1 | ERK signalling | ND | Yes | 138 |
| SMAD4 | Signal transduction | ND | Yes | 139 |
| CDK9 | Transcriptional elongation | Yes | No | 29–30 |
| E2A | B- and T-cell development | ND | No | 140–141 |
| TAL1 | Erythroid differentiation | ND | No | 142 |
| RASSF1 | Microtubule stabilizer | Yes | Yes | 25 |
Requires CKS1 (CDK subunit 1) for binding to SKP2.
Only free (CDK-unbound) cyclin E.
Ubiquitylation seems to promote transcriptional activity. BRCA2, breast cancer associated 2; CDK9, cyclin-dependent kinase 9; CDT1, chromatin licensing and DNA replication factor 1; FOXO1, Forkhead box-containing, O subfamily 1; HPV-E7, human papillomavirus E7 protein; MEFs, mouse embryonic fibroblasts; MEF/ETS, myeloid ELF1-like factor; MKP1, mitogen-activated protein kinase (MAPK) phosphatase 1; MLL, myeloid/lymphoid leukaemia; ND, not determined; ORC1, origin recognition complex 1; RAG2, recombination activating gene 2; RASSF1, Ras association domain family 1; RBL2, retinoblastoma-like 2 (also known as p130); SKP2, S-phase kinase-associated 2; TAL1, T-cell acute lymphocytic leukaemia 1 (also known as SCL); TOB1, transducer of ERBB2; USP18, ubiquitin-specific peptidase 18 (also known as UBP43).
SKP2 as an oncogene
The transformation potential of SKP2 was initially observed in tissue culture systems, in which forced expression of SKP2 in immortalized cells was found to induce degradation of p27, promote entry into S phase and induce growth in the absence of adhesion to the extracellular matrix31. The introduction of a dominant-negative SKP2 mutant into breast cancer cells was shown to result in fewer colonies in soft agar, and accordingly, enforced expression of SKP2 in hormone-dependent breast cancer cells conferred resistance to a G1 arrest mediated by anti-oestrogens and oestrogen deprivation32. Expression of SKP2 was observed to increase with androgen addition in human prostate cells, leading to an increase in p27 degradation and increased proliferation33,34. Moreover, overexpression of SKP2 overcomes cell cycle arrest by androgens33. Together, these results indicate that overexpression of SKP2 (and the subsequent low levels of p27) serves as a growth advantage for cancer cells.
Several mouse models confirm the function of the SKP2–p27 axis in tumorigenesis. For example, in mammary epithelium, p27 deficiency cooperates with the ErbB2 oncogene in breast tumour development. Similarly, overexpression of SKP2 in T cells cooperates with activated NRAS in lymphomagenesis35, and lymphomas arising in mice that are deficient for the transcriptional activator CBP (cAMP-response-element-binding protein (CREB)-binding protein) exhibit reduced levels of p27 and increased levels of SKP2 (REF. 36). Introduction of a p27-null allele into Cbp−/− mice accelerates lymphomagenesis and obviates the need for SKP2 upregulation. In addition, constitutive expression of SKP2 in mouse prostate promoted marked overproliferation, resulting in hyperplasia, dysplasia and low-grade carcinoma of the prostate gland, and, consistent with its crucial role in p27 proteolysis, resulted in a significant downregulation of p27 (REF. 37). Similarly, xenografts of breast cancer cell lines expressing SKP2 grow faster than those expressing lower levels of SKP2 (REF. 38). Analyses of a p27-T187A knock-in mouse, which generates a p27 mutant that cannot be bound by SKP2 owing to the loss of T187 phosphorylation, showed that the SKP2-dependent degradation of p27 is crucial for the progression of colon adenomas to colon carcinomas39. Finally, increased expression of CKS1 in MYC-induced mouse lymphomas correlates with low levels of p27 (REF. 40), in agreement with the function of CKS1 in promoting SKP2-mediated degradation of p27. Importantly, loss of CKS1 in Eμ-Myc B cells impairs the ability of MYC to induce cell proliferation, lymphomagenesis and metastasis.
Numerous studies in patients have shown low levels of p27 rather than complete loss of p27 expression in tumours, and this is in agreement with the notion of p27 as a haploinsufficient tumour suppressor41. The inactivation of p27 in human tumours is rarely due to the loss of one allele or inactivating mutations, and loss of heterozygosity has never been reported. Instead, the low levels of p27 are attributed to a decrease in protein stability17. As such, increases in SKP2 expression (mRNA and protein) are associated with downregulation of p27 in a wide range of human tumours and cell lines. The mechanisms that lead to the upregulation of SKP2 in human cancers have been identified for a small set of tumours (TABLE 2). Notably, in all studied cases of cancer, high levels of SKP2 correlate with poor overall survival. Elevated expression of CKS1 (mRNA and protein) is also observed in some tumours, and, importantly, CKS1 was found to be an independent prognostic marker for breast, colorectal and gastric cancers42–46 (TABLE 2).
Table 2.
Cancers associated with SKP2 deregulation
| Human cancer | Correlation with poor prognosis | Correlation with low p27 levels | Refs |
|---|---|---|---|
| Biliary tract cancer | Yes | No | 143 |
| Breast cancer* | Yes | Yes | 32, 42, 144–145 |
| Cervical cancer | Yes | No | 146 |
| Colon cancer* | Yes | Yes | 39, 43, 147–148 |
| Endometrial cancer | Yes | ND | 149–150 |
| Gastric cancer* | Yes | Yes | 45, 151–152 |
| Glioma/glioblastoma‡ | Yes | Yes | 153–154 |
| Kaposi sarcoma | Yes | No | 155 |
| Lung cancer (NSLC) *‡ | Yes | Yes | 156–158 |
| Lung cancer (SLC)* | Yes | Yes | 157–159 |
| Lymphoma and leukaemia | Yes | No | 40 |
| Multiple myeloma | Yes | Yes | 160–161 |
| Melanoma | Yes | Yes | 162–165 |
| Oral cancers | Yes | Yes | 147, 166–169 |
| Ovarian cancer | Yes | Yes | 170–171 |
| Prostate cancer | Yes | Yes | 34, 172–173 |
CKS1 (CDK subunit 1) also upregulated.
Gene amplification. ND, not determined; NSLC, non-small-cell lung cancer; SKP2, S-phase kinase-associated protein 2; SLC, small-cell lung cancer.
Presumably, SKP2 overexpression or improper temporal expression (such as expression in G1 when it is normally low) confers a growth advantage by enhancing the degradation of p27. However, there are certain cancers in which elevated levels of SKP2 do not correlate with low levels of p27. It is possible that in these tumours p27 is degraded via alternative ubiquitin ligases — through KPC47 or PIRH2 48, for example — and it is important to investigate whether in these same tumours there is a positive selective pressure to enhance the degradation of different SKP2 substrates (such as FOXO1, TOB1, p130 or RASSF1). Experiments that were conducted in prostate carcinoma cells show that SKP2 overexpression eliminates the pro-apoptotic effect of FOXO1, and the levels of FOXO1 and SKP2 are inversely correlated in mouse lymphoma models, suggesting that elevated SKP2 is a major factor responsible for the degradation of FOXO1 in lymphomagenesis. Furthermore, FOXO1 and SKP2 levels were also found to be inversely correlated in endometrial cancer cells49. Notably, FOXO1 regulates cell cycle arrest by transcriptional activation of negative cell cycle regulators, such as p27, p21 and p130 (all of which are also targeted by SKP2). Therefore, characterizing the expression of FOXO1 in human tumours, its correlation with levels of SKP2 and p27, levels of Akt activity and the resulting clinical outcomes will be crucial to fully understanding the role of this tumour suppressor. Finally, a role for RBL2 loss has been suggested for many tumours50–56, and future studies will be needed to determine whether the levels of RBL2 are affected in tumours overexpressing SKP2.
β-TrCP functions in diverse pathways
As mentioned above, mammals express two distinct paralogues of β-TrCP with biochemical properties that are indistinguishable. Work by several groups has demonstrated the versatility of β-TrCP in regulating various cellular processes through mediating the degradation of a variety of targets (TABLE 3). β-TrCP recognizes a DSGXXS destruction motif or its variants (for example, DSG/DDG/EEG/SSGXXS/E/D motifs) in which the serine residues are phosphorylated by specific kinases to allow binding to β-TrCP (TABLE 3). Targets of β-TrCP can be divided into two main groups: cell cycle regulators and pro-apoptotic regulators. Accordingly, inhibition by RNA interference or forced expression of a dominant-negative β-TrCP mutant induces apoptosis in human malignant melanoma and breast cancer cells, and augments the cytotoxic effects of anticancer drugs and ionizing radiation57–59. This effect is probably due to pro-apoptotic factors (for example, IκB (inhibitor of nuclear factor κB), PDCD4 (programmed cell death 4) and others) and CDC25A (which promotes mitotic catastrophe) that accumulate when β-TrCP is inhibited (see below).
Table 3.
Reported substrates of β-TrCP reported substrates
| Reported substrates | Function | Degron in humans | Refs |
|---|---|---|---|
| IκBα | Inhibitor of NFκB | DSGLDS | 71–78 |
| IκBβ | Inhibitor of NFκB | DSGLGS | 72 |
| IκBε | Inhibitor of NFκB | DGSIES | 72 |
| p100 | NFκB signalling | DSAYGS | 174–175 |
| p105 | NFκB signalling | DSGVETS | 176–177 |
| WEE1 | CDK1 inhibitory kinase | DSAFQE/EEGFGS | 64 |
| CDC25A | Phosphatase, CDK1 activator | STDSG | 58, 110 |
| CDC25B | Phosphatase, CDK1 activator | DDGFVD/DSGFCLDS | 110 |
| β-catenin | Wnt signalling | DSGIHS | 76, 99–104 |
| PDCD4 | Protein synthesis | DSGRGDS | 84 |
| Claspin | DNA replication and damage stress Transcriptional repressor | DSGQGS | 62, 63 |
| REST | Transcriptional repressor | DEGIHS/STDSG | 93, 106 |
| ATF4 | Transcription factor | DSGICMS | 178 |
| PRL-R | Growth hormone signalling | DSGRGS | 179 |
| CD4 (HIV VPU)* | Viral replication | ND | 180 |
| IFNR | Cytokine signalling | DSGNYS | 181 |
| DLG | Cell contact and polarity | DSGLPS | 182 |
| EMl1‡ | CDH1 inhibitor | DSGYSS | 60 |
| Snail | Animal patterning | DSGKGS | 75 |
| PER1 | Circadian clock transcription | TSGCSS | 183 |
| PER2 | Circadian clock transcription factor | SSGYGS | 184–185 |
| PC2§ | Calcium signalling | ND | 186 |
| MCL1 | Pro-apoptotic protein | DGSLPS | 187 |
| Pro-caspase 3 | Pro-apoptotic protein | ND | 188 |
| p63 | Epithelial differentiation and apoptosis | ND | 189 |
| GHR | Growth hormone signalling | ND | 190 |
| Bora | PLK1 activator | DSGYNT | 191 |
| STAT1 | Transcription factor | ND | 192 |
CD4 has no DSG motif; VPU (viral protein U) targets CD4 to β-TrCP.
Upregulated in β-Trcp−/−mouse embryonic fibroblasts.
PC2 has no DSG motif; tafazzin targets PC2 to β-TrCP. ATF4, activating transcription factor 4; β-TrCP, β-transducin repeat-containing protein; CDC25A, cell division cycle 25A; CDH1, also known as fizzy/cell division cycle 20 related 1 (FZR1); CDK1, cyclin-dependent kinase 1; DLG, discs large tumour suppressor; EMl1, also known as F-box protein 5; GHR, growth hormone receptor; IκB, inhibitor of NFκB; IFNR, interferon receptor; MCL1, myeloid cell leukaemia 1; ND, not determined; NFκB, nuclear factor κB; p100, NFκB2 protein precursor; p105, NFκB1 protein precursor; PC2, protein polycystin 2; PDCD4, programmed cell death 4; PER1, period homologue 1; PLK1, polo-like kinase 1; PRL-R, protein tyrosine phosphatase 4A3; REST, repressor element 1 (RE1)-silencing transcription factor; STAT1, signal transducer and activator of transcription 1.
β-Trcp1−/− mice have been shown to have impairment in spermatogenesis and reduced fertility without signs of gross tissue abnormalities60,61. Overall viability of these animals is also unaffected. This result is probably due to redundancy with β-TrCP2, which presumably continues to target β-TrCP substrates for degradation. However, MEFs isolated from β-Trcp1−/− animals display centrosome overduplication that is associated with the presence of multipolar metaphase spindles, misaligned chromosomes and a lengthened G2–M transition. The stabilization of claspin62,63 and WEE1 (REF. 64) is probably the main reason that β-Trcp1−/− MEFs progress slower than wild-type cells through the G2–M transition and mitosis.
β-TrCP is an oncoprotein (in some tissues)
Owing to the diversity in its substrates, β-TrCP might be expected both to be oncogenic and display tumour suppressor activity (TABLE 3). However, overwhelming evidence indicates that β-TrCP possesses mainly oncogenic characteristics. Indeed, overexpression of β-TrCP has been reported in many cases. One study, examining colorectal cancer tissues, showed that 56% of the tissues tested had increased β-TrCP1 mRNA and protein levels, and that this increase was associated with decreased apoptosis and poor prognosis65. Furthermore, chemoresistant pancreatic cancer cell lines also display markedly elevated levels of β-TrCP1 and constitutive activation of NFκB (nuclear factor κB; see below)66. β-TrCP1 is also overexpressed in hepatoblastomas67, whereas β-TrCP2 overexpression is observed in some breast cancers68. Similarly, mammary epithelia of female mice exogenously expressing high levels of β-TrCP1 proliferate faster, with 38% of these mice developing carcinomas69. Interestingly, targeted expression of β-TrCP in lymphoid organs produces no phenotype, suggesting that β-TrCP-dependent tumorigenesis is tissue specific69. Together, these observations advocate a role for β-TrCP in promoting tumour development in certain tissues.
β-TrCP targets tumour suppressor proteins for UPS-mediated degradation
NFκB is an inducible, dimeric transcription factor complex composed of members of the Rel family of DNA-binding proteins that activate a large number of genes in response to infection, inflammation and other stresses70. NFκB targets include genes that encode various regulatory cytokines (for example, interleukin and TNFα (tumour necrosis factor-α)), transcription factors, survival factors (for example, BCL-xL), growth factors (for example, interleukin 6 and GMCSF (granulocyte macrophage colony-stimulating factor)), adhesion molecules (for example, MMP9 (matrix metalloproteinase 9)), cell surface receptors and immune modulators. Under normal conditions, inactive NFκB is sequestered in the cytoplasm and a family of inhibitory proteins, the IκBs, actively bind NFκB to mask its nuclear localization signal, thereby preventing nuclear import of NFκB and consequently inhibiting its activity70. The exposure of cells to a variety of stresses leads to the rapid phosphorylation and ubiquitylation of IκB via β-TrCP71–78. The subsequent proteolytic degradation of IκB frees NFκB to translocate to the nucleus, where it can regulate gene transcription. Together, this activation of NFκB through degradation of IκB is considered the classical NFκB signalling pathway.
Constitutive activation of NFκB is observed in many inflammation-associated human cancers, where it contributes to tumorigenesis70. Being a negative regulator of NFκB, IκB functions as a tumour suppressor, and the aberrant activation of NFκB due to defective IκB activity has been demonstrated in several malignancies. For example, NFκB activation is often observed in human hepatocellular carcinomas (HCCs)79. Similarly, MDR2 (multidrug resistance 2; also known as ABCB4)-knock-out mice develop HCC owing to chronic inflammation caused by the accumulation of bile acids. However, the removal of NFκB signalling, following induction of a liver-specific, non-degradable IκB transgene (ΔN-IκB), leads to a dramatic decrease in tumour progression, similar to that observed in NFκB-deficient animals80. Together, these data suggest that aberrant loss of IκB, which might occur in the context of β-TrCP overexpression, might lead to the development of HCC. In fact, this process also appears to occur in melanomas: increased survival of melanoma cells is thought to result primarily from constitutive NFκB activation associated with high levels of β-TrCP and constitutive IκB kinase activity81,82.
Whereas the molecular deregulation of many tumour suppressor genes and/or oncogenes occurs at the transcriptional level, increasing evidence suggests that cellular transformation can originate from altered translational regulation of tumour suppressors and oncogenes. Several eukaryotic translation initiation factors, including eIF4F (eukaryotic translation initiation factor 4F), participate in the regulation of protein translation. Within this complex, the RNA helicase eIF4A catalyses the unwinding and the consequent cap-dependent translation of mRNAs with structured 5′ UTRs (untranslated regions), including mRNAs of proteins that positively regulate cell growth and survival.
PDCD4 is a tumour suppressor that binds to and inhibits eIF4A, subsequently inhibiting translation83. Degradation of PDCD4 through β-TrCP was identified as a key factor in the branch of the mTOR (mammalian target of rapamycin) pathway that controls translation84. In this pathway, S6K1 was shown to phosphorylate PDCD4, marking the protein for β-TrCP-dependent destruction. As PDCD4 blocks translation, suppresses cell growth and promotes apoptosis, the loss of PDCD4 function is thought to contribute to cell transformation. In fact, decreased expression of PDCD4 is observed in advanced carcinomas of the breast and prostate85–87. Similarly, the protein levels of PDCD4 were observed to be downregulated in certain HCCs compared with normal liver88. Recently, the loss of PDCD4 was shown to be an independent risk factor in colorectal cancer and analysis of PDCD4 levels showed that its loss was associated with poor disease-specific survival in some patients and poor overall survival in all patients89. In the lung, loss of PDCD4 expression was widely observed in primary carcinomas of all subtypes, and this expression correlated with higher grade and disease stage, suggesting that the loss of PDCD4 expression is a prognostic factor in lung cancer90. Future studies will be needed to determine whether the levels of PDCD4 are affected in tumours overexpressing β-TrCP and/or displaying overactivation of the PI3K (phosphoinositide 3-kinase)–Akt–mTOR–S6K1 pathway.
A third β-TrCP substrate with oncogenic relevance is REST (repressor element 1 (RE1)-silencing transcription factor), which was originally discovered as a transcriptional repressor of a large number of neuronal differentiation genes in non-neuronal cells and neural stem/progenitor cells91. REST has a dual role as a tumour suppressor (in epithelial cells) and as an oncogenic protein (in neuronal cells; see below)91. Supporting a role for REST in tumour suppression, knockdown of REST using RNA interference has been shown to promote transformation of breast epithelial cells, and deletions encompassing the REST locus have been found in many aggressive human carcinomas92. Exogenous β-TrCP expression transforms human mammary epithelial cells, and reconstitution of REST function by the introduction of a non-degradable mutant of REST abrogates oncogenic transformation by β-TrCP93. The proposed tumour suppressor function of REST seems to lie in its ability to inhibit the PI3K pathway92. If REST function is lost or mutated in cancer cells, activation of PI3K is thought to contribute to cell transformation. Therefore, it will be important to investigate levels of REST and the PI3K pathway in cancers overexpressing β-TrCP.
Defective substrate targeting by β-TrCP in cancer
Although the oncogenic potential of β-TrCP is potentially related to the reduced stability of antiproliferative and pro-apoptotic substrates, defective substrate targeting by β-TrCP might also have a role in oncogenesis. Owing to the redundancy of β-TrCP paralogues, the complete loss of both genes by inactivating mutations and/or deletions is unlikely, and therefore, β-TrCP might have a greater role as an oncogenic protein than as a tumour suppressor. However, rare aberrations that could potentially impede the substrate targeting capabilities of β-TrCP have been found (TABLE 4). A screen for genetic alterations of FBXW11 (which encodes β-TrCP2) in gastric cancer cell lines and primary gastric cancer tissues identified a nucleotide substitution in its seventh WD-repeat domain94. Complementing this study, an analysis of somatic mutations in 95 gastric cancer specimens found five missense mutations in FBXW11, and in these particular tissues, β-catenin levels were higher than controls95. Finally, two mutations in BTRC (encoding β-TrCP1) were found in prostate tissues, and an in-frame insertion in FBXW11 has been identified in one breast tumour96,97.
Table 4.
Cancers associated with β-TrCP deregulation
| Human cancer | Level of upregulation, observed alterations and correlations | Refs |
|---|---|---|
| Cancers displaying high β-TrCP levels | ||
| Breast cancer | Upregulated mRNA levels in primary tumors; mRNA and protein levels in several cell lines | 68 |
| Colon cancer | Upregulated mRNA and protein levels; high NFκB levels; correlation with poor prognosis | 65 |
| Hepatoblastoma | Upregulated mRNA levels; activation of Wnt signalling | 67 |
| Pancreatic cancer | Overexpression in one cell culture line; correlation with NFκB activity and chemoresistance | 66 |
| Melanoma | Upregulated mRNA and protein levels; high NFκB levels; increased survival of cells | 81, 82 |
| Cancers displaying β-TrCP alterations | ||
| Breast cancer | In-frame insertion in FBXW11 | 97 |
| Gastric cancer | Mutation of FBXW11 in one cell culture line and 5 mutations in the 95 gastric cancer tissues tested | 95 |
| Prostate cancer | 2 mutations in BTRC found in the 22 tissues tested | 96 |
β-TrCP, β- transducin repeat-containing protein; FBXW11, F-box and WD repeat domain containing 11 (which encodes β-TrCP2); NFκB, nuclear factor κB.
Mutations in β-TrCP might lead to the stabilization of oncogenic substrates such as β-catenin, which has been implicated in tumorigenesis98. Indeed, high levels of β-catenin in many cancers, such as colorectal cancers, hepatocellular carcinomas and malignant melanomas, are often due to its stabilization, but stabilization, rather than being due to β-TrCP alterations, is frequently due to mutations in the genes encoding APC (adenomatous polyposis coli) and axin, which are crucial to the phosphorylation of β-catenin and its consequent degradation via β-TrCP76,99–104. The stabilization of β-catenin leads to the accumulation of the protein, nuclear translocation and transcription of a number of pro-proliferative genes such as MYC and cyclin D1. Another way for β-catenin to potentially escape degradation is through mutations in the N-terminal phospho-degron. Similarly, some human breast cancers impede the phosphorylation of prolactin receptor — phosphorylation is required for its β-TrCP-dependent degradation, increasing the mitogenic effects of prolactin in mammary tissues105.
Another substrate that evades proteolysis via β-TrCP is REST. As mentioned above, REST behaves as an oncoprotein in neuronal cells. REST is ubiquitylated and targeted for degradation by β-TrCP during G2 to allow the transcriptional derepression of MAD2 (mitotic arrest deficient 2)106, an essential component of the spindle assembly checkpoint. Expression of a stable REST mutant inhibits MAD2 expression in G2 and results in a phenotype that is consistent with faulty activation of the spindle checkpoint. Importantly, an indistinguishable phenotype was observed by expressing REST-FS, an oncogenic frameshift mutant identified in colorectal cancer92 that lacks the β-TrCP-binding domain. Moreover, another study suggests that β-TrCP-mediated degradation of REST is required for proper neural differentiation and that non-degradable REST mutants attenuate differentiation93. Together, these two studies indicate that levels of REST must be accurately controlled to avoid putting neuronal tissues at risk of cancer. Indeed, increased levels of REST due to overproduction107–109 and/or C-terminal truncations (D. Guardavaccaro and M.P., unpublished observations), as observed in human medulloblastomas and neuroblastomas, would inhibit differentiation and generate chromosomal instability, two fundamental mechanisms that contribute to tumorigenesis. Interestingly, REST variants (which lack the β-TrCP-binding motif) are also observed in non-neuronal tumours, but the implication of these findings is not yet understood. It is possible that such truncated variants contribute to cell transformation by promoting aneuploidy and genetic instability in both neuronal and non-neuronal tissues.
Other substrates that are expected to provide an oncogenic gain-of-function when they accumulate in cells harbouring β-TrCP mutations are the Cdc25 dual-specificity phosphatases CDC25A and CDC25B58,110, and EMI1. High levels of Cdc25 have been found in many different human cancers111, including breast, ovarian and colorectal cancer, in which overexpression of CDC25A and CDC25B correlates with clinical outcome112,113. In addition, CDC25A and CDC25B are overexpressed in some non-Hodgkin lymphomas, and these high levels correlate with the aggressiveness of high-grade lymphomas114–116. Although oesophageal, gastric, lung, thyroid, and head and neck cancers also show overexpression of CDC25A and CDC25B, the relationships between β-TrCP expression or mutations and these substrates are unknown, although in some cell lines, CDC25A levels increase because of its stabilization117. The levels of EMI1 transcript and protein have also been found to be upregulated in several human tumour cell lines118 and human malignant cancers119,120. Again, it is not known whether accumulation of EMI1 depends on defective targeting by β-TrCP (either by mutational inactivation of β-TrCP or mutation or truncation of EMI1).
SKP2 and β–TrCP as targets for therapy
The development of pharmaceutical compounds targeting specific SCF ubiquitin ligases is timely and is complemented by basic biochemical studies that have identified substrates for important cellular regulators such as SKP2 and β-TrCP. Many structural biology studies have provided crystallographic, three-dimensional maps of SCF components3. Together with established biochemical data, these resources are ideal for the discovery of small molecules that inhibit SCF ligases either allosterically or by blocking the interaction between F-box proteins and substrate(s).
Previous attempts at targeting components of the degradation machinery have been successful for laboratory and clinical use, as observed with the effectiveness of the proteasome inhibitor bortezomib (velcade) in multiple myeloma121. Proteasome inhibition by bortezomib is achieved by directly binding to the β-subunit of the proteasome, presumably resulting in increased protein stability of factors that promote apoptosis, with resultant increases in sensitivity to chemotherapeutic treatments and radiation therapy. One of the major molecules involved in mediating the effect of proteasome inhibition by bortezomib is NFκB, and as discussed previously, activation of NFκB is dependent on the degradation of IκB following ubiquitylation by β-TrCP. However, whereas drugs such as bortezomib act to stabilize large, nonspecific pools of proteins that are regulated by the proteasome, small molecules designed to prevent degradation of specific proteins by particular ubiquitin ligases, such as SKP2 and β-TrCP, are in development and could prove more efficacious and have fewer side effects. Despite moderate efforts in the industry to develop and utilize such inhibitors, the use of small molecule inhibitors to block the action of SKP2 and β-TrCP has not been reported.
Conclusions and perspectives
The control of regulatory proteins by the UPS is crucial for maintaining the integrity of basic cellular processes such as those governing cell growth, proliferation and survival. This precision is accomplished, in part, through the specific recognition of substrates by the F-box component of SCF ubiquitin ligases. Deregulation of the mechanisms that control protein stability, such as those discussed in this Review, have been shown to contribute to aberrant cellular growth and tumorigenesis. The involvement of the F-box proteins SKP2 and β-TrCP in promoting tumorigenesis is extensive and continues to be elucidated in ongoing studies. The development of small molecule inhibitors against F-box proteins, in particular SKP2 and β-TrCP, is fundamental for future cancer therapy approaches. Additionally, with the majority of the 69 human F-box proteins remaining orphans (that is, unmatched to their substrates), the contribution of SKP2 and β-TrCP in cancer might only be a small indication of a larger trend for this family of proteins. Indeed, this trend is observed for FBXW7, which is a prototypical tumour suppressor gene that is mutated in various human cancers7. Several substrates have been identified for FBXW7, including the oncogenic proteins MYC, cyclin E, NOTCH1, NOTCH4, AURKA (aurora kinase A) and JUN. Moreover, the recent identification of FBXL3 as a component of the circadian clock machinery that targets members of the cryptochrome family for degradation122, together with the increasing links between nightshift work and the incidences of breast and endometrial cancers, suggests that this F-box protein might be involved in processes contributing to cancer123. Another example is FBXL10, a tumour suppressor protein that is resident in the nucleolus in human cells and represses the transcription of ribosomal RNA genes to control cell growth124. Finally, there are F-box-like proteins such as NIPA (nuclear interaction partner of ALK), which is involved in the oncogenic signalling of NPM (nucleophosmin)–ALK (anaplastic lymphoma kinase) and other oncogenic fusions of ALK 125. Future studies identifying the substrates of orphan F-box proteins are poised to advance the field of protein degradation and cancer biology.
DATABASES
Enterz Gene: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=gene
APC | ATM | ATR | AURKA | axin | β-TrCP | BCL-ξL | CBP | CDC25A | CDC25B | CDK1 | CDK2 | CDK9 | Cdkn1b | CDKN1C | CDT1 | CHK1 | CHK2 | CKS1 | claspin | CUL1 | cyclin D1 | eIF4F | EMI1 | ErbB2 | FBXL10 | FBXW7 | FOXO1 | GMCSF | interleukin 6 | JUN | KPC | MAD2 | MMP9 | MYC | NIPA | NOTCH1 | NOTCH4 | NRAS | PDCD4 | PIRH2 | RASSF1 | Rb1 | RBL2 | REST | S6K1 | SKP1 | SKP2 | TNFα | TOB1 | USP18 | WEE1
National Cancer Institute: http://www.cancer.gov breast cancer | colorectal cancer | endometrial cancer | gastric cancer | head and neck cancer | lung cancer | lymphoma | melanoma | oesophageal cancer | pancreatic cancer | thyroid cancer
FURTHER INFORMATION
M. Pagano’s homepage: http://pathology.med.nyu.edu/Pagano
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
Acknowledgements
We thank S. Fuchs, Y. Ben-Neriah, K. Nakayama and J. Skaar for critically reading the manuscript. We apologize to colleagues whose work could not be mentioned owing to space limitations. D.F. is grateful to A. Nans. M.P. is grateful to T. M. Thor for continuous support. Work in the Pagano laboratory is supported by grants from the NIH (R37-CA76,584, R01-GM57,587, R21-CA125,173 and P30-CA01687) and the Multiple Myeloma Research Foundation senior award.
Glossary
- Ubiquitin
A small, 7.5-kDa protein that is ubiquitously expressed in all eukaryotes. Chains of ubiquitin moieties (connected by Lys48) target proteins for proteasomal degradation. Monoubiquitylation or polyubiquitylation through different lysine residues controls the function (not the proteolysis) of various proteins.
- Proteasome
A large multisubunit protein complex (approximately 2.5 MDa) that is found in all eukaryotes and archaea, the main function of which is to degrade excessive, unneeded or damaged proteins by proteolysis using a chemical reaction that breaks peptide bonds in an ATP-dependent manner.
- Ubiquitin-activating enzyme (E1)
An enzyme that activates ubiquitin in a process that requires ATP as an energy source.
- Ubiquitin-conjugating enzyme (E2)
An enzyme that accepts the transfer of ubiquitin from the ubiquitin-activating enzyme (E1) and transfers it to substrates.
- Ubiquitin ligase (E3)
An enzyme that functions as the substrate recognition component of the ubiquitylation machinery. E3 enzymes are capable of interacting with E2 enzymes and substrates to facilitate the transfer of ubiquitin to the selected substrate.
- RING-finger proteins
Proteins that interact with E2 ubiquitin enzymes to serve as an E3 enzyme. They are subdivided structurally into multi-subunit and single-subunit types, including those containing RING-like folds such as the U-box.
- HECT-domain proteins
Proteins that are characterized by the presence of a C-terminal HECT domain, which is a domain of approximately 350 amino acids that is catalytically involved in the attachment of ubiquitin to substrates.
- F-box domain
Originally identified in cyclin F as a stretch of approximately 40 amino acids linking F-box proteins to sKP1 to form the core of the sCF complex.
- Degron
specific sequence of amino acids in a protein substrate typically conserved through evolution that directs the recognition of an E3 ubiquitin ligase.
- Paralogues
Homologous genes that have resulted from a gene duplication event within a single genome. This is in contrast to othologous genes, which are separated by a speciation event.
- C phase
The mammalian cell cycle is divided into four distinct phases called G1, s, G2 and mitosis. C phase is defined as the temporal interval between the G1–s transition and the end of mitosis when CDK activity is present.
- Organomegaly
The abnormal enlargement of organs.
- Mitotic catastrophe
A death resulting from failure of a cell to arrest before mitosis following DNA damage, resulting in severe aberrancies in chromosomal structure and segregation. It might share downstream events with apoptosis.
References
- 1. Hershko A. The ubiquitin system for protein degradation and some of its roles in the control of the cell-division cycle (Nobel lecture) Angew. Chem. Int. Ed. Engl. 2005;44:5932–5943. doi: 10.1002/anie.200501724. An historical perspective about the discovery of the ubiquitin system that describes how E1, E2 and E3 enzymes work together to promote ubiquitin ligation to substrates.
- 2. Petroski MD, Deshaies RJ. Function and regulation of cullin-RING ubiquitin ligases. Nature Rev. Mol. Cell Biol. 2005;6:9–20. doi: 10.1038/nrm1547. An excellent review of cullin RING ubiquitin ligases.
- 3.Cardozo T, Pagano M. The SCF ubiquitin ligase: insights into a molecular machine. Nature Rev. Mol. Cell Biol. 2004;5:739–751. doi: 10.1038/nrm1471. [DOI] [PubMed] [Google Scholar]
- 4.Jin J, et al. Systematic analysis and nomenclature of mammalian F-box proteins. Genes Dev. 2004;18:2573–2580. doi: 10.1101/gad.1255304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cenciarelli C, et al. Identification of a family of human F-box proteins. Curr. Biol. 1999;9:1177–1179. doi: 10.1016/S0960-9822(00)80020-2. [DOI] [PubMed] [Google Scholar]
- 6. Winston JT, Koepp DM, Zhu C, Elledge SJ, Harper JW. A family of mammalian F-box proteins. Curr. Biol. 1999;9:1180–1182. doi: 10.1016/S0960-9822(00)80021-4. References 4–6 classify the mammalian family of F-box proteins.
- 7. Welcker M, Clurman BE. FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nature Rev. Cancer. 2007;8:83–93. doi: 10.1038/nrc2290. An excellent and up-to-date review about FBXW7 and its role in cancer.
- 8.Malumbres M, Barbacid M. Cell cycle kinases in cancer. Curr. Opin. Genet. Dev. 2007;17:60–65. doi: 10.1016/j.gde.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 9.Guardavaccaro D, Pagano M. Stabilizers and destabilizers controlling cell cycle oscillators. Mol. Cell. 2006;22:1–4. doi: 10.1016/j.molcel.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 10.Branzei D, Foiani M. Regulation of DNA repair throughout the cell cycle. Nature Rev. Mol. Cell Biol. 2008;9:297–308. doi: 10.1038/nrm2351. [DOI] [PubMed] [Google Scholar]
- 11.Zhang H, Kobayashi R, Galaktionov K, Beach D. p19Skp1 and p45Skp2 are essential elements of the cyclin A–CDK2 S phase kinase. Cell. 1995;82:915–925. doi: 10.1016/0092-8674(95)90271-6. [DOI] [PubMed] [Google Scholar]
- 12.Carrano AC, Eytan E, Hershko A, Pagano M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nature Cell Biol. 1999;1:193–199. doi: 10.1038/12013. [DOI] [PubMed] [Google Scholar]
- 13.Sutterluty H, et al. p45SKP2 promotes p27Kip1 degradation and induces S phase in quiescent cells. Nature Cell Biol. 1999;1:207–214. doi: 10.1038/12027. [DOI] [PubMed] [Google Scholar]
- 14. Tsvetkov LM, Yeh KH, Lee SJ, Sun H, Zhang H. p27Kip1 ubiquitination and degradation is regulated by the SCFSkp2 complex through phosphorylated Thr187 in p27. Curr. Biol. 1999;9:661–664. doi: 10.1016/s0960-9822(99)80290-5. References 12–14 characterize the function of SKP2 in cell cycle control and the ubiquitin-mediated degradation of the tumour suppressor p27.
- 15.Spruck C, et al. A CDK-independent function of mammalian Cks1: targeting of SCFSkp2 to the CDK inhibitor p27Kip1. Mol. Cell. 2001;7:639–650. doi: 10.1016/s1097-2765(01)00210-6. [DOI] [PubMed] [Google Scholar]
- 16.Ganoth D, et al. The cell-cycle regulatory protein Cks1 is required for SCFSkp2-mediated ubiquitinylation of p27. Nature Cell Biol. 2001;3:321–324. doi: 10.1038/35060126. [DOI] [PubMed] [Google Scholar]
- 17.Bloom J, Pagano M. Deregulated degradation of the cdk inhibitor p27 and malignant transformation. Semin. Cancer Biol. 2003;13:41–47. doi: 10.1016/s1044-579x(02)00098-6. [DOI] [PubMed] [Google Scholar]
- 18. Nakayama K, et al. Targeted disruption of Skp2 results in accumulation of cyclin E and p27Kip1, polyploidy and centrosome overduplication. EMBO J. 2000;19:2069–2081. doi: 10.1093/emboj/19.9.2069. Shows that deletion of SKP2 results in accumulation of p27 in vivo.
- 19. Nakayama K, et al. Skp2-mediated degradation of p27 regulates progression into mitosis. Dev. Cell. 2004;6:661–672. doi: 10.1016/s1534-5807(04)00131-5. Shows that p27 loss reverts most of the phenotypes that are due to SKP2-deficiency and that the SKP2–p27 axis functions not only at G1–S, but also at G2–M.
- 20.Kossatz U, et al. Skp2-dependent degradation of p27kip1 is essential for cell cycle progression. Genes Dev. 2004;18:2602–2607. doi: 10.1101/gad.321004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bornstein G, et al. Role of the SCFSkp2 ubiquitin ligase in the degradation of p21Cip1 in S. phase. J. Biol. Chem. 2003;278:25752–25757. doi: 10.1074/jbc.M301774200. [DOI] [PubMed] [Google Scholar]
- 22. Yu ZK, Gervais JL, Zhang H. Human CUL-1 associates with the SKP1/SKP2 complex and regulates p21CIP1/WAF1 and cyclin D proteins. Proc. Natl Acad. Sci. USA. 1998;95:11324–11329. doi: 10.1073/pnas.95.19.11324. The first evidence that SKP2 targets p21, a tumour suppressor protein, for degradation.
- 23.Kamura T, et al. Degradation of p57Kip2 mediated by SCFSkp2-dependent ubiquitylation. Proc. Natl Acad. Sci. USA. 2003;100:10231–10236. doi: 10.1073/pnas.1831009100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hiramatsu Y, et al. Degradation of Tob1 mediated by SCFSkp2-dependent ubiquitination. Cancer Res. 2006;66:8477–8483. doi: 10.1158/0008-5472.CAN-06-1603. [DOI] [PubMed] [Google Scholar]
- 25.Song MS, et al. Skp2 regulates the antiproliferative function of the tumor suppressor RASSF1A via ubiquitin-mediated degradation at the G(1)–S transition. Oncogene. 2007 Dec 10; doi: 10.1038/sj.onc.1210971. [DOI] [PubMed] [Google Scholar]
- 26.Tedesco D, Lukas J, Reed SI. The pRb-related protein p130 is regulated by phosphorylation-dependent proteolysis via the protein-ubiquitin ligase SCFSkp2. Genes Dev. 2002;16:2946–2957. doi: 10.1101/gad.1011202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huang H, et al. Skp2 inhibits FOXO1 in tumor suppression through ubiquitin-mediated degradation. Proc. Natl Acad. Sci. USA. 2005;102:1649–1654. doi: 10.1073/pnas.0406789102. Identifies FOXO1 as a substrate of SKP2 and suggests SKP2-promoted proteolysis might have a role in tumorigenesis.
- 28.Tokarz S, et al. The ISG15 isopeptidase UBP43 is regulated by proteolysis via the SCFSkp2 ubiquitin ligase. J. Biol. Chem. 2004;279:46424–46430. doi: 10.1074/jbc.M403189200. [DOI] [PubMed] [Google Scholar]
- 29.Garriga J, et al. CDK9 is constitutively expressed throughout the cell cycle, and its steady-state expression is independent of SKP2. Mol. Cell. Biol. 2003;23:5165–5173. doi: 10.1128/MCB.23.15.5165-5173.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kiernan RE, et al. Interaction between cyclin T1 and SCFSKP2 targets CDK9 for ubiquitination and degradation by the proteasome. Mol. Cell. Biol. 2001;21:7956–7970. doi: 10.1128/MCB.21.23.7956-7970.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carrano AC, Pagano M. Role of the F-box protein Skp2 in adhesion-dependent cell cycle progression. J. Cell Biol. 2001;153:1381–1390. doi: 10.1083/jcb.153.7.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Signoretti S, et al. Oncogenic role of the ubiquitin ligase subunit Skp2 in human breast cancer. J. Clin. Invest. 2002;110:633–641. doi: 10.1172/JCI15795. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Waltregny D, et al. Androgen-driven prostate epithelial cell proliferation and differentiation in vivo involve the regulation of p27. Mol. Endocrinol. 2001;15:765–782. doi: 10.1210/mend.15.5.0640. [DOI] [PubMed] [Google Scholar]
- 34.Lu L, Schulz H, Wolf DA. The F-box protein SKP2 mediates androgen control of p27 stability in LNCaP human prostate cancer cells. BMC Cell Biol. 2002;3:22. doi: 10.1186/1471-2121-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Latres E, et al. Role of the F-box protein Skp2 in lymphomagenesis. Proc. Natl Acad. Sci. USA. 2001;98:2515–2520. doi: 10.1073/pnas.041475098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang-Decker N, et al. Loss of CBP causes T cell lymphomagenesis in synergy with p27Kip1 insufficiency. Cancer Cell. 2004;5:177–189. doi: 10.1016/s1535-6108(04)00022-4. [DOI] [PubMed] [Google Scholar]
- 37.Shim EH, et al. Expression of the F-box protein SKP2 induces hyperplasia, dysplasia, and low-grade carcinoma in the mouse prostate. Cancer Res. 2003;63:1583–1588. [PubMed] [Google Scholar]
- 38.Radke S, Pirkmaier A, Germain D. Differential expression of the F-box proteins Skp2 and Skp2B in breast cancer. Oncogene. 2005;24:3448–3458. doi: 10.1038/sj.onc.1208328. [DOI] [PubMed] [Google Scholar]
- 39.Timmerbeul I, et al. Testing the importance of p27 degradation by the SCFskp2 pathway in murine models of lung and colon cancer. Proc. Natl Acad. Sci. USA. 2006;103:14009–14014. doi: 10.1073/pnas.0606316103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keller UB, et al. Myc targets Cks1 to provoke the suppression of p27Kip1, proliferation and lymphomagenesis. EMBO J. 2007;26:2562–2574. doi: 10.1038/sj.emboj.7601691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Philipp-Staheli J, Payne SR, Kemp CJ. p27Kip1: regulation and function of a haploinsufficient tumor suppressor and its misregulation in cancer. Exp. Cell Res. 2001;264:148–168. doi: 10.1006/excr.2000.5143. [DOI] [PubMed] [Google Scholar]
- 42.Slotky M, et al. The expression of the ubiquitin ligase subunit Cks1 in human breast cancer. Breast Cancer Res. 2005;7:R737–R744. doi: 10.1186/bcr1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shapira M, et al. The prognostic impact of the ubiquitin ligase subunits Skp2 and Cks1 in colorectal carcinoma. Cancer. 2005;103:1336–1346. doi: 10.1002/cncr.20917. [DOI] [PubMed] [Google Scholar]
- 44.Shapira M, et al. Alterations in the expression of the cell cycle regulatory protein cyclin kinase subunit 1 in colorectal carcinoma. Cancer. 2004;100:1615–1621. doi: 10.1002/cncr.20172. [DOI] [PubMed] [Google Scholar]
- 45.Masuda TA, et al. Cyclin-dependent kinase 1 gene expression is associated with poor prognosis in gastric carcinoma. Clin. Cancer Res. 2003;9:5693–5698. [PubMed] [Google Scholar]
- 46.Hershko DD, Shapira M. Prognostic role of p27Kip1 deregulation in colorectal cancer. Cancer. 2006;107:668–675. doi: 10.1002/cncr.22073. [DOI] [PubMed] [Google Scholar]
- 47.Kamura T, et al. Cytoplasmic ubiquitin ligase KPC regulates proteolysis of p27Kip1 at G1 phase. Nature Cell Biol. 2004;6:1229–1235. doi: 10.1038/ncb1194. [DOI] [PubMed] [Google Scholar]
- 48.Hattori T, et al. Pirh2 promotes ubiquitin-dependent degradation of the cyclin-dependent kinase inhibitor p27Kip1. Cancer Res. 2007;67:10789–10795. doi: 10.1158/0008-5472.CAN-07-2033. [DOI] [PubMed] [Google Scholar]
- 49.Goto T, et al. Mechanism and functional consequences of loss of FOXO1 expression in endometrioid endometrial cancer cells. Oncogene. 2008;27:9–19. doi: 10.1038/sj.onc.1210626. [DOI] [PubMed] [Google Scholar]
- 50.Bellan C, et al. Missing expression of pRb2/p130 in human retinoblastomas is associated with reduced apoptosis and lesser differentiation. Invest. Ophthalmol. Vis. Sci. 2002;43:3602–3608. [PubMed] [Google Scholar]
- 51.Caputi M, et al. Loss of pRb2/p130 expression is associated with unfavorable clinical outcome in lung cancer. Clin. Cancer Res. 2002;8:3850–3856. [PubMed] [Google Scholar]
- 52.D’Andrilli G, et al. Frequent loss of pRb2/p130 in human ovarian carcinoma. Clin. Cancer Res. 2004;10:3098–3103. doi: 10.1158/1078-0432.ccr-03-0524. [DOI] [PubMed] [Google Scholar]
- 53.Helin K, et al. Loss of the retinoblastoma protein-related p130 protein in small cell lung carcinoma. Proc. Natl Acad. Sci. USA. 1997;94:6933–6938. doi: 10.1073/pnas.94.13.6933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scambia G, Lovergine S, Masciullo V. RB family members as predictive and prognostic factors in human cancer. Oncogene. 2006;25:5302–5308. doi: 10.1038/sj.onc.1209620. [DOI] [PubMed] [Google Scholar]
- 55.Susini T, et al. Expression of the retinoblastoma-related gene Rb2/p130 correlates with clinical outcome in endometrial cancer. J. Clin. Oncol. 1998;16:1085–1093. doi: 10.1200/JCO.1998.16.3.1085. [DOI] [PubMed] [Google Scholar]
- 56.Zamparelli A, et al. Expression of cell-cycle-associated proteins pRB2/p130 and p27kip in vulvar squamous cell carcinomas. Hum. Pathol. 2001;32:4–9. doi: 10.1053/hupa.2001.20371. [DOI] [PubMed] [Google Scholar]
- 57.Soldatenkov VA, Dritschilo A, Ronai Z, Fuchs SY. Inhibition of homologue of Slimb (HOS) function sensitizes human melanoma cells for apoptosis. Cancer Res. 1999;59:5085–5088. [PubMed] [Google Scholar]
- 58.Busino L, et al. Degradation of Cdc25A by β-TrCP during S phase and in response to DNA damage. Nature. 2003;426:87–91. doi: 10.1038/nature02082. [DOI] [PubMed] [Google Scholar]
- 59.Tang W, et al. Targeting β-transducin repeat-containing protein E3 ubiquitin ligase augments the effects of antitumor drugs on breast cancer cells. Cancer Res. 2005;65:1904–1908. doi: 10.1158/0008-5472.CAN-04-2597. [DOI] [PubMed] [Google Scholar]
- 60.Guardavaccaro D, et al. Control of meiotic and mitotic progression by the F box protein β-Trcp 1 in vivo. Dev. Cell. 2003;4:799–812. doi: 10.1016/s1534-5807(03)00154-0. [DOI] [PubMed] [Google Scholar]
- 61.Nakayama K, et al. Impaired degradation of inhibitory subunit of NF-κB (IκB) and β-catenin as a result of targeted disruption of the β-TrCP1 gene. Proc. Natl Acad. Sci. USA. 2003;100:8752–8757. doi: 10.1073/pnas.1133216100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mailand N, Bekker-Jensen S, Bartek J, Lukas J. Destruction of Claspin by SCFβTrCP restrains Chk1 activation and facilitates recovery from genotoxic stress. Mol. Cell. 2006;23:307–318. doi: 10.1016/j.molcel.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 63.Peschiaroli A, et al. SCFβTrCP-mediated degradation of Claspin regulates recovery from the DNA replication checkpoint response. Mol. Cell. 2006;23:319–329. doi: 10.1016/j.molcel.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 64.Watanabe N, et al. M-phase kinases induce phospho-dependent ubiquitination of somatic Wee1 by SCFβ-TrCP. Proc. Natl Acad. Sci. USA. 2004;101:4419–4424. doi: 10.1073/pnas.0307700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ougolkov A, et al. Associations among β-TrCP, an E3 ubiquitin ligase receptor, β-catenin, and NF-κB in colorectal cancer. J. Natl Cancer Inst. 2004;96:1161–1170. doi: 10.1093/jnci/djh219. [DOI] [PubMed] [Google Scholar]
- 66.Muerkoster S, et al. Increased expression of the E3-ubiquitin ligase receptor subunit βTRCP 1 relates to constitutive nuclear factor-κB activation and chemoresistance in pancreatic carcinoma cells. Cancer Res. 2005;65:1316–1324. doi: 10.1158/0008-5472.CAN-04-1626. [DOI] [PubMed] [Google Scholar]
- 67.Koch A, et al. Elevated expression of Wnt antagonists is a common event in hepatoblastomas. Clin. Cancer Res. 2005;11:4295–4304. doi: 10.1158/1078-0432.CCR-04-1162. [DOI] [PubMed] [Google Scholar]
- 68.Spiegelman VS, et al. Induction of homologue of Slimb ubiquitin ligase receptor by mitogen signaling. J. Biol. Chem. 2002;277:36624–36630. doi: 10.1074/jbc.M204524200. [DOI] [PubMed] [Google Scholar]
- 69. Kudo Y, et al. Role of F-box protein βTrcp1 in mammary gland development and tumorigenesis. Mol. Cell. Biol. 2004;24:8184–8194. doi: 10.1128/MCB.24.18.8184-8194.2004. Shows that β-TrCP1 positively controls the proliferation of breast epithelium and its overexpression induces transformation in the breast epithelium.
- 70.Karin M, Greten FR. NF-κB: linking inflammation and immunity to cancer development and progression. Nature Rev. Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 71.Wu C, Ghosh S. β-TrCP mediates the signal-induced ubiquitination of IκBβ. J. Biol. Chem. 1999;274:29591–29594. doi: 10.1074/jbc.274.42.29591. [DOI] [PubMed] [Google Scholar]
- 72.Shirane M, Hatakeyama S, Hattori K, Nakayama K, Nakayama K. Common pathway for the ubiquitination of IκBα, IκBβ, and IκBε mediated by the F-box protein FWD1. J. Biol. Chem. 1999;274:28169–28174. doi: 10.1074/jbc.274.40.28169. [DOI] [PubMed] [Google Scholar]
- 73. Tan P, et al. Recruitment of a ROC1-CUL1 ubiquitin ligase by Skp1 and HOS to catalyze the ubiquitination of IκBα. Mol. Cell. 1999;3:527–533. doi: 10.1016/s1097-2765(00)80481-5. One of the first papers showing that the SCF contains the RING-finger protein RBX1 and that an SCF containing β-TrCP targets IκBα for degradation.
- 74.Kroll M, et al. Inducible degradation of IκBα by the proteasome requires interaction with the F-box protein h-βTrCP. J. Biol. Chem. 1999;274:7941–7945. doi: 10.1074/jbc.274.12.7941. [DOI] [PubMed] [Google Scholar]
- 75.Spencer E, Jiang J, Chen ZJ. Signal-induced ubiquitination of IκBα by the F-box protein Slimb/β-TrCP. Genes Dev. 1999;13:284–294. doi: 10.1101/gad.13.3.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Winston JT, et al. The SCFβ-TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IκBα and β-catenin and stimulates IκBα ubiquitination in vitro. Genes Dev. 1999;13:270–283. doi: 10.1101/gad.13.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yaron A, et al. Identification of the receptor component of the IκBα-ubiquitin ligase. Nature. 1998;396:590–594. doi: 10.1038/25159. [DOI] [PubMed] [Google Scholar]
- 78.Hatakeyama S, et al. Ubiquitin-dependent degradation of IκBα a is mediated by a ubiquitin ligase Skp1 /Cul1 /F-box protein FWD 1. Proc. Natl Acad. Sci. USA. 1999;96:3859–3863. doi: 10.1073/pnas.96.7.3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arsura M, Cavin LG. Nuclear factor-κB and liver carcinogenesis. Cancer Lett. 2005;229:157–169. doi: 10.1016/j.canlet.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 80.Pikarsky E, et al. NF-κB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 81.Dhawan P, Richmond A. A novel NF-κB-inducing kinase-MAPK signaling pathway up-regulates NF-κB activity in melanoma cells. J. Biol. Chem. 2002;277:7920–7928. doi: 10.1074/jbc.M112210200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liu J, et al. Oncogenic BRAF regulates β-Trcp expression and NF-κB activity in human melanoma cells. Oncogene. 2007;26:1954–1958. doi: 10.1038/sj.onc.1209994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang HS, et al. The transformation suppressor Pdcd4 is a novel eukaryotic translation initiation factor 4A binding protein that inhibits translation. Mol. Cell. Biol. 2003;23:26–37. doi: 10.1128/MCB.23.1.26-37.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dorrello NV, et al. S6K1-and βTRCP-mediated degradation of PDCD4 promotes protein translation and cell growth. Science. 2006;314:467–471. doi: 10.1126/science.1130276. [DOI] [PubMed] [Google Scholar]
- 85.Afonja O, Juste D, Das S, Matsuhashi S, Samuels HH. Induction of PDCD4 tumor suppressor gene expression by RAR agonists, antiestrogen and HER-2/neu antagonist in breast cancer cells. Evidence for a role in apoptosis. Oncogene. 2004;23:8135–8145. doi: 10.1038/sj.onc.1207983. [DOI] [PubMed] [Google Scholar]
- 86.Goke R, Barth P, Schmidt A, Samans B, Lankat-Buttgereit B. Programmed cell death protein 4 suppresses CDK1/cdc2 via induction of p21Waf1/Cip1. Am. J. Physiol. Cell Physiol. 2004;287:C1541–C1546. doi: 10.1152/ajpcell.00025.2004. [DOI] [PubMed] [Google Scholar]
- 87.Wen YH, et al. Alterations in the expression of PDCD4 in ductal carcinoma of the breast. Oncol. Rep. 2007;18:1387–1393. [PubMed] [Google Scholar]
- 88.Zhang H, et al. Involvement of programmed cell death 4 in transforming growth factor-β1-induced apoptosis in human hepatocellular carcinoma. Oncogene. 2006;25:6101–6112. doi: 10.1038/sj.onc.1209634. [DOI] [PubMed] [Google Scholar]
- 89.Mudduluru G, et al. Loss of programmed cell death 4 expression marks adenoma-carcinoma transition, correlates inversely with phosphorylated protein kinase B, and is an independent prognostic factor in resected colorectal cancer. Cancer. 2007;110:1697–1707. doi: 10.1002/cncr.22983. [DOI] [PubMed] [Google Scholar]
- 90.Chen Y, et al. Loss of PDCD4 expression in human lung cancer correlates with tumour progression and prognosis. J. Pathol. 2003;200:640–646. doi: 10.1002/path.1378. [DOI] [PubMed] [Google Scholar]
- 91.Majumder S. REST in good times and bad: roles in tumor suppressor and oncogenic activities. Cell Cycle. 2006;5:1929–1935. doi: 10.4161/cc.5.17.2982. [DOI] [PubMed] [Google Scholar]
- 92.Westbrook TF, et al. A genetic screen for candidate tumor suppressors identifies REST. Cell. 2005;121:837–848. doi: 10.1016/j.cell.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 93.Westbrook TF, et al. SCFβ-TRCP controls oncogenic transformation and neural differentiation through REST degradation. Nature. 2008;452:370–374. doi: 10.1038/nature06780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Saitoh T, Katoh M. Expression profiles of βTRCP1 and βTRCP2, and mutation analysis of βTRCP2 in gastric cancer. Int. J. Oncol. 2001;18:959–964. [PubMed] [Google Scholar]
- 95.Kim CJ, et al. Somatic mutations of the β-TrCP gene in gastric cancer. Apmis. 2007;115:127–133. doi: 10.1111/j.1600-0463.2007.apm_562.x. [DOI] [PubMed] [Google Scholar]
- 96.Gerstein AV, et al. APC/CTNNB1 (β-catenin) pathway alterations in human prostate cancers. Genes Chromosomes Cancer. 2002;34:9–16. doi: 10.1002/gcc.10037. [DOI] [PubMed] [Google Scholar]
- 97.Wood LD, et al. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318:1108–1113. doi: 10.1126/science.1145720. [DOI] [PubMed] [Google Scholar]
- 98.Clevers H. Wnt/β-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 99.Liu C, et al. β-Trcp couples β-catenin phosphorylation-degradation and regulates Xenopus axis formation. Proc. Natl Acad. Sci. USA. 1999;96:6273–6278. doi: 10.1073/pnas.96.11.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kitagawa M, et al. An F-box protein, FWD1, mediates ubiquitin-dependent proteolysis of β-catenin. EMBO J. 1999;18:2401–2410. doi: 10.1093/emboj/18.9.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lagna G, Carnevali F, Marchioni M, Hemmati-Brivanlou A. Negative regulation of axis formation and Wnt signaling in Xenopus embryos by the F-box/ WD40 protein βTrCP. Mech. Dev. 1999;80:101–106. doi: 10.1016/s0925-4773(98)00208-1. [DOI] [PubMed] [Google Scholar]
- 102.Hart M, et al. The F-box protein β-TrCP associates with phosphorylated β-catenin and regulates its activity in the cell. Curr. Biol. 1999;9:207–210. doi: 10.1016/s0960-9822(99)80091-8. [DOI] [PubMed] [Google Scholar]
- 103.Latres E, Chiaur DS, Pagano M. The human F box protein β-Trcp associates with the Cul1/Skp1 complex and regulates the stability of β-catenin. Oncogene. 1999;18:849–854. doi: 10.1038/sj.onc.1202653. [DOI] [PubMed] [Google Scholar]
- 104.Marikawa Y, Elinson RP. β-TrCP is a negative regulator of Wnt/β-catenin signaling pathway and dorsal axis formation in Xenopus embryos. Mech. Dev. 1998;77:75–80. doi: 10.1016/s0925-4773(98)00134-8. [DOI] [PubMed] [Google Scholar]
- 105.Li Y, et al. Stabilization of prolactin receptor in breast cancer cells. Oncogene. 2006;25:1896–1902. doi: 10.1038/sj.onc.1209214. [DOI] [PubMed] [Google Scholar]
- 106.Guardavaccaro D, et al. Control of chromosome stability by the β-TrCP–REST–Mad2 axis. Nature. 2008;452:365–369. doi: 10.1038/nature06641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fuller GN, et al. Many human medulloblastoma tumors overexpress repressor element-1 silencing transcription (REST)/neuron-restrictive silencer factor, which can be functionally countered by REST-VP16. Mol. Cancer Ther. 2005;4:343–349. doi: 10.1158/1535-7163.MCT-04-0228. [DOI] [PubMed] [Google Scholar]
- 108.Su X, Kameoka S, Lentz S, Majumder S. Activation of REST/NRSF target genes in neural stem cells is sufficient to cause neuronal differentiation. Mol. Cell. Biol. 2004;24:8018–8025. doi: 10.1128/MCB.24.18.8018-8025.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lawinger P, et al. The neuronal repressor REST/NRSF is an essential regulator in medulloblastoma cells. Nature Med. 2000;6:826–831. doi: 10.1038/77565. [DOI] [PubMed] [Google Scholar]
- 110.Kanemori Y, Uto K, Sagata N. β-TrCP recognizes a previously undescribed nonphosphorylated destruction motif in Cdc25A and Cdc25B phosphatases. Proc. Natl Acad. Sci. USA. 2005;102:6279–6284. doi: 10.1073/pnas.0501873102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Boutros R, Lobjois V, Ducommun B. CDC25 phosphatases in cancer cells: key players? Good targets? Nature Rev. Cancer. 2007;7:495–507. doi: 10.1038/nrc2169. [DOI] [PubMed] [Google Scholar]
- 112.Cangi MG, et al. Role of the Cdc25A phosphatase in human breast cancer. J. Clin. Invest. 2000;106:753–761. doi: 10.1172/JCI9174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kristjansdottir K, Rudolph J. Cdc25 phosphatases and cancer. Chem. Biol. 2004;11:1043–1051. doi: 10.1016/j.chembiol.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 114.Hernandez S, et al. Cdc25 cell cycle-activating phosphatases and c-myc expression in human non-Hodgkin’s lymphomas. Cancer Res. 1998;58:1762–1767. [PubMed] [Google Scholar]
- 115.Hernandez S, et al. Cdc25a and the splicing variant cdc25b2, but not cdc25B1,-B3 or-C, are over-expressed in aggressive human non-Hodgkin’s lymphomas. Int. J. Cancer. 2000;89:148–152. [PubMed] [Google Scholar]
- 116.Ito Y, et al. Cdc25A and cdc25B expression in malignant lymphoma of the thyroid: correlation with histological subtypes and cell proliferation. Int. J. Mol. Med. 2004;13:431–435. [PubMed] [Google Scholar]
- 117.Loffler H, et al. Distinct modes of deregulation of the proto-oncogenic Cdc25A phosphatase in human breast cancer cell lines. Oncogene. 2003;22:8063–8071. doi: 10.1038/sj.onc.1206976. [DOI] [PubMed] [Google Scholar]
- 118.Hsu JY, Reimann JD, Sorensen CS, Lukas J, Jackson PK. E2F-dependent accumulation of hEmi1 regulates S phase entry by inhibiting APCCdh1. Nature Cell Biol. 2002;4:358–366. doi: 10.1038/ncb785. [DOI] [PubMed] [Google Scholar]
- 119.Gutgemann I, Lehman NL, Jackson PK, Longacre TA. Emi1 protein accumulation implicates misregulation of the anaphase promoting complex/ cyclosome pathway in ovarian clear cell carcinoma. Mod. Pathol. 2008;21:445–454. doi: 10.1038/modpathol.3801022. [DOI] [PubMed] [Google Scholar]
- 120.Lehman NL, et al. Oncogenic regulators and substrates of the anaphase promoting complex/ cyclosome are frequently overexpressed in malignant tumors. Am. J. Pathol. 2007;170:1793–1805. doi: 10.2353/ajpath.2007.060767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Adams J, Kauffman M. Development of the proteasome inhibitor Velcade (Bortezomib) Cancer Invest. 2004;22:304–311. doi: 10.1081/cnv-120030218. [DOI] [PubMed] [Google Scholar]
- 122.Busino L, et al. SCFFbxl3 controls the oscillation of the circadian clock by directing the degradation of cryptochrome proteins. Science. 2007;316:900–904. doi: 10.1126/science.1141194. [DOI] [PubMed] [Google Scholar]
- 123.Gallego M, Virshup DM. Post-translational modifications regulate the ticking of the circadian clock. Nature Rev. Mol. Cell Biol. 2007;8:139–148. doi: 10.1038/nrm2106. [DOI] [PubMed] [Google Scholar]
- 124.Frescas D, Guardavaccaro D, Bassermann F, Koyama-Nasu R, Pagano M. JHDM1B/FBXL10 is a nucleolar protein that represses transcription of ribosomal RNA genes. Nature. 2007;450:309–313. doi: 10.1038/nature06255. [DOI] [PubMed] [Google Scholar]
- 125.Bassermann F, et al. NIPA defines an SCF-type mammalian E3 ligase that regulates mitotic entry. Cell. 2005;122:45–57. doi: 10.1016/j.cell.2005.04.034. [DOI] [PubMed] [Google Scholar]
- 126.Amador V, Ge S, Santamaria PG, Guardavaccaro D, Pagano M. APC/CCdc20 controls the ubiquitin-mediated degradation of p21 in prometaphase. Mol. Cell. 2007;27:462–473. doi: 10.1016/j.molcel.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li X, Zhao Q, Liao R, Sun P, Wu X. The SCFSkp2 ubiquitin ligase complex interacts with the human replication licensing factor Cdt1 and regulates Cdt1 degradation. J. Biol. Chem. 2003;278:30854–30858. doi: 10.1074/jbc.C300251200. [DOI] [PubMed] [Google Scholar]
- 128.Mendez J, et al. Human origin recognition complex large subunit is degraded by ubiquitin-mediated proteolysis after initiation of DNA replication. Mol. Cell. 2002;9:481–491. doi: 10.1016/s1097-2765(02)00467-7. [DOI] [PubMed] [Google Scholar]
- 129.Moro L, Arbini AA, Marra E, Greco M. Up-regulation of Skp2 after prostate cancer cell adhesion to basement membranes results in BRCA2 degradation and cell proliferation. J. Biol. Chem. 2006;281:22100–22107. doi: 10.1074/jbc.M604636200. [DOI] [PubMed] [Google Scholar]
- 130.Jiang H, et al. Ubiquitylation of RAG-2 by Skp2-SCF links destruction of the V(D)J. recombinase to the cell cycle. Mol. Cell. 2005;18:699–709. doi: 10.1016/j.molcel.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 131.Liu Y, et al. The ETS protein MEF is regulated by phosphorylation-dependent proteolysis via the protein-ubiquitin ligase SCFSkp2. Mol. Cell. Biol. 2006;26:3114–3123. doi: 10.1128/MCB.26.8.3114-3123.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu H, Cheng EH, Hsieh JJ. Bimodal degradation of MLL by SCFSkp2 and APCCdc20 assures cell cycle execution: a critical regulatory circuit lost in leukemogenic MLL fusions. Genes Dev. 2007;21:2385–2398. doi: 10.1101/gad.1574507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Charrasse S, Carena I, Brondani V, Klempnauer KH, Ferrari S. Degradation of B-Myb by ubiquitin-mediated proteolysis: involvement of the Cdc34-SCFp45Skp2 pathway. Oncogene. 2000;19:2986–2995. doi: 10.1038/sj.onc.1203618. [DOI] [PubMed] [Google Scholar]
- 134.von der Lehr N, Johansson S, Larsson LG. Implication of the ubiquitin/proteasome system in Myc-regulated transcription. Cell Cycle. 2003;2:403–407. [PubMed] [Google Scholar]
- 135.von der Lehr N, et al. The F-box protein Skp2 participates in c-Myc proteosomal degradation and acts as a cofactor for c-Myc-regulated transcription. Mol. Cell. 2003;11:1189–1200. doi: 10.1016/s1097-2765(03)00193-x. [DOI] [PubMed] [Google Scholar]
- 136.Marti A, Wirbelauer C, Scheffner M, Krek W. Interaction between ubiquitin-protein ligase SCFSKP2 and E2F-1 underlies the regulation of E2F-1 degradation. Nature Cell Biol. 1999;1:14–19. doi: 10.1038/8984. [DOI] [PubMed] [Google Scholar]
- 137.Oh KJ, et al. The papillomavirus E7 oncoprotein is ubiquitinated by UbcH7 and Cullin 1-and Skp2-containing E3 ligase. J. Virol. 2004;78:5338–5346. doi: 10.1128/JVI.78.10.5338-5346.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lin YW, Yang JL. Cooperation of ERK and SCFSkp2 for MKP-1 destruction provides a positive feedback regulation of proliferating signaling. J. Biol. Chem. 2006;281:915–926. doi: 10.1074/jbc.M508720200. [DOI] [PubMed] [Google Scholar]
- 139.Liang M, et al. Ubiquitination and proteolysis of cancer-derived Smad4 mutants by SCFSkp2. Mol. Cell. Biol. 2004;24:7524–7537. doi: 10.1128/MCB.24.17.7524-7537.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Huang Z, Nie L, Xu M, Sun XH. Notch-induced E2A degradation requires CHIP and Hsc70 as novel facilitators of ubiquitination. Mol. Cell. Biol. 2004;24:8951–8962. doi: 10.1128/MCB.24.20.8951-8962.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nie L, Xu M, Vladimirova A, Sun XH. Notch-induced E2A ubiquitination and degradation are controlled by MAP kinase activities. EMBO J. 2003;22:5780–5792. doi: 10.1093/emboj/cdg567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nie L, Wu H, Sun XH. Ubiquitination and degradation of Tal1/SCL is induced by Notch signaling and depends on Skp2 and CHIP. J. Biol. Chem. 2007 doi: 10.1074/jbc.M704981200. [DOI] [PubMed] [Google Scholar]
- 143.Sanada T, et al. Skp2 overexpression is a p27Kip1-independent predictor of poor prognosis in patients with biliary tract cancers. Cancer Sci. 2004;95:969–976. doi: 10.1111/j.1349-7006.2004.tb03185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Traub F, Mengel M, Luck HJ, Kreipe HH, von Wasielewski R. Prognostic impact of Skp2 and p27 in human breast cancer. Breast Cancer Res. Treat. 2006;99:185–191. doi: 10.1007/s10549-006-9202-3. [DOI] [PubMed] [Google Scholar]
- 145.Sonoda H, et al. Significance of skp2 expression in primary breast cancer. Clin. Cancer Res. 2006;12:1215–1220. doi: 10.1158/1078-0432.CCR-05-1709. [DOI] [PubMed] [Google Scholar]
- 146.Narayan G, et al. Gene dosage alterations revealed by cDNA microarray analysis in cervical cancer: identification of candidate amplified and overexpressed genes. Genes Chromosomes Cancer. 2007;46:373–384. doi: 10.1002/gcc.20418. [DOI] [PubMed] [Google Scholar]
- 147.Gstaiger M, et al. Skp2 is oncogenic and overexpressed in human cancers. Proc. Natl Acad. Sci. USA. 2001;98:5043–5048. doi: 10.1073/pnas.081474898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nishida N, Nagasaka T, Kashiwagi K, Boland CR, Goel A. High copy amplification of the Aurora-A gene is associated with chromosomal instability phenotype in human colorectal cancers. Cancer Biol. Ther. 2007;6:525–533. doi: 10.4161/cbt.6.4.3817. [DOI] [PubMed] [Google Scholar]
- 149.Kamata Y, et al. High expression of skp2 correlates with poor prognosis in endometrial endometrioid adenocarcinoma. J. Cancer Res. Clin. Oncol. 2005;131:591–596. doi: 10.1007/s00432-005-0671-2. [DOI] [PubMed] [Google Scholar]
- 150.Lahav-Baratz S, et al. Decreased level of the cell cycle regulator p27 and increased level of its ubiquitin ligase Skp2 in endometrial carcinoma but not in normal secretory or in hyperstimulated endometrium. Mol. Hum. Reprod. 2004;10:567–572. doi: 10.1093/molehr/gah084. [DOI] [PubMed] [Google Scholar]
- 151.Ma XM, Liu JH, Guo JW, Liu Y, Zuo LF. Correlation of Skp2 expression in gastric carcinoma to expression of P27 and PTEN. Ai Zheng. 2006;25:56–61. [PubMed] [Google Scholar]
- 152.Ma XM, Liu Y, Guo JW, Liu JH, Zuo LF. Relation of overexpression of S phase kinase-associated protein 2 with reduced expression of p27 and PTEN in human gastric carcinoma. World J. Gastroenterol. 2005;11:6716–6721. doi: 10.3748/wjg.v11.i42.6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Schiffer D, Cavalla P, Fiano V, Ghimenti C, Piva R. Inverse relationship between p27/Kip1 and the F-box protein Skp2 in human astrocytic gliomas by immunohistochemistry and western blot. Neurosci. Lett. 2002;328:125–128. doi: 10.1016/s0304-3940(02)00483-4. [DOI] [PubMed] [Google Scholar]
- 154.Saigusa K, et al. Overexpressed Skp2 within 5p amplification detected by array-based comparative genomic hybridization is associated with poor prognosis of glioblastomas. Cancer Sci. 2005;96:676–683. doi: 10.1111/j.1349-7006.2005.00099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Penin RM, et al. Over-expression of p45SKP2 in Kaposi’s sarcoma correlates with higher tumor stage and extracutaneous involvement but is not directly related to p27KIP1 down-regulation. Mod. Pathol. 2002;15:1227–1235. doi: 10.1097/01.MP.0000036589.99516.D6. [DOI] [PubMed] [Google Scholar]
- 156.Inui N, et al. High expression of Cks1 in human non-small cell lung carcinomas. Biochem. Biophys. Res. Commun. 2003;303:978–984. doi: 10.1016/s0006-291x(03)00469-8. [DOI] [PubMed] [Google Scholar]
- 157.Yokoi S, et al. Amplification and overexpression of SKP2 are associated with metastasis of non-small-cell lung cancers to lymph nodes. Am. J. Pathol. 2004;165:175–180. doi: 10.1016/S0002-9440(10)63286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhu CQ, et al. Skp2 gene copy number aberrations are common in non-small cell lung carcinoma, and its overexpression in tumors with ras mutation is a poor prognostic marker. Clin. Cancer Res. 2004;10:1984–1991. doi: 10.1158/1078-0432.ccr-03-0470. [DOI] [PubMed] [Google Scholar]
- 159.Coe BP, et al. High-resolution chromosome arm 5p array CGH analysis of small cell lung carcinoma cell lines. Genes Chromosomes Cancer. 2005;42:308–313. doi: 10.1002/gcc.20137. [DOI] [PubMed] [Google Scholar]
- 160.Zhan F, et al. CKS1B, overexpressed in aggressive disease, regulates multiple myeloma growth and survival through SKP2-and p27Kip1-dependent and - independent mechanisms. Blood. 2007;109:4995–5001. doi: 10.1182/blood-2006-07-038703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shaughnessy J. Amplification and overexpression of CKS1B at chromosome band 1q21 is associated with reduced levels of p27Kip1 and an aggressive clinical course in multiple myeloma. Hematology. 2005;10:S117–S126. doi: 10.1080/10245330512331390140. [DOI] [PubMed] [Google Scholar]
- 162.Woenckhaus C, et al. Expression of Skp2 and p27KIP1 in naevi and malignant melanoma of the skin and its relation to clinical outcome. Histol. Histopathol. 2005;20:501–508. doi: 10.14670/HH-20.501. [DOI] [PubMed] [Google Scholar]
- 163.Li Q, Murphy M, Ross J, Sheehan C, Carlson JA. Skp2 and p27kip1 expression in melanocytic nevi and melanoma: an inverse relationship. J. Cutan Pathol. 2004;31:633–642. doi: 10.1111/j.0303-6987.2004.00243.x. [DOI] [PubMed] [Google Scholar]
- 164.Katagiri Y, Hozumi Y, Kondo S. Knockdown of Skp2 by siRNA inhibits melanoma cell growth in vitro and in vivo. J. Dermatol. Sci. 2006;42:215–224. doi: 10.1016/j.jdermsci.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 165.Bhatt KV, Hu R, Spofford LS, Aplin AE. Mutant B-RAF signaling and cyclin D1 regulate Cks1/ S-phase kinase-associated protein 2-mediated degradation of p27Kip1 in human melanoma cells. Oncogene. 2007;26:1056–1066. doi: 10.1038/sj.onc.1209861. [DOI] [PubMed] [Google Scholar]
- 166.Fukuchi M, et al. Inverse correlation between expression levels of p27 and the ubiquitin ligase subunit Skp2 in early esophageal squamous cell carcinoma. Anticancer Res. 2004;24:777–783. [PubMed] [Google Scholar]
- 167.Harada K, et al. High expression of S-phase kinase-associated protein 2 (Skp2) is a strong prognostic marker in oral squamous cell carcinoma patients treated by UFT in combination with radiation. Anticancer Res. 2005;25:2471–2475. [PubMed] [Google Scholar]
- 168.Kudo Y, et al. High expression of S-phase kinase-interacting protein 2, human F-box protein, correlates with poor prognosis in oral squamous cell carcinomas. Cancer Res. 2001;61:7044–7047. [PubMed] [Google Scholar]
- 169.Kitajima S, et al. Role of Cks1 overexpression in oral squamous cell carcinomas: cooperation with Skp2 in promoting p27 degradation. Am. J. Pathol. 2004;165:2147–2155. doi: 10.1016/S0002-9440(10)63264-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Shigemasa K, Gu L, O’Brien TJ, Ohama K. Skp2 overexpression is a prognostic factor in patients with ovarian adenocarcinoma. Clin. Cancer Res. 2003;9:1756–1763. [PubMed] [Google Scholar]
- 171.Sui L, et al. Clinical significance of Skp2 expression, alone and combined with Jab 1 and p27 in epithelial ovarian tumors. Oncol. Rep. 2006;15:765–771. [PubMed] [Google Scholar]
- 172.Drobnjak M, et al. Altered expression of p27 and Skp2 proteins in prostate cancer of African-American patients. Clin. Cancer Res. 2003;9:2613–2619. [PubMed] [Google Scholar]
- 173.Yang G, et al. Elevated Skp2 protein expression in human prostate cancer: association with loss of the cyclin-dependent kinase inhibitor p27 and PTEN and with reduced recurrence-free survival. Clin. Cancer Res. 2002;8:3419–3426. [PubMed] [Google Scholar]
- 174.Amir RE, Haecker H, Karin M, Ciechanover A. Mechanism of processing of the NF-κB2 p 100 precursor: identification of the specific polyubiquitin chain-anchoring lysine residue and analysis of the role of NEDD8-modification on the SCFβ-TrCP ubiquitin ligase. Oncogene. 2004;23:2540–2547. doi: 10.1038/sj.onc.1207366. [DOI] [PubMed] [Google Scholar]
- 175.Fong A, Sun SC. Genetic evidence for the essential role of β-transducin repeat-containing protein in the inducible processing of NF-κB2/p 100. J. Biol. Chem. 2002;277:22111–22114. doi: 10.1074/jbc.C200151200. [DOI] [PubMed] [Google Scholar]
- 176.Lang V, et al. βTrCP-mediated proteolysis of NF-κB 1 p 105 requires phosphorylation of p105 serines 927 and 932. Mol. Cell. Biol. 2003;23:402–413. doi: 10.1128/MCB.23.1.402-413.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Orian A, et al. SCFβ-TrCP ubiquitin ligase-mediated processing of NF-κB p 105 requires phosphorylation of its C-terminus by IκB kinase. EMBO J. 2000;19:2580–2591. doi: 10.1093/emboj/19.11.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lassot I, et al. ATF4 degradation relies on a phosphorylation-dependent interaction with the SCFβTrCP ubiquitin ligase. Mol. Cell. Biol. 2001;21:2192–2202. doi: 10.1128/MCB.21.6.2192-2202.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Li Y, Kumar KG, Tang W, Spiegelman VS, Fuchs SY. Negative regulation of prolactin receptor stability and signaling mediated by SCFβ-TrC E3 ubiquitin ligase. Mol. Cell. Biol. 2004;24:4038–4048. doi: 10.1128/MCB.24.9.4038-4048.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Besnard-Guerin C, et al. HIV-1 Vpu sequesters β-transducin repeat-containing protein (βTrCP) in the cytoplasm and provokes the accumulation of β-catenin and other SCFβTrCP substrates. J. Biol. Chem. 2004;279:788–795. doi: 10.1074/jbc.M308068200. [DOI] [PubMed] [Google Scholar]
- 181.Kumar KG, Krolewski JJ, Fuchs SY. Phosphorylation and specific ubiquitin acceptor sites are required for ubiquitination and degradation of the IFNAR1 subunit of type I interferon receptor. J. Biol. Chem. 2004;279:46614–46620. doi: 10.1074/jbc.M407082200. [DOI] [PubMed] [Google Scholar]
- 182.Mantovani F, Banks L. Regulation of the discs large tumor suppressor by a phosphorylation-dependent interaction with the β-TrCP ubiquitin ligase receptor. J. Biol. Chem. 2003;278:42477–42486. doi: 10.1074/jbc.M302799200. [DOI] [PubMed] [Google Scholar]
- 183.Reischl S, et al. β-TrCP1-mediated degradation of PERIOD2 is essential for circadian dynamics. J. Biol. Rhythms. 2007;22:375–386. doi: 10.1177/0748730407303926. [DOI] [PubMed] [Google Scholar]
- 184.Shirogane T, Jin J, Ang XL, Harper JW. SCFβ-TRCP controls clock-dependent transcription via casein kinase 1-dependent degradation of the mammalian period-1 (Per 1) protein. J. Biol. Chem. 2005;280:26863–26872. doi: 10.1074/jbc.M502862200. [DOI] [PubMed] [Google Scholar]
- 185.Eide EJ, et al. Control of mammalian circadian rhythm by CKIε-regulated proteasome-mediated PER2 degradation. Mol. Cell. Biol. 2005;25:2795–2807. doi: 10.1128/MCB.25.7.2795-2807.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tian Y, et al. TAZ promotes PC2 degradation through a SCFβ-Trcp E3 ligase complex. Mol. Cell. Biol. 2007;27:6383–6395. doi: 10.1128/MCB.00254-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ding Q, et al. Degradation of Mcl-1 by β-TrCP mediates glycogen synthase kinase 3-induced tumor suppression and chemosensitization. Mol. Cell. Biol. 2007;27:4006–4017. doi: 10.1128/MCB.00620-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Tan P, et al. Recruitment of a ROC1-CUL1 ubiquitin ligase by Skp1 and HOS to catalyze the ubiquitination of IκBα. Mol. Cell. 1999;3:527–533. doi: 10.1016/s1097-2765(00)80481-5. [DOI] [PubMed] [Google Scholar]
- 189.Gallegos JR, et al. SCF TrCP1 activates and ubiquitylates TAp63γ. J. Biol. Chem. 2008;283:66–75. doi: 10.1074/jbc.M704686200. [DOI] [PubMed] [Google Scholar]
- 190.van Kerkhof P, Putters J, Strous GJ. The ubiquitin ligase SCFβTrCP regulates the degradation of the growth hormone receptor. J. Biol. Chem. 2007;282:20475–20483. doi: 10.1074/jbc.M702610200. [DOI] [PubMed] [Google Scholar]
- 191.Seki A, et al. Plk1-and β-TrCP-dependent degradation of Bora controls mitotic progression. J. Cell Biol. 2008;181:65–78. doi: 10.1083/jcb.200712027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Soond SM, et al. ERK and the E3 ubiquitin ligase βTRCP targets STAT1 for degradation. J. Biol. Chem. 2008 [Google Scholar]