Abstract
Background
We evaluated the cost-effectiveness of Chlamydia screening strategies that use different methods of specimen collection: cervical swabs, urines, and self-obtained vaginal swabs.
Methods
A decision analysis was modeled for a hypothetical cohort of 10,000 per year of women attending sexually transmitted disease (STD) clinics. Incremental cost-effectiveness of 4 screening strategies were compared: 1) Endocervical DNA probe test (PACE2, Gen-Probe), 2) Endocervical AC2 (Aptima Combo 2, Gen-Probe), 3) Self-Obtained Vaginal AC2, and 4) Urine AC2. Sensitivities of the vaginal, urine, and cervical AC2 tests were derived from 324 women attending STD clinics. The primary outcome was cases of pelvic inflammatory disease prevented. The model incorporated programmatic screening and treatment costs and medical cost savings from sequelae prevented.
Results
Chlamydia prevalence in the sampled population was 11.1%. Sensitivities of vaginal, urine, and cervical AC2 were 97.2%, 91.7%, and 91.7%, respectively. The sensitivity of the DNA probe was derived from the literature and estimated at 68.8%. The self-obtained vaginal AC2 strategy was the least expensive and the most cost-effective, preventing 17 more cases of pelvic inflammatory disease than the next least expensive strategy.
Conclusions
Use of a vaginal swab to detect Chlamydia in this STD clinic population was cost-saving and cost-effective.
It has been well established that Chlamydia trachomatis screening among women in most settings is cost-effective.1–8 Populations studied have included women attending family planning clinics,2–5 sexually transmitted disease (STD) clinics,4 emergency departments,1 youth clinics,5 gynecology clinics,5 student health centers,3 and military recruits6,7 and population based screening of 15- to 29-year-old women.8 Most of the analyses assumed asymptomatic status of women.3,5–8 Two analyses, however, compared several strategies, with some of the strategies taking symptom status into account and others screening everyone the same way regardless of symptom status.1,2 Many analyses took the health care system perspective.1–3,5 However, 2 conducted analyses from the military perspective,6,7 and 2 used the societal perspective.4,8
In most previous analyses, either an endocervical specimen was tested4,5 or a urine specimen was tested.1,3,6–8 Consequently, many previous analyses did not evaluate the impact of omitting the speculum examination on the cost-effectiveness outcome.1,3–7
The Aptima Combo 2 (AC2) test is among a new generation of tests that use nucleic acid amplification and is FDA-cleared for use on endocervical, urine, and vaginal specimens. Because most Chlamydia infections are asymptomatic,9–12 many women who seek screening have no symptoms. Unless they are due for an annual Papanicolaou (Pap) smear, asymptomatic women who are screened with a vaginal or urine AC2 test do not require a speculum examination, omission of which can save time and money.
The objective of this study was to evaluate the cost-effectiveness of several Chlamydia screening strategies and to incorporate conditions where a speculum examination may not be necessary.
Methods
Design
A decision analysis was modeled for a hypothetical cohort of 10,000 women per year attending Baltimore STD clinics. Incremental cost-effectiveness of 4 Chlamydia screening strategies were compared: 1) Endocervical DNA Probe (PACE2, Gen- Probe), 2) Endocervical AC2 (Aptima Combo 2, Gen-Probe), 3) Self-Obtained Vaginal AC2, and 4) Urine AC2. Strategies 1 and 2 always required a speculum examination; strategies 3 and 4 only required a speculum examination for symptomatic patients or those due for a Pap smear.
The primary outcome measure was number of cases of pelvic inflammatory disease (PID) prevented. Secondary outcome measures were PID-related sequelae including infertility, ectopic pregnancy, and chronic pelvic pain. The model incorporated programmatic screening and treatment costs and medical costs averted through prevention of PID and its sequelae. The time horizon was 10 years to allow for all PID sequelae to occur. The analyses were conducted from the public health care perspective and included only direct medical costs.
Probability and Cost Estimates
Primary data, unpublished state and local data, and published literature were used to estimate probabilities and costs for the decision analysis. Sensitivities of the vaginal, urine, and endocervical AC2 tests were derived from 324 women (92.6% black) attending Baltimore STD clinics between April 5, 2004 and February 3, 2005. All positive tests were confirmed by retesting the sample using GenProbe’s FDA cleared Aptima C. trachomatis (ACT; Gen-Probe), which has different target sequences than the AC2. Classification as a positive case required at least 1 of the samples (vaginal, urine, or endocervical) to test positive with confirmation. Each woman had all 3 samples collected. Following sample collection, women were asked to rate the 3 sample techniques in order of their preferred method. Written informed consent was obtained from all participants. The study was approved by the Western IRB, Seattle, WA, and the Baltimore City Health Department.
The prevalence of Chlamydia among these women, the proportion of infections that were symptomatic, the proportion of infections that were treated, and the proportion of women who required a Pap smear were all derived from this sample. The sensitivity of the DNA probe13–20 and the estimated proportion of women with untreated Chlamydia cervicitis who would develop PID3,21–23 and its sequelae24–27 were derived from the literature. See Table 1 for additional probability estimates.
Table 1.
Probability Estimates
| Variable | Probability Estimate (%) |
Range (%) |
Reference |
|---|---|---|---|
| Chlamydia prevalence | 11.1 | 7.7–14.5 | Primary data and 95% CI |
| Sensitivity AC2 | |||
| Vaginal | 97.2 | 85.5–99.9 | Primary data and 95% CI |
| First catch urine | 91.7 | 77.5–98.2 | |
| Endocervical | 91.7 | 77.5–98.2 | |
| Specificity AC2 | |||
| Vaginal | 99.5 | 97.1–99.7 | 50 |
| First catch urine | 99.3 | 98.8–99.3 | 50–52 |
| Endocervical | 98.3 | 97.6–98.3 | 50,52,53 |
| Sensitivity DNA probe* (endocervical) | 68.8 | 40–86.5 | 13–20 |
| Specificity DNA probe* (endocervical) | 99.8 | 95.6–100 | 13–20 |
| PID | 30 | 30–41 | 3,21–23 |
| Proportion of PID that is silent | 60 | — | 3,54 |
| Proportion of PID that is overt | 40 | — | 3,54 |
| Inpatient treatment of PID | 12.1 | 5–25 | 24,29,30,38,55,56 |
| Chronic pelvic pain | 18 | — | 24 |
| Ectopic pregnancy | 6 | 3.6–9.1 | 24–27 |
| Infertility | 9 | 7.7–20 | 24,25,27 |
| Infertile women seeking fertility services | 22.4 | — | 57 |
| Proportion of women requiring a Pap smear and/or symptomatic and requiring a pelvic exam to rule out PID |
79.0 | 71.3–86.8 | Primary data and 95% CI |
| Proportion of women requiring a Pap smear and/or reporting abdominal pain and requiring a pelvic exam to rule out PID |
50.5 | 40.9–60.0 | Primary data and CI |
DNA probe was PACE2.
AC2 indicates Aptima Combo 2; PID, pelvic inflammatory disease.
Cost estimates are presented in Table 2. All costs were converted to 2006$ using the medical care portion of the consumer price index. Maryland Medicaid reimbursement rates were used to estimate the cost of the AC2 and DNA Probe tests. Additional cost of a speculum examination was calculated by multiplying an STD clinician’s hourly salary rate by the average amount of time required to perform a speculum examination and adding the cost of a plastic speculum to this estimate. Average time to perform a speculum examination (7 minutes) was calculated from a time-in-motion study of routine patient evaluations that required speculum examinations at the STD clinics. Cost to treat a Chlamydia infection included the Maryland Public Health rate for 1 g of azithromycin and 15 minutes of an STD clinician’s time.
Table 2.
Cost Estimates (2006$)
| Variable | Cost Estimate | Range | Reference |
|---|---|---|---|
| AC2* | $43.42 | — | Maryland State Medicaid reimbursement rate |
| Endocervical DNA probe† cost | $28.02 | — | Maryland State Medicaid reimbursement rate reimbursement STD clicnc |
| Cost to perform pelvic | Speculum, $0.68 | — | Baltimore City STD clinic |
| examination at Baltimore | Clinician time, $4.72 | — | |
| City STD clinic | Total: $5.40 | — | |
| Cost of cervicitis treatment visit at Baltimore City STD clinic |
Clinician visit, $10.12 | — | Baltimore City STD clinic |
| Cost of treatment medication for cervicitis |
Azithromycin 1 g, $15.84 | — | Maryland public health price |
| Inpatient cost for PID treatment |
$8900 | $4277–$13,583 | 29 (HCUP 2003–South region) |
| Outpatient visit cost for PID treatment |
Level 5 initial Office visit, $103 Level 4 F/U Office visit, $70 |
— | Maryland Medicaid rate |
| Cost of treatment medication | Ceftriaxone 250 mg, $20 | — | 58 |
| for PID | Injection admin, $5 | — | Estimate |
| Doxycycline 100 mg BID × 14 d, $3 | — | 59 | |
| Cost for infertility treatment‡ | $5091 | $3915–$13,397 | 29–31 (HCUP 2003–South region) |
| Cost for ectopic pregnancy treatment§ | $6294 | $6294–$7947 | 29,30 (HCUP 2003–South region) |
| Cost for chronic pelvic pain treatment‖ | $8997 | $655–$16,694 | 4,6,24,30,32,33 |
AC2 indicates Aptima Combo 2.
DNA Probe was PACE2.
Estimated delay of 10 years before cost realized.
Estimated delay of 5 years before cost realized.
Estimated delay of 2 years before cost realized.
The cost to treat PID as an outpatient was calculated using the Maryland Medicaid reimbursement rate for a level 5 (40 minute) initial visit and a level 4 (25 minute) follow-up visit.28 The inpatient costs to treat PID29 and the costs associated with PID sequelae4,6,24,29–33 were derived from the literature and the South region of the Health Care Utilization Project. All future costs were discounted at a rate of 3% and cost to charge ratios were used to estimate the costs when only charge data were available.
Analyses
Analyses were conducted using TreeAge Pro 2006 (TreeAge Software, Williamstown, MA) decision analysis software. Incremental cost savings, cases of PID prevented, and incremental cost effectiveness ratios (ICER) were calculated for each screening strategy using DNA probe screening as the comparator strategy. Because the most cost-effective strategy not only prevented more cases of PID but also saved money as compared with the DNA probe screening strategy, ICERs were expressed as negative values. Threshold prevalence was calculated for each strategy to determine at what Chlamydia prevalence the strategy would become the most cost-effective strategy.
Univariate and bivariate sensitivity analyses were conducted for parameters where estimates were uncertain. No published consensus exists on the societal value placed on preventing a case of PID. We, in consensus with other STD experts, decided to use $400 as a conservative estimate of the acceptable cost of preventing a case of PID. Therefore, when univariate sensitivity analyses identified parameter values that increased the cost of the most effective strategy, the parameter value at which the ICER exceeded $400 per case of PID prevented was calculated.
Results
Primary Data
The Chlamydia prevalence in the sampled population of 324 women was 11.1% (95% CI, 7.7%–14.5%). Sixty-six percent of the screened sample were symptomatic with reported genital complaints; however, only 18.8% had abdominal pain. The sensitivities of the vaginal, urine, and endocervical AC2 for detecting Chlamydia in this sample were 97.2%, 91.7%, and 91.7%, respectively. Ninety-four percent of the infected women were successfully treated with antibiotics. Thirty-eight percent of the women were due for a Pap smear on the day of their visit. Forty-six percent of participants preferred vaginal specimen collection, 29% endocervical collection, and 25% urine collection. Ease of vaginal self-collection was reported as “easy” by 80.9%, “OK” by 16.1%, and “hard” by 3.0% of women.
Cost-Effectiveness Analysis
Total costs of each screening strategy, number of cases of PID prevented, cost savings, and ICERs are listed in Table 3. The least expensive and most effective strategy was the self-obtained vaginal AC2 strategy, with programmatic costs savings of $64,000 compared with endocervical DNA probe screening and prevention of 88 additional cases of PID. The other two AC2 strategies prevented fewer cases of PID while costing more money than the self-obtained vaginal AC2 strategy and thus were dominated by the self-obtained vaginal AC2 strategy.
Table 3.
Cost Effectiveness Analysis
| Screening Strategy | Total Cost |
Cases of PID Expected |
Incremental Costs |
Incremental Cases of PID Prevented |
Incremental Cost-Effectiveness Ratio (ICER) |
|---|---|---|---|---|---|
| Endocervical DNA probe* | $636,913 | 115 | — | — | — |
| Endocervical AC2 | $627,204 | 44 | −$9709 | 71 | −$137 |
| First catch urine AC2 | $613,732 | 44 | −$13,472 | 0 | −$13,472† |
| Self obtained vaginal AC2 | $573,205 | 27 | −$40,527 | 17 | −$2384 |
DNA Probe was PACE2 Assay.
Division by 0 results in infinity, therefore, divided −$13,472 by 1.
AC2 indicates Aptima Combo 2.
Prevalence Threshold
The urine AC2 and endocervical AC2 strategies never become the most cost-effective strategies regardless of the Chlamydia prevalence. This is because the cost to process AC2 is the same regardless of specimen used, the cost to obtain a urine or endocervical sample is never less than the cost to obtain a vaginal sample, and the sensitivity of the vaginal AC2 in this study is higher than the sensitivity of either the urine or endocervical AC2. The endocervical DNA probe strategy becomes less expensive than the self-obtained vaginal AC2 strategy when the prevalence is 7.6% or less.
Univariate Sensitivity Analyses
The only variable for which a change in parameter value would result in urine AC2 becoming the most cost saving strategy was a urine AC2 sensitivity greater than the self-obtained vaginal AC2 sensitivity. Similarly, the only variable for which a change in parameter value would result in endocervical AC2 becoming the most cost saving strategy was an endocervical sensitivity greater than 99% or both the urine AC2 and vaginal AC2 sensitivities less than 90%.
One-way sensitivity analyses, using the ranges listed in Table 1, demonstrated that changes in parameter estimates for the following variables were each capable of shifting the most cost-savings strategy from self-obtained vaginal AC2 toward endocervical DNA probe: successful treatment of fewer than 66% of infected women; presumptive treatment of at least 30% of infected women at screening visit; PID sequelae costs less than $1,210; endocervical DNA probe test cost less than $21.60; self-obtained vaginal AC2 test cost more than $49.80; or endocervical DNA probe sensitivity greater than 77.5%.
Given that the self-obtained vaginal AC2 strategy continued to prevent more cases of PID than the endocervical DNA probe strategy even when it costs more, the parameter values of variables at which the ICER for self-obtained vaginal AC2 strategy exceeded $400 were calculated and are presented in Table 4.
Table 4.
Univariate Sensitivity Analyses
| Variable | Alternate Value |
Incremental Cost/Case of PID Prevented (ICER) |
|---|---|---|
| Prevalence | 7.6% | $27 |
| 6.5% | $425 | |
| Proportion treated | 65% | $21 |
| 56% | $406 | |
| Treated day of visit | 30% | $6 |
| 40% | $440 | |
| Cost of PID sequelae | $1210 | $2 |
| $800 | $404 | |
| Cost of endocervical | $21.60 | $5 |
| probe | $18.00 | $410 |
| Cost of self obtained | $49.80 | $1 |
| vaginal AC2 | $53.40 | $406 |
| Sensitivity of endocervical | 77.5% | $5 |
| probe | 80.5% | $415 |
| Sensitivity of self obtained | 88.5% | Break even |
| vaginal AC2 | 85.5% | $415 |
PID indicates pelvic inflammatory disease; ICER, incremental cost effectiveness ratio (compares self-obtained vaginal AC2 with endo-cervical DNA probe); AC2, Aptima Combo 2.
Bivariate Sensitivity Analyses
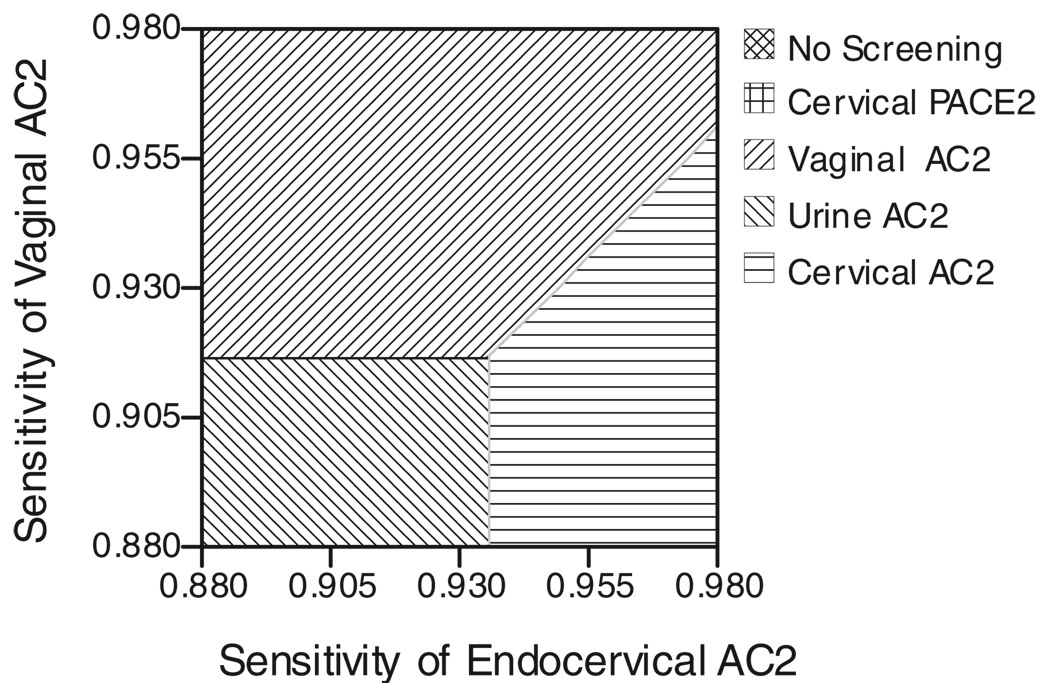
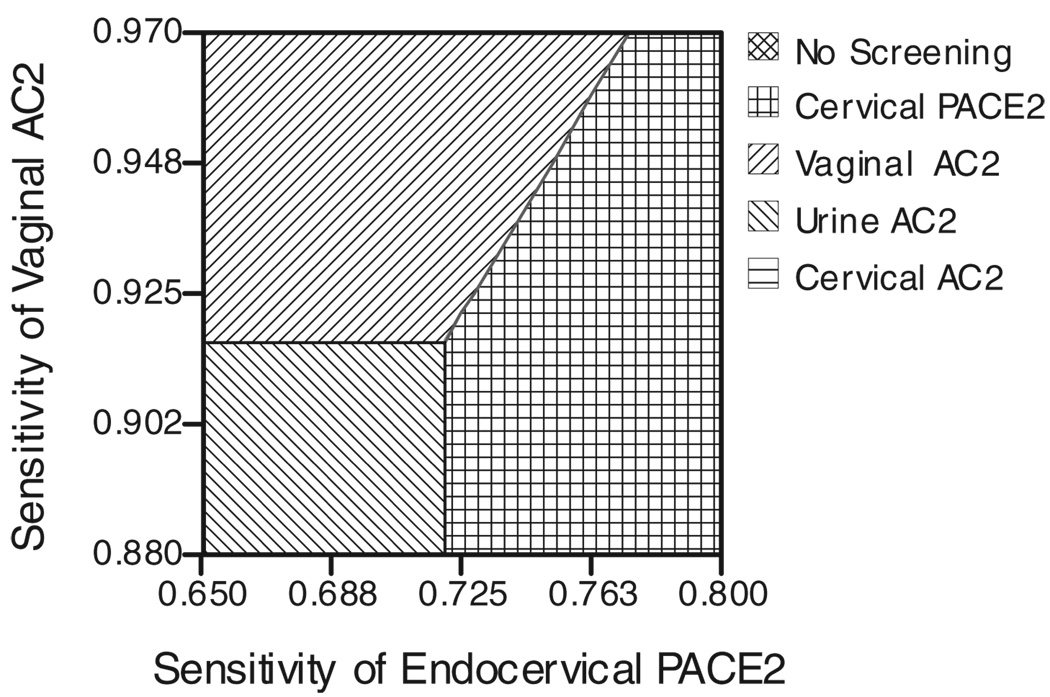
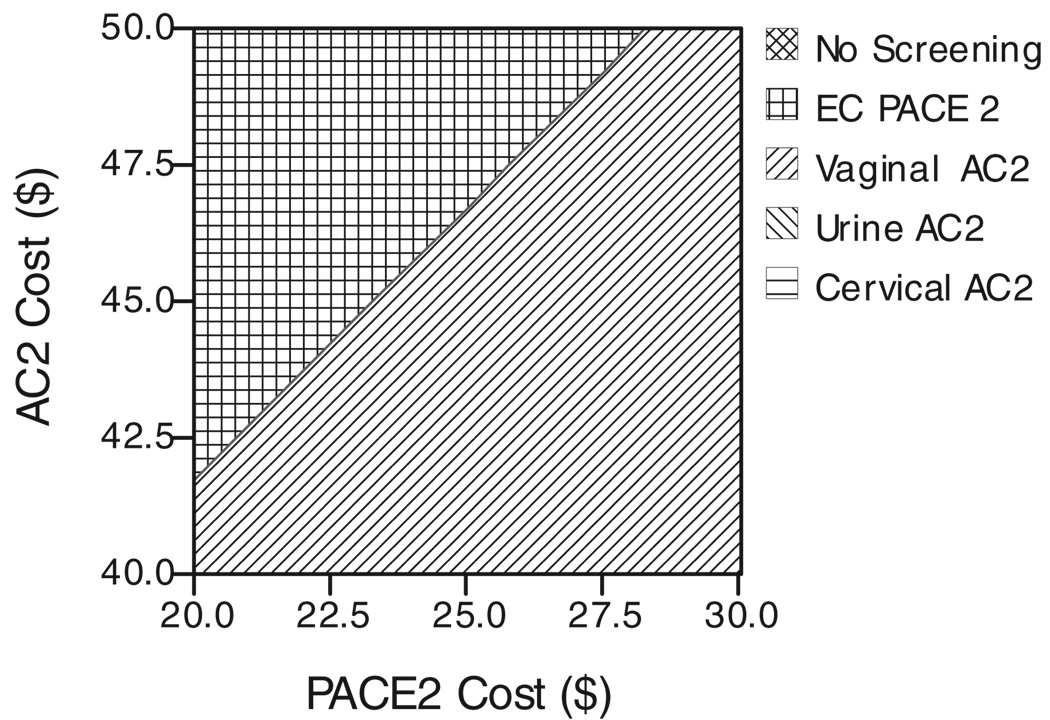
Figure 1 demonstrates the effect of varying both the vaginal AC2 sensitivity and the endocervical AC2 sensitivity. Likewise, Figure 2 demonstrates the effect of varying both the vaginal AC2 sensitivity and the endocervical DNA probe sensitivity. Figure 3 demonstrates the effect of varying both the cost of the AC2 and the cost of the DNA probe.
Fig. 1.
Two-way sensitivity analysis of endocervical AC2 sensitivity versus vaginal AC2 sensitivity. AC2 indicates Aptima Combo 2; PACE2, DNA probe.
Fig. 2.
Two-way sensitivity analysis of endocervical PACE2 sensitivity versus vaginal AC2 sensitivity. PACE2 indicates DNA probe; AC2, Aptima Combo 2.
Fig. 3.
Two-way sensitivity analysis of AC2 cost versus PACE2 cost. AC2 indicates Aptima Combo 2; PACE2, DNA probe; EC, endocervical.
Effect of Reducing Speculum Examinations
If all patients were evaluated with a speculum examination regardless of lack of symptoms and need for Pap smear, then the self-obtained vaginal AC2 strategy saves less money, but is still cost saving. As compared with the endocervical DNA probe strategy, requiring a speculum examination for all patients in the self-obtained vaginal AC2 strategy reduces cost savings from $64,000 to $52,000.
Given that most patients with PID have lower abdominal pain34 and the PID Evaluation and Clinical Health study only enrolled patients who were experiencing pelvic discomfort,35 we looked at the effect of not requiring a speculum examination in patients who do not have abdominal pain. Symptomatic patients who do not have lower abdominal pain can be evaluated for Chlamydia and Gonorrhea with a vaginal or urine AC2 and can be diagnosed with trichomoniasis, vulvovaginal candidiasis, and bacterial vaginosis with a wet mount of a blindly obtained cotton swab vaginal specimen.36 In the sample of 324 women evaluated in this study, 18.8% had abdominal pain. If patients evaluated with the urine AC2 or self-obtained vaginal AC2 strategy only received a speculum examination if they had abdominal pain or needed a Pap smear (50% of sample), then the self-obtained vaginal AC2 strategy savings would rise to $79,000 as compared with the DNA probe comparator strategy.
Discussion
In this population of women attending Baltimore STD clinics, self-obtained vaginal AC2 outperformed the other strategies for detection of Chlamydia infections. As a result, it was more effective than either the urine or endocervical AC2 tests in preventing PID. The additional cost of the AC2 test as compared with the DNA probe test was more than compensated for by the cost savings associated with preventing substantially more cases of PID. Additional savings were realized as a result of the ability to omit the speculum examination in many women tested with self-obtained vaginal AC2. Furthermore, of the 3 sampling techniques experienced by these women, vaginal sampling was preferred by the most women and over 80% reported it was “easy” to perform. Despite a relatively small sample size, the difference in acceptability between vaginal swabs and urine or cervical swabs was statistically significant (P <0.01).
The role of the speculum and bimanual examinations in asymptomatic women, symptomatic women, and STD contacts was evaluated in a recent retrospective chart review of patients attending the same STD clinics where the primary data for this study were collected.37 The investigators found that among symptomatic patients, 5.9% of the syphilis infections and 4.2% of the herpes infections were diagnosed during the speculum examination; however, they did not specify how many of these lesions were internal versus found on the external genitalia, which would require only an external visual inspection.37 Among STD contacts and asymptomatic patients the percentages were lower: 1.7% and 0.6% respectively for syphilis diagnoses and 0.6% and 1.7% respectively for herpes diagnoses.37 Furthermore, the investigators demonstrated that a low percentage of clinically relevant diagnoses (including syphilis, herpes, and PID) would be missed if all patients were screened with self-collected vaginal specimens and serologies for syphilis and herpes simplex virus: 9.3% in symptomatic patients, 3.3% in STD contacts, and 2.3% in asymptomatic patients.37 Whether it is acceptable to miss 9.3% of clinically relevant diagnoses in symptomatic patients is a judgment call. However, the percentage of missed diagnoses would have been less if the symptomatic patients with abdominal pain had received bimanual examinations. It is not possible from the data provided to estimate the exact reduction that this would have led to.37
It is now well accepted that Chlamydia screening among women is cost-effective.1–8 In our cost-effectiveness analysis of 3 AC2 screening strategies we have paid particular attention to the accrual of healthcare system savings when it is possible to omit the speculum examination. Our findings are consistent with those of Shafer et al. and Howell et al. who also concluded that when no other indication exists for a speculum examination, omitting the speculum examination increases the cost-effectiveness of Chlamydia screening.2,38 In Howell’s study, urine specimens were used when there was no indication to perform a speculum examination, but endocervical specimens were used if a speculum examination was already being conducted for other reasons.2 The test used in that study, ligase chain reaction, performed better on endocervical specimens than urine specimens.2 Howell et al. 2 found that the urine test was more cost-effective if there was no other indication to conduct a speculum examination and, not surprisingly, if a speculum examination was already indicated, use of the more sensitive endocervical specimen was more cost-effective. Shafer et al.38 compared use of the urine ligase chain reaction to use of the endocervical ligase chain reaction in asymptomatic adolescents and concluded the same: in asymptomatic females who would not otherwise need a speculum examination, use of the urine specimen was more cost-effective, even though it was slightly less sensitive. In the population we studied, self-obtained vaginal AC2 prevented the most cases of PID, saved the most money, and was preferred by the most women.
Our results may not be generalizable beyond an STD clinic setting. Nevertheless, STD clinics serve women with diverse needs. Some women request only an STD screen and are otherwise healthy. Providing these women with noninvasive screening not only respects the desires of many patients but also saves money from the health care system perspective. Limiting speculum examinations to women who require a Pap smear or present with a gynecologic symptom increases the health care savings of the self-obtained vaginal AC2 screening program by 23%. If however, the speculum examination were limited to women who required a Pap smear or presented with abdominal pain, regardless of genital symptoms, then the health care savings would increase an additional 23% for a total health care savings of 46%.
Other studies have supported the accuracy and acceptability of self-obtained vaginal swabs, when tested by nucleic acid amplification tests, for the detection of Chlamydia infections.39–50 Given rising health care costs and the epidemic of Chlamydia infections in the United States, the provision of an algorithm of using self-obtained vaginal swabs to screen women who do not require a Pap test and who deny having abdominal pain in clinic venues has the potential to be a cost-effective strategy. Vaginal swabs can also be used to diagnose other vaginal and cervical infections such as gonorrhea, trichomonas, yeast infections or bacterial vaginosis. 36,43,44,47 A limitation of this approach is that some clinically important conditions, such as primary syphilitic chancre, HSV lesions, or other medical conditions (i.e., tumor or pregnancy) may be missed. However, many of these other conditions can be diagnosed by alternative means, like serological tests or may be detected during external genital inspection. Future study of this algorithm will provide more information as to the worth of such an approach.
In summary, our results demonstrated that use of self-obtained vaginal swabs was more cost-effective than urine or cervical specimens and patient acceptability of self-obtained vaginal swabs was highest of the 3 specimens evaluated.
Acknowledgments
The authors greatly acknowledge the clinicians of the Baltimore City Health Department, particularly Dr. Emily Erbelding, Treza Salama, and Toni Ingram.
Funding for this study was provided by GenProbe, San Diego, CA, and the HIV Prevention Trials Network (HPTN), sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), National Institute on Drug Abuse (NIDA), National Institute of Mental Health (NIMH), and Office of AIDS Research, of the NIH, DHHS (U01-AI-068613).
Footnotes
Presented at the International Society for Sexually Transmitted Diseases Research meeting, July 10–14, 2005, Amsterdam, The Netherlands.
References
- 1.Mehta SD, Bishai D, Howell MR, et al. Cost-effectiveness of five strategies for Gonorrhea and Chlamydia control among female and male emergency department patients. Sex Transm Dis. 2002;29:83–91. doi: 10.1097/00007435-200202000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Howell MR, Quinn TC, Brathwaite W, et al. Screening women for Chlamydia trachomatis in family planning clinics: The cost-effectiveness of DNA amplification assays. Sex Transm Dis. 1998;25:108–117. doi: 10.1097/00007435-199802000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Paavonen J, Puolakkainen M, Paukku M, et al. Cost-benefit analysis of first-void urine Chlamydia trachomatis screening program. Obstet Gynecol. 1998;92:292–298. doi: 10.1016/s0029-7844(98)00167-7. [DOI] [PubMed] [Google Scholar]
- 4.Marrazzo JM, Celum CL, Hillis SD, et al. Performance and cost-effectiveness of selective screening criteria for Chlamydia trachomatis in women: Implications for a national chlamydia control strategy. Sex Transm Dis. 1997;24:131–141. doi: 10.1097/00007435-199703000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Genc M, Mardh PA. A cost-effectiveness analysis of screening and treatment for Chlamydia trachomatis infection in asymptomatic women. Ann Intern Med. 1996;124(1 pt 1):1–7. doi: 10.7326/0003-4819-124-1_part_1-199601010-00001. [DOI] [PubMed] [Google Scholar]
- 6.Howell MR, Gaydos JC, McKee KT, Jr, et al. Control of Chlamydia trachomatis infections in female army recruits: Cost-effectiveness of screening and treatment in training cohorts to prevent pelvic inflammatory disease. Sex Transm Dis. 1999;26:519–526. doi: 10.1097/00007435-199910000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Howell MR, McKee KT, Jr, Gaydos JC, et al. Point-of-entry screening for C. trachomatis in female army recruits. Who derives the cost savings? Am J Prev Med. 2000;19:160–166. doi: 10.1016/s0749-3797(00)00202-6. [DOI] [PubMed] [Google Scholar]
- 8.Hu D, Hook EW, III, Goldie SJ. Screening for Chlamydia trachomatis in women 15 to 29 years of age: A cost-effectiveness analysis. Ann Intern Med. 2004;141:501–513. doi: 10.7326/0003-4819-141-7-200410050-00006. [DOI] [PubMed] [Google Scholar]
- 9.Mertz KJ, Voigt RA, Hutchins K, et al. Jail STD Prevalence Monitoring Group. Findings from STD screening of adolescents and adults entering corrections facilities: Implications for STD control strategies. Sex Transm Dis. 2002;29:834–839. doi: 10.1097/00007435-200212000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Nsuami M, Cammarata CL, Brooks BN, et al. Chlamydia and Gonorrhea co-occurrence in a high school population. Sex Transm Dis. 2004;31:424–427. doi: 10.1097/01.olq.0000130535.96576.d3. [DOI] [PubMed] [Google Scholar]
- 11.Rogers SM, Miller HG, Miller WC, et al. NAAT-identified and self-reported Gonorrhea and Chlamydial infections: Different at-risk population subgroups? Sex Transm Dis. 2002;29:588–596. doi: 10.1097/00007435-200210000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Kahn R, Moseley K, Thilges J, et al. Community-based screening and treatment for STDs: Results from a mobile clinic initiative. Sex Transm Dis. 2003;30:654–658. doi: 10.1097/01.OLQ.0000083892.66236.7A. [DOI] [PubMed] [Google Scholar]
- 13.Semeniuk H, Zentner A, Read R, et al. Evaluation of sequential testing strategies using non-amplified and amplified methods for detection of Chlamydia trachomatis in endocervical and urine specimens from women. Diagn Microbiol Infect Dis. 2002;42:43–51. doi: 10.1016/s0732-8893(01)00315-7. [DOI] [PubMed] [Google Scholar]
- 14.Wylie JL, Moses S, Babcock R, et al. Comparative evaluation of Chlamydiazyme, PACE 2, and AMP-CT assays for detection of Chlamydia trachomatis in endocervical specimens. J Clin Microbiol. 1998;36:3488–3491. doi: 10.1128/jcm.36.12.3488-3491.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carroll KC, Aldeen WE, Morrison M, et al. Evaluation of the Abbott LCx ligase chain reaction assay for detection of Chlamydia trachomatis and Neisseria gonorrhoeae in urine and genital swab specimens from a sexually transmitted disease clinic population. J Clin Microbiol. 1998;36:1630–1633. doi: 10.1128/jcm.36.6.1630-1633.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lauderdale TL, Landers L, Thorneycroft I, et al. Comparison of the PACE 2 assay, two amplification assays, and Clearview EIA for detection of Chlamydia trachomatis in female endocervical and urine specimens. J Clin Microbiol. 1999;37:2223–2229. doi: 10.1128/jcm.37.7.2223-2229.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Black CM, Marrazzo JM, Johnson RE, et al. Head-to-head multi-center comparison of DNA probe and nucleic acid amplification tests for Chlamydia trachomatis infection in women performed with an improved reference standard. J Clin Microbiol. 2002;40:3757–3763. doi: 10.1128/JCM.40.10.3757-3763.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pollara C, Terlenghi L, De Franscesco MA, et al. Comparative evaluation of BDProbeTec ET, LCx and PACE 2 assays for the detection of Chlamydia trachomatis in urogenital specimens. Eur J Clin Microbiol Infect Dis. 2003;22:512–514. doi: 10.1007/s10096-003-0967-6. [DOI] [PubMed] [Google Scholar]
- 19.Modarress KJ, Cullen AP, Jaffurs WJ, Sr, et al. Detection of Chlamydia trachomatis and Neisseria gonorrhoeae in swab specimens by the Hybrid Capture II and PACE 2 nucleic acid probe tests. Sex Transm Dis. 1999;26:303–308. doi: 10.1097/00007435-199905000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Pasternack R, Vuorinen P, Kuukankorpi A, et al. Detection of Chlamydia trachomatis infections in women by Amplicor PCR: Comparison of diagnostic performance with urine and cervical specimens. J Clin Microbiol. 1996;34:995–998. doi: 10.1128/jcm.34.4.995-998.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paavonen J, Kiviat N, Brunham RC, et al. Prevalence and manifestations of endometritis among women with cervicitis. Am J Obstet Gynecol. 1985;152:280–286. doi: 10.1016/s0002-9378(85)80210-6. [DOI] [PubMed] [Google Scholar]
- 22.Jones RB, Mammel JB, Shepard MK, et al. Recovery of Chlamydia trachomatis from the endometrium of women at risk for chlamydial infection. Am J Obstet Gynecol. 1986;155:35–39. doi: 10.1016/0002-9378(86)90073-6. [DOI] [PubMed] [Google Scholar]
- 23.Stamm WE, Guinan ME, Johnson C, et al. Effect of treatment regimens for Neisseria gonorrhoeae on simultaneous infection with Chlamydia trachomatis. N Engl J Med. 1984;310:545–549. doi: 10.1056/NEJM198403013100901. [DOI] [PubMed] [Google Scholar]
- 24.Haddix AC, Hillis SD, Kassler WJ. The cost effectiveness of azithromycin for Chlamydia trachomatis infections in women. Sex Transm Dis. 1995;22:274–280. doi: 10.1097/00007435-199509000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Westrom L. Incidence, prevalence, and trends of acute pelvic inflammatory disease and its consequences in industrialized countries. Am J Obstet Gynecol. 1980;138:880–892. doi: 10.1016/0002-9378(80)91077-7. [DOI] [PubMed] [Google Scholar]
- 26.Westrom L, Bengtsson LP, Mardh PA. Incidence, trends, and risks of ectopic pregnancy in a population of women. Br Med J (Clin Res Ed) 1981;282:15–18. doi: 10.1136/bmj.282.6257.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Westrom L, Joesoef R, Reynolds G, et al. Pelvic inflammatory disease and fertility. A cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis. 1992;19:185–192. [PubMed] [Google Scholar]
- 28.American Academy of Pediatrics. Elk Grove Village, IL: American Academy of Pediatrics; AAP Medicaid Reimbursement Survey Report. 20042005
- 29.Washington AE, Katz P. Cost of and payment source for pelvic inflammatory disease. Trends and projections, 1983 through 2000. JAMA. 1991;266:2565–2569. [PubMed] [Google Scholar]
- 30.Rein DB, Kassler WJ, Irwin KL, et al. Direct medical cost of pelvic inflammatory disease and its sequelae: Decreasing, but still substantial. Obstet Gynecol. 2000;95:397–402. doi: 10.1016/s0029-7844(99)00551-7. [DOI] [PubMed] [Google Scholar]
- 31.VanderLaan B, Karande V, Krohm C, et al. Cost considerations with infertility therapy: Outcome and cost comparison between health maintenance organization and preferred provider organization care based on physician and facility cost. Hum Reprod. 1998;13:1200–1205. doi: 10.1093/humrep/13.5.1200. [DOI] [PubMed] [Google Scholar]
- 32.van Valkengoed IGM, Postma MJ, Morre SA, et al. Cost effectiveness analysis of a population based screening programme for asymptomatic Chlamydia trachomatis infections in women by means of home obtained urine specimens. Sex Transm Infect. 2001;77:276–282. doi: 10.1136/sti.77.4.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petitta A, Hart SM, Bailey EM. Economic evaluation of three methods of treating urogenital chlamydial infections in the emergency department. Pharmacotherapy. 1999;19:648–654. doi: 10.1592/phco.19.8.648.31534. [DOI] [PubMed] [Google Scholar]
- 34.Blake DR, Fletcher KE, Joshi N, et al. Identification of symptoms that indicate a pelvic examination is necessary to exclude PID in adolescent women. J Pediatr Adolesc Gynecol. 2003;16:25–30. doi: 10.1016/s1083-3188(02)00207-3. [DOI] [PubMed] [Google Scholar]
- 35.Ness RB, Soper DE, Peipert J, et al. Design of the PID evaluation and clinical health (PEACH) study. Control Clin Trials. 1998;19:499–514. doi: 10.1016/s0197-2456(98)00022-1. [DOI] [PubMed] [Google Scholar]
- 36.Blake DR, Duggan A, Quinn TC, et al. Evaluation of vaginal infections in adolescent women: Can it be done without a speculum? Pediatrics. 1998;102:939–944. doi: 10.1542/peds.102.4.939. [DOI] [PubMed] [Google Scholar]
- 37.Singh RH, Erbelding EJ, Zenilman JM, et al. The role of speculum and bimanual examinations when evaluating attendees at a sexually transmitted diseases clinic. Sex Transm Infect. 2007;83:206–210. doi: 10.1136/sti.2006.023309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shafer MA, Pantell RH, Schachter J. Is the routine pelvic examination needed with the advent of urine-based screening for sexually transmitted diseases? Arch Pediatr Adolesc Med. 1999;153:119–125. doi: 10.1001/archpedi.153.2.119. [DOI] [PubMed] [Google Scholar]
- 39.Gaydos CA, Dwyer K, Barnes M, et al. Internet based screening for Chlamydia trachomatis to reach non-clinic populations with mailed self-administered vaginal swabs. Sex Transm Dis. 2006;33:451–457. doi: 10.1097/01.olq.0000200497.14326.fb. [DOI] [PubMed] [Google Scholar]
- 40.Oakeshott P, Hay P, Hay S, et al. Detection of Chlamydia trachomatis infection in early pregnancy using self-administered vaginal swabs and first pass urines: A cross-sectional community-based survey. Br J Gen Pract. 2002;52:830–832. [PMC free article] [PubMed] [Google Scholar]
- 41.Hsieh Y-H, Howell MR, Gaydos JC, et al. Preferency among female army recruits for use of self-administered vaginal swabs or urine to screen for Chlamydia trachomatis genital infections. Sex Transm Dis. 2003;30:769–773. doi: 10.1097/01.OLQ.0000079048.11771.46. [DOI] [PubMed] [Google Scholar]
- 42.Newman SB, Nelson MB, Gaydos CA, et al. Female prisoners’ preference of collection methods for testing for Chlamydia trachomatis and Neisseria gonorrhoeae infection. Sex Transm Dis. 2003;30:306–309. doi: 10.1097/00007435-200304000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Chernesky MA, Hook EW, III, Martin DH, et al. Women find it easy and prefer to collect their own vaginal swabs to diagnose Chlamydia trachomatis or Neisseria gonorrhoeae infections. Sex Transm Dis. 2005;32:729–733. doi: 10.1097/01.olq.0000190057.61633.8d. [DOI] [PubMed] [Google Scholar]
- 44.Holland-Hall CM, Wiesenfeld HC, Murray PJ. Self-collected vaginal swabs for the detection of multiple sexually transmited infections in adolescent girls. J Pediatr Adolesc Gynecol. 2002;15:307–313. doi: 10.1016/s1083-3188(02)00197-3. [DOI] [PubMed] [Google Scholar]
- 45.Richardson E, Sellers JW, Mackinnon S, et al. Prevalence of Chlamydia trachomatis infections and specimen collection preference among women, using self-collected vaginal swabs in community settings. Sex Transm Dis. 2003;30:880–885. doi: 10.1097/01.OLQ.0000091142.68884.2A. [DOI] [PubMed] [Google Scholar]
- 46.Hoebe CJPA, Rademaker CW, Brouwers EE, et al. Acceptability of self-taken vaginal swabs and first-catch urine samples for the diagnosis of urogenital Chlamydia trachomatis and Neisseria gonorrhoeae with an amplified DNA assay in young women attending a public health sexually transmitted disease clinic. Sex Transm Dis. 2006;33:491–495. doi: 10.1097/01.olq.0000204619.87066.28. [DOI] [PubMed] [Google Scholar]
- 47.Wiesenfeld HC, Lowry DLB, Heine RP, et al. Self-collection of vaginal swabs for the detection of Chlamydia, gonorrhea, and trichomonas. Sex Transm Dis. 2001;28:321–325. doi: 10.1097/00007435-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 48.Shafer M-A, Moncada J, Boyer C, et al. Comparing first-void urine specimens, self-collected vaginal swabs, and endocervical specimens to detect Chlamydia trachomatis and Neisseria gonorrhoeae by a nucleic acid amplification test. J Clin Microbiol. 2003;41:4395–4399. doi: 10.1128/JCM.41.9.4395-4399.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schachter J, McCormack Wm, Chernesky MA, et al. Vaginal swabs are appropriate specimens for diagnosis of genital tract infection with Chlamydia trachomatis. J Clin Microbiol. 2003;41:3784–3789. doi: 10.1128/JCM.41.8.3784-3789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schachter J, Chernesky MA, Willis DE, et al. Vaginal swabs are the specimens of choice when screening for Chlamydia trachomatis and Neisseria gonorrhoeae: Results from a multicenter evaluation of APTIMA assays for both infections. Sex Transm Dis. 2005;32:725–728. doi: 10.1097/01.olq.0000190092.59482.96. [DOI] [PubMed] [Google Scholar]
- 51.Gaydos CA, Theodore M, Dalesio N, et al. Comparison of three nucleic acid amplification tests for detection of Chlamydia trachomatis in urine specimens. J Clin Microbiol. 2004;42:3041–3045. doi: 10.1128/JCM.42.7.3041-3045.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gaydos CA, Quinn TC, Willis D, et al. Performance of the APTIMA Combo 2 assay for detection of Chlamydia trachomatis and Neisseria gonorrheae in female urine and endocervical swab specimens. J Clin Microbiol. 2003;41:304–309. doi: 10.1128/JCM.41.1.304-309.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moncada J, Schachter J, Hook EW, III, et al. The effect of urine testing in evaluations of the sensitivity of the Gen-Probe APTIMA Combo 2 assay on endocervical swabs for Chlamydia trachomatis and Neisseria gonorrhoeae. Sex Transm Dis. 2004;31:273–277. doi: 10.1097/01.olq.0000124611.73009.d5. [DOI] [PubMed] [Google Scholar]
- 54.Westrom L, Eschenbach DA. Pelvic inflammatory disease. In: Holmes KK, Sparling PF, Mardh P-A, et al., editors. Sexually Transmitted Diseases. 3rd ed. New York: McGraw-Hill; 1999. pp. 783–809. [Google Scholar]
- 55.Ness RB, Soper DE, Holley RL, et al. Effectiveness of inpatient and outpatient treatment strategies for women with pelvic inflammatory disease: Results from the Pelvic Inflammatory Disease Evaluation and Clinical Health (PEACH) randomized trial. Am J Obstet Gynecol. 2002;186:929–937. doi: 10.1067/mob.2002.121625. [DOI] [PubMed] [Google Scholar]
- 56.Simonsen L, Conn L, Pinner RW, et al. Trends in infectious disease hospitalizations in the United States, 1980–1994. Arch Intern Med. 1998;158:1923–1928. doi: 10.1001/archinte.158.17.1923. [DOI] [PubMed] [Google Scholar]
- 57.Hirsch MB, Mosher WD. Characteristics of infertile women in the United States and their use of infertility services. Fertil Steril. 1987;47:618–625. [PubMed] [Google Scholar]
- 58.Thompson Medical Economics. Drug Topics Redbook Update. 2002;Vo1. 21:10. [Google Scholar]
- 59.The Medical Letter. The choice of antibacterial drugs. Med Lett Drugs Ther. 1999;41:95–104. [PubMed] [Google Scholar]