Introduction
The word nebulizer comes from the Latin ‘nebula’ meaning mist, and was first used in 1872. However, the principles of nebulizer therapy date back over 4000 years. Even Hippocrates supported the use of hot vapours to help the diseases of the throat and chest.1
Nebulizer therapy has altered vastly over the years and cystic fibrosis (CF) caregivers are often bombarded with a large, and often, confusing array of nebulizer systems to choose from.2 The British Thoracic Society and European Respiratory Society formulated nebulizer guidelines in 1994 and 2001, respectively, aiming to give guidance to clinicians prescribing and delivering treatment with nebulizers.3,4
The purpose of this paper is to present practical information regarding the different nebulizer systems available. The aim is to assist the CF clinician to choose the most appropriate nebulizer device for the drug to be nebulized and the individual patient receiving therapy. Where possible information is referenced or taken from the nebulizer device handbooks. However, on occasion, observations are made based on the author's clinical experiences working with adults and children with CF.
Most therapeutic aerosols are heterodisperse, consisting of a range of particle sizes.5 They can be described by the mass median aerodynamic diameter (MMAD) above and below which 50% of the mass of the drug is contained.6
The therapeutic effectiveness of an inhaled drug depends on a number of factors but in particular how much bypasses the oropharynx and deposits in the lungs. There are three main mechanisms of deposition. Inertial impaction occurs in the upper airway and in the first few generations of bronchi where airflow is faster and more turbulent. Impaction also occurs at bends and branches of the airway. Further distally, as the cross‐sectional area of the airways increases, the velocity of airflow decreases and becomes more laminar. In these peripheral airways, particularly if the tidal breath is slow and deep (increasing dwell time), gravitational sedimentation is the main method of deposition. Submicronic particles are more likely to deposit via diffusion providing they are not exhaled.7
Based on industrial hygiene models, particles greater than 10 μm in diameter are demonstrated to deposit in mouth and throat, 5–10 μm deposit in the large conducting airways and oropharanyx, and those between 1–5 μm deposit in the smaller airways and alveoli.8 However, accurately predicting aerosol deposition in real life is challenging, particularly as there are so many patient factors to consider (Table 1).
Table 1.
Factors determining aerosol delivery6
| Aerosol factors | Patient factors |
|---|---|
| Particle size distribution | Adherence with treatments |
| Aerosol density | Tidal volume |
| Hygroscopic properties | Respiratory rate |
| Viscosity and surface tension | Inspiratory flow rate |
| Suspension vs. solution | Breath‐hold time |
| Upper airway anatomy | |
| Lower airway obstruction | |
| Age | |
| Cognitive and physical abilities |
Patient factors
Larger and higher velocity particles have the greatest inertia and the highest probability of impacting on the upper airways. Therefore slower inspirations, larger tidal volumes and lower respiratory rates are recommended to improve lung deposition.6
Worsening lung disease can effect drug deposition. As obstruction increases, aerosol is not distributed evenly and some poorly ventilated areas may not receive any drug.9
Young children have small upper and lower airways, faster respiratory rates and reduced tidal volumes. As such upper airway deposition is high. Most prefer nasal breathing which can filter the aerosol and reduce deposition by half. All of these factors, particularly if the child is crying, dramatically reduce the lung dose. Some authors argue that drug titration for small children is therefore not necessary.10
The patient and parent/carer must have the physical and cognitive ability to use, maintain, clean and disinfect the nebulizer device appropriately.6
Patient adherence with nebulized treatment is probably the most important factor to consider. It is useless having a nebulizer which delivers the desired dose perfectly if it takes too long or is too complex to operate and maintain as the patient is unlikely to use it.
It is also vitally important to regularly watch the CF patient use their nebulizer device to ensure, where possible, they are using an optimum breathing technique and that the nebulizer is suitable for their needs.
Overview of nebulizer systems
Ultrasonic nebulizers
Ultrasonic nebulizers produce aerosol with a rapidly vibrating piezoelectric crystal (1.6–3 MHz). This transfers energy to the liquid medium in an overlying reservoir. It produces particles of 1–6 μm,11 is quiet and is useful for nebulizing large volumes of liquid such as water and saline. It produces a heating effect (1–2°C) which may denature thermally sensitive drugs such as proteins and it may also not be suitable for nebulizing some suspensions.6
Jet nebulizers
Jet nebulizers require a compressed gas source to drive them and create the aerosol. A jet of compressed air is forced through a small hole under pressure and expands rapidly. This causes negative pressure and suction. Fluid is pulled up through a secondary jet and atomized. Liquid droplets hit, or pass around a baffle and are either inhaled or impact on the internal wall of the chamber. Some types of jet nebulizer, such as the Respironics Sidestream® incorporate an open vent. This vent sits in the top of the device and allows the patient to entrain extra air through it which boosts output and pushes more drug particles out of the nebulizer in a given time. The device is continuous and as such more than half of the drug is wasted during exhalation.2 Jet nebulizers are inexpensive, have a fill volume of 2–5 mLs and a residual volume of approximately 0.8 mLs. Eighty percent of the drug output is below 5 μm2 and they are useful for drugs such as bronchodilators, saline and rh‐DNase.
Breath assist open vent jet nebulizers
This is a breath‐enhanced valved system which again utilizes inspiratory flow to increase nebulization rate. However, less drug is lost during expiration as the valve closes. These nebulizers have a greater predicted lung delivery but may have longer treatment times as there is less wastage during expiration.2 They are useful for hypertonic saline, inhaled steroids and nebulized antibiotics (using a filter for the exhaust to prevent environmental contamination). Examples of this device include the PARI LC PLUS®, PARI Sprint® and the Respironics VentStream®.
With all these systems it is important to match the correct nebulizer with the correct flow of driving gas or air from a compressor. Different compressors have varying levels of output. Compressors require regular cleaning and maintenance.
Breath‐actuated jet nebulizers
These only emit aerosol during inhalation and as such cause minimal drug wastage and environmental contamination. Adaptive Aerosol Delivery (AAD® Respironics®) was developed to adapt to the individual patient's breathing pattern by continuously monitoring the pressure changes as the patient is breathing through the mouthpiece. It monitors the preceding three breaths and pulses the aerosol on the fourth breath in the first 50–80% of inspiration. Should the patient adjust their breathing pattern the timing of the pulse is altered to adjust for this change. If the patient stops inhaling, the system stops delivering aerosolized drug. This process continues until a preset dose has been delivered. Marsden et al. compared the Halolite® (first generation AAD® device) to a high output nebulizer (Porta neb®) and found no clinical differences but the Halolite® had higher acceptability and improved true compliance.12
Both the first and second generation AAD devices (Halolite® and Prodose®, respectively) are powered by a portable compressor and cause pneumatic aerosolization. They have been replaced by the I‐neb® (the third generation AAD device) and this will be discussed in detail shortly.
Vibrating mesh nebulizers
The Aerogen Aeroneb® (OnQ technology™) and Pari eFlow® (touch spray technology™) use an annular piezoelectric element circling a domed mesh which causes a to and fro motion acting as a micro pump to create the aerosol. Omron® (MicroAir®) and the I‐neb® (Respironics®) incorporate a piezoelectric element that vibrates a transducer horn, which pulses fluid through a mesh creating an aerosol.6 For the purposes of this paper the practicalities of the Pari eFlow® and Respironics I‐neb AAD system® will be discussed in more detail.
Pari eFlow®
The Pari eFlow® incorporates a touch spray™ metal membrane with 3000 tapered precision‐drilled holes. It vibrates at a frequency of 116 kHz, produces a median particle size of 3–4 μm and is licensed in children over 2 years of age, although many centres are using it successfully in younger children with a mask. The eFlow® operates continuously but due to its ‘holding chamber’ design conserves some drug on expiration. It still requires a filter or exhaust hose for use with antibiotics to prevent environmental contamination.
There are two models of the eFlow®: The eFlow SCF™ is distributed in the USA. It does not incorporate a residual volume (nebulizes to ‘dry’) and therefore drug dose has to be titrated by the prescribing clinician. The eFlow® rapid is distributed in Europe, Israel and Australia. It is designed to have a similar drug delivery, therapeutic safety, efficacy and tolerability to the Pari LC® breath assist jet nebulizer and therefore incorporates a 1.1 mL residual volume.13
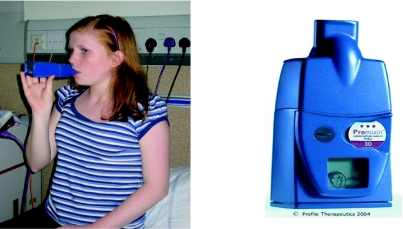
The Pari eFlow® rapid is virtually silent, light-weight and portable. It does not require a compressed air source and can operate by battery or AC power (Figure 1). Inhalation time is much shorter than the Pari LC STAR® as can be seen in the graph in Figure 2.
Figure 1.
PARI eFlow® Rapid
Figure 2.
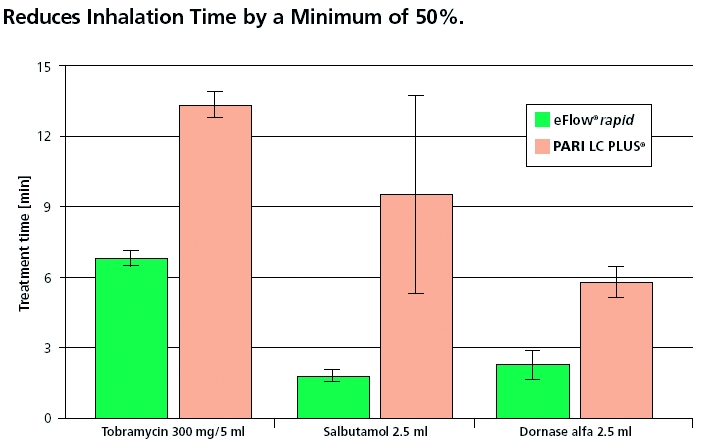
Comparison of inhalation times14
Most drugs can be nebulized through the eFlow® but suspensions should be avoided. In the author's experience some children may complain of medications tasting stronger. It has a fill volume of 2–6 mLs. The eFlow® Rapid currently costs £495 (including two handsets, rechargeable batteries, battery charger and mains adaptor). This is obviously a consideration for the healthcare provider and patient/carer. Currently some companies providing nebulized medications may provide an eFlow® device to accompany new prescriptions. Alternatively charity funding may need to be sought. There are ongoing costs to be recognized: the mesh needs to be replaced every six months at a cost of £38. The handset, including mesh, costs £55 and should be replaced yearly (2008 PARI® price list).
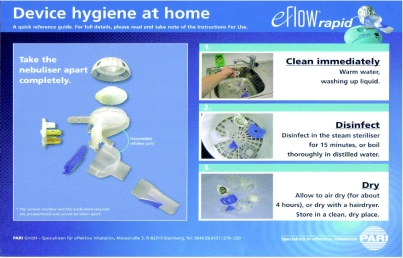
Obviously, cleaning the device appropriately is vitally important (to decontaminate, maximize mesh efficiency and prevent the mesh holes blocking if the patient lives in a ‘hard water area’) and should be done after each use. The method of cleaning and disinfecting is shown in Figure 3. It also includes using the ‘easy care kit’ which is a saline washing device used once a week between the cleaning and disinfecting stages. Disinfection can occur by boiling the device in distilled, de‐mineralized or de‐ionized water or by using a steam sterilizer.
Figure 3.
Cleaning the eFlow® courtesy of PARI®
Patient adherence is reported to be greater using the eFlow® than the PARI LC STAR® and continues over a six‐month period. However, this data is based on self‐reported levels of adherence and therefore must be interpreted with caution.15
Table 2 shows a summary of the pros and cons of the eFlow® Rapid as already discussed.
Table 2.
Summary of the pros and cons of the eFlow® Rapid
| Pros | Cons |
|---|---|
| Fast nebulization: TOBI ® 6–8 mins, Colomycin ® 3–4 mins, hypertonic saline and rh‐DNase 2–3 mins | £495 plus at least £93 replacement parts annually |
| More durable than I‐neb® | Not ‘breath‐activated’ |
| Most drugs (2–6 mLs) can be used (avoid suspensions) | As continuous, some drug is wasted in expiration (have to filter exhaust) |
| Can be used with all ages (mask or mouthpiece) | Cleaning more time‐consuming |
| Virtually silent, lightweight, portable | Holes in mesh can block, increasing treatment time |
| Battery or multivolt power | Poor breathing technique can increase treatment time |
| Slower than the I‐neb ® |
I‐neb® AAD system®
The I‐neb® is the third generation adaptive aerosol delivery system but unlike the Halolite® and Prodose®, which it has superseded, does not require a compressor (Figure 4). The I‐neb® uses Omron® mesh technology.6 It incorporates a piezoelectric element that vibrates a transducer horn which pulses fluid through a mesh consisting of thousands of tapered holes. Liquid in contact with the mesh is forced through the holes by the vibration and droplets of aerosol are formed. As the patient breathes on the mouthpiece, air enters through the air inlet and flap valve, and mixes with the aerosol generated from the mesh.6
Figure 4.
I‐neb AAD System®
Work by Potter, comparing the I‐neb® to the Prodose®, showed them to be very similar in output and particle size and found the antibiotic was not degraded.16
The I‐neb® only operates with a microchip/disc which is programmed to the drug Promixin® (Colistimethate sodium, marketed by Profile Pharma®). This drug is usually prescribed by the patient's general practitioner and is more expensive than Colistin (Colomycin® marketed by Forest®).17
Respironics® deal with the supply, servicing and teaching of the device and send out replacement parts as required, at no additional cost. Some may argue that this level of service goes some way to balancing out the extra cost of Promixin® but there is no health economic data published to date to demonstrate this.
As with other AAD devices it only releases the aerosol when inhalation is detected and therefore allows interruption of treatment and does not require antibiotics to be filtered. The device alerts the patient when inhalation is complete. It is portable and virtually silent. It has a liquid crystal display and inhalation history can be recorded to monitor adherence. It is compatible with the ‘In‐sight’ computer programme and the patient can be coached to improve their breathing technique to make delivery as efficient as possible.
The patient must hold the device horizontally and achieve a good seal around the mouthpiece. It is therefore unsuitable for children under 2 years of age or those who cannot master mouth breathing. Promixin® can be delivered rapidly (approximately 1–2 minutes)18 but longer treatment times may arise from poor breathing technique or mesh holes becoming blocked. The device runs from a rechargeable battery (approximately 40 nebulizations per full charge).
Promixin®, salbutamol and rh‐DNase can be delivered through the I‐neb® using the 1 mL chamber (0.3 mLs aerosolized to the lungs, 0.1 mLs sealed into the chamber and not nebulized, 0.6 mLs left as residual volume). The box of Promixin® usually has extra discs to make nebulizing these other drugs possible. TOBI® (300 mg/5 mLs) and hypertonic saline (7%/4 mLs) can also be taken through the I‐neb® (separate discs required) but must be delivered in a larger lilac chamber and nebulized twice, approximately 6–8 minutes per fill. (0.5 mL aerosolized to the lungs, 0.1 mL sealed into the chamber and not nebulized and the rest left over as residual volume. Therefore when this is done twice the lung dose of 1 mL is equivalentto the dose delivered in the PARI LC PLUS®).19 Tables 3 and 4 show dosing for Promixin®, TOBI® and hypertonic saline.20
Table 3.
Dosing of Promixin® – adapted from the I‐neb® Clinician's Guide20
| Colistin dose | Conventional nebulizer | I‐neb® Promixin® |
|---|---|---|
| 2 MU | 2 MU | 1 MU mix with 1 mL water for injection |
| 1 MU | 1 MU | ½ MU – mix 1 MU vial with 2 mL water for injection and draw out 1 mL to use in I‐neb® |
Table 4.
Dosing of TOBI® and Hypertonic Saline 7%20
| Device | Drug | I‐neb Chamber | Fill volume | Nebulizations (n) |
|---|---|---|---|---|
| Conventional Nebulizer | TOBI® 300 mg/5 mL | N/A | 5 mL | 1 |
| I‐neb AAD | TOBI® 300 mg/5 ml | 0.5 mL (Lilac latched) | 2.5 mL | 2 |
| Conventional Nebulizer | Hypertonic Saline 7%/4 mL | N/A | 4 mL | 1 |
| I‐neb AAD | Hypertonic Saline 7%/4 mL | 0.5 mL (Lilac latched) | 2 mL | 2 |
The I‐neb® has two breathing modes: Tidal Breathing Mode (TBM) and Target Inhalation Mode (TIM) which can each be activated by selecting the appropriate mouthpiece:
TBM – Like the Prodose® and Halolite® this mode uses AAD® as described above;20
TIM – The AAD system analyses the pressure changes relating to airflow and starts delivering the drug during inhalation on the first breath. During inhalation the patient is guided via vibratory feedback to perform slow and deep inhalations up to 8 seconds, pulsing the aerosol for up to 7 seconds and allowing 1 second for deposition in the lungs. A high‐resistance mouthpiece is used to guide the patient to complete these slow, deep inhalations with a maximum inspiratory flow of 20 litres. If the patient cannot extend inhalation to 8 seconds the AAD gradually shortens the target time to match the patients preferred length of inhalation. TIM may increase lung deposition and decrease treatment times but is not suitable for patients with an FEV1 less than 1 litre, or young children.20
Cleaning of the device is vitally important and care must be taken with the delicate mesh (which may not be suitable for all patients/families). Occasionally, if the patient lives in a ‘hard water area’ the holes of the mesh may become blocked which may increase nebulization time (Figure 5).
Figure 5.
Cleaning the I‐neb adapted from Respironics®
Table 5 shows a summary of the pros and cons of the I‐neb® as already discussed.
Table 5.
Summary of the pros and cons of the I‐neb®
| Pros | Cons |
|---|---|
| Fast nebulization: Promixin® (colistin) and rh‐DNase 1–2 mins | Can only be used if can inhale through mouthpiece (>2 years) |
| Virtually silent Lightweight/portable | Only available if on Promixin® |
| Battery or multivolt power | Promixin ® – (generally prescribed by GP) more expensive than colomycin ® (even though ½ strength required, i.e. 2 mu Colomycin ® = 1 mu Promixin ®) |
| Breath‐activated (inhalation only) AAD® | Cleaning time consuming, components delicate |
| No filtering of antibiotics required | Holes in mesh can block increasing treatment time |
| Two breathing modes TBM and TIM | Poor breathing technique can increase treatment time |
| TIM can speed delivery and improve lung deposition (as long as FEV1>1L) | Can only nebulize Promixin®, rh‐DNase, Salbutamol, TOBI ® and hypertonic saline |
| Device, maintenance and replacement parts free | TOBI® and hypertonic saline must be nebulized twice in larger lilac chamber to give 1 mL dose to lung and be equivalent to the PARI LC PLUS ® – longer Rx time |
| 1 MU Colistin in I‐neb ® delivers equivalent of 2 mu via conventional nebulizer | |
| Can download usage data to review compliance and trouble shoot if nebulization time increasing | Some medications may taste stronger |
Conclusion
This brief overview has attempted to provide the CF clinician with the necessary practical information to assist them in choosing the most appropriate nebulizer device to suit their patient. Greater detail has been focused on the newer mesh nebulizers (I‐neb® and eFlow®) as these are being more frequently used at this current time.
Factors which must be taken into account when choosing an appropriate nebulizer include: the age of the patient, the patient's lifestyle, adherence and motivation to treatment, as well as their cognitive and physical ability and that of their carers. The patient's breathing pattern should be considered as well as the nebulized drug characteristics, nebulizer output and cost and durability of the nebulizer device. This choice should be individual to the patient, and discussed with them where possible as each device has different pros and cons, and each patient has different needs. Regular evaluation to ensure the patient continues to have the most appropriate device and that they are using it effectively is essential.
Footnotes
DECLARATIONS —
Competing interests None declared
Funding None
Ethical approval Not applicable
Guarantor NC
Contributorship NC is the sole contributor
Acknowledgements
Pari® and Respironics® for the pictures, tables and graph presented in this paper
References
- 1.Muers M. Overview of nebuliser treatment. Thorax 1997;25 Suppl. 2:S255–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Callaghan C, Barry P. The science of nebulised drug delivery. Thorax 1997;25 Suppl. 2:S31–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Summary of guidelines. Thorax 1997;25 Suppl. 2:S25–35 [Google Scholar]
- 4.European Respiratory Society Guidelines on the use of nebulisers. Eur Respir J 2001;18:228–42 [DOI] [PubMed] [Google Scholar]
- 5.Mercer T. Production and characterisation of aerosols. Arch Intern Med 1973;131:39–50 [PubMed] [Google Scholar]
- 6.Geller D. The science of aerosol delivery in cystic fibrosis. Pediatric Pulmonology 2008;43 Suppl. 9:S5–S17 [Google Scholar]
- 7.Zanen P, Laube B. Targeting the lungs with therapeutic aerosols. In: Bisgaard H, O'Callaghan C, Smalldone G, eds. Drug Delivery to the Lung. New York, NY: Marcel Dekker, Inc.; 2002. p. 211–68 [Google Scholar]
- 8.Swift D, Asgharian B, Kimbell J. Use of mathematical aerosol deposition models in predicting the distribution of inhaled therapeutic aerosols. In: Hickey A, eds. Inhaled Aerosols: Physical and Biological Basis for Therapy. 2nd edn New York, NY: Marcel Dekker, Inc.; 2006 [Google Scholar]
- 9.Coates A, Allen P, MacNeish C, Ho S, Lands L. Effect of size and disease on estimated deposition of drugs administered using jet nebulization in children with CF. Chest 2001;119:1123–30 [DOI] [PubMed] [Google Scholar]
- 10.Barry P, O'Callaghan C. Nebuliser therapy in childhood. Thorax 1997;25 Suppl. 2:S785–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hambleton G, Hung J, Super M. Nebulisers in cystic fibrosis. J R Soc Med 1996;89:14–18 [PMC free article] [PubMed] [Google Scholar]
- 12.Marsden R, Conway S, Dodd M, et al. A multi‐centre, randomised study comparing the Halolite TM Adaptive Aerosol Delivery (AAD TM) system with a high output nebuliser system in patients with Cystic Fibrosis. European Cystic Fibrosis Conference; Genoa, Italy, 2002 [Google Scholar]
- 13.Seemann S, Schmitt A, Waldner R, Hug M, Knoch M. Improving aerosol drug delivery in CF therapy. 28th European Cystic Fibrosis Conference; Crete, Greece, 2005 [Google Scholar]
- 14.Keller M, Hug M, Bitterle E, Bucholski A. Reduced treatment time for colistimethate sodium solutions (colistin CF) aerosolised by eFlow® rapid, a novel electronic nebuliser. 29th European Cystic Fibrosis Conference; Copenhagen, Denmark, 2006 [Google Scholar]
- 15.Reychler G, Lebeque P, Knoop C, Opdekamp Ch. Satisfaction survey concerning vibrating mesh‐nebulizers in CF patients. European Cystic Fibrosis Conference; Belek, Antalya‐Turkey, 2007 [Google Scholar]
- 16.Potter R. Output characteristics of a Prototype I‐neb™ AAD® system. Drug delivery to the lungs 15. London: 2004. December 9–10 [Google Scholar]
- 17.BNF for Children See http://www.bnfc.org
- 18.Langman H, Orr A, McVean R, et al. Delivering a concentrated dose of colistin using AAD® reduces treatment time. Journal of Cystic Fibrosis 2004;3:S57 [Google Scholar]
- 19.Hardaker L, Potter R, Akunda E. Delivery of tobramycin via the I‐neb™ Adaptive Aerosol Delivery (AAD®) System and the Pari LC Plus® nebulizer. 29th European Cystic Fibrosis Conference; Copenhagen, Denmark, 2006 [Google Scholar]
- 20.Respironics® Clinician's Guide I‐neb adaptive aerosol delivery (AAD) system for target inhalation mode (TIM) and tidal breathing mode (TBM). Respironics®