Summary
The product of the trk proto-oncogene encodes a receptor for nerve growth factor (NGF). Here we show that NGF is a powerful mitogen that can induce resting NIH 3T3 cells to enter S phase, grow in semisolid medium, and become morphologically transformed. These mitogenic effects are absolutely dependent on expression of gp140trk receptors, but do not require the presence of the previously described low affinity NGF receptor. gp140trk also serves as a receptor for the related factor neurotrophin-3 (NT-3), but not for brain-derived neurotrophic factor. Both NGF and NT-3 induce the rapid phosphorylation of gp140trk receptors and the transient expression of c-Fos proteins. However, NT-3 appears to elicit more limited mitogenic responses than NGF. These results indicate that the product of the trk proto-oncogene is sufficient to mediate signal transduction processes induced by NGF and NT-3, at least in proliferating cells.
Introduction
Nerve growth factor (NGF) plays an important role in the development and survival of sensory, sympathetic, and certain cholinergic neurons (Levi-Montalcini, 1987). In addition, NGF can induce the differentiation of PC12 pheochromocytoma cells into neuron-like cells (Greene and Tischler, 1976). NGF interacts with at least two types of cell surface receptors, which can be differentiated by their relative (low and high) binding affinities (Sutter et al., 1979). The low affinity receptors (Kd ≈ 1 nM) correspond to an 80,000 dalton glycoprotein (gp80LNGFR) encoded by the LNGFR locus (Hosang and Shooter, 1985; Chao et al., 1986). Molecular characterization of the human (Johnson et al., 1986), rat (Radeke et al., 1987), and chicken (Large et al., 1989) LNGFR genes revealed that the gp80LNGFR NGF receptor is a cell surface glycoprotein with three distinctive domains: an extracellular region rich in cysteine and acidic amino acid residues, a single transmembrane domain, and a short cytoplasmic region that is required for biological activity (Hempstead et al., 1990; Yan et al., 1991). The high affinity receptors (Kd ≈ 10 to 100 PM) were first identified as a molecular species of about 140,000 daltons by cross-linking experiments (Massagué et al., 1981; Hosang and Shooter, 1985), and it is generally accepted that they mediate the known biological properties of NGF (Green et al., 1986).
Recent studies have revealed that NGF can also interact with the product of the trk proto-oncogene, a 140,000 dalton glycoprotein (gp140trk) that possesses the basic structural features of the well-known members of the cell surface receptor family of tyrosine protein kinases (Martin-Zanca et al., 1989). NGF can be cross-linked to the endogenous gp140trk receptors present in the NGF-responsive PC12 cell line (Hempstead et al., 1991; Kaplan et al., 1991a; Klein et al., 1991a). Moreover, NGF binds to gp140trk expressed in heterologous mouse NIH 3T3 and monkey COS cells that do not contain low affinity gp80LNGFR receptors (Hempstead et al., 1991; Klein et al., 1991a). Whether gp140trk represents the functional high affinity receptor or is part of a high affinity receptor complex that may include other molecules such as gp80LNGFR, as recently proposed by Hempstead et al. (1991), remains to be determined.
Regardless of their relative binding affinities, the existence of two distinct classes of NGF receptors raises the question of their relative roles in mediating the signal transduction processes induced by this neurotrophic factor. It is known that ectopic expression of the gp80LNGFR receptor in nonneural cells does not confer NGF responsiveness in spite of efficient NGF binding (Johnson and Taniuchi, 1987; Hempstead et al., 1988). Yet, transfection of the LNGFRgene in certain cell lines of neuronalorigin induces responsiveness to NGF (Hempstead et al., 1989; Matsushima and Bogenmann, 1990; Pleasure et al., 1990), indicating that this receptor may participate in signal transduction upon interaction with certain proteins specifically expressed in these cells. Recent studies have indicated that addition of NGF to PC12 cells results in the rapid phosphorylation of tyrosine residues of gp140trk (Kaplan et al., 1991b; Klein et al., 1991a). Moreover, NGF can activate the intrinsic tyrosine kinase activity of the gp140trk receptors (Kaplan et al., 1991b; Klein et al., 1991a). These observations are reminiscent of the primary responses elicited by well-known mitogens such as platelet-derived growth factor (PDGF), epidermal growth factor (EGF), and fibroblast growth factors (FGFs) when they interact with their functional receptors (Ullrich and Schlessinger, 1990).
We undertook the present studies to investigate whether the gp140trk receptor could mediate NGF-induced biological responses when ectopically expressed in NGF-nonresponsive cells. In addition, we have also investigated whether gp140trk may serve as a receptor for other members of the NGF family of neurotrophic factors, such as brain-derived neurotrophic factor (BDNF) (Barde et al., 1982; Leibrock et al., 1989) and neurotrophin-3 (NT-3) (Hohn et al,, 1990; Jones and Reichardt, 1990; Maisonpierre et al., 1990a; Rosenthal et al., 1990).
Results
Mitogenic Activity of NGF in NIH 3T3 Cells Expressing gp140trk
To ascertain whether gp140trk receptors mediate NGF-induced signal transduction, we first examined whether this neurotrophic factor had mitogenic activity on E25-48 cells, a NIH 3T3–derived cell line expressing gp140trk receptors (Klein et al., 1991a). Our selection of NIH 3T3 cells was based on their lack of endogenous NGF receptors (Klein et al., 1991a) and their capacity to respond to the trk tyrosine kinase, since these cells can be efficiently transformed by trk oncogenes (Barbacid et al., 1991). As shown in Figure 1A, addition of NGF to quiescent NIH 3T3 cells did not elicit detectable mitogenic responses. However, when NGF was added to E25-48 cells, we observed a significant increase in their ability to incorporate [3H]thymidine into their DNA (Figure 1A). The amount of [3H]thymidine incorporated by these cells in response to NGF was comparable to that observed in the presence of PDGF, a well-known mitogen for NIH 3T3 cells (data not shown), and only slightly lower than that induced by 20% calf serum (Figure 1A). In contrast, addition of equal amounts of NGF to another NIH 3T3-derived cell line, B35-41, which expresses the highly related gp145trkB tyrosine protein kinase (Klein et al., 1989; Middlemas et al., 1991), did not induce significant [3H]thymidine incorporation (Figure 1A). Moreover, the mitogenic effect of NGF on gp140trk-expressing E25-48 cells was completely abolished by the addition of a monoclonal antibody elicited against NGF (Figure 1B).
Figure 1. Stimulation of [3H]Thymidine Incorporation by NGF.

(A) Quiescent NIH 3T3, E25-48, and B35-41 cells were incubated with DMEM containing 5 μg/ml insulin (open bars), 20% calf serum (hatched bars), or 30 ng/ml NGF plus 5 μg/ml insulin (filled bars). Results are expressed as the mean of three independent experiments assayed in duplicate (+SEM).
(B) E25-43 cells were stimulated by the indicated amounts of NGF either in the absence (open circles) or presence (filled circles) of a 10-fold molar excess of anti-NGF monoclonal antibody clone 27/21 (Boehringer Mannheim).
Induction of DNA Synthesis
To determine the percentage of E25-48 cells responsive to NGF, we examined DNA synthesis by immunofluorescence analysis of cells incubated in the presence of 5-bromodeoxyuridine (BrdUrd). As shown in Figure 2, addition of 20% calf serum to quiescent NIH 3T3 and E25-48 cells induced most (>70%) of these cells to enter S phase within 24 hr. As a control, insulin, a growth factor that does not have mitogenic properties in NIH 3T3 cells, elicited DNA synthesis in only 1% of NIH 3T3 cells and in 6% of E25-48 cells (Figure 2; Table 1). A similar percentage of NIH 3T3 cells transfected with control pSV2neo plasmid DNA were capable of entering S phase in the absence of added mitogen (data not shown). Addition of saturating amounts of NGF to NIH 3T3 cells failed to elicit any detectable mitogenie response (Figure 2). However, when this neurotrophic factor was added to E25-48 cells, more than 40% of them initiated DNA synthesis (Figure 2; Table 1).
Figure 2. Induction of DNA Synthesis by NGF.
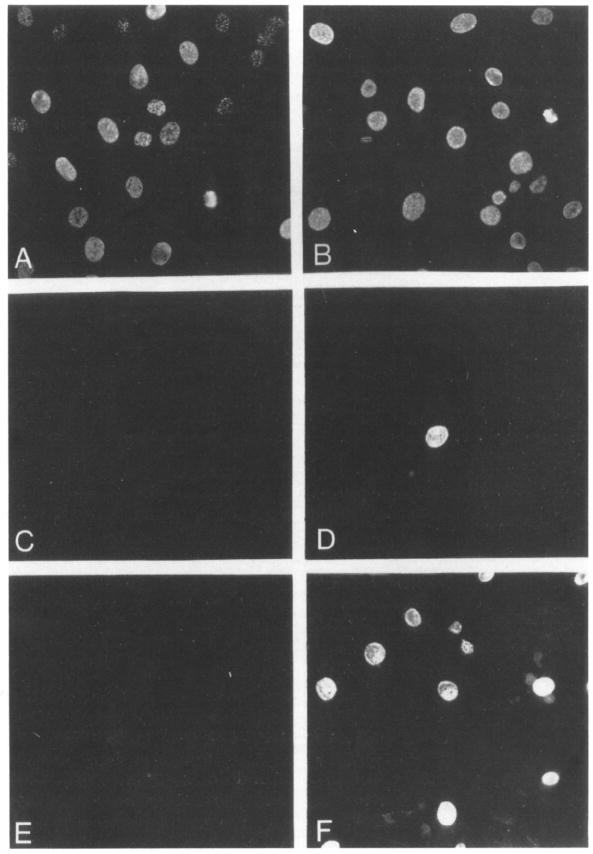
Quiescent NIH 3T3 (A, C, E) and E2546 (B, D, F) cells were incubated in the presence of 100 μM Brdlhd plus either 20% calf serum (A, B), 5 μg/ml insulin (C, D), or 10 ng/ml NGF plus 5 μg/ml insulin (E, F). Cells were fixed after 24 hr and analyzed for immunofluorescence using a mouse anti-BrdUrd monoclonal antibody and donkey anti-mouse IgG conjugated with Texas red. Final magnification 250 x
Table 1.
Induction of DNA Synthesis by NGF
| BrdUrd Incorporation |
|||||
|---|---|---|---|---|---|
| NIH 3T3 Cells |
E25-48 Cells |
||||
| Additionsa | Positive/Total | Percentage Positive | Positive/Total | Percentage Positive | Percentage Positive among gp140trk-Expressing Cellsb |
| None | 4/345 | 1.1% | 22/384 | 5.7% | NDc |
| 20% Calf serum | 330/459 | 71.9% | 241/339 | 71.1% | 100% |
| NGF 10 ng/ml | 3/313 | 0.9% | 139/345 | 40.3% | 86% |
| NGF 50 ng/ml | 2/303 | 0.7% | 149/358 | 41.6% | 89% |
All samples contained 5 μg/ml insulin.
As determined in the experiment described in Figure 3, 47% of E25-48 cells express detectable levels of gp140trk
Not determined.
E25-48 cells were derived from a single neomycin-resistant colony and have maintained their neoR phenotype and consistent levels of gp140trk expression during storage and subsequent culture. Yet, only about half of these cells respond to the mitogenic effect of NGF. Therefore, we decided to examine whether gp140trk was homogeneously expressed in this cell line. For this purpose, E25-48 cells were submitted to immunocytochemical analysis using polyclonal rabbit antibodies elicited against the carboxyl terminus of the trk proto-oncogene product. As shown in Figure 3, only about half of the E25-48 cells expressed detectable levels of gp140trk. More importantly, those cells expressing gp140trk were those that responded to NGF (Figures 3C and 3D). These results indicate that expression of the trk proto-oncogene in heterologous NIH 3T3 cells confers on them mitogenic responsiveness to NGF.
Figure 3. Responsiveness to NGF Correlates with Expression of the trk Proto-Oncogene Product.
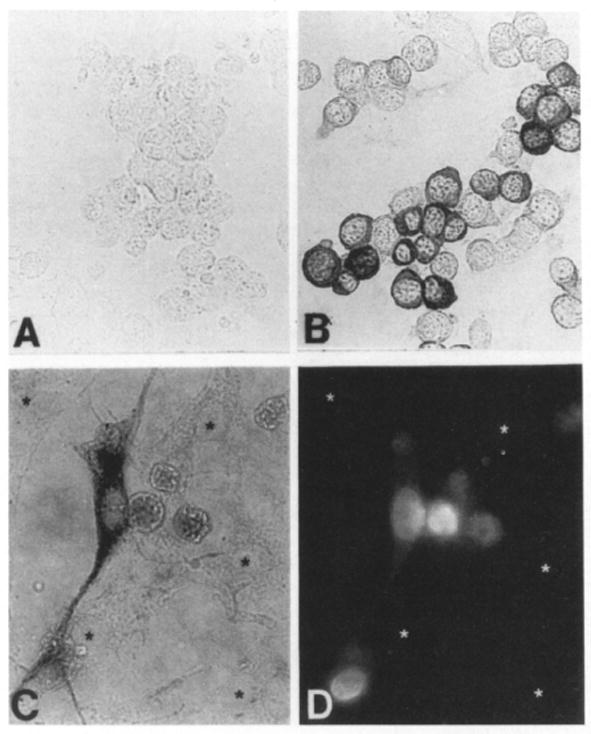
E25-48 cells were assayed for BrdUrd incorporation as described in the legend to Figure 2 and the results photographically recorded. Microslides containing BrdUrd-stained E25-48 cells were then used to immunocytochemically visualize gp140trk expression using the avidin–biotin immunoperoxidase technique. (A) Preimmune rabbit serum. (B, C) Rabbit polyclonal anti-trk antiserum. (D) BrdUrd incorporation in the same field as shown in (C). Final magnifications: 270x (A, B) and 400x (C, D). Asterisks indicate cells without detectable gp140trk expression (C) and without BrdUrd incorporation (D).
NGF Induces Cells to Grow in Semisolid Medium
Although DNA synthesis is often utilized as a parameter to determine mitogenic activity, the above experiments do not resolve whether NGF has a significant effect on the proliferative properties of cells expressing gp140trk. For this purpose, we examined whether NGF could induce E25-48 cells to grow in semisolid medium. As illustrated in Table 2, E25-48 cells in the presence of 5% calf serum exhibited very limited growth under these conditions. Addition of NGF resulted in a 7-to 9-fold induction in the number of E25-48 agar colonies. The percentage of E25-48 cells capable of growth in semisolid medium as a result of the mitogenic properties of NGF was comparable to that of B38-94 cells, a tumorigenic NIH 3T3–derived cell line transformed by a trk oncogene.
Table 2.
Growth in Semisolid Medium of Cells Expressing the gp140trk Receptors
| Cell Line | Number of Cells | Additionsa | Agar Coloniesb | NGF Stimulation |
|---|---|---|---|---|
| NIH 3T3 | 103 | – | 1 | |
| 103 | NGF | 0 | None | |
| 104 | – | 1 | ||
| 104 | NGF | 2 | None | |
| E25-48 | 103 | – | 12 | |
| 103 | NGF | 111 | 9-fold | |
| 104 | – | 79 | ||
| 104 | NGF | 546 | 7-fold | |
| B38-94 | 103 | – | 98 | |
| 103 | NGF | 90 | None | |
| 104 | – | 1604 | ||
| 104 | NGF | 1356 | None |
Cultures were fed every 4 days with 0.5 ml of DMEM containing 5% calf serum in the absence or presence of 30 ng/ml NGF as indicated.
Colonies containing more than 32 cells were scored as positive after 4 weeks of culture.
NGF and Cell Transformation
The effect of NGF on the growth properties of E25-48 cells in semisolid medium raised the possibility that this neurotrophic factor may also confer transforming properties on the product of the trk proto-oncogene. To test this hypothesis, we transfected NIH 3T3 cells with pDM89, a mammalian expression vector containing the human trk proto-oncogene (Martin-Zanca et al., 1989), in either the presence or absence of 30 ng/ml NGF in the cell culture medium. In agreement with previous studies, no significant transforming activity could be observed in the absence of NGF (Table 3) (Oskam et al., 1988). However, expression of the trk proto-oncogene in the presence of NGF induced the transformation of the recipient NIH 3T3 cells with a specific activity of 2 × 104 ffu per μg of pDM89 DNA. This transforming activity is similar to that of most known oncogenes and just 5- to 10-fold lower than that of trk oncogenes isolated from human tumors (Table 3) (Barbacid et al., 1991).
Table 3.
NGF Confers Transforming Activity on the trk Proto-Oncogene Product
| Transforming Activity (Foci per 1.5 × 105 Cells)a |
||||
|---|---|---|---|---|
| trk Gene | Plasmid | Amount of DNA | −NGF | +NGF |
| trk Proto-oncogene | pDM69 | 1 μg | 0 | TMTCb |
| 100 ng | 0 | TMTC | ||
| 10 ng | 0 | 292 | ||
| 1 ng | 0 | 23 | ||
| trk oncogenec | pDM16 | 100 ng | TMTC | TMTC |
| 1 ng | 120 | 113 | ||
Foci were scored 12 days after addition of the DNA.
TMTC, too many to count.
trk oncogene isolated from a human colon carcinoma biopsy (Martin-Zanca et al., 1986).
NT-3 Interacts with gp140trk Receptors
Two NGF-related neurotrophic factors, BDNF and NT-3, have recently been isolated and characterized at the molecular level (Barde et al., 1982; Leibrock et al., 1989; Hohn et al., 1990; Jones and Reichardt, 1990; Maisonpierre et al., 1990a; Rosenthal et al., 1990). To determine whether any of these novel neurotrophic factors also interact with the product of the trk proto-oncogene, we obtained highly purified (>95% homogeneous by SDS–PAGE analysis) preparations of BDNF and NT-3 proteins isolated from supernatantsof Sf9 cells infected with recombinant baculoviruses (see Experimental Procedures). These baculovirus-expressed proteins were tested for their ability to compete with 125I-labeled NGF for binding to the gp140trk receptors expressed in E25-48 cells. As shown in Figure 4, NT-3, but not BDNF, competed with NGF for binding to the trk proto-oncogene product, gp140trk. The observed competition was not complete even at saturating amounts (30 nM) of NT-3 protein, suggesting that this neurotrophic factor recognizes a domain in the gp140trk receptor that overlaps with, but is not identical to, that responsible for NGF binding.
Figure 4. NT-3 Competes with 125I-Labeled NGF for Binding to the trk Receptors Expressed in E25-48 Cells.
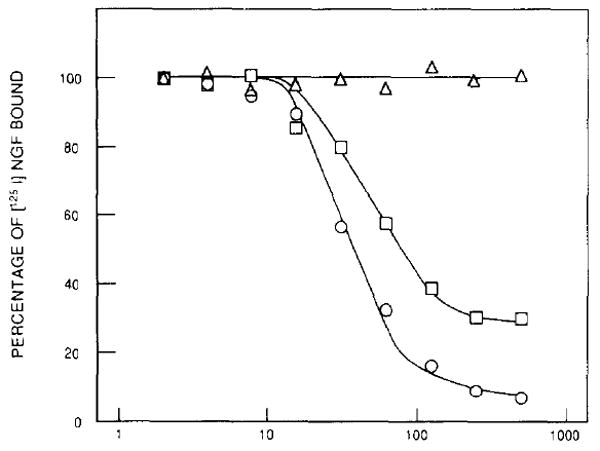
E25-48 cells were preincubated with unlabeled NGF (circles), NT-3 (squares), or BDNF (triangles) at 4°C for 1 hr. 125I-labeled NGF was added to the cultures at a final concentration of 5 ng/ml, and the cells were incubated for an additional 2 hr. The percentage of 125I-labeled NGF bound to the cells was determined as described in Experimental Procedures. Results are expressed as the mean of duplicate samples.
gp140trk Mediates the Mitogenic Properties of NT-3
The interaction of NT-3 with gp140trk may have physiological relevance since NT-3 elicited mitogenic activity in E25-48 cells. As illustrated in Figure 5, addition of NT-3 to gp140trk-expressing E25-48 cells resulted in the induction of DNA synthesis in a significant percentage of cells. No effect was observed when this factor was added to control NIH 3T3 cells (Table 4). Whereas most (>90%) of the E25-48 cells expressing detectable levels of gp140trk entered S phase upon NGF stimulation, only about half of these cells responded to NT-3 (Table 4). These results suggest that NT-3 may exert a more limited mitogenic effect on E25-48 cells than does NGF.
Figure 5. NT-3 Induces DNA Synthesis in E25-48 Cells.
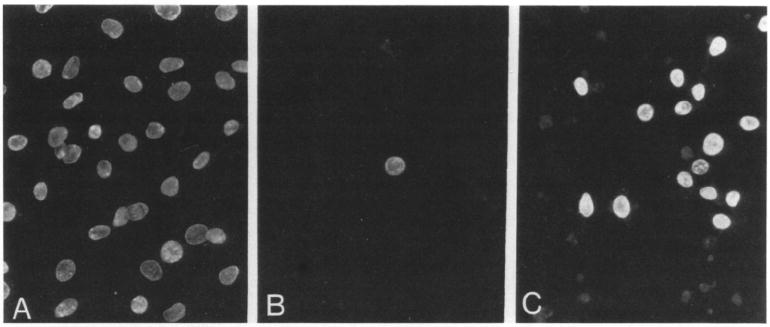
The mitogenic activity of NT-3 was measured by immunofluorescence after BrdUrd incorporation. Cells were stimulated with DMEM containing 20% calf serum (A), 5 μg/ml insulin (B), or 50 ng/ml NT-3 plus 5 μg/ml insulin (C). Final magnification 250x.
Table 4.
Mitogenic Activity of NT-3
| BrdUrd Incorporation |
||||
|---|---|---|---|---|
| NIH 3T3 Cells |
E25-48 Cells |
|||
| Additionsa | Positive/Total | Percentage Positive | Positive/Total | Percentage Positive |
| None | 4/179 | 2.2% | 19/305 | 6.2% |
| 20% Calf serum | 270/341 | 79.2% | 274/352 | 77.8% |
| NGF 50 ng/ml | 13/216 | 6.0% | 126/269 | 46.8% |
| BDNF 50 ng/ml | 4/192 | 2.1% | 34/362 | 9.3% |
| NT-3 50 ng/ml | 2/195 | 1.0% | 68/310 | 22.0% |
All samples contain 5 μg/ml insulin.
To provide a more accurate quantitative analysis of the relative mitogenic properties of NGF and NT-3 on cells expressing gp140trk receptors, we cotransfected NIH 3T3 cells with mammalian expression plasmids encoding the trk proto-oncogene (pDM89) (Martin-Zanca et al., 1989) NGF (pLTRSNGF), and NT-3 (pLL43) (Klein et al., 1991b). Cotransfection of 2 μg of pDM89 DNA with various amounts (2 μg to 2 ng) of pLTRSNGF DNA resulted in the efficient transformation of NIH 3T3 cells (Table 5). The specific transforming activity of pLTRSNGF (in the presence of saturating amounts of pDM89) was found to be 1.6 × 104 ffu/μg, a value very similar to that obtained for pDM69 DNA when transfected into NIH 3T3 cells in the presence of 30 ng/ml NGF in the culture medium (Table 3). When similar experiments were performed in the presence of pDM69 and the NT-3-encoding pLL43 plasmid, we ako observed morphologic transformation of the recipient NIH 3T3 cells. However, the specific transforming activity of pLL43 DNA was only 1.5 × 102 ffu/μg, a value about 100-fold lower than that obtained for pLTRSNGF.
Table 5.
Transformation of NIH 3T3 Cells by Cotransfection of Expression Plasmids Encoding gp140trk, NGF, and NT-3
| Cotransfected DNAs | ||||||
|---|---|---|---|---|---|---|
| gp140trk Receptors |
Neurotrophic Factors |
Transforming Activity (Foci per 1.5 × 105 Cells) |
||||
| Plasmid | DNA (ng) | Plasmida | DNA (ng) | Exp. 1 | Exp. 2 | |
| pDM69 | 2000 | – | – | 0 | 0 | |
| – | – | pLTRSNGF (NGF) | 2000 | 0 | 0 | |
| pDM69 | 2000 | + | pLTRSNGF (NGF) | 2000 | TMTCb | TMTCb |
| pDM69 | 2000 | + | pLTRSNGF (NGF) | 200 | TMTC | TMTC |
| pDM69 | 2000 | + | pLTRSNGF (NGF) | 20 | 350 | 344 |
| pDM69 | 2000 | + | pLTRSNGF (NGF) | 2 | 53 | 32 |
| – | – | pLL43 (NT-3) | 2000 | 0 | 0 | |
| pDM69 | 2000 | + | pLL43 (NT-3) | 2000 | 380 | 210 |
| pDM69 | 2000 | + | pLL43 (NT-3) | 200 | 78 | 44 |
| pDM69 | 2000 | + | pLL43 (NT-3) | 20 | 6 | NDc |
| pDM69 | 2000 | + | pLL43 (NT-3) | 2 | 0 | ND |
| – | – | pLL42 (BDNF) | 2000 | 0 | 0 | |
| pDM69 | 2000 | + | pLL42 (BDNF) | 2000 | 0 | 0 |
| pDM69 | 2000 | + | pLL42 (BDNF) | 200 | 0 | 0 |
The neutrophic factor encoded by each plasmid is indicated in parenthesis.
TMTC, too many to count.
ND, not done.
gp140trk Mediates induction of c-Fos by Neurotrophic Factors
It has been previously shown that addition of NGF to PC12 cells results in the rapid phosphorylation of gp140trk receptors (Kaplan et al., 1991b; Klein et al., 1991a) followed by the induction of c-fos gene expression (Curran and Morgan, 1985; Greenberg et al., 1985; Kruijer et al., 1985). To determine whether the mitogenic effects of NGF and NT-3 on NIH 3T3 cells followed similar signal transduction pathways, we examined the levels of tyrosine phosphorylation of gp140trk in E25-48 cells treated either with purified NGF or with supernatants of Sf9 cells infected with NT-3 recombinant baculoviruses. As shown in Figure 6, both NGF and NT-3 elicited a rapid increase in the phosphorylation levels of gp140trk receptors present in these cells. However, no significant effect was observed with Sf9 supernatants containing BDNF. Similar results were obtained with different preparations of NT-3 and BDNF derived from supernatants of COS cells transiently transfected with CMV/SV40-derived NT-3 and BDNF expression vectors (Figure 6D).
Figure 6. NGF and NT-3 Stimulate Tyrosine Phosphorylation of the trk Proto-Oncogene Product.
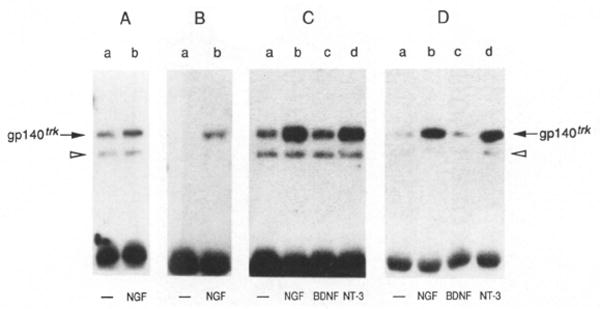
Quiescent E25-48 cells were incubated for 10 min at 37°C with DMEM alone (lanes a) or with DMEM containing 50 ng/ml NGF (lanes b), BDNF (lanes c), or NT-3 (lanes d). Cells were lysed in RIPAE buffer containing 0.5 mM sodium orthovanadate, immunoprecipitated with a rabbit polyclonal antibody elicited against a peptide corresponding to the carboxy-terminal domain of gp140trk (Martin-Zanca et al., 1989) and analyzed by 8% SDS–polyacrylamide gel electrophoresis. The electrophoresed samples were transferred to nitrocellulose filters and blotted with anti-trk antiserum (A) or anti-phosphotyrosine monoclonal antibody 4G10 (UBI) (B–D) as described in Experimental Procedures. (C) depicts tyrosine phosphorylation of gp140trk after stimulation with baculovirus-expressed and BDNF and NT-3, (D) after stimulation with COS cell–expressed BNDF and NT-3. The resulting nitrocellulose filters were exposed to Kodak X-Omat film for 8 hr at −70°C with the help of an intensifying screen.
Addition of NGF and NT-3 to E25-48 cells induced the rapid expression of c-Fos proteins as determined by immunofluorescence analysis (Figure 7; Table 6). No such effect was observed when E25-48 cells were replaced by parental NIH 3T3 cells (data not shown). As summarized in Table 6, NGF was somewhat more effective in inducing c-Fos expression than NT-3. These results provide additional support for the concept that NGF is more efficient than NT-3 in mediating signal transduction through gp140trk receptors.
Figure 7. NGF and NT-3 Induce Expression of c-Fos Protein in E25-48 Cells.
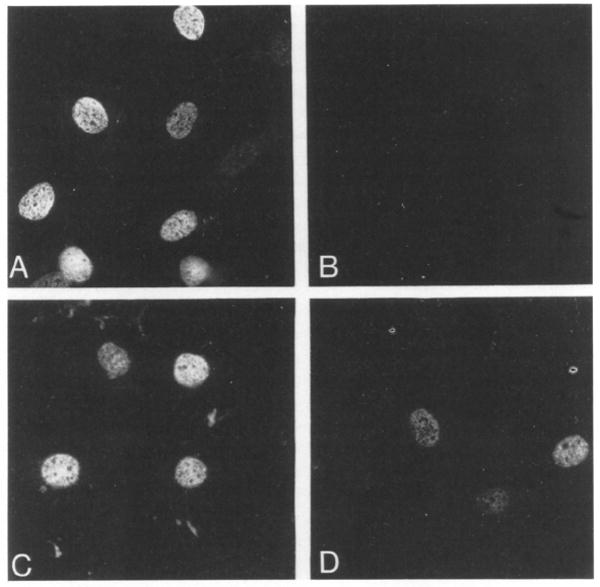
Quiescent E25-48 cells were incubated at 37°C for 90 min in DMEM containing either 20% calf serum (A), 5 μg/ml insulin (B), 50 ng/ml NGF (C), or 50 ng/ml NT-3 (D). After fixation in methanol, immunofluorescence was performed using anti-c-Fos antibodies and anti-rabbit IgG conjugated with rhodamine. Final magnification 390x.
Table 6.
Induction of c-fos Expression by Neurotrophic Factors in gp140trk-expressing E25-48 Cells
| Experiment 1 |
Experiment 2 |
|||
|---|---|---|---|---|
| Additionsa | Positive/Total | Percentage Positive | Positive/Total | Percentage Positive |
| None | 6/257 | 2.3% | 13/216 | 5.7% |
| 20% Calf serum | 190/266 | 71.4% | 143/184 | 77.7% |
| NGF 50 ng/ml | 119/282 | 42.2% | 80/184 | 43.5% |
| BDNF 50 ng/ml | 12/204 | 5.8% | NDb | – |
| NT-3 50 ng/ml | 84/262 | 32.1% | 89/235 | 37.9% |
| NT-3 10 ng/ml | NDb | – | 65/191 | 34.1% |
All samples contain 5 μg/ml insulin.
ND, not determined.
Finally, we conducted kinetic studies of the induction of c-Fos expression in E25-48 cells as a response to NGF. As shown in Figure 8, c-Fos proteins were rapidly synthesized in response to NGF stimulation, reaching maximal expression at 30 to 60 min after addition of the neurotrophic factor. The induced c-Fos proteins disappeared 3 hr after NGF stimulation. Parallel studies with the NGF-responsive PC12 cells revealed similar kinetics (Figure 8). These results suggest that the mitogenic activity of neurotrophic factors in heterologous cells expressing functional gp140trk receptors is transmitted by signal transduction pathways related to those used to exert their neurotrophic properties.
Figure 8. Kinetics of c-Fos Induction by NGF.

Quiescent PC12 (A) and E25-48 (B) cells were stimulated with 100 ng/ml NGF for the indicated times in minutes, pulse labeled for 30 min with 400 μCi/ml [35S]methionine, and the cell lysates immunoprecipitated with rabbit polyclonal antic-Fos antibodies. lmmunoprecipitates were analyzed by 12% SDS–polyacrylamide gel electrophoresis. Gels were subjected to fluorography, dried, and exposed to Kodak X-Omat film for 3 days at −70°C with the help of an intensifying screen. Coelectrophoresed molecular weight markers include phosphorylase B (92,000) albumin (69,000), ovalbumin (46,000) and carbonic anhydrase (30,000).
Discussion
The present studies demonstrate that NGF has strong mitogenic activity in heterologous NIH 3T3–derived E25-48 cells that express the trk tyrosine protein kinase, gp140trk. Addition of NGF to quiescent E25-48 cells resulted in the induction of DNA synthesis in essentially all of the cells that expressed gp140trk receptors. Such a response did not require the presence of additional factors, except insulin. The most dramatic illustration of the mitogenic properties of NGF resides in its ability to induce E25-48 cells to grow in semisolid agar and to induce the morphologic transformation of NIH 3T3 cells transfected with the trk proto-oncogene. These observations indicate that NGF has mitogenic activity comparable to that of growth factors with oncogenic potential, such as v-sis/PDGF, EGF/TGFα, and some of the members of the FGF family (Deuel, 1987; Ross, 1987; Burgess and Maciag, 1989). Removal of NGF from morphologically transformed cultures of NIH 3T3 cells expressing the normal trk proto-oncogene causes the rapid reversion of their transformed phenotype. These results indicate that cellular transformation is a direct response to the mitogenic stimulus of NGF on gp140trk receptors.
It has been recently proposed that the neurotrophic effects of NGF are mediated by heteromeric receptors that encompass the low affinity gp80LNGFR NGF-binding protein and the gp140trk tyrosine kinase (Hempstead et al., 1991). Our studies, however, illustrate that the mitogenic properties of NGF can be solely mediated by gp140trk receptors. It is possible that the proliferative effects of NGF (Aloe and Levi-Montalcini, 1977; Burstein and Greene, 1982; Lillien and Claude, 1985; Otten et al., 1989; Represa and Bernd, 1989; Cattaneo and McKay, 1990) are mediated by signal transduction pathways unrelated to those involved in neuronal differentiation and survival. However, highly transforming oncogenes such as v-src and v-ras induce neuronal differentiation of PC12 cells (Alema et al., 1985; Bar-Sagi and Feramisco, 1985; Noda et al., 1985) suggesting overlapping signal transduction pathways for proliferative and differentiation responses.
Alternatively, NGF may utilize the gp80LNGFR–gp140trk receptor complex to initiate signal transduction in neuronal cells, whereas it may require gp140trk receptors only to elicit mitogenic responses in NIH 3T3 cells. Evidence supporting this hypothesis has been provided by Hempstead et al. (1989, 1990) who reported that a nonresponsive PC12 cell line variant became responsive to NGF upon transfection of the LNGFR gene. Moreover, Yan et al. (1991) have shown that PC12 cells transfected with a chimerit EGF–NGF receptor can be induced to differentiate by EGF in the absence of NGF. These results imply that the short cytoplasmic domain of the low affinity gp80LNGFR receptor must be responsible for inducing the neuronal differentiation of PC12 cells, either alone or upon interaction with gp140trk receptors. Interestingly, since EGF does not bind to gp140trk, such interaction would not require ligand-mediated activation of the gp140trk receptors. However, recent studies by Weskamp and Reichardt (1991) have shown that NGF can induce neurite formation in PC12 cells in the presence of polyclonal antibodies that block binding of NGF to gp80LNGFR receptors. These results imply a direct role of gp140trk in the induction of neural differentiation of PC12 cells by NGF.
The product of the trk proto-oncogene may also serve as a receptor for neurotrophic factors other than NGF. Competition experiments indicated that NT-3, but not BDNF, partially displaced 125I-labeled NGF from binding to gp140trk. Since saturating amounts of NT-3 displaced only about 70% of the bound NGF, these factors may compete for overlapping but not identical binding sites. The interaction of NT-3 with gp140trk receptors elicits a limited functional response. Addition of partially purified NT-3 produced in a baculovirus expression system stimulates thymidine incorporation in E25-48 cells and induces a significant number of these cells to enter S phase. However, the overall mitogenic effect of NT-3 on E25-48 cells appears to be more limited than that of NGF. In agreement with these results, we have observed that NT-3 induces PC12 cells to acquire a neuronal-like phenotype, but they form very small neuritic processes (unpublished data). Whether this neurotrophic effect of NT-3 on PC12 cells is mediated by gp140trk or by another neurotrophic receptor remains to be determined.
The interaction of NGF and NT-3 with gp140trk receptors in heterologous E25-48 cells appears to activate signal transduction pathways similar to those observed in PC12 cells. Addition of either NGF or NT-3 to quiescent E25-48 cells elicits the rapid phosphorylation of tyrosine residues in gp140trk (Kaplan et al., 1991b; Klein et al., 1991a). Moreover, both of these neurotrophic factors induced the rapid expression of the c-fos gene, a hallmark of the biochemical response of PC12 cells to NGF (Curran and Morgan, 1985; Greenberg et al., 1985; Kruijer et al., 1985). Immunoprecipitation analysis of c-Fos proteins in NGF-stimulated PC12 and E25-48 cells revealed similar kinetics, comparable levels of expression, and an indistinguishable pattern of phosphorylation, as deduced from their heterogeneous electrophoretic mobilities. These observations raise the possibility that NGF and NT-3 may utilize similar signal transduction pathways to elicit their mitogenic responses in dividing cells and their neutrophic effects in nondividing neuronal cells.
NGF, NT-3, and BDNF are highly related molecules even in their prohormone forms. As mature neurotrophic factors, they exhibit almost 60% amino acid identity, including their six cysteine residues; the latter presumably play a major role in determining the factors’ tertiary structures (Barde et al., 1982; Leibrock et al., 1989; Hohn et al., 1990; Jones and Reichardt, 1990; Maisonpierre et al., 1990a; Rosenthal et al., 1990). Based on comparison of primary amino acid sequences, each of these factors is equally related to the other two; however, their specificities for the members of the trk family of receptors are significantly different. NGF interacts with gp140trk receptors with high affinity, yet it does not bind to the highly related gp145trkB tyrosine kinase (Klein et al., 1991a). Likewise, BDNF recognizes gp145trkB, but it does not recognize gp140trk receptors (Klein et al., 1991b). In contrast, NT-3 has less selective binding requirements and interacts with similar efficiencies with gp140trk and gp145trkB receptors (Klein et al., 1991b). Whether the broad spectrum of NT-3 interactions with these tyrosine kinases has physiological relevance remains to be determined.
Recent studies have indicated that whereas NT-3 is widely expressed in immature regions of the central nervous system, expression of NGF and BDNF is largely restricted to specific structures (Maisonpierre et al., 1990b; Kaisho et al., 1991). The widely distributed expression of NT-3 dissipates as the developing structures reach maturity. Therefore, it is tempting to speculate that NT-3 may play an important role in the early stages of development, a time in which its broad reactivity might be advantageous to the embryo. Additional biological studies along with a more precise definition of the pattern of gp140trk expression in the embryonic and adult nervous system are required to establish the extent of the involvement of this tyrosine protein kinase in mediating the neurotrophic properties of NGF and NT-3.
Experimental Procedures
Cells and Neurotrophic Factors
Cells, including NIH 3T3 (Jainchill et al., 1969). E25-48, B35-41 (Klein et al., 1991a), and B38-94 cell lines, were grown in Duibecco’s modified Eagle’s medium (DMEM) containing 10% calf serum. B38-94 cells were obtained by transfecting NIH 3T3 cells with pRK25, a mammalian expression plasmid containing the trk5 oncogene (Oskam et al., 1988; Coulier et al., 1990). PC12 cells (Greene and Tischler. 1976) were grown on collagen-coated (Vitrogen) dishes in DMEM containing 10% horse serum and 5% fetal calf serum. NIH 3T3, E25-48. and B38-94 cells were grown in semisolid medium as described elsewhere (Ozanne et al., 1980). Gene transfer assays were performed by the calcium phosphate precipitation technique as previously described (Graham and van der Eb, 1973).
Murine 2.5S NGF was purchased from Upstate Biotechnology, Inc. 125I-labeled NGF was obtained from Amersham (1500 Ci/mmol). Human BDNF and NT-3 were produced using a baculovirus expression system (Summers and Smith, 1987). Human BDNF and NT-3 DNA cassettes containing the coding regions for the preprohormones were generated by PCR amplification of genomic DNA and subcloned into pBluescript KS(–) (Jones and Reichardt, 1990). The 850 bp BDNF and 960 bp NT-3 inserts were released by EcoRI and BamHI digestion and subcloned into EcoRI-and BamHI-linearized pVL 1392 vector to generate pAcS27 (BDNF) and pAcS28 (NT-3). Properly processed, biologically active factors were harvested from 2 liter suspension cultures of Sf9 insect cells 46 hr after infection with either AcS27-6 or AcS28-3 recombinant bacuioviruses. Cell culture supernatants were supplemented with 1 mM EDTA, 1 mM PMSF and batch adsorbed onto CM-52 cation exchanger (Whatman) previously equilibrated with 100 mM sodium phosphate buffer (pH 6.5) containing 10% glycerol, 25 mM NaCl, 1 mM EDTA, and 1 mM PMSF. Bound proteins were eiuted with a linear NaCl gradient. Fractions eluting at 0.4–0.5 M NaCl were collected, dialyzed against 10 mM sodium phosphate buffer (pH 6.8), lyophilized, and reconstituted in 10 mM sodium phosphate buffer (pH 6.8) containing 25 mM NaCl. This procedure yielded >95% pure BDNF and NT-3 proteins as judged by Coomassie blue–stained SDS–polyacrylamide gels. We have established the amino-terminal sequence of NT-3 as YAEHKSHRGEYSV, indicating that the baculovirus expression system is able to process these proteins correctly, at least at the amino terminus (Jones and Reichardt, 1990). Transient expression of BDNF and NT-3 using the COS cell expression system was performed as described elsewhere (Klein et al., 1991b). Mammalian expression plasmids encoding the trk proto-oncogene (pDM69). NGF (pLTRSNGF), BDNF (pLL42), and NT-3 (pLL43) have been described elsewhere (Martin-Zanca et al., 1989; Klein et al., 1991b).
Thymidine Incorporation
Cells were seeded into collagen-coated 6-well plates (Vitrogen) at a density of 5 × 105 per well. After 24 hr, cultures were made quiescent in DMEM containing 0.5% calf serum. After adding the corresponding growth factors, cells were incubated for 18 hr prior to the addition of 1 μCi of [3H]thymidine (85 Ci/mmol; Amersham). Cells were incubated (PBS), trypsinized, and the amount of [3H]thymidine incorporated into DNA determined by filtration through glass filters (Schleicher & Schueli) followed by extensive washing with deionized water. Filter-bound [3H]thymidine was measured by liquid scintillation counting.
lmmunofluorescence Assays
Cells were grown on collagen-coated glass coverslips (Bellco) in 6-well plates at a density of 5 × 105 per well. Cells were made quiescent as described above and incubated with the required factors for 18 hr prior to the addition of 100 μM BrdUrd (Sigma). After an additional 8 hr incubation, cells were fixed with cold methanol (4°C) for 10 min, rehydrated in PBS, and incubated for 30 min in 1.5 M HCl in order to denature the DNA. After washing the coverslips three times in PBS, cells were incubated with a 1:50 dilution of a mouse monoclonal anti-BrdUrd antibody (Becton Dickinson) for 30 min at room temperature, washed with PBS, and incubated with a 1:50 dilution of a donkey polycionai anti-mouse IgG antiserum conjugated with Texas red (Amersham). Finally. cells were washed in PBS–Hoechst dye for 10 min to visualize and to count the nuclei. Coverslips were mounted using Fluoromount G (Southern Biotechnic), and slides were examined under a Zeiss microscope equipped with Ploems epifluorescence optics. A minimum of five random high power fields (400x) were analyzed; results were expressed as number of cells showing positive nuclear fluorescence over the total number of cells counted. NGF-induced c-fos expression was detected by utilizing a similar procedure. Cells were instead incubated with a 1500 dilution of rabbit poiyclonal anti-c-Fos antibodies (Kovary and Bravo, 1991) followed by incubation with swine polyclonai anti-rabbit IgG conjugated with rhodamine (Becton Dickinson).
lmmunocytochemistry
Cells were fixed with methanol and incubated with a 1:1000 dilution of a rabbit polyclonal antiserum raised against a peptide corresponding to the carboxyl terminus of the human gp140trk receptor (Martin-Zanca et al., 1989), followed by a second incubation with a 1:1000 dilution of biotinylated goat anti-rabbit IgG antibodies and a 1:25 dilution of avidin–biotin immunoperoxidase complex (Vector Laboratories). The sites of immunoprecipitate formation were identified using light microscopy following treatment with the chromogen 3,3′-diaminobenzidine tetrahydrochloride (Sigma).
125I-NGF Binding Assays
Cells were harvested and washed once with ice-cold DMEM containing 0.1% BSA (DMEM–BSA). Cells (2 × 105 per sample) were incubated with 80 μl of ice-cold DMEM–BSA containing various concentrations, ranging from 4–500 ng/ml, of unlabeled NGF, BDNF, or NT-3 at 4°C for 1 hr. Twenty microliters of ice-cold DMEM–BSA containing 5 ng/ml or 25 ng/ml 125I-labeled NGF was added to each sample to reach a final concentration of 1 ng/ml or 5 ng/ml 125I-labeled NGF. Cells were further incubated at 4°C for 2 hr, washed four times with ice-cold DMEM–BSA, and iysed in 100 μl of 1 N NaOH. The radioactivity associated with the cells was determined in a G5500 gamma counter (Jing et al., 1990). Nonspecific binding was determined by measuring the radioactivity associated with control NIH 3T3 cells under the same experimental conditions. Nonspecific binding (<10% of the amount of 125I-labeled NGF bound to E25-48 cells in the absence of unlabeled ligand) was subtracted from the results presented.
lmmunoprecipltation Assays and Western Blots
Metabolic labeling of E25-48 and PC12 cells with [35S]methionine and immunoprecipitation analysis with anti-c-Fos antibodies was performed as described (Kovary and Bravo, 1991). Detection of tyrosine phosphorylation of gp140trk receptors by Western blot analysis was done essentially as described (Klein et al., 1991a), with the following modifications: First, mouse anti-phosphotyrosine monoclonal antibody 4G10 (UBI) was used as primary antibody. Second, nitrocellulose filters were subsequently incubated with a secondary rabbit anti-mouse IgG antibody (Dako) before probing with 125I-labeled protein A.
Acknowledgments
We thank Sherri Bryant, Linda K. Long, and Caroline Zarou for excellent technical assistance, Rodrigo Bravo for making available his anti-c-Fos antibodies, and G. Hathaway for determining the amino-terminal sequence of the purified NT-3 protein. K. Ft. J. is supported by an American Cancer Society postdoctoral fellowship. L. F. R. is an Investigator of the Howard Hughes Medical Institute.
References
- Alema S, Casalbore P, Agostini E, Tato F. Differentiation of PC12 phaeochromocytoma cells induced by v-src oncogene. Nature. 1985;316:557–559. doi: 10.1038/316557a0. [DOI] [PubMed] [Google Scholar]
- Aloe L, Levi-Montalcini R. Mast cells increase in tissues of neonatal rats injected with the nerve growth factor. Brain Res. 1977;133:358–366. doi: 10.1016/0006-8993(77)90772-7. [DOI] [PubMed] [Google Scholar]
- Barbacid M, Lamballe F, Pulido D, Klein R. The trk family of tyrosine protein kinase receptors. Biochim Biophys Acta Rev Cancer. 1991 doi: 10.1016/0304-419x(91)90010-i. in press. [DOI] [PubMed] [Google Scholar]
- Barde Y-A, Edgar D, Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982;1:549–553. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Sagi D, Feramisco JR. Microinjection of the ras oncogene protein into PC12 cells induces morphological differentiation. Cell. 1985;42:841–848. doi: 10.1016/0092-8674(85)90280-6. [DOI] [PubMed] [Google Scholar]
- Burgess WH, Maciag T. The heparin-binding (fibroblast) growth factor family of proteins. Annu Rev Biochem. 1989;58:575–606. doi: 10.1146/annurev.bi.58.070189.003043. [DOI] [PubMed] [Google Scholar]
- Burstein DE, Greene LA. Nerve growth factor has both mitogenic and antimitogenic activity. Dev Biol. 1982;94:477–482. doi: 10.1016/0012-1606(82)90364-5. [DOI] [PubMed] [Google Scholar]
- Cattaneo E, McKay R. Proliferation and differentiation of neuronal stem cells regulated by nerve growth factor. Nature. 1990;347:762–765. doi: 10.1038/347762a0. [DOI] [PubMed] [Google Scholar]
- Chao MV, Bothwell MA, Ross AH, Koprowski H, Lanahan AA, Buck CR, Sehgal A. Gene transfer and molecular cloning of the human NGF receptor. Science. 1986;232:518–521. doi: 10.1126/science.3008331. [DOI] [PubMed] [Google Scholar]
- Coulier F, Kumar R, Ernst J, Klein R, Martin-Zanca D, Barbacid M. Human trk oncogenes activated by point mutation, in-frame deletion, and duplication of the tyrosine kinase domain. Mol Cell Biol. 1990;10:4202–4210. doi: 10.1128/mcb.10.8.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran T, Morgan JI. Superinduction of c-fos by nerve growth factor in the presence of peripherally active benzodiazepines. Science. 1985;229:1265–1268. doi: 10.1126/science.4035354. [DOI] [PubMed] [Google Scholar]
- Deuel TF. Polypeptide growth factors: roles in normal and abnormal cell growth. Annu Rev Cell Biol. 1987;3:443–492. doi: 10.1146/annurev.cb.03.110187.002303. [DOI] [PubMed] [Google Scholar]
- Graham FL, van der Eb AJ. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973;52:456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Green SH, Rydel RE, Connolly JL, Greene LA. PC12 cell mutants that possess low- but not high-affinity nerve growth factor receptors neither respond to nor internalize nerve growth factor. J Cell Biol. 1986;102:830–843. doi: 10.1083/jcb.102.3.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg ME, Greene LA, Ziff EB. Nerve growth factor and epidermal growth factor induce rapid transient changes in proto-oncogene transcription in PC12cells. J Biol Chem. 1985;260:14101–14110. [PubMed] [Google Scholar]
- Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempstead BL, Patil N, Olson K, Chao MV. Molecular analysis of the nerve growth factor receptor. Cold Spring Harbor Symp Quant Biol. 1988;53:477–485. doi: 10.1101/sqb.1988.053.01.055. [DOI] [PubMed] [Google Scholar]
- Hempstead BL, Schleifer LS, Chao MV. Expression of functional nerve growth factor receptors after gene transfer. Science. 1989;243:373–375. doi: 10.1126/science.2536190. [DOI] [PubMed] [Google Scholar]
- Hempstead BL, Patil N, Thiel B, Chao MV. Deletion of cytoplasmic sequences of the nerve growth factor receptor leads to loss of high affinity ligand binding. J Biol Chem. 1990;265:9595–9598. [PubMed] [Google Scholar]
- Hempstead BL, Martin-Zanca D, Kaplan DR, Parada LF, Chao MV. High-affinity NGF binding requires coexpression of the trk proto-oncogene and the low-affinity NGF receptor. Nature. 1991;350:678–683. doi: 10.1038/350678a0. [DOI] [PubMed] [Google Scholar]
- Hohn A, Leibrock J, Bailey K, Barde Y-A. Identification and characterization of a novel member of the nerve growth factor/brain-derived neurotrophic factor family. Nature. 1990;344:339–341. doi: 10.1038/344339a0. [DOI] [PubMed] [Google Scholar]
- Hosang M, Shooter EM. Molecular characteristics of nerve growth factor receptors on PC12 cells. J Biol Chem. 1985;260:655–662. [PubMed] [Google Scholar]
- Jainchill JL, Aaronson SA, Todaro GJ. Murine sarcoma and leukemia viruses: assay using clonal lines of contact-inhibited mouse cells. J Virol. 1969;4:549–553. doi: 10.1128/jvi.4.5.549-553.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing S, Spencer T, Miller K, Hopkins C, Trowbridge IS. Role of the human transferrin receptor cytoplasmic domain in endocytosis: localization of a specific signal sequence for internalization. J Cell Biol. 1990;110:283–294. doi: 10.1083/jcb.110.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D, Lanahan A, Buck CR, Sehgal A, Morgan C, Mercer E, Bothwell M, Chao MV. Expression and structure of the human NGF receptor. Cell. 1986;47:545–554. doi: 10.1016/0092-8674(86)90619-7. [DOI] [PubMed] [Google Scholar]
- Johnson EM, Jr, Taniuchi M. Nerve growth factor (NGF) receptors in the central nervous system. Biochem Pharmacol. 1987;36:4189–4195. doi: 10.1016/0006-2952(87)90658-7. [DOI] [PubMed] [Google Scholar]
- Jones KR, Reichardt LF. Molecular cloning of a human gene that is a member of the nerve growth factor family. Proc Natl Acad Sci USA. 1990;87:8060–8084. doi: 10.1073/pnas.87.20.8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaisho Y, Shintani A, Ono Y, Kato K, Igarashi K. Regional expression of the nerve growth factor gene family in rat brain during development. Biochem Biophys Res Commun. 1991;174:379–386. doi: 10.1016/0006-291x(91)90531-b. [DOI] [PubMed] [Google Scholar]
- Kaplan DR, Hempstead BL, Martin-Zanca D, Chao MV, Parada LF. The trk proto-oncogene product: a signal transducing receptor for nerve growth factor. Science. 1991a;252:554–558. doi: 10.1126/science.1850549. [DOI] [PubMed] [Google Scholar]
- Kaplan DR, Martin-Zanca D, Parada LF. Tyrosine phosphorylation and tyrosine kinase activity of the trk proto-oncogene product induced by NGF. Nature. 1991b;350:158–160. doi: 10.1038/350158a0. [DOI] [PubMed] [Google Scholar]
- Klein R, Parada LF, Coulier F, Barbacid M. trkB, a novel tyrosine protein kinase receptor expressed during mouse neural development. EMBO J. 1989;8:3701–3709. doi: 10.1002/j.1460-2075.1989.tb08545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R, Jing S, Nanduri V, O’Rourke E, Barbacid M. The trk proto-oncogene encodes a receptor for nerve growth factor. Cell. 1991a;65:189–197. doi: 10.1016/0092-8674(91)90419-y. [DOI] [PubMed] [Google Scholar]
- Klein R, Nanduri V, Jing S, Lamballe F, Tapley P, Bryant S, Cordon-Cardo C, Jones KR, Reichardt LF, Barbacid M. The trkB tyrosine protein kinase is a receptor for brain-derived neurotrophic factor and neurotrophin-3. Cell. 1991b;66 doi: 10.1016/0092-8674(91)90628-c. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovary K, Bravo R. Expression of different Jun and Fos proteins during the G0-to-G1 transition in mouse fibroblasts: in vitro and in vivo associations. Mol Cell Biol. 1991;11:2451–2459. doi: 10.1128/mcb.11.5.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruijer W, Schubert D, Verma IM. Induction of the proto-oncogene fos by nerve growth factor. Proc Natl Acad Sci USA. 1985;82:7330–7334. doi: 10.1073/pnas.82.21.7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large TH, Weskamp G, Helder JC, Radeke MJ, Misko TP, Shooter EM, Reichardt LF. Structure and developmental expression of the nerve growth factor receptor in the chicken central nervous system. Neuron. 1989;2:1123–1134. doi: 10.1016/0896-6273(89)90179-7. [DOI] [PubMed] [Google Scholar]
- Leibrock J, Lottspeich F, Hohn A, Hofer M, Hengerer B, Masiakowski P, Thoenen H, Barde Y-A. Molecular cloning and expression of brain-derived neurotrophic factor. Nature. 1989;341:149–152. doi: 10.1038/341149a0. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor: thirty-five years later. EMBO J. 1987;6:1145–1154. doi: 10.1002/j.1460-2075.1987.tb02347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillien LE, Claude P. Nerve growth factor is a mitogen for cultured chromaffin cells. Nature. 1985;317:632–634. doi: 10.1038/317632a0. [DOI] [PubMed] [Google Scholar]
- Maisonpierre PC, Belluscio L, Squinto S, Ip NY, Furth ME, Lindsay RM, Yancopoulos GD. Neurotrophin-3: a neurotrophic factor related to NGF and BDNF. Science. 1990a;247:1446–1451. doi: 10.1126/science.247.4949.1446. [DOI] [PubMed] [Google Scholar]
- Maisonpierre PC, Belluscio L, Friedman B, Adlerson RF, Wiegand SJ, Furth ME, Lindsay RM, Yancopoulos GD. NT-3, BDNF, and NGF in the developing rat nervous system: parallel as well as reciprocal patterns of expression. Neuron. 1990b;5:501–509. doi: 10.1016/0896-6273(90)90089-x. [DOI] [PubMed] [Google Scholar]
- Martin-Zanca D, Hughes SH, Barbacid M. A human oncogene formed by the fusion of truncated tropomyosin and protein tyrosine kinase sequences. Nature. 1986;319:743–748. doi: 10.1038/319743a0. [DOI] [PubMed] [Google Scholar]
- Martin-Zanca D, Oskam R, Mitra G, Copeland T, Barbacid M. Molecular and biochemical characterization of the human trk proto-oncogene. Mol Cell Biol. 1989;9:24–33. doi: 10.1128/mcb.9.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J, Guillette BJ, Czech MP, Morgan CJ, Bradshaw RA. Identification of a nerve growth factor receptor protein in sympathetic ganglia membranes by affinity labeling. J Biol Chem. 1981;256:9419–9424. [PubMed] [Google Scholar]
- Matsushima H, Bogenmann E. Nerve growth factor (NGF) induces neuronal differentiation in neuroblastoma cells transfected with the NGF receptor cDNA. Mol Cell Biol. 1990;10:5015–5020. doi: 10.1128/mcb.10.9.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlemas DS, Lindberg RA, Hunter T. trkB, aneural receptor protein-tyrosine kinase: evidence for a full-length and two truncated receptors. Mol Cell Biol. 1991;11:143–153. doi: 10.1128/mcb.11.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M, Ko M, Ogura A, Liu DG, Amano T, Takano T, Ikawa Y. Sarcoma viruses carrying ras oncogenes induce differentiation-associated properties in a neuronal cell line. Nature. 1985;318:73–75. doi: 10.1038/318073a0. [DOI] [PubMed] [Google Scholar]
- Oskam R, Coulier F, Ernst M, Martin-Zanca D, Barbacid M. Frequent generation of oncogenes by in vitro recombination of TRK protooncogene sequences. Proc Natl Acad Sci USA. 1988;85:2964–2968. doi: 10.1073/pnas.85.9.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten U, Ehrhard P, Peck R. Nerve growth factor induces growth and differentiation of human B lymphocytes. Proc Natl Acad Sci USA. 1989;86:10059–10063. doi: 10.1073/pnas.86.24.10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozanne B, Fulton RJ, Kaplan PL. Kirsten murine sarcoma virus transformed cell lines and a spontaneously transformed rat cell-line produce transforming factors. J Cell Physiol. 1980;105:163–180. doi: 10.1002/jcp.1041050118. [DOI] [PubMed] [Google Scholar]
- Pleasure SJ, Reddy UR, Venkatakrishnan G, Roy AK, Chen J, Ross AH, Trojanowski JQ, Pleasure DE, Lee VM. Introduction of nerve growth factor (NGF) receptors into a medulloblastoma cell line results in expression of high- and low-affinity NGF receptors but not NGF-mediated differentiation. Proc Natl Acad Sci USA. 1990;87:8496–8500. doi: 10.1073/pnas.87.21.8496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radeke MJ, Misko TP, Hsu C, Herzenberg LA, Shooter EM. Gene transfer and molecular cloning of the rat nerve growth factor receptor. Nature. 1987;325:593–597. doi: 10.1038/325593a0. [DOI] [PubMed] [Google Scholar]
- Represa J, Bernd P. Nerve growth factor and serum differentially regulate development of the embryonic otic vesicle and cochleovestibular ganglion in vitro. Dev Biol. 1989;134:21–29. doi: 10.1016/0012-1606(89)90074-2. [DOI] [PubMed] [Google Scholar]
- Rosenthal A, Goeddel DV, Nguyen T, Lewis M, Shih A, Laramee GR, Nikolics K, Winslow JW. Primary structure and biological activity of a novel human neurotrophic factor. Neuron. 1990;4:767–773. doi: 10.1016/0896-6273(90)90203-r. [DOI] [PubMed] [Google Scholar]
- Ross R. Platelet-derived growth factor. Annu Rev Med. 1987;38:71–79. doi: 10.1146/annurev.me.38.020187.000443. [DOI] [PubMed] [Google Scholar]
- Summers MD, Smith GE. Bulletin No. 1555. College Station, Texas: Texas Agricultural Experiment Station and Texas A&M University; 1987. A manual of methods for baculovirus vectors and insect cell culture procedures; pp. 10–39. [Google Scholar]
- Sutter A, Riopelle RJ, Harris-Warrick RM, Shooter EM. Nerve growth factor receptors. Characterization of two distinct classes of binding sites on chick embryo sensory ganglia cells. J Biol Chem. 1979;254:5972–5982. [PubMed] [Google Scholar]
- Ullrich A, Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990;61:203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Weskamp G, Reichardt LF. Evidence that biological activity of NGF is mediated through a novel subclass of high affinity receptors. Neuron. 1991;6:649–663. doi: 10.1016/0896-6273(91)90067-a. [DOI] [PubMed] [Google Scholar]
- Yan H, Schlessinger J, Chao MV. Chimeric NGF–EGF receptors define domains responsible for neuronal differentiation. Science. 1991;252:561–563. doi: 10.1126/science.1850551. [DOI] [PubMed] [Google Scholar]


