Abstract
The chemokine receptor CXCR4 and its ligand, stromal cell derived factor-1α (SDF1α) regulate neuroblast migration towards the ischemic boundary after stroke. Using loss-and gain-function, we investigated the biological effect of CXCR4/SDF1α on neural progenitor cells. Neural progenitor cells, from the subventricular zone (SVZ) of the adult rat, were transfected with rat CXCR4-pLEGFP-C1 and pSIREN-RetroQ-CXCR4-siRNA retroviral vectors. Migration assay analysis showed that inhibition of CXCR4 by siRNA significantly reduced cell migration compared to the empty vector, indicating that CXCR4 mediated neural progenitor cell motility. When neural progenitor cells were cultured in growth medium containing bFGF (20 ng/ml), over-expression of CXCR4 significantly reduced the cell proliferation as measured by the number of bromodeoxyuridine+ (BrdU+) cells (26.4%) compared with the number in the control group (54.0%). Addition of a high concentration of SDF1α (500 ng/ml) into the progenitor cells with over-expression of CXCR4 reversed the cell proliferation back to the control levels (57.6%). Immunostaining analysis showed that neither over-expression nor inhibition of CXCR4 altered the population of neurons and astrocytes, when neural progenitor cells were cultured in differentiation medium. These in vitro results suggest that CXCR4/SDF1α primarily regulates adult neural progenitor cell motility but not differentiation, while over-expression of CXCR4 in the absence of SDF1α decreases neural progenitor cell proliferation.
Keywords: stromal cell derived factor-1α, CXCR4, subventricular zone, neural progenitor cells, migration, proliferation
1. Introduction
Focal cerebral ischemia promotes subventricular zone-derived neuroblast proliferation and migration to the ischemic boundary region where these neuroblasts differentiate into mature neurons (Arvidsson A 2002; Parent JM 2002; Jin K 2003; Zhang RL 2003; Zhang R 2004b, a). Among other factors, recent studies demonstrate that stromal cell derived factor-1α (SDF1α, CXCL12), a CXC chemokine, and its receptor CXCR4 play an important role in regulating neuroblast migration after stroke (Imitola J 2004; Robin et al. 2006; Thored et al. 2006). SDF1α secreted by activated astrocytes and endothelial cells in the ischemic boundary region attracts neuroblasts expressing CXCR4 in the subventricular zone (SVZ) towards the boundary (Imitola J 2004). Blockage of SDF1α /CXCR4 signaling with ADM3100 attenuates neuroblast migration (Robin et al. 2006).
SDF1α /CXCR4 signaling mediates hematopoietic stem cell homing, retention, and engraftment into the bone marrow (Christopherson et al. 2004). Studies of CXCR4 knock-out mice reveal malformation of various regions of the brain, including the granule cell layers of cerebellum (Vilz et al. 2005), dentate gyrus (Bagri et al. 2002; Lu et al. 2002), and cortical interneurons (Stumm et al. 2003; Tiveron MC 2006). These defects are presumably due to abnormal migration and proliferation of progenitor cells. Both SDF1α and CXCR4 are expressed by SVZ neural progenitor cells in the rodent and human (Peng et al. 2004; Tran et al. 2004). To further investigate the effect of SDF1α /CXCR4 signaling on biological function of adult neural progenitor cells, we performed a series of gain-and loss-of-function experiments on rat SVZ neural progenitor cells. We demonstrate that in addition to migration, SDF1α /CXCR4 signaling is involved in neural progenitor cell proliferation.
2. Results
2.1. Construction of CXCR4 siRNA and CXCR4 cDNA plasmids
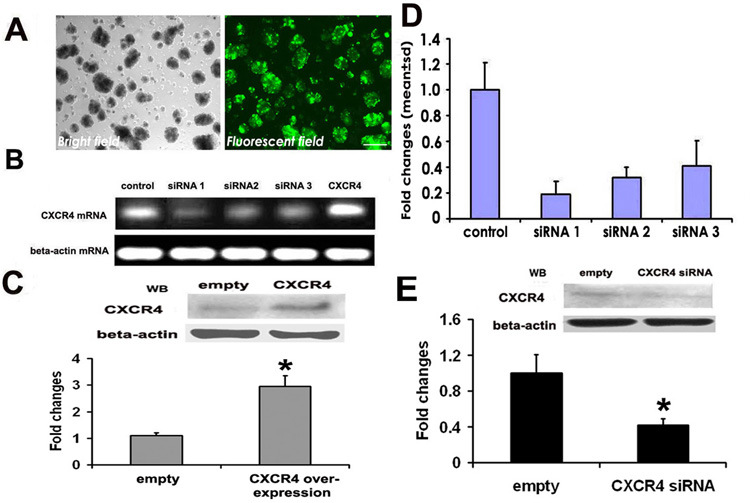
To perform gain-and loss-of-function experiments, we employed pLEGFP-C1 vector to over-express CXCR4 and pSIREN-RetroQ-siRNA vector which contains CXCR4 small interference RNA (siRNA) to block CXCR4 (Fig. 1). Neural progenitor cells infected with the control pLEGFP-C1 vector exhibited strong GFP signals 16 h after infection (Fig. 2A) and approximately 90% of the cells were infected based on GFP signals (Fig. 2A). SVZ neural progenitor cells infected with the control pLEGFP-C1 vector exhibited migration, proliferation, and differentiation, which are comparable to that observed in non-infected neural progenitor cells, suggesting that the vector itself does not affect biological function of neural progenitor cells. We then applied this protocol to infect neural progenitor cells with the CXCR4-pLEGFP-C1 vector. Real-time RT-PCR and Western-blot analysis revealed that the CXCR4-pLEGFP-N1 vector substantially increased mRNA (Fig. 2B) and protein (Fig. 2C) levels of CXCR4, respectively, indicating that CXCR4 was over-expressed. To block endogenous CXCR4, we constructed three pSIREN-RetroQ-siRNA-CXCR4 vectors and found that all three vectors attenuated endogenous CXCR4 mRNA levels with a maximum blockage of 80% mRNA by the pSIREN-RetroQ-siRNA-CXCR4-1 vector (Fig. 2B and 2D). The pSIREN-RetroQ-siRNA-CXCR4-1 vector also abolished CXCR4 protein compared with the control vector (Fig. 2E). Thus, this vector was selected for the following experiments in the present study.
Figure 1.
The design and construction of CXCR4 siRNA sequences. Panel A shows the siRNAs location in the plasmid. Panels B to D show the three siRNA stem loop sequences and predicted CXCR4 stem loops.
Figure 2.
Attenuation and over-expression of CXCR4 by vectors carrying siRNA-CXCR4 and CXCR4 cDNA, respectively. Panel A shows approximately 90% of the adult neural progenitor cells exhibited GFP signals 24h after transfection with siRNA-CXCR4 (A, GFP) compared with the total number of cells under the bright field (A, bright). Over-expression of CXCR4 in adult neural progenitor cells increased CXCR4 mRNA (B, CXCR4) and protein (C, CXCR4). In contrast, transfection with three siRNA-CXCR4 vectors resulted in substantial reduction of endogenous mRNA levels with a maximum blockage of 80% mRNA by the pSIREN-RetroQ-siRNA-CXCR4-1 vector (B and D). Panel E shows siRNA-CXCR4 suppressed CXCR4 protein on Western blots. Beta-actin was used as an internal control. *p<0.05, N=3/group. Scale bar: 100µm.
2.2. CXCR4 gain-function promotes neural progenitor cell migration
To examine the effects of over-expression of CXCR4 on neural progenitor cell migration, a neurosphere assay was employed (Wang et al, 2006). Neural progenitor cells with over-expression of CXCR4 exhibited a significant increase migration distance compared with the distance in the cells infected with the empty vector (Fig.3A–3E). The motility of the progenitor cells with over-expression of CXCR4 was further enhanced when SDF1α at a concentration of 100 ng/ml was added, although this concentration of SDF1 α did not significantly increase migration of the progenitor cells with the empty vector (Fig. 3E), suggesting that SDF1α can reinforce the cell motility through over-expression of CXCR4. In parallel, over-expression of CXCR4 led to a significant increase of the number of migrating cells in a blind-well migration chamber system (Fig. 3F, p<0.05). These data suggest that over-expression of CXCR4 promotes neural progenitor cell migration.
Figure 3.
The effects of over-expression of CXCR4 and siRNA-CXCR4 on neural progenitor cell migration. Panels A to D show single SVZ progenitor cells infected by empty retrovirus (control, A), retrovirus with CXCR4 cDNA (CXCR4, B), retrovirus with CXCR4 cDNA treated with SDF1α (CXCR4+SDF, C), and siRNA-CXCR4 retrovirus (siRNA, D). Panel E shows quantitative data. Over-expression of CXCR4 increased the migration of adult SVZ progenitor cells (B and E, CXCR4) compared with the control (A and E, CON), while addition of SDF-1α (100ng/ml) further increased the migration (C and E, CXCR4+SDF1α). In contrast, siRNA-CXCR4 significantly blocked cell migration (D and E, siRNA-CXCR4) and SDF1α (100 ng/ml) did not enhance the migration (E, siRNA+SDF1α). *p<0.05 versus the control group, # p<0.05 versus the CXCR4 group, N=8/group. Over-expression of CXCR4 significantly increased the number of migrating cells and knockdown of CXCR4 with siRNA decreased the number of migrating cells in a blind-well chamber system (F). N=6/group for blind-well chamber migration. *p<0.05 and **p<0.01 compared with control group. Error bar means SD. Scale bar = 100µm.
In contrast, neural progenitor cells infected with the pSIREN-RetroQ-siRNA-CXCR4 exhibited a significant reduction of migration in the neurosphere assay (Fig. 3C–3E, p<0.05) and the blind-well migration chamber system (Fig. 3F)
2.3. CXCR4 gain-function decreases the cell proliferation
We then examined the effects of over-expression of CXCR4 on neural progenitor cell proliferation and differentiation. When neural progenitor cells were cultured in the growth medium, over-expression of CXCR4 resulted in a significant (p<0.05) reduction of the number of bromodeoxyuridine (BrdU) positive cells compared with the number in the cells infected with the empty vector (Fig. 4A). However, the presence of SDF1α at a dose of 500 but not 100 ng/ml, abolished the effect of over-expression of CXCR4 on reduction of BrdU positive cells (Fig. 4A). In contrast to proliferation, over-expression of CXCR4 did not alter the number of TuJ1 and GFAP positive cells when neural progenitor cells were cultured in the differentiation medium (Fig. 4B). . Attenuation of endogenous CXCR4 expression with CXCR4 siRNA did not alter the number of BrdU+ cells and the number of TuJ-1 and GFAP positive cells (Fig. 4B). Microarray analysis revealed that over-expression CXCR4 in the presence of growth medium dramatically down-regulated expression of TGFβ1, TGFβ2, TGFβ2 receptor and TGFβ3 genes in neural progenitor cells, whereas SDF1α at a dose of 500ng/ml reversed TGFβ2 receptor gene expression (Fig. 5). These data suggest that over-expression of CXCR4 affects proliferation but not differentiation of neural progenitor cells.
Figure 4.
The effects of over-expression of CXCR4 and knockdown of siRNA-CXCR4 on neural progenitor cell proliferation and differentiation. Panel A demonstrates the quantification of BrdU and GFP positive cells infected by siRNA and over-expression CXCR4 retrovirus. Panel B shows CXCR4 over-expression and downregulation did not change the differentiation of SVZ cells into astrocytes and neuroblast cells. *p<0.05 versus control group, #p<0.05 versus CXCR4 group. N=6/group for proliferation and N=4/group for differentiation study.
Figure 5.
Microarray analysis revealed that CXCR4 over-expression downregulated TGFβ1 (B, red oval), TGFβ2 (B, blue rectangle), TGFβ3 gene (B, orange rounded rectangle) and TGFβ2 receptor (B, yellow diamond) expression in adult SVZ neurospheres compared with adult SVZ neurospheres infected with empty retrovirus (A). However, incubation of SDF1α (500 ng/ml) for extra 24 hour reversely up-regulated TGFβ2 receptor expression in adult SVZ neurospheres (C and E) compared with CXCR4 over-expressed neurospheres without treatment with SDF1α (B and E). Panels D and E are quantitative data of gene transcripts in SVZ neural progenitor cells. Control, CXCR4, and CXCR4+SDF1α represent neural progenitor cells infected with empty vector, CXCR4 over-expression, and CXCR4 over-expression treated with SDF1α (500 ng/ml), respectively.
3. Discussion
The present study demonstrates that superabundant expression of CXCR4 in adult neural progenitor cells promoted cell migration but decreased cell proliferation. Addition of SDF1α further enhanced cell motility and reversed cell proliferation reduced by over-expression of CXCR4. Microarray analysis revealed that over-expression of CXCR4 down-regulated TGFβ2 receptor expression which was partially rescued by the presence of SDF1α. Attenuation of endogenous CXCR4 by siRNA blocks neural progenitor cell motility. These data suggest that in addition to cell motility, SDF1α/CXCR4 signaling is involved in adult neural progenitor cell proliferation.
SDF1α/CXCR4 signaling is important for neural progenitor cell guidance and orientation in the developing mammalian brain (Ma et al. 1998; Zou et al. 1998; Suzuki et al. 2001; Bagri et al. 2002; Lu et al. 2002). We and others demonstrated that SDF1α provides cues for neuroblast migration towards the ischemic boundary (Imitola J 2004; Robin et al. 2006; Thored et al. 2006). Consistent with these findings, the present study shows that neural progenitor cells with over-expression of CXCR4 promoted the cell migration and additional SDF1α further enhanced migration, whereas attenuation of endogenous CXCR4 with siRNA blocked migration of neural progenitor cells. We previously demonstrated that the majority of cells which migrate out of the neurosphere express CXCR4 and migrating neuroblast phenotype (Robin et al. 2006). Therefore, over-expression of CXCR4 in neural progenitor cells could facilitate neuroblast migration towards the ischemic boundary where levels of SDF1α are elevated (Jin K 2003; Imitola J 2004; Miller JT 2005).
In addition to regulating cell motility, SDF1α/CXCR4 signaling mediates cell proliferation (Kahn et al. 2004; Bowie MB 2006; Lisignoli G 2006). SDF1α promotes hematopoietic progenitor cell proliferation (Lataillade et al. 2000). CXCR4 knock-out mice exhibit malformation of interneurons (Stumm et al. 2003). The present study shows that incubation of adult neural progenitor cells with SDF1α increased the progenitor cell proliferation in a dose dependent manner, which is consistent with previous findings in human neural stem cells (Imitola J 2004). Interestingly, forced over-expression of CXCR4, in absence of exogenous SDF1α, substantially decreased neural progenitor cell proliferation and addition of SDF1α reversed the action of over-expressed CXCR4 on cell proliferation. These data suggest that elevation of CXCR4 by itself has the effect on neural progenitor cell proliferation. Others have shown that expression of CXCR4 in human embryonic neural progenitor cells is upregulated during neuronal but not astrocytic differentiation (Peng et al, 2007). Although it is not known how over-expression of CXCR4 suppresses cell proliferation, our microarray data suggest that downregulation of TGFβ and its receptor, which are related to cell proliferation (Langer JC 2004; Chen et al. 2006; Sawada 2006), induced by over-expression of CXCR4 is associated with reduction of the cell proliferation, whereas addition of SDF1α is able to reverse the effect of over-expression of CXCR4 on TGFβ2 receptor expression. However, the role of TGFβ family genes in neural progenitor cells with over-expression of CXCR4 warrants further investigation.
In summary, our data suggest that SDF1α/CXCR4 signaling mediates adult neural progenitor cell motility but not differentiation. Over-expression of CXCR4 in the absence of SDF1α decreases neural progenitor cells proliferation.
4. Experiment procedure
4.1. Culture of Neurospheres
Neural progenitor cells were dissociated from the SVZ of normal Wistar rats aged 3 to 4 months, as previously reported (Morshead et al. 1994; Chiasson et al. 1999; Wang et al. 2006b; Liu et al. 2007a; Liu et al. 2007b). These SVZ cells have been well documented to have characteristics of neural progenitor cells (Morshead et al. 1994; Chiasson et al. 1999; Wang et al. 2006b; Liu et al. 2007a; Liu et al. 2007b). SVZ cells were suspended at a density of 10,000 to 20,000 cells per milliliter in 6mL of serum-free growth medium containing 10 µg/mL insulin (Biological Industries, Beit Haemek, Israel), 200 µg/mL transferrin (Sigma, St. Louis, MO, USA), 0.1 mM 2-mercaptoethanol, 2 mM L-glutamine (Biological Industries), 100 µg/mL streptomycin (Biological Industries), and 10 mM HEPES (N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid) buffer (pH=7.3; Biological Industries), 20 ng/mL of epidermal growth factor (EGF, R&D Systems, Minneapolis, MN, USA) and basic fibroblast growth factor (bFGF, R&D Systems), which we termed as a growth medium. SVZ cells were cultured for 14 to 21 days until neurosphere size approached 200 to 400 µm in diameter. Passage 1 to 5 SVZ cells were used in the present study.
4.2. Viral vector construction and production
The rat CXCR4-pEYFP-N1 vector was kindly provided by Dr. Richard Miller (Milan, Italy) and digested with Age I (New England Biolabs, Ipswich, MA, USA) and Hind III enzyme (Invitrogen Corporation, Carlsbad, CA, USA). The fragment containing CXCR4 was linked to pLEGFP-C1 retroviral vector (Clontech, Mountain View, CA, USA) with the CMV promoter to generate a self-inactivating (SIN) vector. The control vector lacks the CXCR4 gene and expresses only EGFP. To construct the recombinant pSIREN-RetroQ-siRNA, three complementary DNA oligonucleotides were chemically synthesized, which contains 5'-BamH I (Invitrogen), 3'-EcoR I (Invitrogen) sites for insertion, and one additional restriction (Mlu I, Invitrogen) site for positive clone selection. Oligonucleotides were annealed, and inserted between the BamH I and EcoR I sites immediately downstream of the H1 promoter in RNAi-Ready pSIREN-RetroQ vector (Clontech). Three siRNA target sequences were designed by means of online tool provided by the company (Clontech, http://bioinfo.clontech.com/rnaidesigner).
4.3. Cell Culture and Transfections
Retrovirus was generated by transient transfection of the 293 Ecopack packaging cell line (Clontech) by means of Lipofection 2000 transfection reagent (Invitrogen Corporation). Forty-eight hours after transfection, the viral supernatant was harvested, spinned down at 800g for 5min, and used for transduction of target cells. The retrovirus titer was determined using 3T3 cell line (ATCC, Manassas, VA, USA) based on a previous report (Pear et al, 1993). SVZ progenitor cells were infected with both pLEGFP-C1 and pSIREN-RetrQ-ZsGreen retroviruses by spinning for 90 min at 2,000 rpm at 30°C (Cherry et al. 2000; Werner M 2004). The virus was removed 12 h after the infection and fresh growth medium was added.
4.4. Assays for neural progenitor cell proliferation, differentiation and migration
Neural progenitor cells proliferate in the growth medium (Dictus et al. 2007). To examine the effect of CXCR4 on proliferation of neural progenitor cells, 48 h after virus infection, virus infected SVZ cells were cultured at a density of 105/ml in 8-well chambers (Nalge Nunc International, Rochester, NY, USA) with 500µl of the growth medium. BrdU, (10µg/mL, Sigma), a thymidine analog that is incorporated into the DNA of dividing cells during S-phase, was added into the growth medium 6 h prior to termination of experiments. The SVZ cells were cultured for 24 h and immunostaining was performed to measure the number of BrdU positive cells.
Neural progenitor cells differentiate into neurons and glial cells after withdrawing growth factors in medium (Consiglio A 2004). To examine the effect of CXCR4 on the differentiation of neural progenitor cells, virus infected SVZ cells were plated directly onto laminin coated 8-well chambers in DMEM/F-12 medium containing 2% fetal bovine serum without EGF and bFGF, which is referred to as a differentiation medium. Every 4 days, one-half of the medium was replaced with fresh medium. Incubation was terminated 14 days after plating, and immunostaining was performed to identify phenotype of neural progenitor cells.
Neural progenitor cells migrate out of neurospheres when they are cultured in matrigel (BD Bioscience, San Jose, CA, USA) (Aarum J 2003; Wang et al. 2006a). To examine whether CXCR4/SDF1α regulates neural progenitor cell motility, we aspirated a single neurosphere with GFP signal (approximately 80 µm in diameter) under the fluorescent microscope and plated it on matrigel coated 96 well plates. Migration distances of cells out of neurospheres were measured at 0h and 72h after plating.
4.5. Blind-well chamber assay
Blind-well migration chambers (BW200S) and polycarbonate filters (PFA5) were acquired through Neuroprobe Incorporation (Gaithersburg, MD, USA). Briefly, 5µm pore-size polycarbonate filters were coated with growth factor-reduced matrigel basement membrane matrix (BD Biosciences) and positioned between a chamber containing serum-free media with SDF1α (100 and 500 ng/ml, R & D Systems) and another chamber containing 50,000 virus infected SVZ cells suspended in serum-free media. Chambers were incubated for 18 hours. Using cotton-tipped applicators, matrigel was carefully wiped away from the filter and filters were stained for 20 minutes at room temperature in 4% paraformaldehyde. Migrating cells caught in the membrane were then stained using Hematoxlin-Mayer’s and Eosin (Anatech Ltd, Denver, NC). The number of cells was measured.
4.6. Immunocytochemistry and Quantification
Single or double immunofluorescent staining of cultured cells was performed as previously described (Zhang et al. 2001; Zhang RL 2003). The following primary antibodies were used in the present study: mouse anti-BrdU (1:100, Boehringer Mannheim, Indianapolis, IN, USA), mouse anti-β-tubulin III (Tuj-1, 1:1,000, Covance Incorporation., Princeton, NJ, USA), rabbit anti-GFAP (1:500, Dako Cytomation California Incorporation., Carpinteria, CA, USA), goat anti-GFAP (1:500, Santa Cruz Biotechnology, Incorporation., CA, USA); mouse anti-nestin (1:100, BD Biosciences Pharmingen San Diego, CA, USA). Cultured cells were fixed in 4% paraformaldehyde for 15 to 20 mins at room temperature. Nonspecific binding sites were blocked with 5% normal goat serum in phosphated buffer saline (PBS) medium for 30 min at room temperature. The cells were then incubated with the primary antibodies listed above and with FITC-or Cy3-conjugated secondary antibodies. Nuclei were counterstained with 40, 60-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, CA, USA). The numbers of BrdU, TuJ1, GFAP, and nestin positive cells and total number of DAPI nuclei were counted under a 20× objective lens and the percentage of each cell type was determined.
4.7. RNA isolation and Real-time RT-PCR
Total RNA from cells was isolated using RNeasy Mini Kit (Qiagen Incorporation) and followed by reverse-transcription (Liu et al. 2006; Liu et al. 2007b). Quantitative RT-PCR was performed on an ABI 7000 PCR instrument (Applied Biosystems) using 3-stage program parameters provided by the manufacturer, as follows; 2 min at 50°C to require optimal AmpErase uracil-N-glycosylase activity, 10 min at 95°C to activate AmpliTaq Gold DNA polymerase, and then each cycle 15 s at 95°C, 1 min at 60°C for 50 cycles. 2 min at 50°C, 10 min at 95°C, and then 40 cycles of 15 s at 95°C and 1 min at 60°C. Each sample was tested in triplicate and data obtained from three independent experiments were expressed as a subtraction of the quantity of specific transcripts to the quantity of the control gene β-actin in mean arbitrary units. CT values were quantified by the 2−ΔΔCt method (Livak KJ 2001). The primer of β-actin: Forward: 5’-CCATCATGAAGTGTGACGTTG, Reverse: 5’-CAATGATCTTGATCTTCATGGTG, CXCR4 primers are: 5’-CTCCAAGCTGTCACACTCCA, 5’-TCCCCACGTAATACGGTAGC.
4.8. Microarray hybridization
Using Qiagen RNaesy purification kit, total RNA was isolated from SVZ cells infected with CXCR4-pLEGFP-C1 vector 48h after incubation in the growth medium in the presence or absence of SDF1α (500ng/ml). The non-radioactive OligoGEArray filters (ORN-024.2; SuperArray Incorporation) were used and hybridization procedures were as described by the manufacturer (Liu et al. 2007a). The biotin dUTP-labeled cDNA probes (biotin UTP-labeled oligo probes were applied in Oligo GEArray) were specifically generated in the presence of a designed set of gene-specific primers using total RNA (4 µg) and 1µl reverse transcriptase. The array filters were hybridized with biotin-labeled probes at 60°C for 17 h. The filters were then washed twice with 2 × saline sodium citrate buffer (SSC)/1% sodium dodecyl sulfate (SDS) and then twice with 0.1 × SSC/1% SDS at 60°C for 15 min each. Chemiluminescent detection steps were performed by subsequent incubation of the filters with alkaline phosphatase-conjugated streptavidin and CDP-Star substrate (Superarray Incorporation).
4.9. Western blot
Cultures were rinsed with PBS and proteins were extracted in 200 µl RIPA lysis buffer. Lysates from neural progenitor cells were sonicated for 10s and centrifuged at 14000g for 20 min. Protein concentration of cell extract was determined using a BCA protein assay kit (Pierce, Rockford, IL). Equal amounts of proteins were loaded on 10% SDS-polyacrylamide gel. After electrophoresis, the proteins were transferred to nitrocellulose membranes and the blots were subsequently probed with the following antibodies: CXCR4 (Cell Signaling Technology Incorporation, Beverly, MA, USA) and β-actin (Chemicon Incorporation). For detection, horseradish peroxidase (HRP-) conjugated secondary antibodies (Jackson ImmunoRes Incorporation, West Grove, PA) were used (1:2000) followed by enhanced chemiluminescence (ECL) development (Amersham, Buckinghamshire, United Kingdom). The optical density was quantified using an image processing and analysis program (Scion image, Ederick, MA).
4.10. Statistical analysis
The data are presented as means ± SD, unless otherwise stated. One way analysis of variance (ANOVA) or paired t-test was used for multiple or two-group comparisons, respectively. Data obtained from Blind-well chamber assay were analyzed with Nonparamteric Exact Kruskal-Wallis Test followed by Wilcoxon test for group comparison, if the overall group effect was detect at 0.05 level. A value of p<0.05 was taken as significant.
Acknowledgements
This work was supported by NINDS grants PO1 NS23393, PO1 NS42345, RO1NS38292.
Abbreviation
- SVZ
subventricular zone
- SDF1α
stromal derived factor-1α
- BrdU
Bromodeoxyuridine
- EGF
epidermal growth factor
- bFGF
basic fibroblast growth factor
- siRNA
small inference RNA
- PBS
phosphated buffer saline
- SDS
sodium dodecyl sulfate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aarum JSK, Haeberlein SL, Persson MA. Migration and differentiation of neural precursor cells can be directed by microglia. Proc Natl Acad Sci U S A. 2003;100:15983–15988. doi: 10.1073/pnas.2237050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidsson ACT, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Bagri A, Gurney T, He X, Zou YR, Littman DR, Tessier-Lavigne M, Pleasure SJ. The chemokine SDF1 regulates migration of dentate granule cells. Development. 2002;129:4249–4260. doi: 10.1242/dev.129.18.4249. [DOI] [PubMed] [Google Scholar]
- Bowie MBMK, Kent DG, McCaffrey L, Hoodless PA, Eaves CJ. Hematopoietic stem cells proliferate until after birth and show a reversible phase-specific engraftment defect. J Clin Invest. 2006;116:2808–2816. doi: 10.1172/JCI28310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Choo A, Chin A, Oh SK. TGF-beta2 allows pluripotent human embryonic stem cell proliferation on E6/E7 immortalized mouse embryonic fibroblasts. J Biotechnol. 2006;122:341–361. doi: 10.1016/j.jbiotec.2005.11.022. [DOI] [PubMed] [Google Scholar]
- Cherry SR, Biniszkiewicz D, van Parijs L, Baltimore D, Jaenisch R. Retroviral expression in embryonic stem cells and hematopoietic stem cells. Mol Cell Biol. 2000;20:7419–7426. doi: 10.1128/mcb.20.20.7419-7426.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiasson BJ, Tropepe V, Morshead CM, van der Kooy D. Adult mammalian forebrain ependymal and subependymal cells demonstrate proliferative potential, but only subependymal cells have neural stem cell characteristics. J Neurosci. 1999;19:4462–4471. doi: 10.1523/JNEUROSCI.19-11-04462.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopherson KW, 2nd, Hangoc G, Mantel CR, Broxmeyer HE. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 2004;305:1000–1003. doi: 10.1126/science.1097071. [DOI] [PubMed] [Google Scholar]
- Consiglio AGA, Dolcetta D, Follenzi A, Bordignon C, Gage FH, Vescovi AL, Naldini L. Robust in vivo gene transfer into adult mammalian neural stem cells by lentiviral vectors. Proc Natl Acad Sci U S A. 2004;101:14835–14840. doi: 10.1073/pnas.0404180101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dictus C, Tronnier V, Unterberg A, Herold-Mende C. Comparative analysis of in vitro conditions for rat adult neural progenitor cells. J Neurosci Methods. 2007;161:250–258. doi: 10.1016/j.jneumeth.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Imitola JRK, Park KI, Mueller FJ, Nieto M, Teng YD, Frenkel D, Li J, Sidman RL, Walsh CA, Snyder EY, Khoury SJ. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A. 2004;101:18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin KSY, Xie L, Peel A, Mao XO, Batteur S, Greenberg DA. Directed migration of neuronal precursors into the ischemic cerebral cortex and striatum. Mol Cell Neurosci. 2003;24:171–189. doi: 10.1016/s1044-7431(03)00159-3. [DOI] [PubMed] [Google Scholar]
- Kahn J, Byk T, Jansson-Sjostrand L, Petit I, Shivtiel S, Nagler A, Hardan I, Deutsch V, Gazit Z, Gazit D, Karlsson S, Lapidot T. Overexpression of CXCR4 on human CD34+ progenitors increases their proliferation, migration, and NOD/SCID repopulation. Blood. 2004;103:2942–2949. doi: 10.1182/blood-2003-07-2607. [DOI] [PubMed] [Google Scholar]
- Langer JCHE, Orenstein J, Snoeck HW. Quantitative trait analysis reveals transforming growth factor-beta2 as a positive regulator of early hematopoietic progenitor and stem cell function. J Exp Med. 2004;199:5–14. doi: 10.1084/jem.20030980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lataillade JJ, Clay D, Dupuy C, Rigal S, Jasmin C, Bourin P, Le Bousse-Kerdiles MC. Chemokine SDF-1 enhances circulating CD34(+) cell proliferation in synergy with cytokines: possible role in progenitor survival. Blood. 2000;95:756–768. [PubMed] [Google Scholar]
- Lisignoli GTS, Piacentini A, Cristino S, Grassi F, Cavallo C, Facchini A. CXCL12 (SDF-1) and CXCL13 (BCA-1) chemokines significantly induce proliferation and collagen type I expression in osteoblasts from osteoarthritis patients. J Cell Physiol. 2006;206:78–85. doi: 10.1002/jcp.20435. [DOI] [PubMed] [Google Scholar]
- Liu XS, Zhang ZG, Zhang L, Morris DC, Kapke A, Lu M, Chopp M. Atorvastatin downregulates tissue plasminogen activator-aggravated genes mediating coagulation and vascular permeability in single cerebral endothelial cells captured by laser microdissection. J Cereb Blood Flow Metab. 2006;26:787–796. doi: 10.1038/sj.jcbfm.9600227. [DOI] [PubMed] [Google Scholar]
- Liu XS, Zhang ZG, Zhang RL, Gregg SR, Meng H, Chopp M. Comparison of in vivo and in vitro gene expression profiles in subventricular zone neural progenitor cells from the adult mouse after middle cerebral artery occlusion. Neuroscience. 2007a;146:1053–1061. doi: 10.1016/j.neuroscience.2007.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XS, Zhang ZG, Zhang RL, Gregg S, Morris DC, Wang Y, Chopp M. Stroke induces gene profile changes associated with neurogenesis and angiogenesis in adult subventricular zone progenitor cells. J Cereb Blood Flow Metab. 2007b;27:564–574. doi: 10.1038/sj.jcbfm.9600371. [DOI] [PubMed] [Google Scholar]
- Livak KJST. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu M, Grove EA, Miller RJ. Abnormal development of the hippocampal dentate gyrus in mice lacking the CXCR4 chemokine receptor. Proc Natl Acad Sci U S A. 2002;99:7090–7095. doi: 10.1073/pnas.092013799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci U S A. 1998;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JTBJ, Wimborne HJ, Walker AL, Hess DC, Hill WD, Carroll JE. The neuroblast and angioblast chemotaxic factor SDF-1 (CXCL12) expression is briefly up regulated by reactive astrocytes in brain following neonatal hypoxic-ischemic injury. BMC Neurosci. 2005;6:63. doi: 10.1186/1471-2202-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, van der Kooy D. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–1082. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Parent JMVZ, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann. Neurol. 2002;52:802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- Peng H, Huang Y, Rose J, Erichsen D, Herek S, Fujii N, Tamamura H, Zheng J. Stromal cell-derived factor 1-mediated CXCR4 signaling in rat and human cortical neural progenitor cells. J Neurosci Res. 2004;76:35–50. doi: 10.1002/jnr.20045. [DOI] [PubMed] [Google Scholar]
- Peng H, Kolb R, Kennedy JE, Zheng J. Differential expression of CXCL12 and CXCR4 during human fetal neural progenitor cell differentiation. J Neuroimmune Pharmacol. 2007;2:251–258. doi: 10.1007/s11481-007-9081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin AM, Zhang ZG, Wang L, Zhang RL, Katakowski M, Zhang L, Wang Y, Zhang C, Chopp M. Stromal cell-derived factor 1alpha mediates neural progenitor cell motility after focal cerebral ischemia. J Cereb Blood Flow Metab. 2006;26:125–134. doi: 10.1038/sj.jcbfm.9600172. [DOI] [PubMed] [Google Scholar]
- Sawada RIT, Tsuchiya T. Changes in expression of genes related to cell proliferation in human mesenchymal stem cells during in vitro culture in comparison with cancer cells. J Artif Organs. 2006;9:179–184. doi: 10.1007/s10047-006-0338-z. [DOI] [PubMed] [Google Scholar]
- Stumm RK, Zhou C, Ara T, Lazarini F, Dubois-Dalcq M, Nagasawa T, Hollt V, Schulz S. CXCR4 regulates interneuron migration in the developing neocortex. J Neurosci. 2003;23:5123–5130. doi: 10.1523/JNEUROSCI.23-12-05123.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Rahman M, Mitsuya H. Diverse transcriptional response of CD4(+) T cells to stromal cell-derived factor (SDF)-1: cell survival promotion and priming effects of SDF-1 on CD4(+) T cells. J Immunol. 2001;167:3064–3073. doi: 10.4049/jimmunol.167.6.3064. [DOI] [PubMed] [Google Scholar]
- Thored P, Arvidsson A, Cacci E, Ahlenius H, Kallur T, Darsalia V, Ekdahl CT, Kokaia Z, Lindvall O. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006;24:739–747. doi: 10.1634/stemcells.2005-0281. [DOI] [PubMed] [Google Scholar]
- Tiveron MCRM, Moepps B, Zhang YL, Seidenfaden R, Favor J, Konig N, Cremer H. Molecular interaction between projection neuron precursors and invading interneurons via stromal-derived factor 1 (CXCL12)/CXCR4 signaling in the cortical subventricular zone/intermediate zone. J Neurosci. 2006;26:13273–13278. doi: 10.1523/JNEUROSCI.4162-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran PB, Ren D, Veldhouse TJ, Miller RJ. Chemokine receptors are expressed widely by embryonic and adult neural progenitor cells. J Neurosci Res. 2004;76:20–34. doi: 10.1002/jnr.20001. [DOI] [PubMed] [Google Scholar]
- Vilz TO, Moepps B, Engele J, Molly S, Littman DR, Schilling K. The SDF-1/CXCR4 pathway and the development of the cerebellar system. Eur J Neurosci. 2005;22:1831–1839. doi: 10.1111/j.1460-9568.2005.04378.x. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang ZG, Zhang RL, Gregg SR, Hozeska-Solgot A, LeTourneau Y, Wang Y, Chopp M. Matrix metalloproteinase 2 (MMP2) and MMP9 secreted by erythropoietin-activated endothelial cells promote neural progenitor cell migration. J Neurosci. 2006a;26:5996–6003. doi: 10.1523/JNEUROSCI.5380-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhang ZG, Zhang RL, Jiao ZX, Wang Y, Pourabdollah-Nejad DS, LeTourneau Y, Gregg SR, Chopp M. Neurogenin 1 mediates erythropoietin enhanced differentiation of adult neural progenitor cells. J Cereb Blood Flow Metab. 2006b;26:556–564. doi: 10.1038/sj.jcbfm.9600215. [DOI] [PubMed] [Google Scholar]
- Werner MKJ, Baum C, Brocker T. B-cell-specific transgene expression using a self-inactivating retroviral vector with human CD19 promoter and viral post-transcriptional regulatory element. Gene Ther. 2004;11:992–1000. doi: 10.1038/sj.gt.3302255. [DOI] [PubMed] [Google Scholar]
- Zhang RZZ, Wang L, Wang Y, Gousev A, Zhang L, Ho KL, Morshead C, Chopp M. Activated neural stem cells contribute to stroke-induced neurogenesis and neuroblast migration toward the infarct boundary in adult rats. J. Cereb. Blood. Flow. Metab. 2004a;24:441–448. doi: 10.1097/00004647-200404000-00009. [DOI] [PubMed] [Google Scholar]
- Zhang RZZ, Zhang C, Zhang L, Robin A, Wang Y, Lu M, Chopp M. Stroke transiently increases subventricular zone cell division from asymmetric to symmetric and increases neuronal differentiation in the adult rat. J. Neurosci. 2004b;24:5810–5815. doi: 10.1523/JNEUROSCI.1109-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RL, Zhang ZG, Zhang L, Chopp M. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience. 2001;105:33–41. doi: 10.1016/s0306-4522(01)00117-8. [DOI] [PubMed] [Google Scholar]
- Zhang RLZL, Zhang ZG, Morris D, Jiang Q, Wang L, Zhang LJ, Chopp M. Migration and differentiation of adult rat subventricular zone progenitor cells transplanted into the adult rat striatum. Neuroscience. 2003;116:373–382. doi: 10.1016/s0306-4522(02)00696-6. [DOI] [PubMed] [Google Scholar]
- Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]