Introduction
Allergic bronchopulmonary aspergillosis (ABPA) is a recognized disorder in patients with cystic fibrosis. It is characterized clinically by wheeze, productive cough and pulmonary infiltrates associated with a decline in lung function. The diagnosis is classically made using a combination of clinical, immunological and microbiological criteria. The most commonly used treatment is at present corticosteroids, with their attendant side-effects. The case described illustrates some of the pitfalls in making the diagnosis and outlines the use of two potential emerging therapies in the form of intravenous Methylprednisolone and the recombinant anti-IgE monoclonal antibody Omalizumab.
Case description
An 11-year-old boy with cystic fibrosis (CF) presented with acute dyspnoea. CF was diagnosed shortly after birth following presentation with meconium ileus. His genotype was delta508: delta508. He had had two previous isolations of Pseudomonas aeruginosa, the last being at the age of 4 years. His height was 155 cm (91st centile) and his weight was 45 kg (91st centile). His forced expiratory volume per second (FEV1) was 2.8 litres (98% predicted) and his forced vital capacity (FVC) was 3.2 litres (98% predicted) one month prior to his presentation.
He had a two-day prodromal coryzal illness and presented with a dry cough.
On admission he was hypoxic, tachypnoeic and severely dyspnoeic. He was unable to perform spirometry. His oxygen saturation was 88% while breathing room air and his respiratory rate was 30 breaths per minute. He had bilateral crepitations audible on examination.
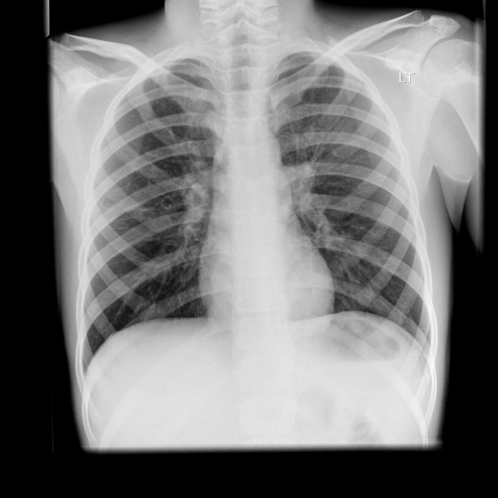
The admission chest radiograph is shown in Figure 1. No organisms were isolated from the initial sputum samples taken. The admitting diagnosis was of an infective exacerbation of his cystic fibrosis. He was treated with intravenous Cefuroxime, physiotherapy and supplementary oxygen.
Figure 1.
Chest radiograph on admission
Wheeze developed 48 hours after admission and he was commenced empirically on oral Prednisolone 40 mgs once daily. Despite this he deteriorated, needing a period of continuous BIPAP ventilation for 4 days, with the maximum pCO2 being 7.02 kPa (N 4.5–6 kPa). His antibiotics were changed to Ceftazidime and Tobramycin with additional oral Azithromycin to cover Pseudomonal and atypical infection.
After seven days, Prednisolone was stopped as the total IgE from the day of admission, was only 179 kU/l (N <70 kU/l). Further investigations were undertaken looking for evidence of atypical infection and immunological causes. These are shown in Table 1.
Table 1.
Evidence of atypical infection and immunological causes
| Immunoglobulins |
| IgG 7.54 g/L (N 3.8–15.2 g/L) |
| IgA 2.62 g/L (N 0.64–2.58 g/L) |
| IgM 0.89 g/L (N 0.43–1.9 g/L) |
| IgE 9178 kU/L (N <70 kU/L) |
| Specific RAST to Aspergillus 16.7 kU/L Grade III response (N <0.35 kU/L) |
| Eosinophil count 1.95 ×109/l (N 0.04–0.4 ×109/L) |
| Complement function |
| C3 2.03 g/L (0.68–1.8 g/L) |
| C4 0.28 g/l (0.18–0.6 g/L) |
| CH100 – Normal |
| Alternate pathway – Normal |
| pANCA/cANCA – Negative |
| ASOT <200 units/ml (N <200 units/mL) |
| Neutrophil oxidative burst – Normal pattern |
| Nitroblue Tetrazolium test – <10 % unstimulated, >90% stimulated |
| Serology |
| Influenza A 1:32 |
| Influenza B 1:64 |
| Coxiella burnetti P2 <1:32 |
| RSV 1:64 |
| Mycoplasma pneumoniae <1:32 |
| Adenovirus <1:32 |
| Psitticosis/LGV <1:32 |
| PCP culture – Negative |
| AFB culture – Negative |
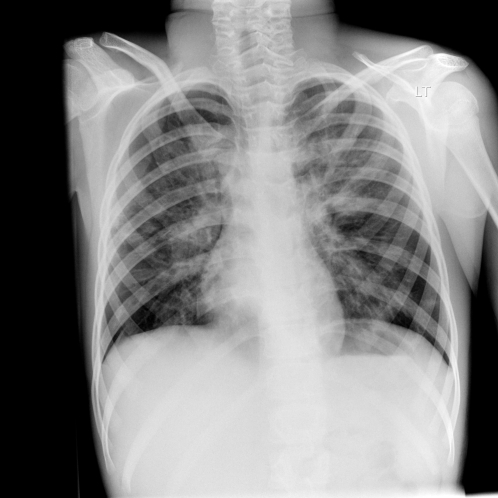
A chest X-ray taken on the 14th day of admission (
Figure 2.
Chest X-ray on 14th day of admission
Figure 2) revealed extensive peribronchial thickening and bilateral infiltrates. A repeat IgE was taken on day 15. This was now 9178 kU/L (N < 70 kU/L) with specific aspergillus RAST 16.7 kU/L (N <0.35 kU/L) and a peripheral blood eosinophillia of 1.95 × 109/L (N 0.04–0.4 ×109/L). The combination of acute clinical deterioration, increased total IgE, the presence of Aspergillus specific IgE and progressive chest radiological changes was highly suggestive of allergic bronchopulmonary aspergillosis. None of the other listed investigations provided a positive diagnosis.
In view of the severity of his illness and his failure to respond to oral Prednisolone in the early phase of the illness he was treated with IV Methylprednisolone. Initially at a dose of 20 mg/kg for three days before being halved for a further three days. This was followed by a maintenance dose of 40 mg of oral Prednisolone daily.
He improved, with a reduction in the severity of his cough and resolution of his dyspnoea at rest. IgE peaked five days after starting Methylprednisolone at 12,673 kU/L and was 3650 kU/L a week postdischarge. He was discharged approximately two weeks after re-starting steroid treatment on overnight home oxygen. Chest radiograph changes resolved after six weeks. Home oxygen was discontinued completely within a month of his discharge. Lung function took longer to return to previous levels, with an FEV1 of 1.7 litres at 8 weeks post admission. A further month later it had improved to 2.2 L compared to premorbid 2.8 L.
Following an episode of shingles three months after this initial presentation attempts were made to reduce the dose of steroids but this led to a return of symptoms. As a result Voriconazole was added as an oral antifungal agent. This allowed weaning of the steroid dose to a lowest point of 10 mg of Prednisolone on alternate days, six months after his symptoms first began. The Voriconazole was stopped after the development of a severe blistering rash four months after it was initiated. Nebulized Amphotericin was tried as an alternative but the patient was unable to tolerate this. Oral Itraconazole was commenced instead and therapeutic serum levels were achieved.
Approximately 10 months after his initial illness, he developed symptoms suggestive of a reactivation of his ABPA. His IgE rose to 1975 kU/L (from a previous level of 1125 kU/L) and he had a reduced exercise tolerance and productive cough. His FEV1 fell to 2.0 L from 2.3 L. He had failed to respond to a treatment course of intravenous antibiotics. Prednisolone was increased to 40 mg daily in order to prevent further deterioration.
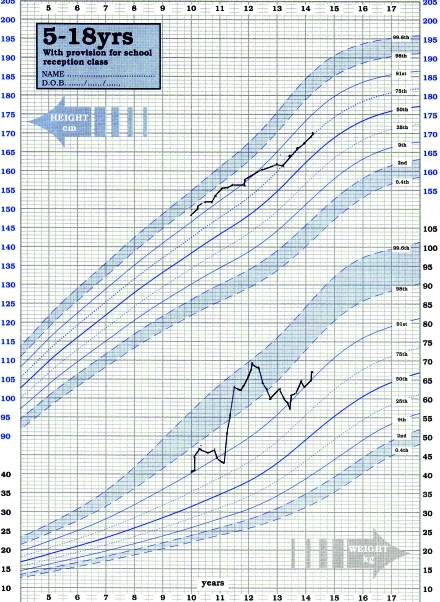
By this stage he had developed side‐effects of long-term steroid treatment: impaired glucose tolerance; a distressing Cushingoid appearance; and significant weight gain (Figure 3).
Figure 3.
Graph showing the patient’s weight gain
In view of these effects a therapeutic trial of the anti-IgE monoclonal antibody Omalizumab was embarked on. It was given at a dose of 375 mg fortnightly as a subcutaneous injection for a 16-week period. Within two weeks of starting, his lung function improved to 2.8 L and his symptoms resolved. His steroids were weaned to 10 mg alternate days without recurrence of symptoms.
Twelve months after his initial course a second course was given due to a rise in IgE and development of symptoms, before any deterioration in lung function took place. This was successful and avoided an increase in steroid dosage. He has had no clinically obvious side-effects from either treatment course thus far. His weight fell back to his previous centile, his facial appearance improved and his blood glucose profile normalized.
He is currently well with lung function just below the mean for his height.
Discussion
Allergic bronchopulmonary aspergillosis was first described as a potential complication of cystic fibrosis in 1965.1 The prevalence of ABPA has been noted to vary considerably between different centres. In a study based on the Epidemiologic Registry of Cystic Fibrosis, it was reported to be between 2.1 % and 13.6 % across Europe.2 Factors associated with increased prevalence of ABPA and CF include age, atopy, severity of lung disease and colonization with Pseudomonas.3
Diagnostically, ABPA presents difficulties because of significant overlap in symptoms and presentation compared with other clinical exacerbations of cystic fibrosis. This is compounded by a wide variability in diagnostic criteria and treatment used in different centres. This is illustrated by the results of a retrospective descriptive postal questionnaire survey of the 58 specialist centres in the UK.4
Minimal diagnostic criteria for ABPA based on consensus conference recommendations3 are outlined in Table 2.
Table 2.
Minimal diagnostic criteria for ABPA based on consensus conference recommendations
| 1. Acute or subacute clinical deterioration (cough, wheeze, exercise intolerance, exercise-induced asthma, change in pulmonary function or increased sputum production) not attributable to another etiology |
| 2. Total serum IgE concentration of >500 IU/mL (1200 ng/mL). If ABPA is suspected and the total IgE level is 200–500 IU/mL, repeat testing in 1–3 months is recommended. If patient is taking steroids, repeat when steroid treatment is discontinued |
| 3. Immediate cutaneous reactivity to Aspergillus (prick skin test wheal of >3 mm in diameter with surrounding erythema, while the patient is not being treated with systemic antihistamines) or in vitro demonstration of IgE antibody to A. fumigatus |
4. One of the following:
|
Our case highlights some of the difficulties in this area. He fulfilled all the diagnostic criteria outlined above, however these were only met by day 15 of the admission, by which point he had deteriorated to the point of dependence on non-invasive ventilation. Furthermore, an initial steroid treatment course at a dose equivalent to 1 mg/kg of Prednisolone had failed to prevent his deterioration. Aside from an acceptance that clinical symptoms may predate changes in total IgE and specific IgE antibody to Aspergillus there appears to be a paucity of evidence for the quantification of any delay, although this potentially has very significant clinical implications.
One striking feature of our case was the severity of his disease. A small number of comparable cases requiring non-invasive ventilation and intensive care support are reported in the literature.5,6 The original case reports1 of what was later defined as ABPA in CF describe spontaneous resolution and recovery of lung function. Our case also regained his lung function, albeit after a prolonged period of treatment and convalescence. However, there is evidence suggestive that over time ABPA is associated with decreasing lung function over and above that from CF alone,7 highlighting the need for timely diagnosis and treatment.
The immunopathogenesis of ABPA in CF is slowly being elucidated. It is associated with an antigen-specific, Th2-like T–cell immune response.8 At present, systemic oral corticosteroids are recognized as the preferable first-line therapy. They are thought to work by inhibition of phospholipase A2 activity, arachadonic acid metabolism, chemotaxis, cell adhesion, tissue infiltration of inflammatory cells, and production of IL-1 and TNF.3 Their use leading to a reduction in serum IgE and eosinophilia, clearing of pulmonary infiltrates and reduction in bronchospasm.3
Difficulties with both the overlap between ABPA and other clinical entities in CF and lack of universally applied diagnostic criteria have meant that evidence for different treatment regimes is based on small numbers of patients and may not be directly comparable. As a result, data on the treatment of relapsing patients and those suffering significant side‐effects are even rarer.
Methylprednisolone has been found to be a successful treatment in a range of different autoimmune diseases. To our knowledge, there are only two reports of its usage in CF in the literature.9,10 The first was a series of four patients treated in New Zealand with between 15 and 20 mg/kg of intravenous methylprednisolone,9 the second a group of nine patients treated with 10 mg/kg compared to a group of five controls treated with oral corticosteroids in Israel.10 Both groups show evidence of improvement in both lung function, decline in IgE levels and improvement in symptoms.
Furthermore, both groups report an overall reduction in steroid‐related side-effects but report flushing, myalgia and malaise as direct side-effects of the pulse, sufficient to lead to discontinuation of the treatment of one patient in the New Zealand group. Our patient developed transient hypertension associated with the first dose of Methylprednisolone, but this resolved without treatment. A similar reaction was also observed in one of the New Zealand group. Neither group refers to Methylprednisolone usage in the setting of acute life-threatening ABPA, which limits the comparability with our case. However, the possibility of reduction of steroid-related side-effects is worthy of further attention given the significant vulnerability of CF patients to problems with glucose metabolism and bone mineralization.
Omalizumab is a recombinant humanized monoclonal antibody. It was designed to utilize the high affinity receptor for IgE and as a result it binds circulating free IgE without affecting cell bound IgE. It has been shown to be effective in treatment of allergic asthma, improving asthma symptoms scores, airway inflammation and reducing need for corticosteroid treatment.11–13 Its usage in cystic fibrosis has been limited to small number of case reports within the literature. Our experience of improved lung function, better symptom control and, most importantly, successful weaning of steroid dosage fit with those other reported cases, further highlighting the potential of anti-IgE agents as an adjunctive in steroid‐dependent cases of ABPA. In addition, our case did not develop any clinically significant side-effects during either course of treatment. We look forward to future randomized control trials, which will determine its true efficacy.
In summary we present a case of life-threatening allergic bronchopulmonary aspergillosis in a previously well child with cystic fibrosis. It highlights the need to remain vigilant for confirmatory evidence of the condition although this may only be obvious as the illness evolves. Furthermore, it illustrates the usage of alternative strategies for management, which may prevent the accrual of steroid-related side-effects in those steroid-dependent patients.
Footnotes
DECLARATIONS —
Competing interests None declared
Funding None
Ethical approval Not applicable
Guarantors MFT
Contributorship MFT is the sole contributor
Acknowledgements
The author acknowledges the child and his family for their permission to publish this case
References
- 1.Mearns M, Young W, Baten J. Transient pulmonary infiltrations in cystic fibrosis due to allergic aspergillosis. Thorax 1965;20:385–92 [Google Scholar]
- 2.Mastella G, Rainisio M, Harms HK, et al. Allergic bronchopulmonary aspergillosis in cystic fibrosis. A European epidemiological study. Epidemiologic Registry of Cystic Fibrosis. Eur Respir J 2000;16:464–71 [DOI] [PubMed] [Google Scholar]
- 3.Stevens DA, Moss RB, Kurup VP, et al. Allergic bronchopulmonary aspergillosis – state of the art: Cystic Fibrosis Foundation Consensus Conference. Clin Infect Dis 2003;37Suppl.:225–64 [DOI] [PubMed] [Google Scholar]
- 4.Cunningham S, Madge SL, Dinwiddie R. Survey of criteria used to diagnose allergic bronchopulmonary aspergillosis in cystic fibrosis. Arch Dis Child 2001;84:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skowronski E, Fitzgerald D. Life-threatening allergic bronchopulmonary aspergillosis in a well child with cystic fibrosis. Med J Aus 2005;182:482–4 [DOI] [PubMed] [Google Scholar]
- 6.Brynes CA, Jaksic M, Tate JC. 21% prevalence of ABPA in a regional paediatric cystic fibrosis clinic – how high is too high?. J Cystic Fibrosis 2008;7:S58 [Google Scholar]
- 7.Kraemer R, Delosea N, Ballinari P, Gallati S, Crameri R. Effect of allergic bronchopulmonary aspergillosis on lung function in children with cystic fibrosis. Am J Respir Crit Care Med 2000;175:967. [DOI] [PubMed] [Google Scholar]
- 8.Skov M, Poulsen LK, Koch C. Increased antigen-specific Th-2 response in allergic bronchopulmonary aspergillosis in patients with cystic fibrosis. Paediatr Pulmonol 1999;27:74–9 [DOI] [PubMed] [Google Scholar]
- 9.Thomson JM, Wesley A, Brynes C, Nixon G. Pulse intravenous methylprednisolone for resistant allergic bronchopulmonary aspergillosis in cystic fibrosis. Paediatr Pulmonol 2006;41:164–70 [DOI] [PubMed] [Google Scholar]
- 10.Cohen‐Cymberknoh M, Blau H, Shoseyov D, Efrati O, Armoni S, Kerem E. Intravenous methylprednisolone for allergic bronchopulmonary aspergillosis in CF. J Cystic Fibrosis 2008;7:S58. [DOI] [PubMed] [Google Scholar]
- 11.Milgrom H, Flick RB, Su JQ, et al. Treatment of allergic asthma with monoclonal anti-IgE antibody. N Engl J Med 1999;341:1966–73 [DOI] [PubMed] [Google Scholar]
- 12.Djukanovic R, Wilson SJ, Kraft M, et al. Effects of treatment with ant-immunoglobulin E antibody omalizumab on airway inflammation in allergic asthma. Am J Respir Crit Care Med 2005;171:88–9 [DOI] [PubMed] [Google Scholar]
- 13.Walker S, Monteil M, Phelan K, Lasserson TJ, Walters EH. Anti IgE for chronic asthma in adults and children. Cochrane Database Syst Rev 2006;2. [DOI] [PubMed] [Google Scholar]
- 14.van der Ent CK, Hoekstra H, Rijkers GT. Successful treatment of allergic bronchopulmonary aspergillosis with recombinant anti-IgE antibody. Thorax 2007;62:276–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zirbes JM, Milla CE. Steroid-sparing effect of Omalizumab for allergic bronchopulmonary aspergillosis and cystic fibrosis. Paediatr Pulmonol 2008;43:607–10 [DOI] [PubMed] [Google Scholar]