Abstract
Synchronized firing in neural populations has been proposed to constitute an elementary aspect of the neural code, but a complete understanding of its origins and significance has been elusive. Synchronized firing has been extensively documented in retinal ganglion cells, the output neurons of the retina. However, differences in synchronized firing across species and cell types have led to varied conclusions about its mechanisms and role in visual signaling. Recent work on two identified cell populations in the primate retina, the ON and OFF parasol cells, permits a more unified understanding. Intracellular recordings reveal that synchronized firing in these cell types arises primarily from common synaptic input to adjacent pairs of cells. Statistical analysis indicates that local pairwise interactions can explain the pattern of synchronized firing in the entire parasol cell population. Computational analysis reveals that the impact of synchronized firing on the visual signal is substantial. Thus, in the parasol cells, the origins and impact of synchronized firing on the neural code may be understood as locally shared input which influences the visual signals transmitted from eye to brain.
More than the sum of its parts
The collective function of neurons in a circuit is typically not predictable based on recordings from individual neurons. Instead, in many circuits – including the olfactory bulb [53], hippocampus [5], retina [25, 13], somatosensory [39], auditory [44] and visual cortices [36] – neural activity exhibits striking interactions, i.e. the firing of one neuron is not statistically independent of the firing of others. These interactions make it difficult to understand the activity of the entire network, one of the great challenges in neuroscience. A complete understanding of neural interactions in a circuit requires that one understand their impact on patterned neural activity, their underlying mechanisms, and their role in information processing. This article reviews recent progress in the retina, an experimentally tractable neural circuit with known biological function.
Visual information is transmitted from the eye to the brain in the spiking activity of populations of retinal ganglion cells (RGCs) [25, 13]. RGCs frequently exhibit synchronized firing, i.e. a tendency for two or more cells to fire nearly simultaneously more often than expected by chance [3, 26, 7, 16, 14, 30, 4, 1, 15, 12, 2, 20, 21, 22, 23]. In some cases, a majority of the action potentials generated by a RGC are synchronized with those of other nearby RGCs [43]. Synchronized firing patterns can include at least 10 RGCs [43, 40, 46], although their full extent is unknown.
Although synchronized firing is widespread amongst RGCs, its impact on patterned activity, its mechanistic origin, and its functional role remain incompletely understood, for several reasons. First, few studies have explored synchronized firing in more than two cells. Second, multiple mechanisms, including direct and indirect gap junction coupling and common synaptic input, have been implicated [9, 14, 17]. Finally, previous work supports a wide range of interpretations of the impact of synchronized firing on visual function: communicating a unique visual signal to the brain [26, 43], introducing redundancy in visual signals [37], or producing a negligible impact on downstream processing [30].
One way to achieve a more unified understanding is to focus on a single, tractable and functionally important cell population. In the primate retina, roughly 20 distinct types of RGCs independently sample visual space, receive unique synaptic inputs, and project to distinct targets in the brain [10]. Recently, substantial progress has been made on understanding synchronized firing in two cell types, the ON and OFF parasol cells, which form the dominant input to the dorsal stream pathway in the central visual system [27]. Individual parasol cells can be identified readily by their morphology [17], entire populations of parasol cells can be recorded using new technologies [19], and parasol cell light responses can be captured accurately using newly developed models [34, 35]. These advances set the stage for understanding the full pattern of synchronized firing, the mechanisms subserving it, and its impact on visual signaling. Hopefully, larger lessons about neural interactions in other types of RGCs and other neural circuits can be gleaned from examining this thoroughly characterized neural population.
Synchronized firing in parasol cells of primate retina
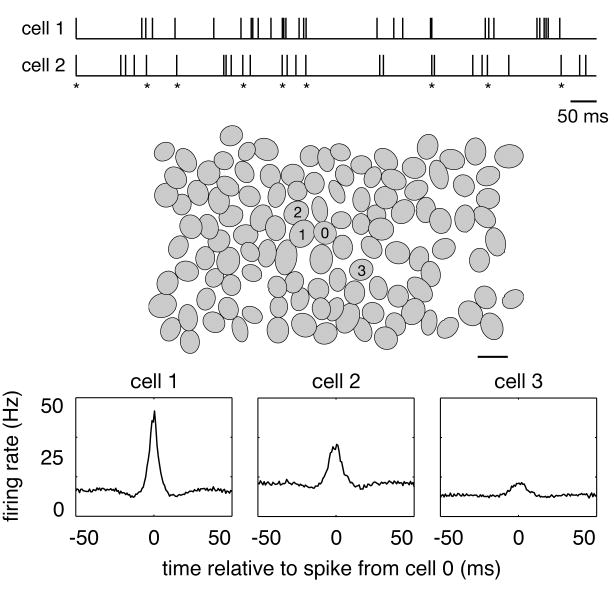
Synchronized firing among RGCs has been documented in many species (e.g. goldfish [3], salamander [26, 7, 40, 45], frog [16], rat [14], mouse [30], rabbit [4, 1, 15, 12, 2], guinea pig [40, 45], cat [20, 21, 22, 29, 28] and monkey [46]). In macaque monkey, multi-electrode recordings reveal a tendency for nearby parasol cells to fire synchronously (Figure 1, top, asterisks). The magnitude and time scale of this synchronized firing can be summarized in the cross-correlogram, which shows the firing rate of one cell as a function of time relative to the occurrence of a spike in a second cell (Figure 1, bottom panels) [33]. For cells that fire independently, the cross-correlogram should be flat. Instead, the cross-correlograms for nearby parasol cells of the same sign (ON, or OFF) show a pronounced peak near the origin, even when the visual stimulus is unchanging over time. In the case of Figure 1 (bottom left), the peak represents a 3-fold increase in firing rate of the reference cell within ±5 ms of a spike in an adjacent cell in the mosaic.
Figure 1.
Synchronized firing in primate retinal ganglion cells [46]. The top traces represent spike trains from two ON parasol cells. Asterisks mark moments in time when both cells fired nearly simultaneously (±5 ms). The middle panel shows a recording of a complete population of ON parasol cells. Each oval represents the 0.9 standard deviation contour of a Gaussian fit to the receptive field of one cell (scale bar, 200μm). The magnitude and time scale of synchronized firing between the center cell (0) and three neighbors (1,2,3) is revealed by their cross-correlograms (bottom panels), which shows the firing rate of center cell as a function of time relative to the occurrence of a spike in the other cell. The height and width of the central peak indicate the magnitude and time scale of synchronized firing between each pair of cells. Note that the magnitude of synchronized firing decreases systematically with the distance between cells.
All pairs of neighboring parasol cells of the same sign exhibit clear synchronized firing on a ±5 ms time scale [46]. The degree of synchrony, measured by the peak of the cross-correlogram, falls off systematically as a function of the distance between cells, such that cells separated by more than a few receptive field diameters are essentially independent. On average, ∼20% of the spikes generated by an individual ON parasol cell contribute to synchronous activity patterns over and above what would be expected by chance. Synchrony is stronger in ON cells than in OFF cells; this difference could be caused by differences in the circuitry producing synchronized firing or by differences in the signals traversing otherwise similar circuitry.
Structure of synchronized firing
The cross-correlogram reveals interactions between a pair of cells, but not in larger groups of cells. Firing events consisting of more than two cells occur far more frequently than expected from statistical independence (Figure 2) (see [43]), raising the possibility that synchronized firing in pairs of cells is merely a window onto more complex patterns of activity in the population [24, 43]. However, recent work has shown that synchronized firing in larger groups is largely accounted for by pairwise interactions between adjacent cells, and thus does not imply complex or long-range interactions.
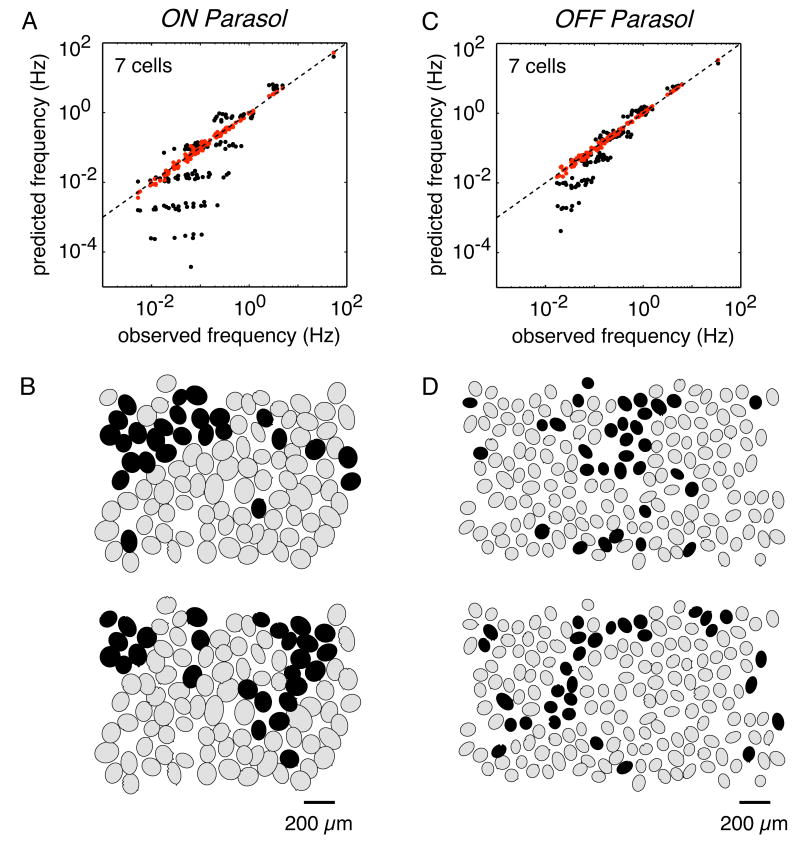
Figure 2.
Multi-neuron firing patterns in primate retinal ganglion cells [46]. (a,c) The frequency of all simultaneous firing patterns was measured in the presence of steady, spatially uniform illumination (10ms time bins). In a group of 7 cells, there are 27 possible firing patterns, ranging from no cells firing (0000000) to all cells firing (1111111). The observed frequency of each firing pattern was compared to predictions from statistical independence (black points). Many firing patterns with multiple synchronized spikes occurred far more often than expected by chance (below dashed line of equality) indicating significant multi-neuron synchronized firing. A statistical model that accounts for synchrony between pairs of adjacent neurons successfully predicted all multi-neuron firing patterns (red points). (b,d) A visualization of synchronized firing at select moments in time (cells firing represented as black). These plots indicate that synchronized firing can encompass well over 7 cells in contiguous regions. It is unknown whether synchrony between adjacent neurons can account for these large patterns of activity.
To illustrate the approach, consider 3 cells (A, B and C), each of which fires a spike (e.g. A = 1) or does not (e.g. A = 0). Suppose that P(A,B,C) represents the frequency of all 8 possible patterns of firing in these three cells. The simplest model is that any given pattern of firing, e.g. all three cells firing simultaneously, occurs with a frequency predicted from statistical independence, e.g. P(A = 1,B = 1,C = 1) = P(A = 1)P(B = 1)P(C = 1). This model fails, which is not surprising because it fails to describe activity in just two cells, e.g. P(A = 1,B = 1) ≠ P(A = 1)P(B = 1) (see Figure 1). Given that the independent model fails, the next simplest model is a pairwise model, in which firing patterns in all pairs of cells, i.e. P(A,B), P(A,C) and P(B,C), are sufficient to predict the full pattern of activity in all three cells, P(A,B,C).
However, as stated, the pairwise model is mathematically underconstrained, because P(A,B), P(A,C) and P(B,C) do not provide a unique prediction of P(A,B,C). To solve this problem, one can select the model distribution P(A,B,C) with the highest entropy, or randomness, given the observed pairwise probabilities (for discussion, see [42]). One can then empirically test whether the pairwise model accounts for the observed patterns of activity in all three cells. This maximum entropy approach can also be used to probe the patterns of firing in larger groups of cells. Two recent studies indicate that a pairwise model explains the observed patterns of activity in up to 7-10 cells with high accuracy [46, 40]. Furthermore, in groups of up to 7 parasol cells, over 99% of synchronized firing structure is predicted from a pairwise model which is determined purely by measured interactions between adjacent cells in the mosaic (Figure 2) [46], arguably the simplest possible pairwise model. A limitation of these studies is that synchronized firing patterns can extend to more than 7-10 cells (see Figure 2) and it is unclear whether these larger activity patterns are captured by a pairwise model.
The above findings suggest that higher-order interactions contribute minimally if at all to synchronized firing, and that the aggregate effect of pairwise interactions on patterned activity in the population can be powerful. Indeed, some multi-neuronal firing patterns can occur with a frequency more than one million times higher than expected based on independent firing, while other patterns occur hundreds of times less frequently than expected [40]. If such large patterns could be predicted based on pairwise measurements, then the firing patterns displayed by the network may be highly constrained, perhaps even affording error-correcting properties [40] (but see [31, 6]).
Mechanisms of synchronized firing
The observation that higher-order correlations are negligible suggests that understanding the biophysical mechanisms of synchronized firing in a pair of adjacent cells might suffice to explain the patterns of activity of the complete population. In principle, synchronized firing could be produced by two broad classes of mechanisms: reciprocal coupling, wherein activity in one cell influences activity in another, or common input, wherein the two cells receive input from a shared set of presynaptic cells (Figure 3a). These classes of mechanisms suggest different functions: reciprocal connections may imply redundant signals [23], while common inputs could produce a multiplexed code [24].
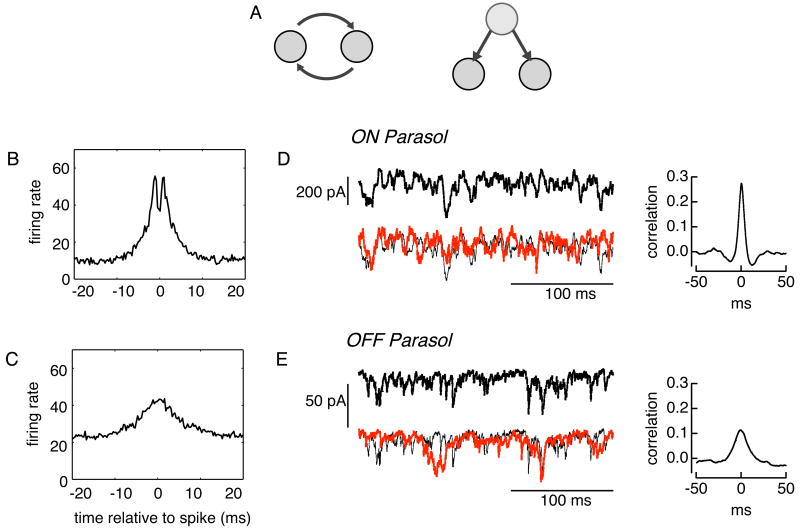
Figure 3.
Mechanisms underlying synchronized firing in primate retinal ganglion cells (Rieke, Shlens & Chichilnisky, unpublished results). (a) Schematic representation of two kinds of connectivity that could mediate synchronized firing. (b) The cross-correlogram between adjacent ON parasol cells often exhibits a bimodal shape. This feature suggests reciprocal coupling, potentially mediated by gap junctions. (c) The cross-correlogram between adjacent OFF parasol cells does not exhibit a bimodal shape, suggesting that common input may dominate synchronized firing. (d,e) Intracellular recordings from pairs of ON and OFF parasol cells indicate that synaptic currents are highly correlated. Each cell was voltage-clamped at the resting potential of inhibition to isolate excitatory synaptic currents. The cross-correlation of the intracellular currents indicate that the time scale and magnitude of the correlated synaptic inputs roughly match the observed synchronized firing (b,c). Thus, common synaptic input appears to account for a major component of synchronized firing in both ON and OFF parasol cells.
Experiments using tracer injection [51, 9, 15] and electrical stimulation [23, 15, 14] support the role of reciprocal connections via gap junctions in several species. Bimodal cross-correlograms – indicating that spikes in two cells are frequently separated by a stereotyped delay consistent with signal propagation – also suggest reciprocal connections [23, 15]. In rabbit, OFF but not ON alpha cells are reciprocally connected [15], one of several interesting asymmetries between ON and OFF cells of the same type (e.g. [8]). In primate, adjacent ON-parasol and OFF-parasol cells are tracer-coupled, perhaps via intermediate amacrine cells [9], but only ON parasol cells exhibit bimodal cross-correlograms at fine time scales (Figure 3b,c).
Although the evidence for reciprocal coupling is srong, several results indicate that it is not sufficient to explain synchronized firing. First, cross-correlograms can show structure on several time scales [20, 21, 22], with the slowest components sensitive to block of chemical synaptic transmission [7]. Second, the probability that a spike elicited in one cell produces a spike in the second cell varies from 1-3% in cat [22] to 20% in rabbit [15]. The efficiency observed in cat is not sufficient to explain the observed peak in the cross-correlogram. More directly, recent paired voltage-clamp recordings indicate that reciprocal coupling is weak in ON parasol cells and absent in OFF parasol cells [18]. OFF parasol cells, despite their apparent lack of reciprocal connectivity, exhibit clear synchronized firing (see also [46]). This synchrony can be explained by strongly correlated synaptic input that is present in adjacent parasol cells of both signs, with a time scale and magnitude matching those of synchronized firing (Figure 3d,e).
Impact on visual signals
Does synchronized firing alter the signaling of visual information from the eye to the brain [38]? Some studies have approached this problem by computationally reconstructing the stimulus from the activity of pairs of neurons, and comparing the fidelity of the reconstructed stimulus to that obtained from independent, single neuron responses [41, 37, 30, 11, 52]. This strategy has fostered the development of new analytical methods, but it has been limited to analyzing pairs of cells, largely because of prohibitive data requirements. Because synchronized firing extends across larger groups of neurons (Figure 2c,d), this technical limitation leaves open the larger question of how interactions in the entire network influence visual signals.
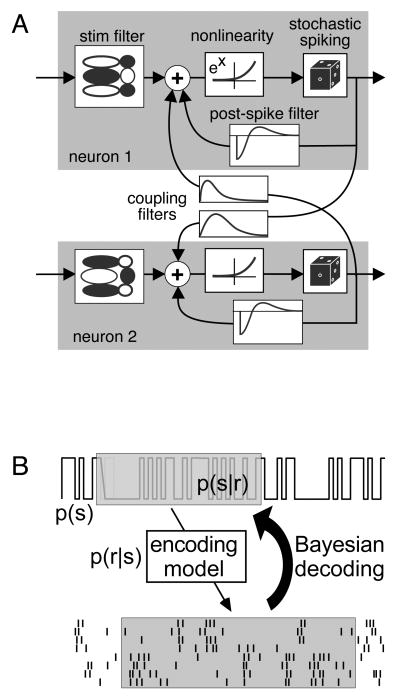
To overcome this limitation, a recent study employed a parametric statistical model (generalized linear model) of the population response of ON and OFF parasol cells that captures all synchronized firing, spike train structure, and stimulus dependencies in the entire population [47, 32]. The model is unusually tractable in that all model parameters can be inferred from experimental data using standard techniques (Figure 4a). Importantly, because the model contains a tractable mathematical expression for the population activity, one can effectively invert it to decode optimally the stimulus from the population activity (Figure 4b). In the case of collections of ON and OFF parasol cells completely covering a region of visual space, 20% more information about the stimulus was extracted from spike trains when synchronized firing was exploited in the process of decoding [35]. Thus, the impact of synchronized firing on the visual signal in parasol cells is significant.
Figure 4.
Decoding the stimulus from population activity [35]. (a) A parametric model (generalized linear model) can capture the activity of a complete population of ON and OFF parasol retinal ganglion cells. Each model neuron spikes stochastically, with a probability equal to the exponentiated summed drive of a linearly filtered stimulus, a post-spike feedback current, and cross-currents from spikes in coupled cells. Because a tractable mathematical expression expresses the likelihood of a population response to any stimulus, P(response|stimulus), one can invert the expression using Bayes rule to optimally reconstruct the stimulus. A model which includes coupling filters captures synchronized firing more accurately, and is able to extract 20% more information about a stimulus, than a model which does not include coupling filters.
Conclusions & Future
Retinal ganglion cells, like neurons in many other circuits, exhibit substantial synchronized firing. Synchronized firing in primate ON and OFF parasol cells is dictated by their regular geometric spatial organization: cells fire in tandem with surrounding cells largely as a consequence of common synaptic input. Synchronized firing in small groups of cells can be predicted from the interactions between immediately adjacent pairs of cells in the mosaic, and can substantially affect the visual signal transmitted to the brain. This work has advanced our understanding of the structure, origin and impact of synchronized firing in two important cell types, and may provide a framework for examining other neural circuits. It also raises many questions.
Pairwise interactions between adjacent cells can account for the patterns of activity in up to 7 parasol cells. Can the structure of synchronized firing in the entire parasol cell population be accounted with the same simple model? In some species, specific stimulus features such as motion reversal [45] and looming [16] can elicit strong synchronous activity, potentially revealing a specialized circuit and a unique symbol in the neural code of the retina [24]. Can synchronous activity elicited by such stimuli be accounted for by the pairwise model? More generally, can the pairwise model explain the interaction of synchronized firing with the visual signal encoded by spike trains?
Synchronized firing in parasol cells arises largely from common synaptic input to neighboring cells. This raises the possibility of multiplexed coding, wherein signals from the source of common input are encoded by synchronized activity in RGCs [26, 43]. How does synchronization depend on receptive field or dendritic field overlap, and what does this dependence indicate about underlying circuitry? How does synchronized synaptic input interact with reciprocal coupling to produce synchronized firing?
Modeling work indicates that 20% more visual information is available to the brain when synchronized activity is taken into account in interpreting the retinal output. Does the improvement in decoding suggest a synergistic neural code, or does it reflect compensation for redundancy in the code [37]? What mechanisms could downstream structures use to exploit synchronized firing in reconstructing the stimulus (see [49, 48, 50])? Does the additional information in synchronized firing reflect particular stimulus features, and can it be attributed to specific circuit elements?
Acknowledgments
The authors were supported by Miller Institute for Basic Research in Science, University of California, Berkeley (JS), NIH grants EY017736 (EJC) and EY11850 (FMR), and the Howard Hughes Medical Institute (FMR).
Footnotes
Highly Recommended References
[23]. Review of seminal papers documenting synchronized firing in cat. Author discusses cell type specificity and potential mechanisms of synchronized firing.
Recommended References
[45]. Demonstration that synchronized firing in salamander selectively signals motion reversal. Motion reversal response arises from nonlinear processing in RGC receptive fields.
[16]. Demonstration that synchronized firing encodes a unique behaviorally relevant signal. A looming stimulus selectively generates synchronous activity in a class of frog RGCs. Pharmacaological modulation of synchronous activity in retina suppressed or enhanced escape behavior elciited by looming stimulus.
[46]. First characterization of synchronized firing in primate RGCs. Structure of synchronized is highly dictated by cell type and firing patterns of up to 7 neurons are well characterized by interactions between adjacent cells.
[35]. Authors demonstrate that tractable statistical model of primate RGCs capture all network dynamics. Optimal decoding exploiting statistical model reveals that 20% of visual information is conveyed through correlations.
[40]. Demonstration that pairwise synchrony accurately characterizes firing patterns in up to 10 RGCs. Based on scaling properties of pairwise model, authors propose that large networks of RGCs might be highly constrained by correlated activity.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ackert JM, Wu SH, Lee JC, Abrams J, Hu EH, Perlman I, Bloomfield SA. Light-induced changes in spike synchronization between coupled ON direction selective ganglion cells in the mammalian retina. J Neurosci. 2006;26(16):4206–4215. doi: 10.1523/JNEUROSCI.0496-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amthor FR, Tootle JS, Grzywacz NM. Stimulus-dependent correlated firing in directionally selective retinal ganglion cells. Vis Neurosci. 2005;22(6):769–787. doi: 10.1017/S0952523805226081. [DOI] [PubMed] [Google Scholar]
- 3.Arnett D. Statistical dependence between neighboring retinal ganglion cells in goldfish. Exp Brain Res. 1978;32(1):49–53. doi: 10.1007/BF00237389. [DOI] [PubMed] [Google Scholar]
- 4.Arnett D, Spraker TE. Cross-correlation analysis of the maintained discharge of rabbit retinal ganglion cells. J Physiol. 1981;317:29–47. doi: 10.1113/jphysiol.1981.sp013812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Best PJ, White AM, Minai A. Spatial processing in the brain: the activity of hippocampal place cells. Annu Rev Neurosci. 2001;24:459–486. doi: 10.1146/annurev.neuro.24.1.459. [DOI] [PubMed] [Google Scholar]
- 6.Bethge M, Berens P. Near-maximum entropy models for binary neural representations of natural images. Adv Neural Information Processing Systems. 2007;20:1–8. [Google Scholar]
- 7.Brivanlou IH, Warland DK, Meister M. Mechanisms of concerted firing among retinal ganglion cells. Neuron. 1998;20(3):527–539. doi: 10.1016/s0896-6273(00)80992-7. [DOI] [PubMed] [Google Scholar]
- 8.Chichilnisky EJ, Kalmar RS. Functional asymmetries in ON and OFF ganglion cells of primate retina. J Neurosci. 2002;22(7):2737–2747. doi: 10.1523/JNEUROSCI.22-07-02737.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dacey DM, Brace S. A coupled network for parasol but not midget ganglion cells in the primate retina. Vis Neurosci. 1992;9(34):279–290. doi: 10.1017/s0952523800010695. [DOI] [PubMed] [Google Scholar]
- 10.Dacey DM, Peterson BB, Robinson FR, Gamlin PD. Fireworks in the primate retina: in vitro photodynamics reveals diverse LGN-projecting ganglion cell types. Neuron. 2003;37(1):15–27. doi: 10.1016/s0896-6273(02)01143-1. [DOI] [PubMed] [Google Scholar]
- 11.Dan Y, Alonso JM, Usrey WM, Reid RC. Coding of visual information by precisely correlated spikes in the lateral geniculate nucleus. Nat Neurosci. 1998;1(6):501–507. doi: 10.1038/2217. [DOI] [PubMed] [Google Scholar]
- 12.DeVries SH. Correlated firing in rabbit retinal ganglion cells. J Neurophysiol. 1999;81(2):908–920. doi: 10.1152/jn.1999.81.2.908. [DOI] [PubMed] [Google Scholar]
- 13.Field GD, Chichilnisky EJ. Information processing in the retina: Circuitry, coding, and mechanism. Ann Reviews in Neuroscience. 2007;30 doi: 10.1146/annurev.neuro.30.051606.094252. [DOI] [PubMed] [Google Scholar]
- 14.Hidaka S, Akahori Y, Kurosawa Y. Dendrodendritic electrical synapses between mammalian retinal ganglion cells. J Neurosci. 2004;24(46):10553–10567. doi: 10.1523/JNEUROSCI.3319-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu EH, Bloomfield SA. Gap junctional coupling underlies the short-latency spike synchrony of retinal alpha ganglion cells. J Neurosci. 2003;23(17):6768–6777. doi: 10.1523/JNEUROSCI.23-17-06768.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishikane H, Gangi M, Honda S, Tachibana M. Synchronized retinal oscillations encode essential information for escape behavior in frogs. Nat Neurosci. 2005;8(8):1087–1095. doi: 10.1038/nn1497. [DOI] [PubMed] [Google Scholar]
- 17.Jacoby RA, Stafford D, Kouyama N, Marshak D. Synaptic inputs to ON parasol ganglion cells in the primate retina. J Neurosci. 1996;16(24):8041–8056. doi: 10.1523/JNEUROSCI.16-24-08041.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khuc Trong P, Rieke F. Origin of correlated activity between parasol retinal ganglion cells. Nature Neurosci. 2008 doi: 10.1038/nn.2199. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Litke AM. The retinal readout system: a status report. Nucl Instr and Meth A. 1999;435:242–249. [Google Scholar]
- 20.Mastronarde DN. Correlated firing of cat retinal ganglion cells. i. spontaneously active inputs to x- and y-cells. J Neurophysiol. 1983;49(2):303–324. doi: 10.1152/jn.1983.49.2.303. [DOI] [PubMed] [Google Scholar]
- 21.Mastronarde DN. Correlated firing of cat retinal ganglion cells. II. responses of x- and y-cells to single quantal events. J Neurophysiol. 1983;49(2):325–349. doi: 10.1152/jn.1983.49.2.325. [DOI] [PubMed] [Google Scholar]
- 22.Mastronarde DN. Interactions between ganglion cells in cat retina. J Neurophysiol. 1983;49(2):350–365. doi: 10.1152/jn.1983.49.2.350. [DOI] [PubMed] [Google Scholar]
- 23.Mastronarde DN. Correlated firing of retinal ganglion cells. Trends Neurosci. 1989;12(2):75–80. doi: 10.1016/0166-2236(89)90140-9. [DOI] [PubMed] [Google Scholar]
- 24.Meister M. Multineuronal codes in retinal signaling. Proc Natl Acad Sci USA. 1996;93(2):609–614. doi: 10.1073/pnas.93.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meister M, Berry MJ. The neural code of the retina. Neuron. 1999;22(3):435–450. doi: 10.1016/s0896-6273(00)80700-x. [DOI] [PubMed] [Google Scholar]
- 26.Meister M, Lagnado L, Baylor DA. Concerted signaling by retinal ganglion cells. Science. 1995;270(5239):1207–1210. doi: 10.1126/science.270.5239.1207. [DOI] [PubMed] [Google Scholar]
- 27.Merigan WH, Maunsell JH. How parallel are the primate visual pathways? Annu Rev Neurosci. 1993;16:369–402. doi: 10.1146/annurev.ne.16.030193.002101. [DOI] [PubMed] [Google Scholar]
- 28.Neuenschwander S, Castelo-Branco M, Singer W. Synchronous oscillations in the cat retina. Vision Res. 1999;39(15):2485–2497. doi: 10.1016/s0042-6989(99)00042-5. [DOI] [PubMed] [Google Scholar]
- 29.Neuenschwander S, Singer W. Long-range synchronization of oscillatory light responses in the cat retina and lateral geniculate nucleus. Nature. 1996;379(6567):728–732. doi: 10.1038/379728a0. [DOI] [PubMed] [Google Scholar]
- 30.Nirenberg S, Carcieri SM, Jacobs AL, Latham PE. Retinal ganglion cells act largely as independent encoders. Nature. 2001;411(6838):698–701. doi: 10.1038/35079612. [DOI] [PubMed] [Google Scholar]
- 31.Nirenberg SH, Victor JD. Analyzing the activity of large populations of neurons: how tractable is the problem? Curr Opin Neurobiol. 2007 doi: 10.1016/j.conb.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paninski L. Convergence properties of three spike-triggered analysis techniques. Network. 2003;14(3):437–464. [PubMed] [Google Scholar]
- 33.Perkel DH, Gerstein GL, Moore GP. Neuronal spike trains and stochastic point processes. II. simultaneous spike trains. Biophys J. 1967;7(4):419–440. doi: 10.1016/S0006-3495(67)86597-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pillow JW, Paninski L, Uzzell VJ, Simoncelli EP, Chichilnisky EJ. Prediction and decoding of retinal ganglion cell responses with a probabilistic spiking model. J Neurosci. 2005;25(47):11003–11013. doi: 10.1523/JNEUROSCI.3305-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pillow JW, Shlens J, Paninski L, Sher A, Litke AM, Chichilnisky EJ, Simoncelli EP. Spatio-temporal correlations and visual signalling in a complete neuronal population. Nature. 2008;454(7207):995–999. doi: 10.1038/nature07140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Priebe NJ, Ferster D. Inhibition, spike threshold, and stimulus selectivity in primary visual cortex. Neuron. 2008;57(4):482–497. doi: 10.1016/j.neuron.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 37.Puchalla JL, Schneidman E, Harris RA, Berry MJ. Redundancy in the population code of the retina. Neuron. 2005;46(3):493–504. doi: 10.1016/j.neuron.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 38.Rieke F, Warland D, de Ruyter van Steveninck RR, Bialek W. Spikes: exploring the neural code. MIT Press; Cambridge, MA: 1997. [Google Scholar]
- 39.Romo R, Salinas E. Flutter discrimination: neural codes, perception, memory and decision making. Nat Rev Neurosci. 2003;4(3):203–218. doi: 10.1038/nrn1058. [DOI] [PubMed] [Google Scholar]
- 40.Schneidman E, Berry MJ, Segev R, Bialek W. Weak pairwise correlations imply strongly correlated network states in a neural population. Nature. 2006;440(7087):1007–1012. doi: 10.1038/nature04701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schneidman E, Bialek W, Berry MJ. Synergy, redundancy, and independence in population codes. J Neurosci. 2003;23(37):11539–11553. doi: 10.1523/JNEUROSCI.23-37-11539.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schneidman E, Still S, Berry MJ, Bialek W. Network information and connected correlations. Phys Rev Lett. 2003;91:238701. doi: 10.1103/PhysRevLett.91.238701. [DOI] [PubMed] [Google Scholar]
- 43.Schnitzer MJ, Meister M. Multineuronal firing patterns in the signal from eye to brain. Neuron. 2003;37(3):499–511. doi: 10.1016/s0896-6273(03)00004-7. [DOI] [PubMed] [Google Scholar]
- 44.Schreiner CE, Winer JA. Auditory cortex mapmaking: principles, projections, and plasticity. Neuron. 2007;56(2):356–365. doi: 10.1016/j.neuron.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwartz G, Taylor S, Fisher C, Harris R, Berry MJ., 2nd Synchronized firing among retinal ganglion cells signals motion reversal. Neuron. 2007;55(6):958–969. doi: 10.1016/j.neuron.2007.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shlens J, Field GD, Gauthier JL, Grivich MI, Petrusca D, Sher A, Litke AM, Chichilnisky EJ. The structure of multi-neuron firing patterns in primate retina. J Neurosci. 2006;26(32):8254–8266. doi: 10.1523/JNEUROSCI.1282-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Truccolo W, Eden UT, Fellows MR, Donoghue JP, Brown EN. A point process framework for relating neural spiking activity to spiking history, neural ensemble, and extrinsic covariate effects. J Neurophysiol. 2005;93(2):1074–1089. doi: 10.1152/jn.00697.2004. [DOI] [PubMed] [Google Scholar]
- 48.Usrey WM, Reid RC. Synchronous activity in the visual system. Annu Rev Physiol. 1999;61:435–456. doi: 10.1146/annurev.physiol.61.1.435. [DOI] [PubMed] [Google Scholar]
- 49.Usrey WM, Reppas JB, Reid RC. Paired-spike interactions and synaptic efficacy of retinal inputs to the thalamus. Nature. 1998;395(6700):384–387. doi: 10.1038/26487. [DOI] [PubMed] [Google Scholar]
- 50.Usrey WM, Reppas JB, Reid RC. Specificity and strength of retinogeniculate connections. J Neurophysiol. 1999;82(6):3527–3540. doi: 10.1152/jn.1999.82.6.3527. [DOI] [PubMed] [Google Scholar]
- 51.Vaney DI. Many diverse types of retinal neurons show tracer coupling when injected with biocytin or neurobiotin. Neurosci Lett. 1991;125(2):187–190. doi: 10.1016/0304-3940(91)90024-n. [DOI] [PubMed] [Google Scholar]
- 52.Warland DK, Reinagel P, Meister M. Decoding visual information from a population of retinal ganglion cells. J Neurophysiol. 1997;78(5):2336–2350. doi: 10.1152/jn.1997.78.5.2336. [DOI] [PubMed] [Google Scholar]
- 53.Wilson RI, Mainen ZF. Early events in olfactory processing. Annu Rev Neurosci. 2006;29:163–201. doi: 10.1146/annurev.neuro.29.051605.112950. [DOI] [PubMed] [Google Scholar]