Summary
We present genetic evidence that integrins regulate epithelial–mesenchymal interactions during organogenesis. Mice with a mutation in the α8 gene do not express the integrin α8β1 and exhibit profound deficits in kidney morphogenesis. In wild-type animals, inductive interactions between the ureteric epithelium and metanephric mesenchyme are essential for kidney morphogenesis. In α8 mutant homozygotes, growth and branching of the ureteric bud and recruitment of mesenchymal cells into epithelial structures are defective. Consistent with these phenotypes, α8 expression is induced in mesenchymal cells upon contact with the ureter. Since none of its previously identified ligands appears likely to mediate the essential functions of α8β1 in kidney morphogenesis, we have used an α8β1–alkaline phosphatase chimera to localize novel ligand(s) in the growing ureter. The distribution of these ligand(s) makes them strong candidates for regulators of kidney morphogenesis.
Introduction
The development of an organism requires precise spatiotemporal coordination of cell behavior and is therefore highly dependent on communication among cells. Reciprocal inductive interactions among cells or groups of cells are of particular importance during embryogenesis because they result in cellular differentiation and formation of tissues and organs (Spemann and Mangold, 1924; reviewed in Gurdon, 1992). Morphogenesis of the kidney serves as an excellent model system to analyze the cellular and molecular mechanisms underlying organogenesis (reviewed in Grobstein, 1956; Saxen, 1987). Kidney development depends on a series of reciprocal inductive events between the ureteric epithelium and the metanephric mesenchyme. In the mouse, the ureteric bud emanates from the Wolffian duct on embryonic day (E) 10.5–E11 (Figure 1). Subsequently, the ureteric bud grows toward and invades the metanephric mesenchyme. Mesenchymal cells condense around the tips of the growing and branching ureter and are transformed in a sequential order into pretubular aggregates, renal vesicles, comma-shaped bodies, and S-shaped bodies that ultimately give rise to the epithelia of the tubules and glomeruli of the mature kidney. The many branches of the ureter form the collecting duct tree of the kidney.
Figure 1. Schematic Representation of Kidney Development.

(A) At E10.5–E11.5 the ureteric bud (utb) emanates from the Wolffian duct (wd).
(B) The ureteric bud invades the metanephric mesenchyme (um) and branches. The mesenchyme condenses (cm) around the tip of the ureter branches (ub).
(C) The mesenchyme is transformed via intermediates such as pretubular aggregates (pa) and comma-shaped bodies (c).
(D) The mesenchyme has transformed into an epithelium consisting of distal tubules (dt) and proximal tubules (pt) that will mature into the excretory nephron. st, ureter stalk.
Scale bars, 10 μm.
Molecular genetic analyses in mice have revealed a complex network of regulatory proteins that control kidney organogenesis. These include the secreted signaling molecules glial cell line–derived neurotrophic factor (GDNF), wnt-4, bone morphogenetic protein 7 (BMP7), and platelet-derived growth factor B (PDGF-B) and their receptors such as the c-ret and PDGFβ tyrosine kinases (Soriano, 1994; Leveen et al., 1994; Stark et al., 1994; Dudley et al., 1995; Luo et al., 1995; Moore et al., 1996; Pichel et al., 1996; Sanchez et al., 1996; Schuchardt et al., 1996). A number of transcription factors, including WT-1, Pax-2, and BF-2, are also required for kidney development (Kreidberg et al., 1993; Torres et al., 1995; Hatini et al., 1996). Transcription factors and signaling molecules must regulate expression levels and activities of downstream targets that execute the imposed developmental program. Among strong candidates for downstream targets are integrins, a family of heterodimeric cell surface proteins that serve as receptors for extracellular matrix (ECM) molecules and counterreceptors on adjacent cells (reviewed in Hynes, 1992). Experiments with kidney organ cultures have provided evidence that integrin receptors and their ligands regulate the development of polarized epithelia from metanephric mesenchymal cells following induction by the ureteric epithelium (reviewed in Ekblom, 1996).
We now demonstrate that integrins are required in vivo for kidney morphogenesis. We have previously shown that the integrin α8 subunit forms heterodimers exclusively with the integrin β1 subunit and that these heterodimers serve as receptors for the ECM molecules fibronectin (FN), vitronectin (VN), and tenascin-C (TN-C) (Müller et al., 1995; Schnapp et al., 1995; Varnum-Finney et al., 1995). We show here that integrin α8β1 is expressed in many developing organs and particularly in the kidney, in mesenchymal cells bordering on epithelial cell sheets that undergo branching morphogenesis. Mice carrying a targeted mutation in the integrin α8 gene show severe deficits in kidney morphogenesis due to reduced growth of the ureteric bud toward the metanephric mesenchyme, reduced branching of the ureteric epithelium within the mesenchyme, and defective epithelialization of kidney mesenchymal cells. We colocalize a novel ligand to the surface of the branching ureter that is likely a mediator of the effects on kidney morphogenesis.
Results
Integrin α8 Expression Is Induced in Mesenchymal Cells Surrounding the Growing and Branching Ureter
To determine the expression patterns of the integrin α8 subunit during mouse development, we cloned the mouse α8 cDNA and raised in rabbits an antibody against a peptide encompassing the cytoplasmic domain of α8. A second antibody was raised against the extracellular domain of the α8 subunit expressed as a soluble secreted protein in COS cells. Both antibodies were affinity purified, revealed identical expression patterns for the α8 subunit, and did not cross-react with other integrins in Western blots performed with extracts from various mouse tissues (data not shown). In agreement with earlier observations in chicken (Bossy et al., 1991), immunoprecipitation analyses employing extracts from various mouse tissues revealed that the α8 subunit was associated exclusively with the integrin β1 subunit (data not shown). At all developmental stages analyzed, the integrin α8 subunit, reflecting the presence of the α8β1 receptor, was highly expressed in mesenchymal but not epithelial cells in developing organs such as the gut, the lung, the gonads, and the nephrogenic cord (Figures 2 and 3 and data not shown).
Figure 2. Immunohistochemical Localization of the Integrin α8 Subunit in the Developing Kidney.
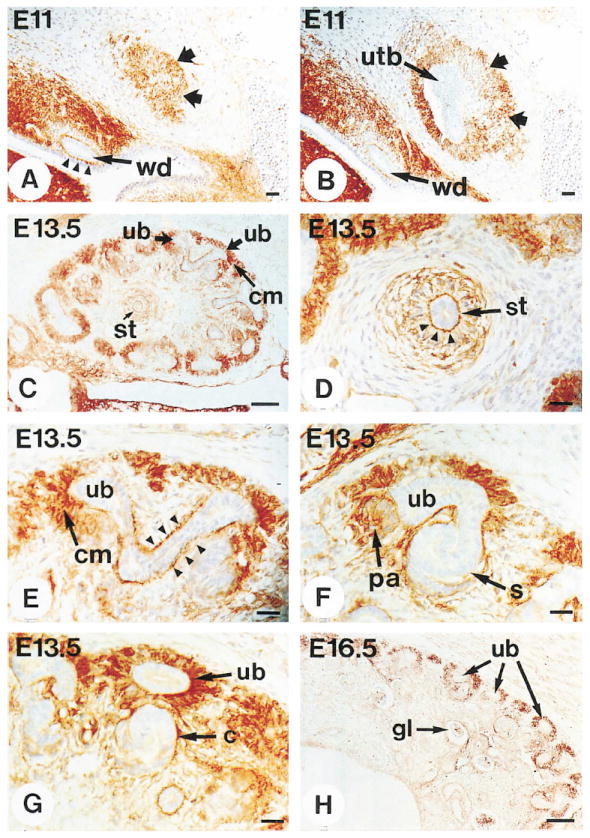
Sections of wild-type embryos were stained with antibody to the extracellular domain of the integrin α8 subunit. Abbreviations as in Figure 1.
(A and B) At E11, expression of the α8 subunit is evident in mesenchymal cells surrounding the Wolffian duct, with high concentrations at the interface between the duct epithelium and the surrounding mesenchymal cells (arrowheads). The α8 subunit is also expressed in mesenchymal cells above the tip of the growing ureteric bud (A, wide arrows) and surrounding the ureteric bud (B, wide arrows). (C–G) At E13.5, the α8 subunit is expressed in the condensing mesenchyme surrounding the tips of the branching ureter (C and E) and in pretubular aggregates (F) but not in comma-shaped bodies (G) or S-shaped (s) bodies (F). The α8 subunit is expressed at high levels in mesenchymal cells bordering on the ureteric epithelium, as shown in a transverse section (D, arrowheads) and longitudinal section (E, arrowheads) through the ureter.
(H) At E16.5, expression of the α8 subunit is seen in the outer layer of the kidney on condensing mesenchymal cells but not more internally on differentiated structures such as the glomerulus (gl).
Scale bars, 100 μm (C and H) and 10 μm (A, B, and D–G).
Figure 3. Targeting of the α8 Locus.
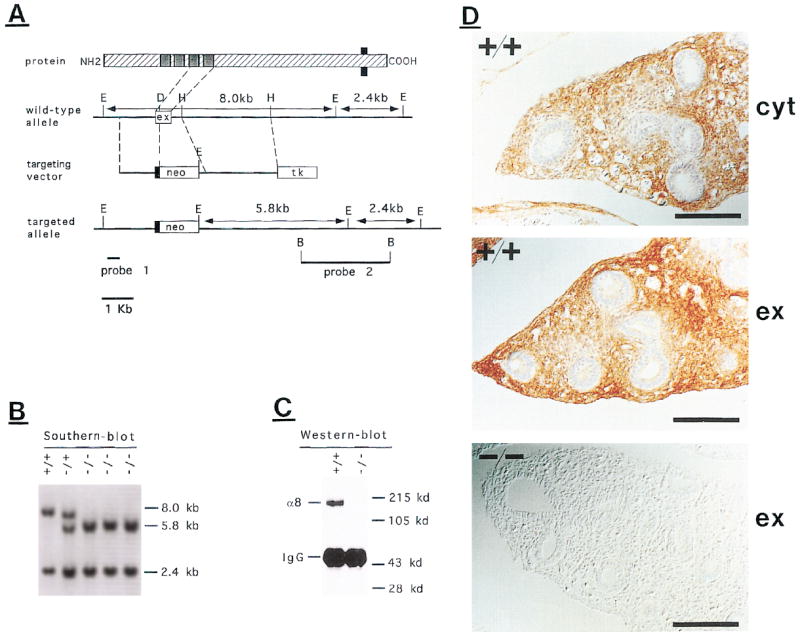
(A) Schematic representation of the α8 protein and restriction map of the genomic α8 clone. The configuration of the targeting construct, the targeted allele, the probes used for screening, and the expected Southern blot fragments are indicated. E, EcoRI; H, HindIII; D, DpnI; B, BamHI. For DpnI only one of many restriction sites is shown.
(B) Mouse tail DNA from offspring of an intercross between heterozygous mice was digested with EcoRI and analyzed by Southern blotting with probe 2. The 8.0 kb band reflects the wild-type α8 allele, and the 5.8 kb band reflects the targeted α8 locus. A 2.4 kb band is detected in both genotypes.
(C) Extracts were prepared from lung tissue of newborn wild-type or homozygous mutant mice. α8 protein was immunoprecipitated with an antiserum specific for the extracellular domain of α8, followed by western blot with a second antiserum against the extracellular domain. Proteins were visualized by chemiluminescence. The positions of the α8 protein and the immunoglobulin G heavy chain are indicated.
(D) Sections through the lung of wild-type and homozygous mutant P0 animals were stained with an antibody against the cytoplasmic (cyt) or extracellular (ex) domain of integrin α8. Scale bars, 50 μm.
The spatiotemporal expression patterns of the integrin α8 subunit in the developing kidney were studied in detail. Figure 1 summarizes different stages of development of the metanephric kidney, and Figure 2 shows the corresponding expression patterns for the integrin α8 subunit. At E11, the integrin α8 subunit was expressed throughout the mesenchyme of the nephrogenic cord. Very high expression was observed in mesenchymal cells immediately surrounding the Wolffian duct (Figure 2A). Prior to formation of the ureteric bud, no α8 expression was evident within the mesenchyme that separates the urogenital ridge from the metanephric mesenchyme and within the metanephric mesenchyme itself. Upon branching of the ureteric bud from the Wolffian duct, expression of the integrin α8 subunit was induced in cells surrounding the growing ureteric epithelium as it extended toward the metanephric mesenchyme (Figures 2A and 2B). At E13.5, the α8 subunit was expressed within the metanephric mesenchyme in mesenchymal condensates around the branching tips of the ureter (Figures 2C and 2E) and in pretubular aggregates (Figure 2F) but not on comma-shaped bodies (Figure 2G) or S-shaped bodies (Figure 2F). Thus, expression of the α8 subunit was first induced in mesenchymal cells upon contact with the ureter but was subsequently down-regulated upon epithelialization of these cells. This was particularly evident in sections from E16.5 animals (Figure 2H), where α8 was expressed in condensing mesenchymal cells in the outer marginal zone of the kidney but not in more internal parts that contained more differentiated structures.
In contrast to the transient expression of the α8 subunit in mesenchymal cells that differentiated into epithelium, α8 expression remained high in the mesenchymal cells that bordered on the ureteric epithelium (Figures 2D and 2E). The strong polarized and continuous expression of the α8 subunit on the cell surface of mesenchymal cells facing toward the ureter epithelium suggests that α8β1 heterodimers may become localized by a ligand expressed on the ureteric epithelium.
Integrin α8–Deficient Mice Show at Birth Renal Agenesis or Dysgenesis
To determine the essential functions of the integrin α8β1 receptor, we inactivated the gene encoding the α8 subunit by gene targeting in mice (Figure 3). Heterozygous animals carrying one mutated α8 locus showed no overt abnormal phenotype. Homozygous mutant animals developed to birth with approximately the expected Mendelian frequency (33 of 163 animals), but most homozygous mutants died by the first or second day after birth. Western blot analysis as well as immunohistochemistry confirmed that we had generated a null allele, because α8 protein was undetectable in homozygous mutants by either technique (Figures 3C and 3D).
Analysis of homozygous mutant animals at birth revealed severe kidney abnormalities (Figure 4 and Table 1). In contrast to wild-type animals (Figure 4A), 54% of the mutant animals were born without ureters or kidneys (Figure 4B). In 40% of the animals a ureter had formed unilaterally and had invaded the metanephric mesenchyme (Figures 4C and 4D). Once the ureter had invaded the mesenchyme, either it did not branch and no nephrons were evident (14% of the mutants), or more substantial branching occurred and excretory nephrons were evident (26% of the mutants; Figures 4C and 4D). In fact, the latter structures were similar in shape but typically smaller than wild-type kidneys (data not shown). In a few mutant animals (6%), two ureters had formed, had invaded the metanephric mesenchyme, and had branched to varying degrees. In a preliminary analysis, we did not observe major abnormalities in organs other than the kidneys.
Figure 4. Kidney Phenotype in α8-Deficient Mice at P0.

The urogenital tract was dissected from wild-type (A) or α8-deficient (B–D) mice.
(A) In wild-type mice the adrenal glands (ad), kidneys (k), ureter (u), bladder (b), and testes (t) were well developed.
(B) The most seriously affected mutants had no ureter and kidney, but the bladder and adrenal glands were unaffected. Note that the dorsal aorta (da) and uterine horns (uh) were not removed in this dissection.
(C) One small kidney and one rudiment (boxed) from a mutant animal.
(D) High magnification view of the rudiment (boxed in C). The star indicates the undifferentiated mesenchyme between the adrenal gland and the ureter. Scale bars, 100 μm.
Table 1.
Kidney Deficiencies in Newborn Animals
| Genotype | +/+ | +/− | −/− |
|---|---|---|---|
| 0 Kidneys | 0 | 0 | 31 |
| 1 Rudiment | 0 | 0 | 8 |
| 1 Kidney | 0 | 0 | 15a |
| Kidney + rudiment | 0 | 0 | 2a |
| 2 Kidneys | 30 | 105 | 1a |
The urogenital tract was dissected from newborn animals derived from intercrosses of mice heterozygous for a mutant α8 allele. The number of animals with the indicated number of kidneys or kidney rudiments was determined, and the genotype of the offspring was confirmed by Southern blot analysis.
Mutant kidneys were in most cases substantially smaller than wild-type kidneys.
Some of the homozygous mutant animals survived for several weeks or months after birth. All surviving animals had one kidney, suggesting that the cause of death in mutant animals was kidney agenesis or dysgenesis. The presence of animals with single kidneys demonstrates that stochastic factors contribute to the variability in phenotype. To test for potential genetic contributions, we interbred homozygous mutants that had survived for several months. Sixty-five of the 97 offspring (67%) of these crossings survived with one or two small kidneys. Because the original heterozygous founders are a genetic mix of 129 and C57Bl/6 mice, the data suggest that a genetic modifier locus (or multiple loci) present in one or both of the two genetic backgrounds modulates the penetrance of the α8-dependent phenotype (see Discussion).
Integrin α8β1 Is Required for Growth and Branching of the Ureter and for Development of Epithelial Specializations
As discussed above, we showed that expression of the integrin α8 subunit is induced in mesenchymal cells bordering on the growing and branching ureteric bud and on condensing mesenchymal cells in the metanephric mesenchyme. The expression patterns suggested that integrin α8β1 may be required for formation, growth, and branching of the ureter; for formation of epithelial structures from mesenchymal cells; or for both. To distinguish among these possibilities, we analyzed by standart histological techniques the progressive development of the kidney in α8-deficient embryos (Figure 5 and Table 2). We focused on E11.5, when the ureteric bud invades the metanephric mesenchyme, and on E13.5 and E16.5, when the ureter in wild-type animals has branched within the mesenchyme and signs of epithelial differentiation are visible.
Figure 5. Kidney Phenotype in α8-Deficient Mice at E11.5 and E13.5.
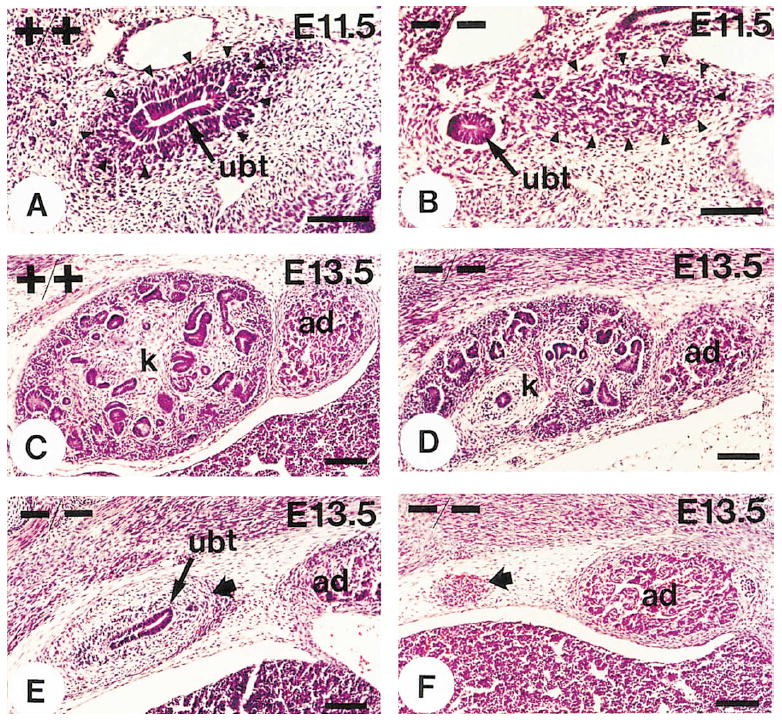
Sections were stained with hematoxylin and eosin.
(A and B) Sections from E11.5 wild-type and mutant kidney regions. The arrowheads demarcate the metanephric mesenchyme. In wild-type animals (A) the ureteric bud (ubt) had invaded the metanephric mesenchyme. In α8-deficient mice (B) the ureteric bud had developed but had not invaded the metanephric mesenchyme.
(C) Section from a wild-type metanephros at E13.5.
(D–F) Sections showing the extent of metanephric development at E13.5 in α8-deficient animals. In some animals the ureter had branched within the mesenchyme (D); in some it had invaded the mesenchyme (wide arrow) but had not branched (E); and in others it had not reached the mesenchyme (wide arrow), which was in the process of degeneration (F).
k, kidney; ad, adrenal gland. Scale bars, 100 μm.
Table 2.
Analysis of Metanephric Development
| Developmental age | E11.5 | E13.5 | P0 |
|---|---|---|---|
| Wild type | n = 10 | n = 10 | n = 60 |
| Ureter, branches | 100% | 100% | 100% |
| α8 Mutant | n = 8 | n = 28 | n = 114 |
| No ureter | 0% | 29% | 75% |
| Ureter, not in mesenchyme | 100% | 25% | 0% |
| Ureter, in mesenchyme | 0% | 29% | 9% |
| Ureter, branches | 0% | 18% | 17% |
Wild-type or mutant embryos (E11.5 or E13.5) were sectioned, stained with hematoxilin and eosin, and analyzed for progression of metanephric development. P0 animals were analyzed by dissection and visual inspection.
n, number of ureters analyzed.
In all wild-type and heterozygous animals, the ureteric bud had invaded the metanephric mesenchyme at E11.5 (Figure 5A). In contrast, in all α8-deficient mice, the ureteric bud had formed but had not yet invaded the metanephric mesenchyme (Figure 5B). No defects were observed in the development of the mesonephros or the Wolffian duct (data not shown). At E13.5 in wild-type animals, the ureter had branched and epithelialization of mesenchymal cells was progressing (Figure 5C); in homozygous mutant animals, kidney development was affected to varying degrees (Figures 5D–5F and Table 2). In 54% of the developing nephric systems, the metanephric mesenchyme was degenerating and no or only a short ureter was visible (Figure 5F). In the remaining cases, a ureter had invaded the metanephric mesenchyme, but branching and formation of epithelial specializations were evident in only 18% of cases (Figures 5D–5F). Branching and epithelialization was less substantial than in wild-type or heterozygous littermates (compare Figure 5C to Figures 5D and 5E).
We conclude that in the absence of integrin α8β1 the ureteric bud forms properly, but growth of the ureteric bud toward the metanephric mesenchyme and growth and branching of the ureteric epithelium within the metanephric mesenchyme are defective. In addition, recruitment of mesenchymal cell into epithelial structures is impaired.
Expression of p75 and Pax-2 Is Induced in Metanephric Mesenchyme of Mutant Animals
Conversion of the metanephric mesenchyme to epithelial structures is impaired in mutant animals. One explanation may be that the delayed growth of the ureter leads to delayed invasion of the mesenchyme at a time when it has lost its competence to respond to inductive signals or the signals are no longer present. Alternatively, integrin α8β1 may have a direct role in regulating migration of mesenchymal cells away from the ureteric epithelium after they have received the inductive stimulus. Finally, α8β1 may have a role in the epithelialization process itself. To distinguish among these possibilities, we analyzed the expression of markers for early inductive events within the mutant mesenchyme (Figure 6). We focused on the transcription factor Pax-2 and the low affinity neurotrophin receptor p75NTR, both of which are induced in the condensing mesenchyme. Pax-2 is also expressed in the ureteric epithelium and p75NTR in differentiating renal vesicles (Figure 6; Dressler et al., 1990; Sariola et al., 1991).
Figure 6. Analysis of p75NTR and Pax-2 Expression at E13.5 and E15.5.
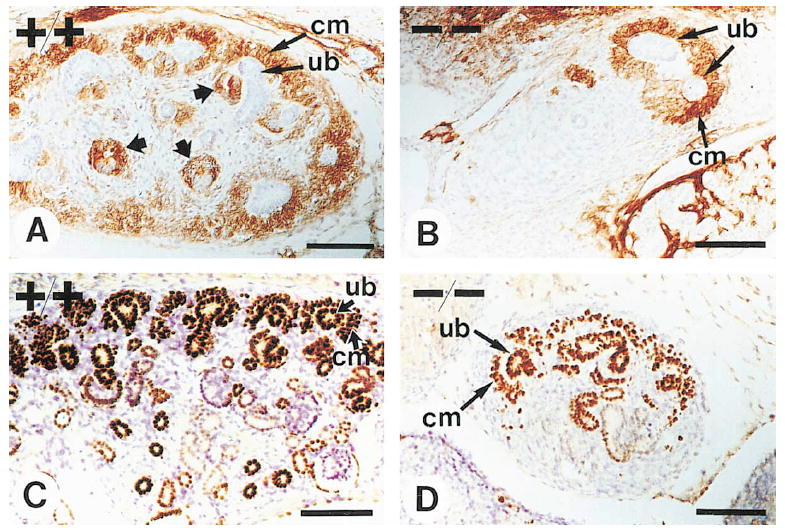
(A and B) Sections from E13.5 wild-type (A) and mutant (B) animals were stained with antibodies to the p75NTR receptor. Expression was apparent in wild-type and mutant animals in condensing mesenchymal cells (cm) surrounding the ureter branches (ub). In wild-type animals, p75NTR expression was also observed in renal vesicles (A, wide arrows). (C and D) Sections from E15.5 wild-type (C) and mutant (D) animals were stained with antibodies to Pax-2, which is expressed both in the ureteric epithelium and upon induction in the condensing mesenchyme. Only a small part of a section from the wild-type kidney is shown, whereas the whole rudiment of an α8-deficient animal is visible. Note that in mutant animals the ureter had formed very few branches but Pax-2 expression was induced. Scale bars, 50 μm.
Induction was evaluated in 14 mutant mesenchyma between E13.5 and E15.5. The ureteric bud had invaded the mesenchyme in 5 cases. Expression of the markers was induced in all 5 mesenchyma that had been invaded by the ureter, and it was evident in cells that were not in direct contact with the ureter (Figures 6B and 6D). We conclude that the mutant metanephric mesenchyme was able to responde to ureter-derived inductive signals and that mesenchymal cells were migrating after induction. Formation of mesenchymal condensates was also evident, but the kidney rudiments appeared less well organized than wild-type kidneys, and few if any comma-shaped and S-shaped bodies were visible (compare Figures 6A and 6C to Figures 6B and 6D). While at E13.5 the ureter had invaded 47% of the mutant mesenchyma and induction of Pax-2 and p75NTR was evident in all mesenchyma invaded by the ureter, only 17% of the animals showed at birth signs of epithelialization of metanephric mesenchymal cells (Table 2 and Figure 6). Thus we conclude that in α8-deficient animals, epithelialization of mesenchymal cells is defective.
The Developing Kidney Contains a Novel Ligand for Integrin α8β1
Integrin α8β1 serves as a receptor for FN, VN, and TN-C, but none of these ligands is expressed in the correct patterns to account for the kidney phenotype in α8-deficient mice (see Discussion). To characterize the distribution of potential α8β1 ligands, we stained whole-mount kidneys and kidney sections with a truncated recombinant integrin α8β1 heterodimer tagged with alkaline phosphatase (α8β1-AP). Similar chimeras have been generated with receptor tyrosine kinases to identify their ligands (Cheng and Flanagan, 1994). To obtain this heterodimer, the extracellular domain of the integrin β1 subunit was fused to an alkaline phosphatase tag that replaced the transmembrane and cytoplasmic domains. To facilitate purification, the extracellular domain of the α8 subunit was expressed with a C-terminal polyhistidine-myc tag. Expression vectors containing these chimera were cotransfected into COS cells and secreted α8β1-AP heterodimers were purified from the supernatants. In enzyme-linked immunosorbent assays, the α8β1-AP heterodimers bound specifically to FN, VN, and TN-C, the three known ligands for integrin α8β1 (S. D. et al., unpublished data).
Whole-mount kidneys were stained with antibodies to the integrin α8 subunit or with α8β1-AP. As predicted, the α8 antibody stained condensing mesenchymal cells surrounding ureteric branches on the outside of the kidney (Figure 7A). The soluble recombinant α8β1-AP heterodimer revealed a complementary pattern (Figure 7B), staining the tips of the branching ureter. In prolonged staining reactions, additional diffuse staining was observed over most kidney mesenchymal cells (data not shown). The diffuse staining was most likely due to interaction of α8β1-AP with its ligands FN and TN-C. Less pronounced staining for these ligands may reflect their diffuse distribution, may indicate low expression levels, or may be a consequence of the stringent fixation and washing conditions during the staining procedure, which may disrupt some but not all of the receptor–ligand interactions. Colocalization of integrin α8β1 with a novel ligand was also evident in kidney sections where α8β1-AP stained the surface of the branching ureteric epithelium (Figures 7D, 7F, and 7H), in an overlapping but more confined pattern compared with that seen using antibodies against the integrin α8 subunit (Figures 7E and 7G). Staining with α8β1-AP was specific, as it was blocked by an antibody to the β1 chain but not by a control antibody (data not shown). In agreement with our earlier observations that integrin α8β1 binds in a cation-dependent manner to an RGD motif within its ligands (Müller et al., 1995), staining was also dependent on divalent cations and completely blocked by RGD but not RGE peptides (Figures 7I and 7K).
Figure 7. Expression of a Novel Integrin α8β1 Ligand within the Kidney.
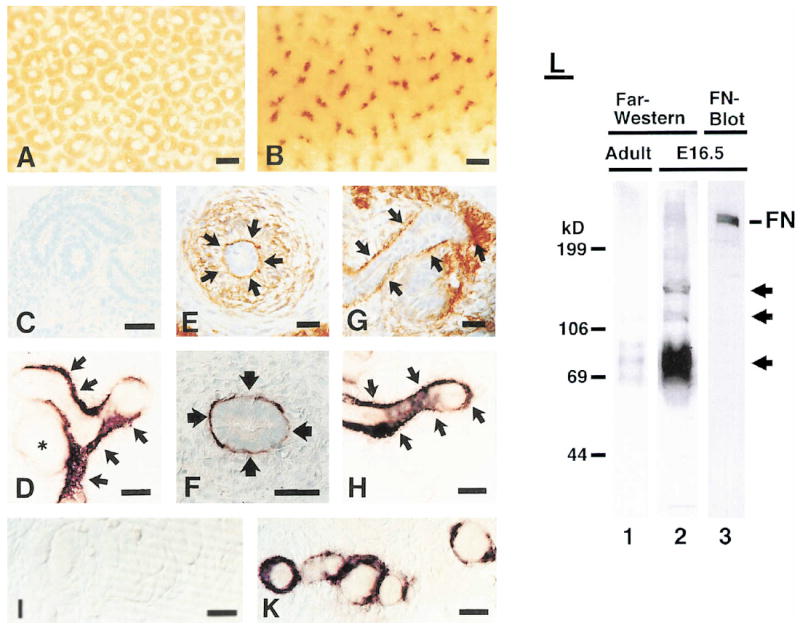
(A and B) Whole-mount kidneys were stained with an antibody specific for the extracellular domain of integrin α8 (A) or with α8β1-AP (B). (C–I and K) Sections from an E13.5 kidney were stained with a control AP fusion protein (C), with an antibody to α8 (E and G), or with α8β1-AP (D, F, H, I, and K). Staining was carried out in the presence of RGD (I) or RGE (K) peptides. The arrows indicate areas where colocalization between α8β1 and its novel ligand is evident. The asterisk in (D) marks a comma-shaped body. (L) Extracts were prepared from embryonic and adult kidneys. Proteins were separated on 6% polyacrylamide gels, transferred to nitrocellulose, and probed with either α8β1-AP or an antibody to FN. The arrows indicate novel proteins that interact in an RGD-dependent manner with α8β1-AP.
Scale bars, 50 μm (A and B) and 10 μm (C–K).
To provide further evidence for a novel α8β1 ligand, we analyzed extracts from embryonic and adult kidneys by Far-Western blot using α8β1-AP as a probe (Figure 7L). Proteins with molecular weights of about 220, 150, 110, and 60–90 kDa were detected in extracts from embryonic kidney. Very low amounts of the 60–90 kDa species were also present in adult kidney. Interactions of α8β1-AP with these proteins were abolished by RGD but not RGE peptides (data not shown). Western blot analysis suggested that the 220 kDa species was most likely FN (Figure 7L). However, the 150 and 110 kDa proteins and at least some of the 60–90 kDa proteins did not comigrate with FN, VN, or TN-C (Figure 7L and data not shown). We conclude that the embryonic kidney contains novel ligands for integrin α8β1. Because some of these proteins are expressed in embryonic but not adult kidney, they are strong candidates for mediators of integrin α8β1 functions during kidney development.
Discussion
Integrin α8β1 Is Critically Important for Epithelial–Mesenchymal Interactions during Kidney Morphogenesis
We demonstrate here that integrin α8β1 plays a crucial role in epithelial–mesenchymal interactions during kidney morphogenesis. Expression of integrin α8β1 is induced in mesenchymal cells bordering on the growing and branching ureter epithelium. Inactivation of the α8β1 receptor in mice leads to deficits in growth of the ureteric bud as it extends toward the metanephric mesenchyme and to reduced branching of the ureteric epithelium within the metanephric mesenchyme. In addition, epithelialization of the metanephric mesenchyme is impaired. By using an α8β1-AP chimera, we provide evidence for a novel ligand coexpressed with α8β1 at the border between epithelial and mesenchymal cells. This ligand is a strong candidate for mediator of the effects of mesenchymally expressed integrin α8β1 on ureter development and kidney morphogenesis.
Animals lacking integrin α8β1 are born with variable kidney phenotypes: some lack kidneys and ureters; some have ureters attached to kidney rudiments; and others have one or two ureters attached to small, functional kidneys. The latter can survive to adulthood. Analysis of the establishment of the phenotype during development provides an explanation for this variability. At E11.5, when the ureteric bud has formed and has initiated invasion of the metanephric mesenchyme in wild-type embryos, both the ureteric bud and metanephric mesenchyme are present, but invasion has not commenced in mutants. In some mutants, invasion is never successfully initiated, leading to renal agenesis and ureteric bud regression; in others, invasion is initiated at later times, but branching morphogenesis of the ureter and epithelialization of the metanephric mesenchyme are severely compromised. Thus, in many mutants, only kidney rudiments, consisting of undifferentiated mesenchyme attached to the ureter, are present at birth; in others, morphogenesis at a reduced rate generates small kidneys.
It is likely that the variability in the manifestation of the phenotype is partially a consequence of differences in the genetic background of the animals analyzed. Individual α8-deficient mice contain varying chromosomal contributions from both 129 and C57Bl/6 inbred mice. Some parameter of ureter growth such as replication rate of epithelial cells may vary in different genetic backgrounds. In support of a genetic contribution, 67% of the offspring of intercrosses of homozygous mutant survivors developed one or two kidneys (in contrast to 18% in the crosses between heterozygous animals). In the future, it will be necessary to cross the α8 allele into different genetic backgrounds to map potential modifier loci.
Adhesive Interactions Mediated by Integrin α8β1
It is well established that branching morphogenesis of epithelial cell sheets in developing organs is highly dependent on interactions with surrounding mesenchymal cells (reviewed in Grobstein, 1956; Saxen, 1987; Gurdon, 1992; Ekblom, 1996). When the ureteric epithelium is cultivated in vitro in the absence of mesenchyme, it flattens and spreads and no tubular growth occurs (reviewed by Grobstein, 1956). Tubular growth and branching are observed in cocultures with mesenchymal cells, and both proliferation and branching of the ureteric bud are affected by local environmental factors (Grobstein, 1953; reviewed in Saxen, 1987). An influence of the surrounding mesenchyme on epithelial growth has also been demonstrated for the Wolffian duct. Holtfreter (1944) suggested that the mesenchyme may guide the forming duct while it extends rostrocaudally. Subsequent transplantation experiments have shown that duct formation and growth depend on adhesive interactions between duct cells and mesenchymal cells (Ti-Chow-Tung and Su-Hwei-Ku, 1944; Poole and Steinberg, 1981, 1982).
Results presented above demonstrate that integrin α8β1 is one of the molecules that mediates interactions between the ureter and mesenchyme. It has previously been shown that integrin α8β1 binds to FN, VN, and TN-C (Müller et al., 1995; Schnapp et al., 1995; Varnum-Finney et al., 1995). None of these molecules exhibits an appropriate pattern of spatiotemporal expression to account for the kidney phenotype of α8-deficient mice. FN is expressed in the metanephric mesenchyme prior to invasion by the ureteric bud and is down-regulated upon induction (Ekblom, 1981; Aufderheide et al., 1987). VN is not expressed in the embryonic kidney (Seiffert et al., 1991). TN-C expression is first evident at E13.5 and is restricted to early stages of the stromal cell differentiation pathway. Its expression increases thereafter, much too late to account for the phenotype in α8β1-deficient animals (Aufderheide et al., 1987). Consistent with these observations, mice with targeted mutations in the VN or TN-C genes develop without kidney abnormalities (Saga et al., 1992; Zheng et al., 1995). Embryos homozygous for a targeted mutation in the FN gene die too early to analyze kidney development, but antibodies to FN do not perturb kidney development in organ cultures (Klein et al., 1988; Sariola et al., 1988; George et al., 1993). Abundant binding sites for a soluble α8β1-AP chimera were localized to the interface between the branching ureter and surrounding mesenchymal cells, suggesting that a novel ligand for integrin α8β1 is appropriately localized to mediate the interactions essential for normal kidney morphogenesis. Candidate ligand(s) expressed in embryonic but not adult kidney have also been identified in blots using α8β1-AP as a probe. These ligand(s) may mediate adhesive interactions between the ureter epithelium and mesenchymal cells expressing integrin α8β1. In the absence of integrin α8β1, adhesive interactions that are required for growth and branching of the ureter epithelium may be disrupted, leading to a reduced rate of kidney morphogenesis and renal agenesis or dysgenesis.
Participation of Integrin α8β1 in Inductive Interactions
In the simplest model of the reciprocal interactions that mediate kidney morphogenesis, formation of the metanephric kidney involves two signals, one derived from the metanephric mesenchyme inducing growth and branching of the ureter, and a second derived from the ureter inducing epithelialization of the mesenchyme. Integrins activate cellular signal transduction mechanism upon ligand binding and regulate actin cytoskeletal organization and dynamics (reviewed in Clark and Brugge, 1995; Schwartz et al., 1995). It is therefore likely that integrin α8β1 plays a direct role in the signaling systems that coordinate development of epithelial and mesenchymal cells. In the following sections we discuss the role of integrin α8β1 in epithelial–mesenchymal interactions in light of the signaling systems that are likely to be disrupted in α8β1-deficient animals.
Disruption of Metanephric Mesenchyme–Derived Inductive Signals
The earliest abnormality observed in α8β1-deficient mice is decreased growth of the ureteric bud, suggesting a deficit in the transduction of a signal derived from the metanephric mesenchyme. Recent experiments in organ culture and analyses of mice carrying targeted mutations have shown that GDNF is one of the mesenchyme-derived secreted signals required for growth and branching of the ureter. Activation of the c-ret receptor tyrosine kinase in ureteric epithelial cells mediates the response of these cells to GDNF (Moore et al., 1996; Pichel et al., 1996; Sanchez et al., 1996; Schuchardt et al, 1996; Vega et al., 1996). This is apparently not the only signaling system inducing ureteric bud growth, because a subset of mice lacking c-ret still form a ureter (Schuchardt et al., 1996). Strikingly, in both α8- and c-ret–deficient mice, similar phenotypic variability is apparent, ranging from lack of kidneys to small kidneys. At this point it is not known whether defects in ureteric bud growth in α8β1-deficient mice reflect disruption in the GDNF/c-ret signaling cascade, inhibition of ureteric bud responsiveness to this cascade, or disruption of another signaling cascade that accounts for residual kidney morphogenesis in c-ret mutants.
It was not anticipated that integrin α8β1 receptor expressed by mesenchymal cells is involved in signaling from the mesenchyme to the ureteric bud. Recent results obtained in kidney organ cultures may provide an explanation. Antisense oligonucleotides against c-ret not only reduce levels of c-ret expression in the ureter and disrupt branching morphogenesis; they also lead to a drastic reduction in the expression of numerous ECM molecules such as laminin, collagen IV, and perlecan (Liu et al., 1996). As one possible scenario, in c-ret–deficient animals, expression of an α8β1 ligand may be absent; in α8 mutants, the ligand may not be appropriately assembled into a basement membrane. Since the ECM has been shown to enhance efficiency of growth factor signaling pathways by immobilization or presentation of these factors or by activation of synergistic cosignaling pathways (reviewed in Clark and Brugge, 1995; Schwartz et al. 1995), ECM disorganization in the α8β1 mutant animals may disrupt transmission of inductive signals, disrupting proliferative or migratory responses of ureteric bud epithelial cells.
Disruption of Ureter-Derived Inductive Signals
The impaired epithelialization of the metanephric mesenchyme in α8 mutants suggests that α8β1 may also regulate signal transmission from the ureteric bud to the metanephric mesenchyme. Although little is known about the signaling molecules involved, it is clear that induction requires close proximity between the ureteric epithelium and metanephric mesenchyme (reviewed in Saxen, 1987). Subsequently, cells that have received the inductive signal migrate away from the ureteric epithelium, leading to spreading of the signal over several cell diameters (Saxen, 1987). Potential candidates for the inductive signal include members of the wnt and TGF-β superfamilies (reviewed in Parr and McMahon, 1994; Roelink, 1996). Both wnt-11 and BMP7 are expressed in the developing kidney, and wnt-11 is concentrated in the tip of the ureteric bud (Lyons et al., 1995; Kispert et al., 1996). Mice with targeted mutations in BMP7 and wnt-4 show defects in kidney development (Stark et al., 1994; Dudley et al., 1995; Luo et al., 1995). Integrin α8β1 is unlikely to mediate initial inductive events because expression of this integrin within the mesenchyme is an early response to ureter-derived inducers. Indeed, we have strong evidence that some initial signals are transmitted, because both Pax-2 and p75NTR are induced in mutant mesenchymal cells. Integrin α8β1 also is not essential for subsequent migration of mesenchymal cells, because expression of Pax-2 and p75NTR is observed in mesenchymal cells several cell diameters away from the ureter.
Still, morphogenetic events following induction are defective in integrin α8β1–deficient mice. This is most strikingly demonstrated by the observation that in some mutant animals an unbranched ureter attached to an undifferentiated mesenchyme is visible at birth or even months after birth. Integrins play a central role in ECM assembly, and these matrices are essential to generate the polarity of epithelial and endothelial cells (reviewed in Ekblom, 1996). Organ culture experiments have shown that formation of polarized epithelia from the kidney mesenchyme is dependent on proper basement membrane assembly, a process that is dependent on integrin α6β1 expressed in the newly generated epithelial cells (reviewed in Ekblom, 1996). It is conceivable that integrin α8β1 plays a role in early steps of matrix assembly and that it may exert its effect on epitheliogenesis by helping to organize a matrix that localizes short-range signaling molecules such as wnts and BMPs.
The activation of α8 expression in the mesenchyme by the ureter is presumably caused at the transcriptional level. Transcription factor Pax-2 is also induced in the metanephric mesenchyme upon contact with the ureter (Dressler et al., 1990), and Pax-2 is required for kidney development (Torres et al., 1995). This raises the possibility that Pax-2 may regulate α8 expression. Future analysis of the mechanism that leads to induction of α8 within mesenchymal cells should help in the identification of the inductive signal and its mode of action.
A General Role for Integrins in Epithelial–Mesenchymal Interactions
The data presented here strongly support the possibility that integrin α8β1 plays a critical role in epithelial–mesenchymal interactions. It is well established that growth of epithelial tubules and branching morphogenesis of epithelial cell sheets in forming organs are dependent on epithelial–mesenchymal interactions. During embryogenesis, integrin α8β1 is expressed in the mesenchyme of many duct-forming organs. However, no major deficits were observed in α8β1-deficient mice in other tissues besides the kidney. It is conceivable that α8β1 functions in other tissues are masked by additional members of the integrin family that may also regulate epithelial–mesenchymal interactions. Indeed, α6-containing integrins regulate branching morphogenesis in the submandibular gland in organ culture (Kadoya et al., 1995). Mice carrying a targeted mutation in the integrin α3 gene have altered branching of glomerular capillary loops and of bronchi (Kreidberg et al., 1996). In Caenor-habditis elegans, mutations within an integrin β-chain gene lead to apparent deficits in epithelialization of distal tip cells and ovarian sheet cells (Gettner, 1994). Future analyses of mice carrying targeted mutations in multiple integrin α genes will be important for directly investigating potential redundant integrin functions in epithelial–mesenchymal interactions.
Experimental Procedures
DNA Constructs
A cDNA encoding the mouse α8 protein was isolated (S. D. et al., unpublished data) and sequenced. An α8 genomic clone was isolated by screening a 129SVJ genomic library (Stratagene) with a mouse α8 cDNA probe. DNA fragments containing α8 exons were identified by DNA sequencing. An exon encoding amino acids 362–424 was interrupted by the PGK-neo selectable marker (Figure 2A). A genomic α8 DNA fragment extending from the SacI site to the DpnI site was isolated and shortened from the SacI site by 200 bp with Bal31, Klenow fragment blunted, and fused to the Klenow-blunted HindIII site in PGK-neo to generate PGK-neo-1.2. A 3.1 kb HindIII genomic DNA fragment 3′ of the α8 exon was inserted into the HindIII site of PGK-tk, and an XhoI fragment containing the HindIII fragment and the PGK-tk expression cassette was cloned into the XhoI site of PGK-neo-1.2. For Southern blots, probe 1 was obtained by subcloning a 200 bp genomic 5′ to the the targeting vector, and probe 2 was obtained by subcloning a genomic BamHI fragment near the 3′ end (Figure 2A).
Cloning of β1-AP and α8-HIS-myc, COS-cell expression, and protein purification will be described elsewhere (S. D. et al., unpublished data).
Generation of α8-Deficient Mice
The JM1 embryonic stem cells (J. J. M. and R. A. P., unpublished data) were grown on mitotically inactivated STO cells and electroporated with 25 μg of linearized targeting vector. After 8–10 days in selective medium (300 μg/ml G418 and 0.2 μM FIAU), colonies were picked, expanded, and screened by polymerase chain reaction and Southern blot analysis and used for injection of C57Bl/6 blastocysts (Joyner, 1993). To obtain germline transmission, chimeric male mice were mated to C57Bl/6 females.
Histological Methods
Histological methods were carried out as described previously (Jones et al., 1994). Primary antibodies were used as follows: affinity-purified antibody to the α8 extracellular or cytoplasmic domains at 10 and 25 μg/ml, respectively; to p75NTR at 10 μg/ml (Weskamp and Reichardt, 1991); and to Pax-2 at 15 μg/ml (a gift from G. Dressler).
For whole-mount staining with α8β1-AP, kidneys were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS); blocked with 1% bovine serum albumin (BSA), 0.1% Triton X-100 in PBS; washed with buffer J (0.1% BSA, 100 mM NaCl, 1 mM MnCl2, 0.1% Triton X-100, 25 mM Tris-HCl [pH 7.5]); and incubated with 3 μg/ml α8β1-AP in buffer J. Where indicated, GRGDSP or GRGESP peptides (50–100 μg/ml) were included. Samples were washed three times with buffer J, once with 100 mM NaCl, 1 mM MnCl2, 0.1% Triton X-100, 25 mM Tris-HCl (pH 7.5); fixed with 60% acetone, 3% formaldehyde in 20 mM HEPES (pH 7.0); washed with 150 mM NaCl, 20 mM HEPES (pH 7.0); heated for 1 hr at 65°C; rinsed with AP buffer (100 mM NaCl, 5 mM MgCl2, 100 mM Tris-HCl [pH 9.5]); and developed with substrate solution (0.17 mg/ml 5-bromo-4-chloro-3-indolylyphosphate, 0.33 mg/ml nitro blue tetrazolium in AP buffer). For antibody staining, fixed samples were blocked with 1% BSA, 0.1% Triton X-100, and 1% H2O2 in PBS; washed with buffer D (1% BSA, 0.1% Trition X-100, 0.02% sodium azide in PBS); incubated with 10 μg/ml α8 antibody; washed with buffer D; and detected by the ABC method (Vector). Staining of sections with α8β1-AP will be described elsewhere (S. D. et al., unpublished data).
Far-Western Blots
Kidneys were homogenized in PBS in a dounce homogenizer and lysed in 150 mM NaCl, 1% Nonidet P-40, 0.5% deoxycholic acid, 0.1% SDS, 50 mM Tris-HCl (pH 8.0). Proteins were separated on 6% SDS-polyacrylamide gels. After transfer of protein to nitrocellulose, filters were blocked for 1 hr with 3% BSA in PBS and developed as described above for tissue sections.
Acknowledgments
We thank C. Backus and A. Spindle for help with mouse husbandry; I. Farinas and K. Jones for technical advice; G. R. Dressler for Pax-2 antibodies; and J. G. Flanagan for AP fusion vectors. Thanks go to E. Huang for suggesting the use of AP fusion proteins. We thank members of the Reichardt laboratory for discussions and A. Kralli, R. Brandenberger, and A. Patapoutian for critical reading of the manuscript. This work was supported by United States Public Health grant P01–16033 (to L. F. R.) and P01-HD26732 (to R. A. P.); and by the Howard Hughes Medical Institute (to L. F. R). L. F. R. is an investigator and U. M. an associate of the Howard Hughes Medical Institute.
References
- Aufderheide E, Chiquet-Ehrismann R, Ekblom P. Epithelial-mesenchymal interactions in the developing kidney lead to expression of tenascin in the mesenchyme. J Cell Biol. 1987;105:599–608. doi: 10.1083/jcb.105.1.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossy B, Bossy-Wetzel E, Reichardt LF. Characterization of the integrin alpha 8 subunit: a new integrin beta 1-associated subunit, which is prominently expressed on axons and on cells in contact with basal laminae in chick embryos. EMBO J. 1991;10:2375–2385. doi: 10.1002/j.1460-2075.1991.tb07776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HJ, Flanagan JG. Identification and cloning of ELF-1, a developmentally expressed ligand for the Mek4 and Sek receptor tyrosine kinases. Cell. 1994;79:157–168. doi: 10.1016/0092-8674(94)90408-1. [DOI] [PubMed] [Google Scholar]
- Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- Dressler GR, Deutsch U, Chowdhury K, Nornes HO, Gruss P. Pax2, a new murine paired-box-containing gene and its expression in the developing excretory system. Development. 1990;109:787–795. doi: 10.1242/dev.109.4.787. [DOI] [PubMed] [Google Scholar]
- Dudley AT, Lyons KM, Robertson EJ. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev. 1995;9:2795–2807. doi: 10.1101/gad.9.22.2795. [DOI] [PubMed] [Google Scholar]
- Ekblom P. Formation of basement membranes in the embryonic kidney: an immunohistological study. J Cell Biol. 1981;91:1–10. doi: 10.1083/jcb.91.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblom P. Receptors for laminins during epithelial morphogenesis. Curr Opin Cell Biol. 1996;8:700–706. doi: 10.1016/s0955-0674(96)80112-8. [DOI] [PubMed] [Google Scholar]
- George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- Gettner S. PhD thesis. University of California; San Francisco: 1994. BETApat-3, a C. elegans integrin beta subunit. [Google Scholar]
- Grobstein C. Inductive epithelio-mesenchymal interaction in cultured organ rudiments of the mouse. Science. 1953;118:52–55. doi: 10.1126/science.118.3054.52. [DOI] [PubMed] [Google Scholar]
- Grobstein C. Inductive tissue interaction in development. Adv Cancer Res. 1956;4:187–236. doi: 10.1016/s0065-230x(08)60725-3. [DOI] [PubMed] [Google Scholar]
- Gurdon JB. The generation of diversity and pattern in animal development. Cell. 1992;68:185–199. doi: 10.1016/0092-8674(92)90465-o. [DOI] [PubMed] [Google Scholar]
- Hatini V, Huh SO, Herzlinger D, Soares VC, Lai E. Essential role of stromal mesenchyme in kidney morphogenesis revealed by targeted disruption of Winged Helix transcription factor BF-2. Genes Dev. 1996;10:1467–1478. doi: 10.1101/gad.10.12.1467. [DOI] [PubMed] [Google Scholar]
- Holtfreter J. Experimental studies on the development of the pronephros. Rev Can Biol. 1944;3:220–250. [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Jones KR, Farinas I, Backus C, Reichardt LF. Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell. 1994;76:989–999. doi: 10.1016/0092-8674(94)90377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner AL. Gene Targeting: A Practical Approach. Oxford: IRL Press; 1993. [Google Scholar]
- Kadoya Y, Kadoya K, Durbjee M, Holmvall K, Sorokin L, Ekblom P. Antibodies against domain E3 of laminin-1 and integrin α6 perturb branching epithelial morphogenesis of submandibular gland but by different modes. J Cell Biol. 1995;129:521–534. doi: 10.1083/jcb.129.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kispert A, Vainio S, Shen L, Rowitch DH, McMahon AP. Proteoglycans are required for maintenance of wnt-11 expression in the ureter tip. Development. 1996;122:3627–3637. doi: 10.1242/dev.122.11.3627. [DOI] [PubMed] [Google Scholar]
- Klein G, Langegger M, Timpl R, Ekblom P. Role of laminin A chain in the development of epithelial cell polarity. Cell. 1988;55:331–341. doi: 10.1016/0092-8674(88)90056-6. [DOI] [PubMed] [Google Scholar]
- Kreidberg JA, Donovan MJ, Goldstein SL, Rennke H, Shepherd K, Jones RC, Jaenisch R. Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development. 1996;122:3537–3547. doi: 10.1242/dev.122.11.3537. [DOI] [PubMed] [Google Scholar]
- Kreidberg JA, Sariola H, Loring JM, Maeda M, Pelletier J, Housman D, Jaenisch R. WT-1 is required for early kidney development. Cell. 1993;74:679–691. doi: 10.1016/0092-8674(93)90515-r. [DOI] [PubMed] [Google Scholar]
- Leveen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev. 1994;8:1875–1887. doi: 10.1101/gad.8.16.1875. [DOI] [PubMed] [Google Scholar]
- Liu ZZ, Wada J, Kumar A, Carone FA, Takahashi M, Kanwar YS. Comparative role of phosphotyrosine kinase domains of c-rosand c-ret protooncogenes in metanephric development with respect to growth factors and matrix morphogens. Dev Biol. 1996;178:133–148. doi: 10.1006/dbio.1996.0204. [DOI] [PubMed] [Google Scholar]
- Luo G, Hofmann C, Bronckers AL, Sohocki M, Bradley A, Karsenty G. BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev. 1995;9:2808–2820. doi: 10.1101/gad.9.22.2808. [DOI] [PubMed] [Google Scholar]
- Lyons KM, Hogan BLM, Robertson EJ. Colocalization of BMP-7 and BMP-2 RNAs suggests that these factors cooperatively mediate tissue interactions during murine development. Mech Dev. 1995;50:71–83. doi: 10.1016/0925-4773(94)00326-i. [DOI] [PubMed] [Google Scholar]
- Moore MW, Klein RD, Farinas I, Sauer H, Armanini M, Phillips H, Reichardt LF, Ryan AM, Carver-Moore K, Rosenthal A. Renal and neuronal abnormalities in mice lacking GDNF. Nature. 1996;382:76–79. doi: 10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- Müller U, Bossy B, Venstrom K, Reichardt LF. Integrin alpha 8 beta 1 promotes attachment, cell spreading, and neurite outgrowth on fibronectin. Mol Biol Cell. 1995;6:433–48. doi: 10.1091/mbc.6.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr BA, McMahon AP. Wnt genes and vertebrate development. Curr Opin Gen Dev. 1994;4:523–528. doi: 10.1016/0959-437x(94)90067-d. [DOI] [PubMed] [Google Scholar]
- Pichel JG, Shen L, Sheng HZ, Granholm AC, Drago J, Grinberg A, Lee EJ, Huang SP, Saarma M, Hoffer BJ, et al. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature. 1996;382:73–76. doi: 10.1038/382073a0. [DOI] [PubMed] [Google Scholar]
- Poole TJ, Steinberg MS. Amphibian pronephric duct morphogenesis; segregation, cell rearrangement and directed migration of the Ambystoma duct rudiment. J Embryol Exp Morphol. 1981;63:1–16. [PubMed] [Google Scholar]
- Poole TJ, Steinberg MS. Evidence of the guidance of pronephric duct migration by a craniocaudally traveling adhesion gradient. Dev Biol. 1982;92:144–158. doi: 10.1016/0012-1606(82)90159-2. [DOI] [PubMed] [Google Scholar]
- Roelink H. Tripartite signaling of pattern: interactions between hedgehogs, BMPs and wnts in the control of vertebrate development. Curr Opin Neurobiol. 1996;6:33–40. doi: 10.1016/s0959-4388(96)80006-7. [DOI] [PubMed] [Google Scholar]
- Saga Y, Yagi T, Ikawa Y, Sakakura T, Aizawa S. Mice develop normally without tenascin. Genes Dev. 1992;6:1821–1831. doi: 10.1101/gad.6.10.1821. [DOI] [PubMed] [Google Scholar]
- Sanchez MP, Silos-Santiago I, Frisen J, He B, Lira SA, Barbacid M. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature. 1996;382:70–73. doi: 10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- Sariola H, Auferheide E, Bernhard H, Henke-Fahle S, Dippold W, Ekblom P. Antibodies to cell surface ganglioside GD3 perturb inductive epithelial–mesenchymal interactions. Cell. 1988;54:235–245. doi: 10.1016/0092-8674(88)90556-9. [DOI] [PubMed] [Google Scholar]
- Sariola H, Saarma M, Sainio K, Arumae U, Palgi J, Vaahtokari A, Thesleff I, Karavanov A. Dependence of kidney morphogenesis on the expression of nerve growth factor receptor. Science. 1991;254:571–573. doi: 10.1126/science.1658930. [DOI] [PubMed] [Google Scholar]
- Saxen L. Organogenesis of the Kidney. Cambridge, UK: Cambridge University Press; 1987. [Google Scholar]
- Schnapp LM, Hatch N, Ramos DM, Klimanskaya IV, Sheppard D, Pytela R. The human integrin alpha 8 beta 1 functions as a receptor for tenascin, fibronectin, and vitronectin. J Biol Chem. 1995;270:23196–23202. doi: 10.1074/jbc.270.39.23196. [DOI] [PubMed] [Google Scholar]
- Schuchardt A, D’Agati V, Pachnis V, Costantini F. Renal agenesis and hypodysplasia in ret mutant mice result from defects in ureteric bud development. Development. 1996;122:1919–1929. doi: 10.1242/dev.122.6.1919. [DOI] [PubMed] [Google Scholar]
- Schwartz MA, Schaller MD, Ginsberg M. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- Seiffert D, Keeton M, Eguchi Y, Sawdey M, Loskutoff DJ. Detection of vitronectin mRNA in tissues and cells of the mouse. Proc Natl Acad Sci USA. 1991;88:9402–9406. doi: 10.1073/pnas.88.21.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Abnormal kidney development and hematological disorderes in PDGF β-receptor mutant mice. Genes Dev. 1994;8:1888–1896. doi: 10.1101/gad.8.16.1888. [DOI] [PubMed] [Google Scholar]
- Spemann H, Mangold H. Über Induktionen von Embryonalanlagen durch Implantation artfremder Organisatoren. Arch Entw Mech Org. 1924;100:599–638. [Google Scholar]
- Stark K, Vainio S, Vassileva G, McMahon AP. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature. 1994;372:679–683. doi: 10.1038/372679a0. [DOI] [PubMed] [Google Scholar]
- Ti-Chow-Tung, Su-Hwei-Ku Experimental studies on the development of the pronephric duct in anuran embryos. J Anat. 1944;78:52–57. [PMC free article] [PubMed] [Google Scholar]
- Torres M, Gomez-Pardo E, Dressler GR, Gruss P. Pax-2 controls multiple steps of urogenital development. Development. 1995;121:4057–4065. doi: 10.1242/dev.121.12.4057. [DOI] [PubMed] [Google Scholar]
- Varnum-Finney B, Venstrom K, Müller U, Kypta R, Backus C, Chiquet M, Reichardt LF. The integrin receptor alpha 8 beta 1 mediates interactions of embryonic chick motor and sensory neurons with tenascin-C. Neuron. 1995;14:1213–1222. doi: 10.1016/0896-6273(95)90268-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega QC, Worby CA, Lechner MS, Dixon JE, Dressler GR. Glial cell line-derived neurotrophic factor activates the receptor tyrosine kinase RET and promotes kidney morphogenesis. Proc Natl Acad Sci USA. 1996;93:10657–10661. doi: 10.1073/pnas.93.20.10657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weskamp G, Reichardt LF. Evidence that biological activity of NGF is mediated through a novel subclass of high affinity receptors. Neuron. 1991;6:649–663. doi: 10.1016/0896-6273(91)90067-a. [DOI] [PubMed] [Google Scholar]
- Zheng X, Saunders TL, Camper SA, Samuelson LC, Ginsburg D. Vitronectin is not essential for normal mammalian development and fertility. Proc Natl Acad Sci USA. 1995;92:12426–12430. doi: 10.1073/pnas.92.26.12426. [DOI] [PMC free article] [PubMed] [Google Scholar]


