Abstract
Both the growth and respiration of Candida albicans are sensitive to extracts of Allium sativum and investigations into the anticandidal activities are now focussing on the purified constituents to determine the targets of inhibition. Of particular interest is allyl alcohol (AA), a metabolic product that accumulates after trituration of garlic cloves. Putative targets for AA were investigated by monitoring changes in intracellular responses after exposure of C. albicans cells to AA or a commercially available garlic extract. Two-photon laser scanning microscopy and other techniques were used. Changes typical of oxidative stress – NADH oxidation and glutathione depletion, and increased reactive oxygen species – were observed microscopically and by flow cytometry. Known targets for AA are alcohol dehydrogenases Adh1 and 2 (in the cytosol) and Adh3 (mitochondrial), although the significant decrease in NAD(P)H after addition of AA is indicative of another mechanism of action.
INTRODUCTION
Garlic (Allium sativum) has been used medicinally since before the time of the Sumerian civilization (2600–2100 BC), by when it was already widely cultivated in India and China (Harris et al., 2001). Traditionally used as an antimicrobial agent, garlic has been reported also to modulate cardiovascular and immune functions as well as having antioxidant and anticancer properties. Garlic constituents were first investigated by Wertheim (1844); later studies showed that steam distillation gave diallyl disulphide, whereas more gentle extraction using ethanol at 25 °C or at subzero temperature released allicin and alliin respectively (Cavallito et al., 1944). That the latter were examples of a number of cysteine sulphoxides and corresponding thiosulphinates was more recently demonstrated (Stoll & Seebeck, 1950, 1951; Lawson, 1996). Although the bioactive effects of garlic are attributed to the sulphur-containing molecules, other smaller metabolic breakdown products of these molecules have received increasing attention for their antimicrobial efficacy (Harris et al., 2000; Banerjee et al., 2003).
Allyl alcohol (AA), the focus of the present study, is produced from garlic in two ways: firstly by a self-condensation reaction of allicin and secondly by the reaction between alliin, the precursor of allicin, and water (Lawson, 1996). It is released after ingestion of garlic (Egen-Schwind et al., 1992) and is present in exhaled air after ingestion of all garlic products, the highest concentration being after ingestion of freeze-dried garlic tablets (Laasko et al., 1989). Mechanisms, first proposed by Block (1985), for the formation of AA in aqueous environments such as those found in vivo result from the transformation of the thiosulphinates such as diallyl trisulphide and diallyl disulphide (Block, 1985; Block et al., 1992), with up to one-third of the ‘lost’ allyl groups during the transformation producing AA (Lawson, 1996). Recent investigations with rats reported that similar degradative metabolites are formed in vivo (Germain et al., 2002).
Garlic as an antibacterial (Sharma et al., 1977; Skyrme, 1997) has been the focus of detailed investigations recentzly on Helicobacter pylori (O’Gara et al., 2000; Ross et al., 2001). Differential inhibitory effects against Escherichia coli and Lactobacillus casei, whereby a 10-fold greater sensitivity was evident in the former (Rees et al., 1993) have been studied in more detail with respect to the different membrane structures of Gram-positive and -negative bacteria (Cottrell, 2003). Antiprotozoal studies include those on Entamoeba histolytica (Mirelman et al., 1987; Reuter, 1994) and Giardia intestinalis (Harris et al., 2000); the anticandidal effects (Ghannoum, 1988, 1990; Lemar et al., 2002, 2003) of garlic similarly include a wide range of ultrastructural lesions affecting cytoplasmic membranes, organelles and cytoskeletal organization. The widespread efficacy of this plant extract as an antimicrobial has been linked to the ease by which these molecules pass through cell membranes and react biologically at the low level of thiol bonds in amino acids (Miron et al., 2000). That no example of acquired microbial resistance to garlic has been reported may also stem from its diverse modes of action and the multiplicity of intracellular targets that each bioactive component inactivates. Work with G. intestinalis (Harris et al., 2000) and Candida albicans (Lemar et al., 2002) has suggested that two of the simplest garlic constituents, diallyl disulphide and AA, are amongst the most potent; the former can be isolated by a process of steam distillation (Wertheim, 1844). More recent investigations, however, show that diallyl trisulphide is still more potent (Davis, 2005). A well-researched mechanism of AA toxicity is by its inhibition of alcohol dehydrogenase after its conversion to the toxic aldehyde acrolein (Rando, 1974). Acrolein (crotonaldehyde) and AA were the most toxic of 20 low-boiling-point compounds tested on the yeast Candida utilis (Sestakova et al., 1976). More recent investigations have indicated that AA itself is not toxic to cells of the methylotrophic yeast Pichia pastoris, but it is toxic when oxidized via an alcohol oxidase pathway to acrolein (Johnson et al., 1999). In rats, in vivo, AA is metabolized by liver alcohol dehydrogenase to acrolein (Mapoles et al., 1994). Severe damage to the microtubules of rat hepatocyte mitochondria after exposure to AA (Vengerovskii et al., 1989) was observed concomitantly with the depletion of glutathione (Nagelkerke et al., 1991). However, more recent studies revealed that 100 µl AA per kg body weight in rats fed by gastric tube was not toxic to hepatocytes as determined by RNA extraction (Tygstrup et al., 1997). These studies do not preclude this derivative from possible clinical use at the in vivo concentration required to be an effective antifungal. Effects of AA on Saccharomyces cerevisiae include disruption of cellular kinetics, redox balance and electrophoretic mobility (Wills & Phelps, 1978). Acrolein is also known to deplete intracellular stores of glutathione and cause peroxidation of cellular lipids. This affects the permeability of the membrane and consequently undermines the viability of the cell (Glascott et al., 1996).
In this study we investigated the biocidal mechanisms of AA on C. albicans by assessing its effects on cell physiology, and also on morphology using optical and electron imaging.
METHODS
Organism and culture
Candida albicans (Berkhout) 5134 was grown in YPD medium [Bacto-peptone 2% (w/v), Difco; yeast extract 1% (w/v), Oxoid; and glucose 2% (w/v)]. An early-stationary-phase culture was used as an inoculum (1:100 dilution in 50 ml) in 100 ml conical flasks. Growth was aerobic at 30 °C in a rotary shaker (150 r.p.m.). Cell numbers were determined with a haemocytometer slide (Fuschs-Rosenthal) after dilution in sterile medium. Optical density (OD450) was measured using a Pye-Unicam SP-1400 spectrophotometer. Stock cultures of C. albicans were grown overnight at 30 °C and maintained at 4 °C on YPD agar containing 2% (w/v) agar (Oxoid), in the presence or absence of garlic preparation.
Garlic preparations
Garlic powder (provided by Cultech Biospeciality Products, Swansea, Wales, UK) was prepared to the required concentration in sterile growth medium. After 30 min the garlic powder extract (GPE) was centrifuged at 3900 g for 10 min and the supernatant passed through a sterile 0·2 µm filter (Millipore). Stock suspensions were prepared on the day of testing in PBS (pH 5·6) or appropriate media as stated. Concentration ranges for biocide assays for AA (0–10 mM) were prepared in sterile growth medium (AA was purchased from Sigma-Aldrich). MIC values were determined by the microtitre broth dilution method (Cottrell, 2003)
Ultrastructural investigations
Scanning electron microspcopy
Cells were fixed in 3 % (v/v) glutaraldehyde in PBS (pH 7·4) and dehydrated in increasing concentrations of ethanol (10%, v/v, increments, to 100 %). A drop was placed onto a round glass coverslip for critical-point drying (CPD 030; Balzers). The sample was mounted onto an aluminium slab (10 × 10 mm JEOL type; Agar Scientific) using silver paint (Agar Scientific). Sputter coating was performed in a sputter coater (S150B; Edwards) and the image viewed using a scanning electron microscope (5200LV; JEOL).
Transmission electron microscopy
Cells were fixed in cacodylate buffer, pH 6·9, containing 1 % (v/v) paraformaldehyde and 2 % (v/v) glutaraldehyde, at 4 °C for 1 h. The cells were centrifuged and dehydrated in successive washes of ethanol for 30 min. The pellet of cells was embedded in Spurr resin and ultrathin sections obtained (Ultratome III; LKB). The sections were mounted onto a 0.5 % Pioloform (in chloroform)-coated 3·05 mm copper grid then stained with 2% aqueous uranyl acetate and 2 % lead citrate before imaging on a transmission electron microscope (1210; JEOL).
Whole-cell respiration
Organisms in growth medium were harvested in exponential phase. Cells were washed and resuspended in 500 µl PBS pH 6·4. Measurement of glucose-supported O2 consumption was performed at 30 °C in a thermostatically controlled closed electrode system (Rank) using a Teflon membrane electrode. Cell suspensions (40 µl) were injected into 2 ml PBS (pH 5·6) and continually stirred at 200 r.p.m.
Redox investigation: preincubation and conditions for microscopic examination
Early-stationary-phase cells were harvested and resuspended in PBS (pH 5·6). Samples (0·5 ml) were preloaded with 200 nM tetramethylrhodamine ethyl ester (TMRE) at 37 °C for 30 min before sample removal to the observation chamber and supplemented with 10 mM glucose. The dish containing the yeasts was equilibrated with unrestricted access to atmospheric oxygen on the stage of a Nikon E600FN upright microscope which was maintained at 30 °C. AA or GPE was subsequently added to the incubation mixture.
Fluorescent probes for two-photon laser scanning microscopy
The cationic potentiometric fluorescent dye TMRE was used to monitor changes in mitochondrial membrane potential, Δψm. The large negative potential gradient across the inner mitochondrial membrane results in the accumulation of TMRE within the matrix compartment according to its Nernst potential (Loew et al., 1993). Production of reactive oxygen species (ROS) was monitored with the ROS-sensitive fluorescent probe 5-(-6)-chloromethyl-2′,7′-dichlorohydrofluorescein diacetate (CM-H2DCFDA, 10 µM). The acetate group of CM-H2DCFDA is hydrolysed by esterases when it enters the cell and the molecule is trapped inside as the non-fluorescent 5-(-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein (CM-H2DCFH). CM-H2DCFH was chosen because, unlike underivatized dichlorodihydrofluorescein, it is well retained in cells (Xie et al., 1999) and, in this case, in the mitochondrial matrix (Aon et al., 2003). Oxidation of CM-H2DCFH by ROS, particularly by hydrogen peroxide (H2O2) and hydroxyl radical (Vanden Hoek et al., 1997a, b), yields the fluorescent product carboxymethyldichlorofluorescein (CM-DCF), and indirectly measures mitochondrially produced that has dismutated to H2O2 through the action of superoxide dismutase (Chance et al., 1979; Turrens et al., 1985).
Glutathione was monitored intracellularly by production of the fluorescent adduct glutathione-bimane (GSB) (Kosower & Kosower, 1987) as a result of the reaction of the cell permeant monochlorobimane (MCB, 50 µM) with reduced glutathione (GSH) catalysed by glutathione-S-transferase (GST) (Cortassa et al., 2004). Reduced nicotinamide nucleotides were monitored by their autofluorescence under the imaging conditions optimized previously (Aon et al., 2003).
Image acquisition and analysis
Images were recorded using a two-photon laser scanning microscope (Bio-Rad MRC-1024MP) with excitation at 740 nm (Tsunami Ti: Sapphire laser, Spectra-Physics) as described before (Aon et al., 2003). Briefly, owing to the overlap in the cross-sections for two-photon excitation of the three fluorophores of interest (Xu et al., 1996) (TMRE, CM-DCF, and NADH or GSB) this wavelength permitted recording of Δψm, ROS production, and NAD(P)H or GSH simultaneously. The red emission of TMRE was collected at 605 ± 25 nm; the green emission of CM-DCF was recorded at 525 ± 25 nm; and the blue emission of GSB detected at 480 ± 20 nm. NADH emission was collected as the total fluorescence <490 nm. At 3·5 s or 30 s intervals as indicated, 512 × 512 pixel 8-bit greyscale images of the three emission channels were collected simultaneously and stored.
While determining kinetics of GSB production in cells, about 10 images were recorded to get the cellular background of NADH before acute addition of 50 µM MCB. This was necessary because the NAD(P)H and GSB emissions were collected at similar wavelengths, i.e. 480–490 nm (Aon et al., 2004; Cortassa et al., 2004). At steady state, the NAD(P)H fluorescence levels (n = 14) represented 31 ± 1·2 % of the maximal GSB fluorescence levels attained (n=14), indicating an approximately threefold increase with respect to the background. Images were analysed offline using ImageJ software (Wayne Rasband, National Institutes of Health; http://rsb.info.nih.gov/ij/).
Fluorometric kinetic studies
The effect of AA and GPE on purified GST (from rabbit liver, Sigma cat. no. G8261, 6 U ml−1 final concentration) as a function of GSH concentration was investigated. Increasing concentrations of GSH (in PBS pH 5·6) in the absence and presence of AA (0·5 or 1·0 mM) and GPE (5 or 10 mg ml−1) were added to 3 ml PBS (pH 5·6); a subsequent addition of 50 µl GST was used to commence the reaction. Monochlorobimane (MCB, 2 µM final concentration; λmax excitation 391 nm; λmax emission 491 nm) was used as previously described (Aon et al., 2003), and fluorescence measured using a Cary Eclipse fluorescence spectrophotometer (Varian).
Analysis of intracellular ROS using flow cytometry
Fluorescence distributions were obtained from 100 000 events per cell sample through a bandpass filter FL1 using a FACScalibur flow cytometer (BD Biosciences). Forward light scatter and side scatter were measured and used for gating the data collection. The fluorescence of gated cell populations was analysed using validated analysis software, WinMDI 2.8. Intracellular events in a population of C. albicans were analysed using CM-H2DCFH as a marker for ROS as described previously (Aon et al., 2003).
Materials
TMRE, CM-H2DCFDA and MCB were purchased from Molecular Probes. All other reagents, including enzymes, were from Sigma-Aldrich unless specified.
RESULTS
Growth inhibition
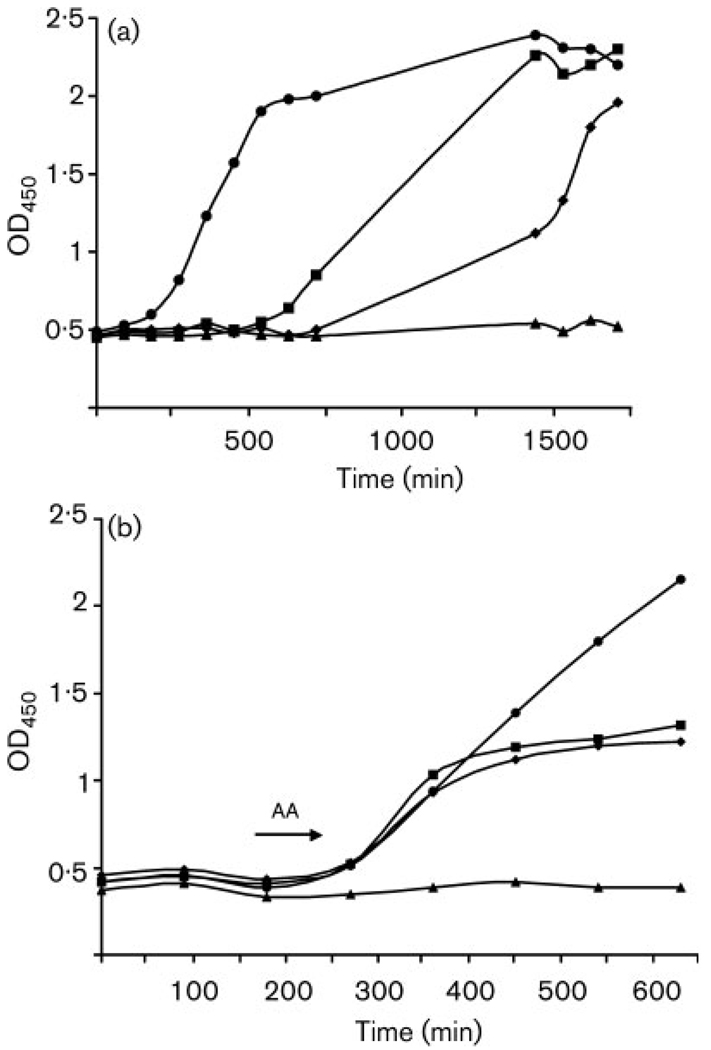
Incubation with AA resulted in an extended lag phase and inhibition of growth during exponential phase (Fig. 1). Low concentrations of AA present throughout the experiment extended the time which the culture took to establish; increases in AA concentration resulted in decreases in growth rates – doubling times were as follows: control cultures (0 mM AA), 4 h; 0·25 mM AA (14·5 µg ml−1), 8·3 h; 0·5 mM AA (29 µg ml−1), 10·5 h. However, these AA-inhibited cultures eventually achieved the same maximum cell numbers as the controls (Fig. 1a). Doses of AA greater than 0·5 mM (29 µg ml−1) resulted in complete inhibition (data not shown). Established cultures of C. albicans were able to tolerate higher concentrations of AA; when AA was added in mid-exponential phase, inhibition of growth was observed only at concentrations ⩾ 1 mM AA (29 µg ml−1) (Fig. 1b). The IC50 (the concentration at which 50 % of the population is killed) for AA was 1 mM (58 µg ml−1) at 10 h, and the MIC value was 0·1 mM (5·8 µg ml−1). Colony growth on YPD medium indicated that AA is fungistatic at concentrations above 1 mM but fungicidal at concentrations above 2 mM (116 µg ml−1).
Fig. 1.
Effect of AA on growth of C. albicans after addition at early stationary phase and mid-exponential phase. (a) Early stationary phase: ●, 0 mM; ■, 0·25 mM (14·5 µg ml−1); ◆ 0·5 mM (29 µg ml−1); ▲, uninoculated control. (b) Mid-exponential phase (as indicated by arrow): ●, 0 mM; ■, 1·0 mM (58 µg ml−1); ◆, 1·5 mM (87 µg ml−1); ▲, uninoculated control. Results are representative of triplicate experiments. The discrepancies in the time scales between the panels is due to extension of the lag phase on exposure to AA.
Ultrastructural effects
Scanning electron microscopy
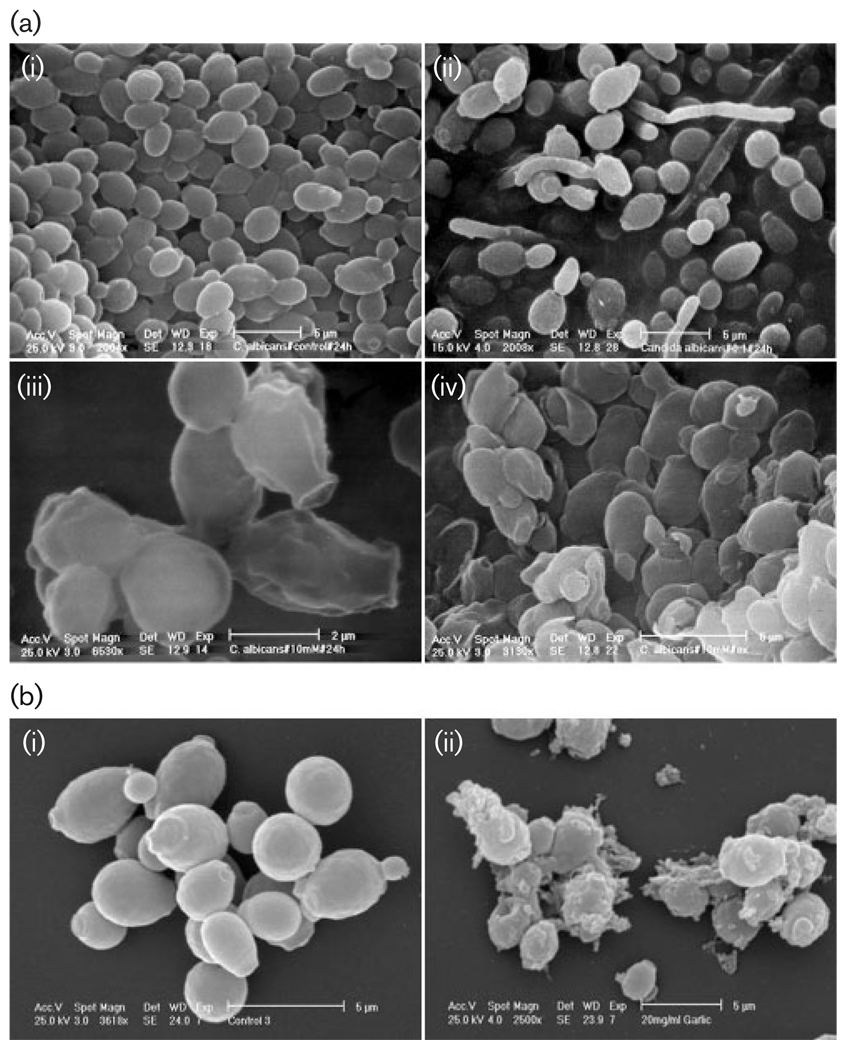
Aerobic growth of C. albicans cells in YPD medium at 30 °C gave typical yeast cells displaying the characteristic bud scars (Fig. 2ai). Development of pseudohyphae (chains of elongated unseparated blastospores) was only observed in cultures containing low concentrations of AA (Fig. 2aii). The external morphology of the cells after addition of 0·1 mM AA (5·8 µg ml−1) (MIC) did not appear as smooth as that of untreated cells; this indicates a possible loss of cytosolic volume (Fig. 2aiii). Increasing the AA concentration further distorted the cell wall surface (Fig. 2aiv). Pseudohyphae were not observed at higher concentrations of AA. Neither untreated nor treated cultures produced true hyphae (presence of septa between elongated cells). Cells treated with GPE showed distortion of their outer surface, possibly due to loss of cytosolic volume; the collection of debris on the surface was also evident (Fig. 2b).
Fig. 2.
Scanning electron micrographs of C. albicans treated with AA or GPE (a) AA: (i) untreated culture, (ii) 0·1 mM AA (5·8 mg ml−1), (iii) 1·0 mM (58 µg ml−1) AA, (iv) 10 mM (580 µg ml−1; high concentration used to induce extreme effects). (b) GPE (i) untreated control, (ii) 20 mg GPE ml−1.
Transmission electron microscopy
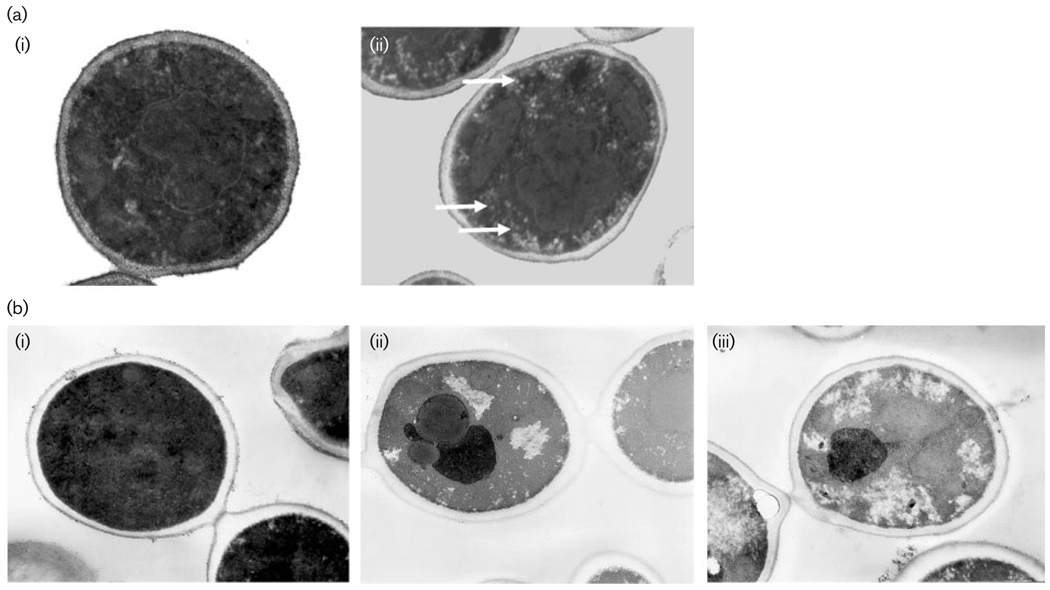
Incubation of C. albicans in the presence of AA or GPE caused notable alterations in the internal morphology as compared to control cells (Fig. 3ai, bi). At high concentrations of AA (10 mM; 580 µg ml−1) (Fig. 3aii), peripheral vacuoles were evident that were not visible in organisms from the control culture (Fig. 3ai). Treatment with GPE resulted in increased granulation of the cytoplasm and diminished cell membrane definition on increasing GPE concentration (Fig. 3bii, biii) in comparison with the control (Fig. 3bi).
Fig. 3.
Transmission electron micrographs of C. albicans treated with AA or GPE. (a) AA: (i) untreated control, (ii) 10 mM AA; note increased granulation of cytosol and appearance of peripheral vacuoles (white arrows) in the presence of AA. (b) GPE: (i) untreated control, (ii) 1 mg GPE ml−1, (iii) 30 mg GPE ml−1; pictures taken at magnification of × 18000.
Cellular respiration
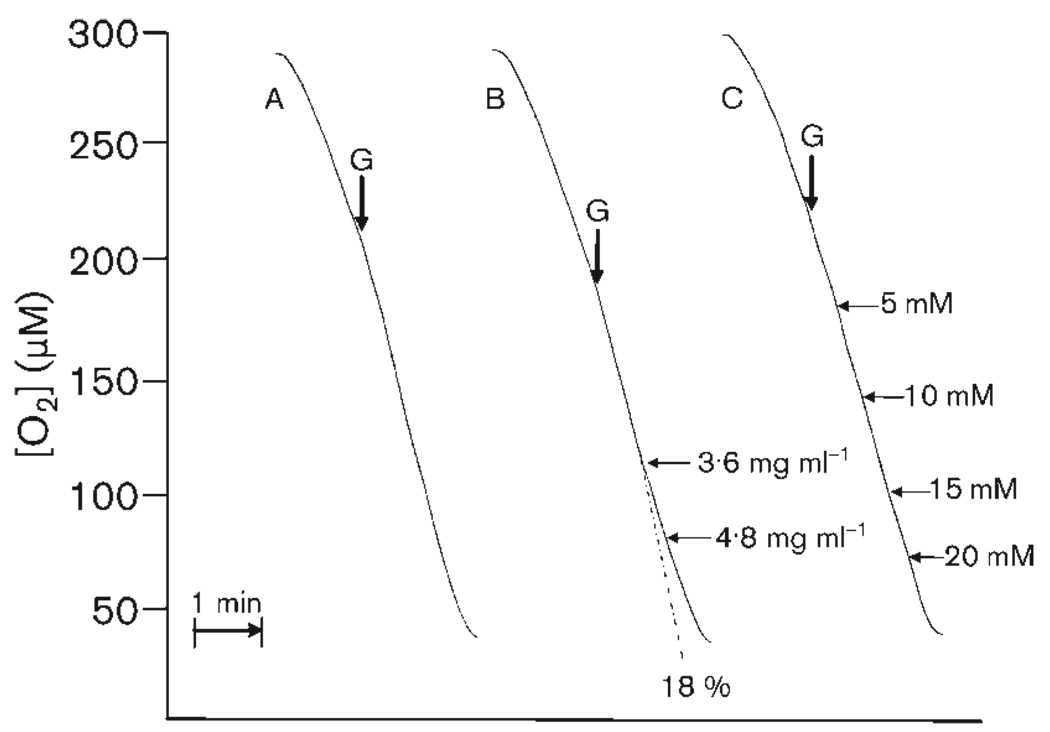
Oxygen consumption rates increased as oxygen concentration decreased, down to about 50 % of air saturation value (Fig. 4). Successive additions of 5 mM AA (29 µg ml−1) to the suspension of exponential-phase cells of C. albicans utilizing glucose supported respiration did not alter the rate of oxygen consumption. The final concentration was 20 mM AA (1·16 µg ml−1), 200 times the MIC for AA. Successive additions of 120 µg GPE ml−1 (exponential-phase cells, glucose-supported respiration) had no effect up to a concentration of 2·4 mg ml−1. Inhibition of respiration was observed at concentrations ⩾3·6 mg ml−1. A final concentration of 4·8 mg GPE ml−1 caused 18 % reduction of the rate of oxygen consumption.
Fig. 4.
Oxygen consumption of organisms harvested from an exponentially growing culture of C. albicans. Endogenous rates (addition of 100 mM glucose indicated by arrows ‘G’) are shown for control (A), garlic powder extract (B) and AA (C). Horizontal arrows indicate additions of GPE or AA; adjacent numbers indicate concentration. Percentage inhibition at a final concentration of 4·8 mg GPE ml−1 is indicated below trace B. The dotted line indicates rate of oxygen consumption if no inhibition had occurred. Cell density was 8 × 106 cells ml−1. Traces are typical of results with three batches of cells.
Confocal imaging of redox mechanisms
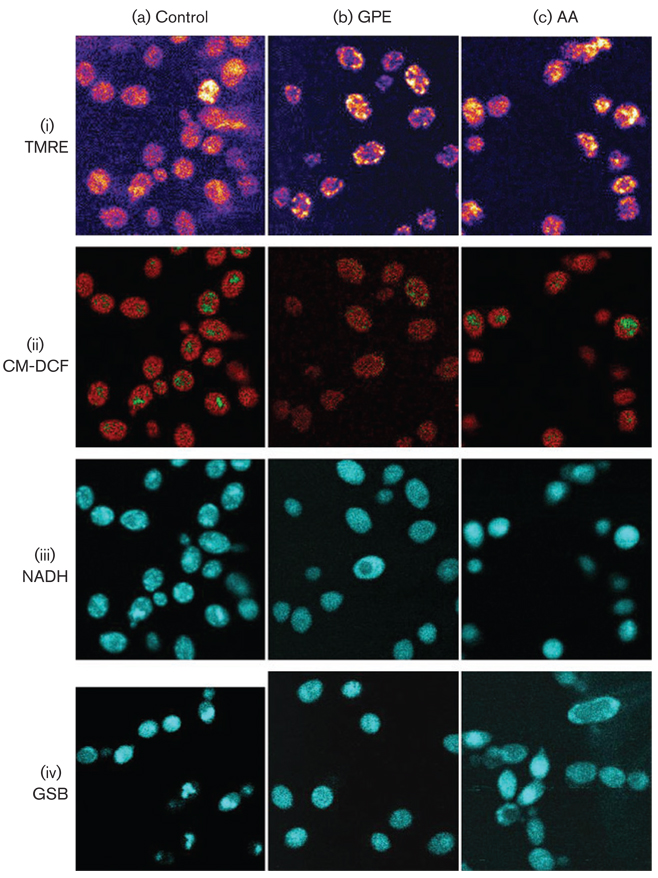
The key intracellular variables mitochondrial membrane potential (Δψm), ROS and reduced glutathione (GSH) were monitored with fluorescent probes (TMRE, CM-DCF and MCB); the NAD(P)H redox status was imaged using autofluorescence (Fig. 5). A significant increase in Δψm was evident after treatment with 1 mM AA as compared to controls or exposure or 5 mg GPE ml−1 (Fig. 5i). A pattern of effects characteristic of oxidative stress was induced (i.e. high ROS, and a low level of GSH) was induced by addition of 1 mM AA (the IC50 value for this compound) to early-stationary-phase cells (Fig. 5). Addition of 5 mg GPE ml−1 caused a similar increase in ROS and depletion of GSH, but a decrease in NADH autofluorescence was also observed. A similar response was exhibited by exponential-phase cells (not shown). Low levels of ROS were localized in the mitochondria in control cells (Fig. 5aii); increased CM-DCF fluorescence was observed in both mitochondrial and cytoplasmic compartments after treatment with either GPE or AA (Fig. 5bii and 5cii). Autofluorescence of mitochondrial NAD(P)H was intense in control samples, but this signal was attenuated by oxidation after addition of GPE and AA (Fig. 5biii and 5ciii). Autofluorescence from mitochondrial and cytosolic NADH after AA addition was quantified as mean values of the pixel intensities. The decrease of NAD(P)H autofluorescence intensity for both cytosolic and mitochondrial compartments was significant after addition of AA (P<0·001; n=25).
Fig. 5.
Fluorescent monitoring by two-photon scanning laser microscopy of C. albicans control and treated cells (1 mM AA or 5 mg GPE ml−1): (i) mitochondrial membrane potential, monitored with TMRE; (ii) ROS, monitored with CM-DCF; (iii) NAD(P)H, monitored by autofluorescence; (iv) GSH, monitored by fluorescence of GSB produced from added MCB. Images of stationary-phase cells were obtained as described in Methods. Laser conditions were as follows. (i) Untreated cells, 5 × accumulated image, 80% laser intensity; 1 mM AA, no accumulation, 40 % laser intensity; 5 mg GPE ml−1, 3 × accumulated image, 40% laser intensity. (ii, iii, iv) Untreated, 1 mM AA and 5 mg ml−1 GPE all 3 × accumulated image, 40% laser intensity.
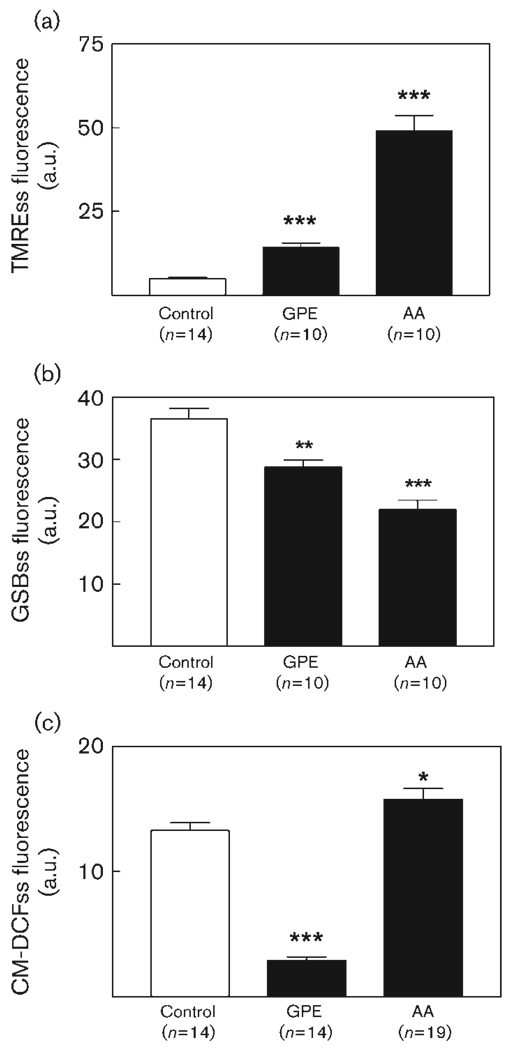
Formation of GSB, used to monitor glutathione levels before and after addition of challenge agents, indicated a general decrease in cell fluorescence (Fig. 5iv); AA gave the most significant decrease in GSB fluorescence (21 ± 0·7 a.u., n=10), to below the levels of the NAD(P)H autofluorescence control (55±0·9 a.u., n=14). The effect of GPE was less significant (Fig. 6).
Fig. 6.
Effects of garlic components on fluorescence signals from two-photon microscopy images. C. albicans cells were harvested in early stationary phase and exposed to different garlic components at room temperature. The steady-state (ss) levels of fluorescence (as the mean pixel intensity of a normal distribution, in arbitrary units) from TMRE, GSB and CM-DCF probes after 30 min treatment with 1 mM AA or 5 mg GPE ml−1 were determined. Asterisks indicate the level of significance: ***, P<0·001; **, P<0·01; *, P<0·05 versus control.
Glutathione-S-transferase activity
That GST was not a target for AA or GPE was confirmed by fluorimetric analysis of the reaction of the purified enzyme. Inhibition of GST was not evident on increasing concentrations of either AA or GPE (results not shown). This suggests that GST is not one of the intracellular targets of AA or GPE.
Flow cytometric analysis
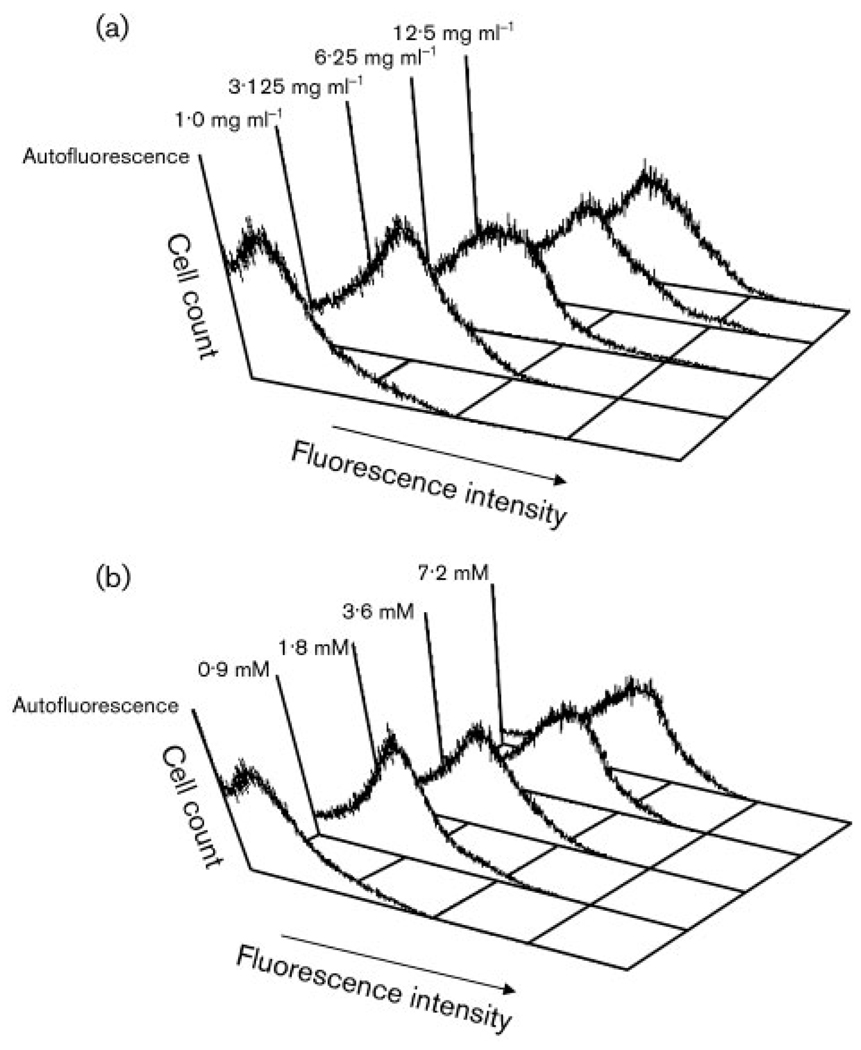
The fluorogenic compound CM-H2DCFH is oxidized by ROS to give a fluorescent product (see Methods). Control suspensions of C. albicans loaded with this fluorogen showed only autofluorescence at 530 nm, whereas cells treated with AA and GPE fluoresced much more brightly. The fluorescence intensities increased as the concentration of either challenge agent was increased (Fig. 7).
Fig. 7.
Fluorescence of C. albicans cells treated with (a) GPE or (b) AA at the concentrations indicated.
DISCUSSION
These results further confirm that some garlic components possess significant anticandidal properties (Lemar et al., 2002) and that AA, a metabolic breakdown product of allicin, plays a major role in the inhibition of growth of C. albicans.
Allicin is considered one of the principal antimicrobial constituents of triturated garlic cloves (Cavallito et al., 1944), and although it has microbicidal activity in vitro (Ankri & Mirelman, 1999), its half-life in tissues is too short to ensure appreciable pathogen damage (Koch, 1996). Systemic levels of the metabolic products of allicin are reported to be relatively high (Egen-Schwind et al., 1992), and researchers have now turned their interest to these constituents as possessing considerable antimicrobial properties. AA is found in exhaled air after ingestion of freeze-dried garlic tablets (Laasko et al., 1989). As it is a known hepato-toxicant, its use in pure form would have to be administered carefully; however, dosage up to 100 µl AA per kg body weight in rats after administration by gastric tube presented no toxicity to hepatocytes (Tygstrup et al., 1997). These studies do not preclude the use of this constituent for clinical administration at the in vivo concentration required to produce an antifungal effect. Conversion of AA to acrolein can lead to subsequent inhibition of alcohol dehydrogenases (Rando, 1974). S. cerevisiae has 20 alcohol dehydrogenases (Kruckerberg & Dickinson, 2004), but the best understood and possibly the most important enzyme targets involved in AA toxicity are the cytosolically located Adh1 and 2, involved in ethanol formation and growth on ethanol respectively, and Adh3, which is mitochondrial. The functions of Adh3 are not entirely understood, but it has been postulated to be involved in a redox shuttle, transferring mitochondrial NADH to the cytosol (Bakker et al., 2000). Improvements in methods for measuring mitochondrial redox state (see Chance, 2004, for a review) have allowed us to detect a decrease in both cytosolic and mitochondrial NADH. Results suggest that although it is possible that all three of these enzymes are targeted by AA, another site of action is involved to produce this effect (Fig. 5ciii). Depletion of NAD(P)H, the co-substrate for glutathione reductase, would diminish the recovery of the GSH pools in both compartments. As AA depletes glutathione (Glascott et al., 1996), one might predict an increase in ROS. Confocal imaging and quantitative analysis does indeed indicate the depletion of glutathione and increase in ROS when cells are treated with AA (Figs 5, 6 and 7). Electron microscopy of cells treated with AA also indicates that mitochondria may be a target for AA (Fig. 3). However, decreased respiration, as observed with GPE and other garlic constituents (K. M. Lemar and others, unpublished), was not observed for AA. This may be explained by the fact that the alcohol dehydrogenase function is not implicated in glucose respiration, as when organisms are grown in the presence of excess glucose, alcohol dehydrogenase is inactivated (Gonzalez et al., 2000). The conversion of AA to the toxic aldehyde acrolein would therefore not occur. Exposure to AA may thus impair C. albicans viability through mechanisms not directly associated with mitochondrial function.
The enzyme GST, associated with maintaining redox balance, is a target of a different garlic constituent, diallyl disulphide (DADS) (K. M. Lemar and others, unpublished). Our experiments demonstrated that it is not a target for AA. This is perhaps unsurprising, as the two compounds have quite different structures. However, this observation assists in our understanding of why GPE has such a broad spectrum of activity. The multiple metabolic constituents produced by the plant (many of which remain to be explored), may have many different targets, and hence produce diverse effects on cell processes. Its extensive repertoire of inhibitory constituents makes garlic an interesting potential alternative to single-site-specific antibiotics or synthetic organic compounds for combating C. albicans infections. However, the presence of toxic metabolites, such as AA itself, may limit the dose that can be administered. Previous investigations concerning the antifungal properties of whole garlic extracts compared to some of its breakdown products have shown that they have similar MICs, with similar mechanisms of action, such as glutathione depletion and reaction with thiol groups (Davis et al., 2003). AA may well be an exception due to the activity of the acrolein/alcohol dehydrogenase pathway activity; however its antifungal efficacy in vitro would have to be determined by MIC studies.
Abbreviations
- Δψm
mitochondrial membrane potential
- AA
allyl alcohol
- CM-DCF
carboxymethyldichlorofluorescein
- CM-H2DCFDA
5-(-6)-chloromethyl-2′,7′-dichlorohydrofluorescein diacetate
- CM-H2DCFH
5-(-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein
- GPE
garlic powder extract
- GSB
glutathione-bimane
- GST
glutathione-S-transferase
- MCB
monochlorobimane
- ROS
reactive oxygen species
- TMRE
tetramethylrhodamine ethyl ester
REFERENCES
- Ankri S, Mirelman D. Antimicrobial properties of allicin from garlic. Microbes Infect. 1999;1:125–129. doi: 10.1016/s1286-4579(99)80003-3. [DOI] [PubMed] [Google Scholar]
- Aon MA, Cortassa S, Marban E, O’Rourke B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J Biol Chem. 2003;278:44735–44744. doi: 10.1074/jbc.M302673200. [DOI] [PubMed] [Google Scholar]
- Aon MA, Cortassa S, O’Rourke B. Percolation and criticality in a mitochondrial network. Proc Natl Acad Sci U S A. 2004;101:4447–4452. doi: 10.1073/pnas.0307156101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker BM, Bro C, Kotter P, Luttik MA, van Dijken JP, Pronk JT. The mitochondrial alcohol dehydrogenase Adh3p is involved in a redox shuttle in Saccharomyces cerevisiae. J Bacteriol. 2000;182:4730–4737. doi: 10.1128/jb.182.17.4730-4737.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee SK, Mukherjee PK, Maulik SK. Garlic as an antioxidant: the good, the bad and the ugly. Phytother Res. 2003;17:97–106. doi: 10.1002/ptr.1281. [DOI] [PubMed] [Google Scholar]
- Block E. The chemistry of garlic and onions. Sci Am. 1985;252:114–119. doi: 10.1038/scientificamerican0385-114. [DOI] [PubMed] [Google Scholar]
- Block E, Putnam D, Zhao SH. Allium chemistry: GC-MS analysis of thiosulfinates and related compounds from onion, leek, scallion, shallot, chive, and Chinese chive. J Agric Food Chem. 1992;40:2431–2438. [Google Scholar]
- Cavallito C, Bailey JH, Buck JS. Allicin, the antibacterial principle of Allium sativum II. Its precursor and “essential oil” of garlic. J Am Chem Soc. 1944;67:1032–1033. [Google Scholar]
- Chance B. Mitochondrial NADH redox state, monitoring discovery and deployment in tissue. Methods Enzymol. 2004;385:361–370. doi: 10.1016/S0076-6879(04)85020-1. [DOI] [PubMed] [Google Scholar]
- Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Cortassa S, Aon MA, Winslow RL, O’Rourke B. A mitochondrial oscillator dependent on reactive oxygen species. Biophys J. 2004;87:2060–2073. doi: 10.1529/biophysj.104.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell S. PhD thesis. Cardiff School of Biosciences, Cardiff University; 2003. An investigation into the antibacterial effects of Allium sativum (garlic) [Google Scholar]
- Davis SR. An overview of the antifungal properties of allicin and its breakdown products - the possibility of a safe and effective antifungal prophylactic. Mycoses. 2005;48:95–100. doi: 10.1111/j.1439-0507.2004.01076.x. [DOI] [PubMed] [Google Scholar]
- Davis SR, Perrie R, Apitz-Castro R. The in vitro susceptibility of Scedosporium prolificans to ajoene, allitridium and a raw extract of garlic (Allium sativum) J Antimicrob Chemother. 2003;51:593–597. doi: 10.1093/jac/dkg144. [DOI] [PubMed] [Google Scholar]
- Egen-Schwind C, Eckard R, Jekat FW, Winterhoff H. Pharmacokinetics of vinyldithiins, transformation products of allicin. Planta Med. 1992;58:8–13. doi: 10.1055/s-2006-961379. [DOI] [PubMed] [Google Scholar]
- Germain E, Auger J, Ginies C, Siess MH, Teyssier C. In vivo metabolism of diallyl disulphide in the rat: identification of two new metabolites. Xenobiotica. 2002;32:1127–1138. doi: 10.1080/0049825021000017902. [DOI] [PubMed] [Google Scholar]
- Ghannoum MA. Studies on the anticandidal mode of action of Allium sativum (garlic) J Gen Microbiol. 1988;134:2917–2924. doi: 10.1099/00221287-134-11-2917. [DOI] [PubMed] [Google Scholar]
- Ghannoum MA. Inhibition of Candida adhesion to buccal epithelial cells by an aqueous extract of Allium sativum (garlic) J Appl Bacteriol. 1990;68:163–169. doi: 10.1111/j.1365-2672.1990.tb02562.x. [DOI] [PubMed] [Google Scholar]
- Glascott PA, Jr, Gilfor E, Serroni A, Farber JL. Independent antioxidant action of vitamins E and C in cultured rat hepatocytes intoxicated with allyl alcohol. Biochem Pharmacol. 1996;52:1245–1252. doi: 10.1016/0006-2952(96)00478-9. [DOI] [PubMed] [Google Scholar]
- Gonzalez E, Fernandez MR, Larroy C, Sola L, Pericas MA, Pares X, Biosca JA. Characterization of a (2R,3R)-2,3-butanediol dehydrogenase as the Saccharomyces cerevisiae YAL060W gene product. Disruption and induction of the gene. J Biol Chem. 2000;275:35876–35885. doi: 10.1074/jbc.M003035200. [DOI] [PubMed] [Google Scholar]
- Harris JC, Plummer S, Turner MP, Lloyd D. The microaerophilic flagellate Giardia intestinalis: Allium sativum (garlic) is an effective antigiardial. Microbiology. 2000;146:3119–3127. doi: 10.1099/00221287-146-12-3119. [DOI] [PubMed] [Google Scholar]
- Harris JC, Cotrell SL, Plummer S, Lloyd D. Antimicrobial properties of Allium sativum (garlic) Appl Microbiol Biotechnol. 2001;57:282–286. doi: 10.1007/s002530100722. [DOI] [PubMed] [Google Scholar]
- Johnson MA, Waterham HR, Ksheminska GP. Positive selection of novel peroxisome biogenesis-defective mutants of the yeast Pichia pastoris. Genetics. 1999;151:1379–1391. doi: 10.1093/genetics/151.4.1379. 7 other authors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch H. Biopharmaceutics of garlic’s effective compounds. In: Koch HP, Lawson LD, editors. Garlic: the Science and Therapeutic Application of Allium sativum L and related species. Baltimore: Williams & Wilkins; 1996. pp. 213–220. [Google Scholar]
- Kosower NS, Kosower EM. Thiol labeling with bromobimanes. Methods Enzymol. 1987;143:76–84. doi: 10.1016/0076-6879(87)43015-2. [DOI] [PubMed] [Google Scholar]
- Kruckerberg AL, Dickinson JR. Carbon metabolism. In: Schweizer JRDM, editor. Metabolism and Molecular Physiology of Saccharomyces cerevisiae. Boca Raton, FL: CRC Press; 2004. [Google Scholar]
- Laasko I, Seppanen-Laasko T, Hiltunen R, Muller B, Jansen H, Knoblock K. Volatile garlic odour components: gas phases and absorbed exhaled air analysed by headspace gas chromatography-mass spectrometry. Planta Med. 1989;55:257–261. doi: 10.1055/s-2006-961998. [DOI] [PubMed] [Google Scholar]
- Lawson LD. The composition and chemistry of garlic cloves and processed garlic. In: Koch HP, Lawson LD, editors. Garlic: The Science and Therapeutic Application of Allium sativum and Related Species. Baltimore: Williams & Wilkins; 1996. pp. 37–107. [Google Scholar]
- Lemar KM, Turner MP, Lloyd D. Garlic (Allium sativum) as an anti-Candida agent: a comparison of the efficacy of fresh garlic and freeze-dried extracts. J Appl Microbiol. 2002;93:398–405. doi: 10.1046/j.1365-2672.2002.01707.x. [DOI] [PubMed] [Google Scholar]
- Lemar KM, Muller CT, Plummer S, Lloyd D. Cell death mechanisms in the human opportunistic pathogen Candida albicans. J Eukaryot Microbiol. 2003;50(Suppl):685–686. doi: 10.1111/j.1550-7408.2003.tb00687.x. [DOI] [PubMed] [Google Scholar]
- Loew LM, Tuft RA, Carrington W, Fay FS. Imaging in five dimensions: time-dependent membrane potentials in individual mitochondria. Biophys J. 1993;65:2396–2407. doi: 10.1016/S0006-3495(93)81318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapoles JE, Iwahashi M, Lucas D, Zimmerman BT, Simon FR. Acetaldehyde exposure causes growth inhibition in a Chinese hamster ovary cell line that expresses alcohol dehydrogenase. Alcohol Clin Exp Res. 1994;18:632–639. doi: 10.1111/j.1530-0277.1994.tb00922.x. [DOI] [PubMed] [Google Scholar]
- Mirelman D, Monheit D, Varon S. Inhibition of growth of Entamoeba histolytica by allicin, the active principle of garlic extract (Allium sativum) J Infect Dis. 1987;156:243–244. doi: 10.1093/infdis/156.1.243. [DOI] [PubMed] [Google Scholar]
- Miron T, Rabinkov A, Mirelman D, Wilchek M, Weiner L. The mode of action of allicin: its ready permeability through phospholipid membranes may contribute to its biological activity. Biochim Biophys Acta. 2000;1463:20–30. doi: 10.1016/s0005-2736(99)00174-1. [DOI] [PubMed] [Google Scholar]
- Nagelkerke JF, van de Water B, Twiss IM, Zoetewey JP, de Bont HJ, Dogterom P, Mulder GJ. Role of microtubuli in secretion of very-low-density lipoprotein in isolated rat hepatocytes: early effects of thiol reagents. Hepatology. 1991;14:1259–1268. [PubMed] [Google Scholar]
- O’Gara EA, Hill DJ, Maslin DJ. Activities of garlic oil, garlic powder, and their diallyl constituents against Helicobacter pylori. Appl Environ Microbiol. 2000;66:2269–2273. doi: 10.1128/aem.66.5.2269-2273.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando RR. Allyl alcohol-induced irreversible inhibition of yeast alcohol dehydrogenase. Biochem Pharmacol. 1974;23:2328–2331. doi: 10.1016/0006-2952(74)90563-2. [DOI] [PubMed] [Google Scholar]
- Rees L, Minney SF, Plummer NT, Slater JH, Skyrme DA. A quantitative assessment of the anti-microbial activity of garlic (Allium sativum) World J Microbiol Biotechnol. 1993;9:303–307. doi: 10.1007/BF00383068. [DOI] [PubMed] [Google Scholar]
- Reuter R. Allium sativum and Allium ursinium: part 2-pharmacology and medical application. Phytomedicine. 1994;2:73–91. doi: 10.1016/S0944-7113(11)80052-8. [DOI] [PubMed] [Google Scholar]
- Ross ZM, O’Gara EA, Hill DJ, Sleightholme HV, Maslin DJ. Antimicrobial properties of garlic oil against human enteric bacteria: evaluation of methodologies and comparisons with garlic oil sulfides and garlic powder. Appl Environ Microbiol. 2001;67:475–480. doi: 10.1128/AEM.67.1.475-480.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestakova M, Adamek L, Stros F. Effect of crotonaldehyde on the metabolism of Candida utilis during the production of single cell protein from ethanol. Folia Microbiol. 1976;21:444–454. doi: 10.1007/BF02876935. [DOI] [PubMed] [Google Scholar]
- Sharma VD, Sethi MS, Kumar A, Rarotra JR. Antibacterial property of Allium sativum Linn. in vivo and in vitro studies. Indian J Exp Biol. 1977;15:466–468. [PubMed] [Google Scholar]
- Skyrme D. PhD thesis. University of Wales, College of Cardiff; 1997. An investigation into the anti-microbial activity of Allium sativum. [Google Scholar]
- Stoll A, Seebeck E. Synthesis of natural alliin. Experientia. 1950;6:330. doi: 10.1007/BF02152860. [DOI] [PubMed] [Google Scholar]
- Stoll A, Seebeck E. The specificity of the alliinase from Allium sativum. C R Hebd Seances Acad Sci. 1951;232:1441–1442. [PubMed] [Google Scholar]
- Turrens JF, Alexandre A, Lehninger AL. Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch Biochem Biophys. 1985;237:408–414. doi: 10.1016/0003-9861(85)90293-0. [DOI] [PubMed] [Google Scholar]
- Tygstrup N, Jensen SA, Krog B, Dalhoff K. Expression of liver functions following sub-lethal and non-lethal doses of allyl alcohol and acetaminophen in the rat. J Hepatol. 1997;27:156–162. doi: 10.1016/s0168-8278(97)80296-6. [DOI] [PubMed] [Google Scholar]
- Vanden Hoek TL, Li C, Shao Z, Schumacker PT, Becker LB. Significant levels of oxidants are generated by isolated cardiomyocytes during ischemia prior to reperfusion. J Mol Cell Cardiol. 1997a;29:2571–2583. doi: 10.1006/jmcc.1997.0497. [DOI] [PubMed] [Google Scholar]
- Vanden Hoek TL, Shao Z, Li C, Schumacker PT, Becker LB. Mitochondrial electron transport can become a significant source of oxidative injury in cardiomyocytes. J Mol Cell Cardiol. 1997b;29:2441–2450. doi: 10.1006/jmcc.1997.0481. [DOI] [PubMed] [Google Scholar]
- Vengerovskii AI, Sedykh IM, Saratikov AS. Effectiveness of hepatoprotective agents in experimental allyl alcohol poisoning. Gig Tr Prof Zabol. 1989:46–47. [PubMed] [Google Scholar]
- Wertheim T. Protozoology. London: Bailliére, Tindall & Cassel; 1844. [Google Scholar]
- Wills C, Phelps J. Functional mutants of yeast alcohol dehydrogenase affecting kinetics, cellular redox balance, and electrophoretic mobility. Biochem Genet. 1978;16:415–432. doi: 10.1007/BF00484208. [DOI] [PubMed] [Google Scholar]
- Xie Z, Kometiani P, Liu J, Li J, Shapiro JI, Askari A. Intracellular reactive oxygen species mediate the linkage of Na+/K+-ATPase to hypertrophy and its marker genes in cardiac myocytes. J Biol Chem. 1999;274:19323–19328. doi: 10.1074/jbc.274.27.19323. [DOI] [PubMed] [Google Scholar]
- Xu C, Zipfel W, Shear JB, Williams RM, Webb WW. Multiphoton fluorescence excitation: new spectral windows for biological nonlinear microscopy. Proc Natl Acad Sci U S A. 1996;93:10763–10768. doi: 10.1073/pnas.93.20.10763. [DOI] [PMC free article] [PubMed] [Google Scholar]