Abstract
BACKGROUND
Inappropriate antibacterial treatment of ventilator-associated pneumonia (VAP) due to multidrug-resistant (MDR) pathogens is associated with increased mortality. Endotracheal aspirate (ETA) surveillance cultures potentially identify MDR pathogens, particularly MDR Pseudomonas aeruginosa, resulting in improved selection of therapy in patients who subsequently develop VAP.
OBJECTIVE
To investigate the role of ETA surveillance cultures in the identification of MDR P. aeruginosas newly intubated adults who subsequently develop VAP.
METHODS
Daily ETA surveillance cultures for P. aeruginosa were collected in all adults newly intubated for 48 hours or more. Patients with preexisting lung disease or colonization or infection with P. aeruginosa were excluded. Risk factors and outcomes of patients newly colonized with MDR P. aeruginosa were assessed.
RESULTS
Seventy-five patients newly colonized with P. aeruginosa were identified. Twenty (27%) of these patients were colonized with a P. aeruginosa isolate that was MDR (resistant to ≥3 classes of antibiotics). Six patients were colonized by an isolate resistant to all tested classes of antibiotics. Forty-five percent of patients colonized with MDR P. aeruginosa subsequently developed VAP. Prior receipt of fluoroquinolones was an independent predictor of colonization with MDR P. aeruginosa (OR 11.82; 95% CI 2.10 to 66.46; p = 0.005).
CONCLUSIONS
Performance of routine surveillance cultures may aid in the early detection of MDR P. aeruginosa, improving the initiation of early and appropriate antibiotic therapy for patients who subsequently develop VAP.
Keywords: antibiotics, multidrug resistance, Pseudomonas aeruginosa, ventilator-associated pneumonia
Nosocomial pneumonia is the leading cause of death from hospital-acquired infections.1 Nearly one-third of patients intubated for 48 hours or more develop ventilator-associated pneumonia (VAP).2 VAP is associated with prolonged mechanical ventilation and increased hospital costs; mortality ranges from 17% to 50%.3,4 Patients with VAP due to Pseudomonas aeruginosa have an attributable mortality exceeding 40%.5 Failure to institute appropriate antimicrobial therapy in a timely fashion has been consistently associated with increased mortality.1,6–8 According to the United States National Nosocomial Infections Surveillance System data from 2003, 21.1% of P. aeruginosa isolates from US intensive care units (ICUs) were resistant to imipenem and 29.5% were resistant to fluoroquinolones.9 This represents a 15% and 9% increase, respectively, in resistance compared with the prior 5-year period.
Further complicating VAP management is the relatively slow finalization of culture and antibiotic susceptibility results. Identification of a causative organism can take up to 72 hours, with an additional 24 hours needed to determine susceptibility. However, delay in the initiation of antimicrobial therapy increases VAP mortality,1,6,7 and postponing therapy in order to perform diagnostic studies is not recommended.10 National guidelines recommend empiric therapy targeted against the most likely infecting organism based on known risk factors for a multidrug-resistant (MDR) pathogen.10 Surveillance cultures using endotracheal aspirates (ETAs) or bronchoalveolar lavage have been suggested as a mechanism to improve selection of appropriate empiric antimicrobial regimens.11–13 However, routine use of surveillance cultures remains controversial.
The purpose of this study was to evaluate ETA colonization with P. aeruginosa and subsequent evolution of VAP. Associated patient outcomes and risk factors for MDR P. aeruginosa were assessed.
Methods
PATIENTS
The study was a prospective observational cohort investigation of mechanically ventilated patients at the University of California, San Francisco (UCSF) Medical Center from October 2002 through April 2006. UCSF Medical Center is a 547-bed tertiary care teaching hospital with 60 critical care beds made up of a medical ICU, surgical ICU, and cardiac ICU.
All patients 18 years or older admitted to any of the ICUs and mechanically ventilated for 48 hours or more were screened daily for P. aeruginosa using ETAs, without regard for signs or symptoms of infection. Surveillance cultures were performed on all patients for research purposes only. A waiver of patient consent was obtained for the collection of the surveillance cultures. Patients were asked to consent to the study and were enrolled with the isolation of the first positive P. aeruginosa culture. In the event that the patient was unable to provide informed consent (eg, due to intubation or sedation), informed consent was obtained from the patient’s legally authorized representative. To identify patients at lower risk for colonization or infection with P. aeruginosa, patients with known risk factors for P. aeruginosa infection or colonization were excluded. These included patients with cystic fibrosis or bronchiectasis, chronic tracheostomy, previous intubation during the current hospitalization, or a previous blood or respiratory culture positive for P. aeruginosa during the current hospitalization or any past hospitalizations. The study was approved by the UCSF Institutional Review Board.
MICROBIOLOGY AND ANTIBIOTIC SUSCEPTIBILITY TESTING
Daily ETAs were screened for P. aeruginosa on Pseudomonas-selective medium (Hardy Diagnostics, Santa Maria, CA) per standard microbiologic techniques. Antibiotic susceptibility testing was performed on the first positive isolate recovered using microbroth dilution (Dade Behring, Deerfield, IL) and results were interpreted using Clinical and Laboratory Standards Institute guidelines.14 Antibiotics tested included ceftazidime, cefepime, aztreonam, piperacillin/tazobactam, ticarcillin/clavulanic acid, imipenem, ciprofloxacin, levofloxacin, gentamicin, and tobramycin. Antibiotics were grouped into the following 5 categories: (1) cephalosporins/monobactams (ceftazidime, cefepime, aztreonam), (2) β-lactamase inhibitor combinations (piperacillin/tazobactam, ticarcillin/clavulanic acid), (3) fluoroquinolones (ciprofloxacin, levofloxacin), (4) aminoglycosides (gentamicin, tobramycin), and (5) carbapenems (imipenem). Organisms were classified as either susceptible or resistant to the antibiotics tested; strains with intermediate susceptibility were considered resistant. P. aeruginosa isolates were defined as MDR if the organism was resistant to 3 or more of the 5 antibiotic categories.
DATA COLLECTION AND ENDPOINTS
Data on patient demographics, hospital and ICU admission dates, chest radiograph findings, microbiology results, ICU admitting diagnosis, days of mechanical ventilation, and antibiotics received 21 days prior to the first positive P. aeruginosa culture were collected. APACHE II (Acute Physiology and Chronic Health Evaluation), SAPS II (Simplified Acute Physiology Score), and lung injury scores were calculated for all patients on the day of enrollment. Patients were followed for 28 days postenrollment until ICU discharge or death, whichever occurred first. Patients were classified as having either MDR Pseudomonas or non–MDR Pseudomonas based on the antibiotic susceptibility results of the first positive surveillance isolate. Data were entered into an ACCESS database (Microsoft, 2002).
DEFINITIONS
VAP was defined as any lower respiratory infection that developed after 2 days of mechanical ventilation. Criteria for VAP were presence or progression of a new infiltrate identified by chest radiograph plus 2 of the following 3 clinical criteria: (1) temperature greater than 38 °C, (2) white blood cell count greater than 12,000 cells/μL or less than 4,000 cells/μL, and (3) purulent secretions and 106 cfu/mL or greater P. aeruginosa from the ETA and/or 104 cfu/mL or greater from bronchoalveolar lavage (BAL).15
STATISTICAL ANALYSIS
Normal or approximately normally distributed continuous variables were described by means and standard deviations and were compared using unpaired Student’s t-test. Normalcy of the data was determined using the Shapiro-Wilk W test. Medians with 25th to 75th interquartile ranges and Mann-Whitney U tests were used for the skewed continuous variables. Categorical variables were compared using χ2 tests or Fisher’s exact tests, as appropriate. A 2-sided p value less than 0.05 was considered statistically significant. Backward, stepwise multiple logistic regression models were used to determine the possible influence of recent antibiotic therapy and the isolation of MDR P. aeruginosa. Any variable with a p value less than or equal to 0.2 by univariate analysis was also included in the full model. Statistical analysis was performed using STATA version 9.0 (STATA Corp., College Station, TX).
Results
PATIENT CHARACTERISTICS
A total of 1868 patients were screened over a 3½ year period; of these, 157 patients newly colonized with P. aeruginosa with associated antibiotic susceptibility data were identified. Eighty-two patients had either a prior positive culture for P. aeruginosa or a preexisting respiratory condition predisposing them to Pseudomonas infection or colonization (ie, cystic fibrosis, chronic ventilation, or preexisting pneumonia) or refused participation in the study; these patients were excluded per the study protocol. Thus, 75 patients were included in this study (Table 1). VAP developed in 33 (44%) of these patients. Twenty-two of the 33 patients with VAP were confirmed with BAL. Twenty-six patients developed VAP due to P. aeruginosa alone; 5 patients developed polymicrobial VAP including P. aeruginosa. Two patients developed VAP due to bacteria other than P. aeruginosa. Twenty (27%) patients were newly colonized with MDR P. aeruginosa (Table 1), and 6 patients had an isolate resistant to all 5 classes of antibiotics. Patients with MDR Pseudomonas had a significantly greater number of days of mechanical ventilation, ICU days, and hospital days compared with the non–MDR Pseudomonas group.
Table 1.
Characteristics at Enrollment of Patients Newly Colonized with Pseudomonas aeruginosa
| Patient Characteristic | MDR Pseudomonas (n = 20) | Non-MDR Pseudomonas (n = 55) | p Value |
|---|---|---|---|
| Age, y (mean ± SD) | 61 ± 14 | 63 ± 13 | 0.45 |
| Male (%) | 13 (65) | 23 (42) | 0.08 |
| ICU (%) | |||
| medical | 12 (60) | 19 (35) | 0.15 |
| surgical, scheduled | 6 (30) | 25 (45) | |
| surgical, nonscheduled | 2 (10) | 11 (20) | |
| Chronic respiratory disease | 5 (25) | 11 (20) | 0.75 |
| Diabetes mellitus | 5 (25) | 8 (15) | 0.31 |
| Cancer | 3 (15) | 12 (22) | 0.75 |
| Immunosuppression | 4 (20) | 4 (7) | 0.20 |
| Days of hospitalization prior to ICU admission, median (25–75% percentile) | 0 (0–1) | 0 (0–2) | 0.70 |
| Days of hospitalization prior to first positive culture, median (25–75% percentile) | 12.5 (5–31) | 9 (3–15) | 0.052 |
| Days of ventilation prior to first positive culture, median (25–75% percentile) | 10 (5–23.5) | 5 (3–11) | 0.015 |
| APACHE II at ICU admission (mean ± SD) | 26 ± 8 | 23 ± 7 | 0.12 |
| SAPS II at ICU admission (mean ± SD) | 46 ± 14 | 44 ± 16 | 0.60 |
| ARDS at enrollment | 3 (15) | 2 (4) | 0.11 |
| ICU days, median (25–75% percentile) | 30 (17–55) | 20 (11–32) | 0.02 |
| Hospital days, median (25–75% percentile) | 37 (28.5–72) | 34 (21–47) | 0.046 |
APACHE = Acute Physiology and Chronic Health Evaluation; ARDS = acute respiratory distress syndrome; ICU = intensive care unit; MDR = multidrug-resistant; SAPS = Simplified Acute Physiology Score.
CLINICAL OUTCOMES
Although VAP occurred more frequently in the MDR Pseudomonas colonized group, this difference was not significant (45% vs 40%; p = 0.7) (Table 2). Once patients became colonized with P. aeruginosa, VAP developed rapidly in our cohort, at a median of 2.5 days from the time of the first positive ETA culture in the non–MDR Pseudomonas group compared with only 1 day in the MDR Pseudomonas group. Two of the 20 patients colonized with MDR Pseudomonas developed VAP due to other bacteria (Acinetobacter baumanii; Escherichia coli). VAP developed at a median of 16 days from ICU admission (range 7–32) in the MDR Pseudomonas group compared with 10 days (range 6–14) in the non–MDR Pseudomonas group (p = 0.08). Length of ICU stay was significantly higher in the MDR Pseudomonas group (30 days) compared with the non–MDR Pseudomonas group (20 days) (p = 0.02). There were no significant differences between the groups for either 28-day or crude mortality (Table 2). Patients who developed VAP had significantly higher APACHE II scores at admission compared with patients who did not develop VAP (27 ± 7 vs21 ± 7;p = 0.002).
Table 2.
Outcomes of Patients Newly Colonized with Pseudomonas aeruginosa
| Patient Outcome | MDR Pseudomonas (n = 20) | Non-MDR Pseudomonas (n = 55) | p Value |
|---|---|---|---|
| VAP due to P. aeruainosa, n (%)a | 9(45) | 22 (40) | 0.70 |
| Non-Pseudomonas VAP, n (%)a | 2(10) | 0(0) | 0.07 |
| Bacteremia with P. aeruginosa | 2(10) | 2(4) | 0.29 |
| Days from first positive culture to development of VAP, median | 1 (1–5) | 2.5(1–5) | 0.92 |
| Sepsis within 28 days after enrollment, n (%) | 4(20) | 11 (20) | 1.0 |
| Mortality, n (%) | (35) | 16(29) | 0.62 |
MDR = multidrug-resistant; VAP = ventilator-associated pneumonia.
Within 28 days after enrollment or until discharged from the intensive care unit.
PSEUDOMONAS AERUGINOSA SUSCEPTIBILITY DATA AND ANTIBIOTIC UTILIZATION
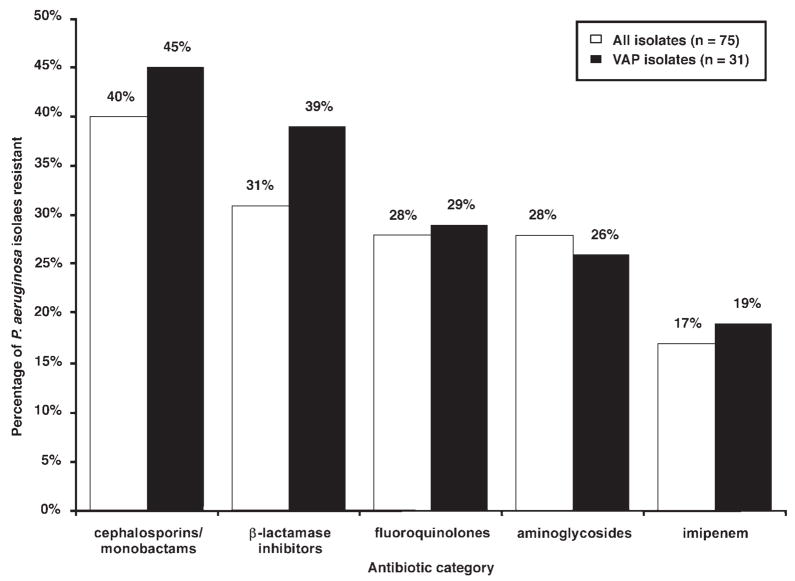
The antibiotic resistance profile of the 75 P. aeruginosa isolates is shown in Figure 1. Antibiotics received in the 21 days prior to the first positive endotracheal surveillance culture are presented in Table 3. Seven patients (5 in the non–MDR Pseudomonas group, 2 in the MDR Pseudomonas group) received no antibiotics during the 21 days prior to enrollment. Interestingly, 29 (53%) patients in the non–MDR Pseudomonas group, but only 5 (25%) patients in the MDR Pseudomonas group, received at least one antipseudomonal antibiotic prior to enrollment (p = 0.04). When stratified by antibiotic category, only fluoroquinolones were used more frequently prior to enrollment in the MDR Pseudomonas group compared with the non–MDR Pseudomonas group (35% vs 4%; p = 0.001).
Figure 1.
Antibiotic resistance profile of Pseudomonas aeruginosa Isolated from all patients and among patients who subsequently developed ventilator-associated pneumonia (VAP).
Table 3.
Antibiotics Received in the 21 Days Preceding First Positive Endotracheal Aspirate Culture
| Antibiotic, n (%) | Overall (n = 75) | MDR Pseudomonas (n = 20) | Non-MDR Pseudomonas(n = 55) | p Value |
|---|---|---|---|---|
| Any | 68 (91) | 18(90) | 50(91) | 1.0 |
| Any active against Pseudomonas aeruginosa at enrollment | 34 (45) | 5(25) | 29 (53) | 0.04 |
| Fluoroquinolones | 9(12) | 7(35) | 2 (4) | 0.001 |
| Aminoglycosides | 11 (15) | 2(10) | 9(16) | 0.72 |
| Imipenem | 4(5) | 1 (5) | 3(5) | 1.0 |
| Cephalosporins/monobactams | 13(17) | 4(20) | 9(16) | 0.74 |
| β-lactamase inhibitors | 33 (44) | 9(45) | 24 (44) | 0.92 |
MDR = multidrug-resistant.
Risk factors for MDR Pseudomonas were evaluated using a backward stepwise regression model including the following variables: prior exposure to fluoroquinolones, cephalosporins/monobactam, imipenem, aminoglycosides, and β-lactamase inhibitors in addition to ICU admission category, immunosuppression, days of hospitalization prior to enrollment, days of ventilation prior to enrollment, APACHE II score at ICU admission, and acute respiratory distress syndrome at enrollment. Only prior treatment with a fluoroquinolone (OR 11.82; 95% CI 2.10 to 66.46; p = 0.005) and duration of hospitalization prior to enrollment (OR 1.05; 95% CI 1.002 to 1.10; p = 0.04) were independent risk factors for colonization with MDR Pseudomonas.
Discussion
In this prospective study, daily ETA surveillance cultures identified patients newly colonized with P. aeruginosa. Importantly, many of these patients had no traditional risk factors for P. aeruginosa, and colonization occurred very soon after initiation of mechanical ventilation. A large percentage of patients were colonized with MDR P. aeruginosa, half of whom went on to develop VAP. Development of VAP was rapid in MDR isolates, occurring a median of only one day after colonization. Prior receipt of a fluoroquinolone was the sole antibiotic associated with colonization with MDR Pseudomonas.
Our finding that prior use of fluoroquinolones was independently associated with development of MDR Pseudomonas is in accord with the findings of 2 previous studies.16,17 Trouillet et al.17 found that prior fluoroquinolone exposure was the sole antibiotic associated with development of piperacillin-resistant P. aeruginosa VAP (OR 4.6; 95% CI 1.7 to 12.7; p = 0.003). In a separate study, the same authors16 showed that receipt of a fluoroquinolone in the 15 days prior to VAP diagnosis was associated with the development of “potentially resistant” bacteria compared with “other” bacteria (OR 14.9; p = 0.001). Our study confirms this association between prior fluoroquinolone use and the development of MDR P. aeruginosa VAP.
Three studies have examined the role of routine respiratory surveillance cultures in the management of VAP.13,18,19 ETAs taken twice weekly identified the causative organism in 34 of 41 (83%) patients with BAL-confirmed VAP.13 P. aeruginosa was the most commonly isolated organism (24%), and 58% were ticarcillin-resistant. All occurred in late-onset VAP (eg, VAP occurring ≥7 days of mechanical ventilation). Hayon et al.18 prospectively evaluated 125 consecutive patients with BAL-confirmed VAP. Prior surveillance respiratory cultures (ie, ETA, BAL, protected brush specimen) data were available for 109 VAP episodes. Surveillance cultures identified at least one, but not all, causative organisms in only 27% of VAP episodes and identified all of the causative organisms in only 35% of VAP episodes. The positive predictive value of respiratory surveillance cultures was greatest when the organism was isolated for less than 72 hours prior to the diagnosis compared with organisms isolated greater than 72 hours (56% vs 13%; p = 0.001). Another trial performed tracheal aspirate and protected brush catheter cultures in 356 intubated heart surgery patients 3 days after surgery, weekly, and at extubation. Despite a low overall rate of VAP, P. aeruginosa was the most common organism isolated. The mean time between positive surveillance culture and the diagnostic VAP culture was 4.3 ± 1.8 days (range 2–7). The most recent study used 3 times weekly urinary surveillance cultures in addition to once weekly oral, nasal, and rectal cultures and 3 times weekly tracheal aspirates to predict VAP due to MDR pathogens.20 In this last study, MDR pathogens were defined as methicillin-resistant Staphylococcus aureus, extended-spectrum β-lactamase–producing Enterobacteriaceae, or A. baumanii, Stenotrophomonas maltophilia, and P. aeruginosa resistant only to 1 or more of the following 3 antibiotics: ceftazidime, piperacillin, or imipenem. ETA surveillance cultures predicted VAP secondary to MDR organisms in 69% of patients. However, the investigators’ definition of MDR may have led to an overestimate of MDR gram-negative pathogens.
Our study differs from these previous investigations in that ETA surveillance cultures were performed on a daily basis. We documented a much shorter time from positive surveillance culture (1 day) to development of VAP than previous studies (4–5 days). Our findings of Pseudomonas VAP, including MDR Pseudomonas, developing only 1 day after colonization is worrisome, particularly since we limited our study population to patients with no prior history of P. aeruginosa colonization or infection. It is possible that the use of daily ETAs allowed for earlier identification of the causative VAP pathogen. Our colonized patients were more likely to develop VAP compared with those in earlier surveillance studies. In addition, MDR Pseudomonas was more common than that observed with previous investigations. Earlier studies reported a greater incidence of MDR organisms with “late-onset” VAP (>5–7 days), compared with “early-onset” VAP (<5–7 days). The median time of mechanical ventilation prior to positive culture was significantly longer in our MDR Pseudomonas group (10 vs 5 days; p = 0.015). However, studies have also shown that both early- and late-onset VAP and nosocomial pneumonia can be caused by MDR pathogens.21–23 This finding further underscores the potential for active surveillance toward the selection of more appropriate empiric antibiotic therapy, particularly at institutions with a high prevalence of antibiotic-resistant pathogens. At UCSF Medical Center, 38% of all Pseudomonas isolates are resistant to fluoroquinolones and 24% are resistant to imipenem. Interestingly, 53% of the patients in the non–MDR Pseudomonas group received at least one antibiotic with activity against P. aeruginosa prior to enrollment compared with only 25% of patients in the MDR Pseudomonas group. This may be due to the eradication of sensitive organisms in the non–MDR Pseudomonas group.
Our study is associated with some limitations. The lack of significant difference in mortality between the non-MDR and MDR groups may be due to small sample size. Because this was an observational investigation evaluating the role of daily surveillance cultures and the risk factors for MDR Pseudomonas, we systematically cultured all patients intubated for 48 hours or more, and the results of the surveillance cultures were not available to the treating clinician. Thus, antibiotic treatment remained empirical. This is in accord with previously published studies on the value of surveillance cultures in the management of VAP.12,18–20 Prospective, controlled trials with associated cost–benefit analyses should take place to fully clarify the role of daily ETA in mechanically ventilated patients. Finally, we performed surveillance cultures only for P. aeruginosa. Further studies must confirm whether our results can be generalized to other organisms known to cause VAP.
The high mortality associated with inadequately treated VAP, particularly due to P. aeruginosa, highlights the need to commence timely and appropriate antibiotic therapy.15 According to the 2005 American Thoracic Society guidelines for treatment of nosocomial pneumonia, empiric antibiotic therapy for VAP should be dictated by local susceptibility patterns and initiated promptly, taking care to avoid antibiotics that the patient had recently received.10 In addition, patients with VAP should be treated for potentially drug-resistant organisms, regardless of the timing of the pneumonia during the course of hospitalization. The high incidence of MDR Pseudomonas in our study suggests that broad-spectrum antibiotics should be considered in any patient suspected to have VAP, particularly in institutions in which MDR pathogens are common. Inadequate empiric antimicrobial therapy has been consistently linked with increased mortality.1,6,7 However, multiple studies also confirm that modification of an initially inactive antibiotic regimen based on the results of antibiotic susceptibility tests does not subsequently improve mortality.7,24,25 This further underscores the importance of administering the right drug at the first suspicion of VAP. Considering the very short time from positive surveillance culture to development of VAP observed in our cohort, the initial empirical choice is critical.
Conclusions
VAP due to P. aeruginosa is associated with high mortality. Performing daily surveillance cultures can aid in the early detection of patients colonized with P. aeruginosa, particularly MDR Pseudomonas. This, in turn, may lead to the more timely selection of appropriate empiric antibiotic therapy in patients who subsequently develop VAP.
Acknowledgments
The project described was supported by grant number KL2 RR024130 from the National Center for Research Resources (NCRR) (Dr. Yang) and grant numbers PH 50HL74005 and HL69809 (Drs. Wiener-Kronish and Zhuo).
We thank Amy J Markowitz JD for editorial assistance.
Footnotes
The content of this paper is solely the responsibility of the authors and does not necessarily represent the official view of the NCRR or the National Institutes of Health.
This study was presented in part at the International Conference of the American Thoracic Society, San Francisco, CA, May 2007 (abstract 955098).
Contributor Information
Katherine Yang, Assistant Clinical Professor, Department of Clinical Pharmacy, School of Pharmacy, University of California, San Francisco, CA.
Hanjing Zhuo, Associate Specialist, Cardiovascular Research Institute, School of Medicine, University of California, San Francisco.
B Joseph Guglielmo, Professor and Chair, Department of Clinical Pharmacy, School of Pharmacy, University of California, San Francisco.
Jeanine Wiener-Kronish, Anesthetist-in-Chief, Department of Anesthesia and Critical Care, Massachusetts General Hospital, Boston, MA.
References
- 1.Mehta RM, Niederman MS. Nosocomial pneumonia in the intensive care unit: controversies and dilemmas. J Intensive Care Med. 2003;18:175–88. doi: 10.1177/0885066603254249. [DOI] [PubMed] [Google Scholar]
- 2.Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165:867–903. doi: 10.1164/ajrccm.165.7.2105078. [DOI] [PubMed] [Google Scholar]
- 3.Rello J, Ollendorf DA, Oster G, et al. Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest. 2002;122:2115–21. doi: 10.1378/chest.122.6.2115. [DOI] [PubMed] [Google Scholar]
- 4.Rello J, Lorente C, Diaz E, et al. Incidence, etiology, and outcome of nosocomial pneumonia in ICU patients requiring percutaneous tracheotomy for mechanical ventilation. Chest. 2003;124:2239–43. doi: 10.1378/chest.124.6.2239. [DOI] [PubMed] [Google Scholar]
- 5.Fagon JY, Chastre J, Hance AJ, Montravers P, Novara A, Gibert C. Nosocomial pneumonia in ventilated patients: a cohort study evaluating attributable mortality and hospital stay. Am J Med. 1993;94:281–8. doi: 10.1016/0002-9343(93)90060-3. [DOI] [PubMed] [Google Scholar]
- 6.Iregui M, Ward S, Sherman G, Fraser VJ, Kollef MH. Clinical importance of delays in the initiation of appropriate antibiotic treatment for ventilator-associated pneumonia. Chest. 2002;122:262–8. doi: 10.1378/chest.122.1.262. [DOI] [PubMed] [Google Scholar]
- 7.Kollef MH, Ward S. The influence of mini-BAL cultures on patient outcomes: implications for the antibiotic management of ventilator-associated pneumonia. Chest. 1998;113:412–20. doi: 10.1378/chest.113.2.412. [DOI] [PubMed] [Google Scholar]
- 8.Kollef MH, Sherman G, Ward S, Fraser VJ. Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest. 1999;115:462–74. doi: 10.1378/chest.115.2.462. [DOI] [PubMed] [Google Scholar]
- 9.National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32:470–85. doi: 10.1016/S0196655304005425. [DOI] [PubMed] [Google Scholar]
- 10.Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 11.Crnich CJ, Proctor RA. Ventilator-associated pneumonia: does surveillance have a role in its management? Crit Care Med. 2003;31:2411–2. doi: 10.1097/01.CCM.0000090807.72720.93. [DOI] [PubMed] [Google Scholar]
- 12.Depuydt PO, Blot SI, Benoit DD, et al. Antimicrobial resistance in nosocomial bloodstream infection associated with pneumonia and the value of systematic surveillance cultures in an adult intensive care unit. Crit Care Med. 2006;34:653–9. doi: 10.1097/01.CCM.0000201405.16525.34. [DOI] [PubMed] [Google Scholar]
- 13.Michel F, Franceschini B, Berger P, et al. Early antibiotic treatment for BAL-confirmed ventilator-associated pneumonia: a role for routine endotracheal aspirate cultures. Chest. 2005;127:589–97. doi: 10.1378/chest.127.2.589. [DOI] [PubMed] [Google Scholar]
- 14.16th informational supplement, M100-S16. Wayne, PA: Clinical and Laboratory Standards Insititute; 2006. Performance standards for antimicrobial susceptibility testing. [Google Scholar]
- 15.Garnacho-Montero J, Sa-Borges M, Sole-Violan J, et al. Optimal management therapy for Pseudomonas aeruginosa ventilator-associated pneumonia: an observational, multicenter study comparing monotherapy with combination antibiotic therapy. Crit Care Med. 2007;35:1888–95. doi: 10.1097/01.CCM.0000275389.31974.22. [DOI] [PubMed] [Google Scholar]
- 16.Trouillet JL, Chastre J, Vuagnat A, et al. Ventilator-associated pneumonia caused by potentially drug-resistant bacteria. Am J Respir Crit Care Med. 1998;157:531–9. doi: 10.1164/ajrccm.157.2.9705064. [DOI] [PubMed] [Google Scholar]
- 17.Trouillet JL, Vuagnat A, Combes A, Kassis N, Chastre J, Gibert C. Pseudomonas aeruginosa ventilator-associated pneumonia: comparison of episodes due to piperacillin-resistant versus piperacillin-susceptible organisms. Clin Infect Dis. 2002;34:1047–54. doi: 10.1086/339488. [DOI] [PubMed] [Google Scholar]
- 18.Hayon J, Figliolini C, Combes A, et al. Role of serial routine microbiologic culture results in the initial management of ventilator-associated pneumonia. Am J Respir Crit Care Med. 2002;165:41–6. doi: 10.1164/ajrccm.165.1.2105077. [DOI] [PubMed] [Google Scholar]
- 19.Bouza E, Perez A, Munoz P, et al. Ventilator-associated pneumonia after heart surgery: a prospective analysis and the value of surveillance. Crit Care Med. 2003;31:1964–70. doi: 10.1097/01.ccm.0000084807.15352.93. [DOI] [PubMed] [Google Scholar]
- 20.Depuydt P, Benoit D, Vogelaers D, et al. Systematic surveillance cultures as a tool to predict involvement of multidrug antibiotic resistant bacteria in ventilator-associated pneumonia. Intensive Care Med. 2007;34:675–82. doi: 10.1007/s00134-007-0953-z. [DOI] [PubMed] [Google Scholar]
- 21.Ibrahim EH, Ward S, Sherman G, Kollef MH. A comparative analysis of patients with early-onset vs late-onset nosocomial pneumonia in the ICU setting. Chest. 2000;117:1434–42. doi: 10.1378/chest.117.5.1434. [DOI] [PubMed] [Google Scholar]
- 22.Giantsou E, Liratzopoulos N, Efraimidou E, et al. Both early-onset and late-onset ventilator-associated pneumonia are caused mainly by potentially multiresistant bacteria. Intensive Care Med. 2005;31:1488–94. doi: 10.1007/s00134-005-2697-y. [DOI] [PubMed] [Google Scholar]
- 23.Verhamme KM, De Coster W, De Roo L, et al. Pathogens in early-onset and late-onset intensive care unit–acquired pneumonia. Infect Control Hosp Epidemiol. 2007;28:389–97. doi: 10.1086/511702. [DOI] [PubMed] [Google Scholar]
- 24.Rello J, Gallego M, Mariscal D, Sonora R, Valles J. The value of routine microbial investigation in ventilator-associated pneumonia. Am J Respir Crit Care Med. 1997;156:196–200. doi: 10.1164/ajrccm.156.1.9607030. [DOI] [PubMed] [Google Scholar]
- 25.Sanchez-Nieto JM, Torres A, Garcia-Cordoba F, et al. Impact of invasive and noninvasive quantitative culture sampling on outcome of ventilator-associated pneumonia: a pilot study. Am J Respir Crit Care Med. 1998;157:371–6. doi: 10.1164/ajrccm.157.2.97-02039. [DOI] [PubMed] [Google Scholar]