Abstract
Immuno-spin trapping is a highly sensitive method for detecting DNA radicals in biological systems. This technique involves three main steps: (i) in situ and real-time trapping of DNA radicals with the nitrone spin trap 5,5-dimethyl-1-pyrroline-N-oxide (DMPO), thus forming DMPO–DNA nitrone adducts (referred to here as nitrone adducts); (ii) purification of nitrone adducts; and (iii) analysis of nitrone adducts by heterogeneous immunoassays using Abs against DMPO. In experiments, DMPO is added prior to the formation of free radicals. It diffuses easily through all cell compartments and is present when DNA free radicals are formed as a result of oxidative damage. Due to its low toxicity, DMPO can be used in cells at high enough concentrations to out-compete the normal reactions of DNA radicals, thus ensuring a high yield of DNA nitrone adducts. Because both protein and DNA nitrone adducts are formed, it is important that the DNA be pure in order to avoid misinterpretations. Depending on the model under study, this protocol can be completed in as few as 6 h.
INTRODUCTION
Reactive oxygen species (ROS) include radical (e.g., superoxide radical anion and hydroxyl radical) and non-radical (e.g., hydrogen peroxide (H2O2), hypochlorous acid and peroxynitrite) oxidizing agents1,2. Many human diseases and responses of tissues to toxicants involve an increased production of ROS that overwhelms the cells’ antioxidant defenses, which is a state known as oxidative stress2–4. Oxidative stress produces modifications of the structure and function of biomolecules that can end in modulation of signal transduction2,5, mutagenesis and/or tissue damage2. Cellular targets for oxidatively generated damage by ROS include nucleic acids, lipids and proteins. Their susceptibility to modification depends on the location of ROS production, the availability of metal ions6 and the relative availability of the target to be oxidized3,4.
Among the cellular targets of ROS, DNA oxidation is important because DNA damage must be repaired to ensure the continuity of the cell’s life6,7, and radical formation in DNA can have serious phenotypic consequences. Oxidatively generated damage to DNA generally starts with an initial abstraction of an electron or hydrogen atom, or the electrophilic addition of the hydroxyl radical to a base, thus producing DNA-centered radicals8,9. These often react with oxygen10, resulting in the formation of oxidized end products1 and fragmentation of the DNA molecules11. The final oxidation products and consequences depend on whether the radical is located on the base or the sugar. Several comprehensive review articles7,8,12 and a book9 describe the radical damage to DNA components, radical intermediates and their oxidation products.
Most of the techniques used to study oxidatively generated DNA damage are based on the detection of final oxidation products7, such as 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxo-dG)13. Because of its relative abundance, 8-oxo-dG is one of the most studied products of free-radical damage to guanine in the DNA and is thus the most commonly used biomarker in many human diseases involving oxidatively generated DNA damage13,14. However, 8-oxo-dG can be repaired by cell glycosylases or excreted, and its usefulness as a biomarker has been questioned due to its artifactual generation during sample work-up and analysis13,15.
The direct detection of DNA radicals (Fig. 1) with electron spin resonance (ESR), which relies only on physics, is a priori the best method for detecting DNA radicals16. However, free radicals are short-lived species (nanoseconds to milliseconds) due to their high reactivity9,17, and even with chemically purified DNA treated with oxidizing agents, the detection of DNA radicals using ESR requires specialized conditions and equipment11,16. Consequently, ESR has never been able to detect DNA radicals formed inside cells or tissues.
Figure 1.
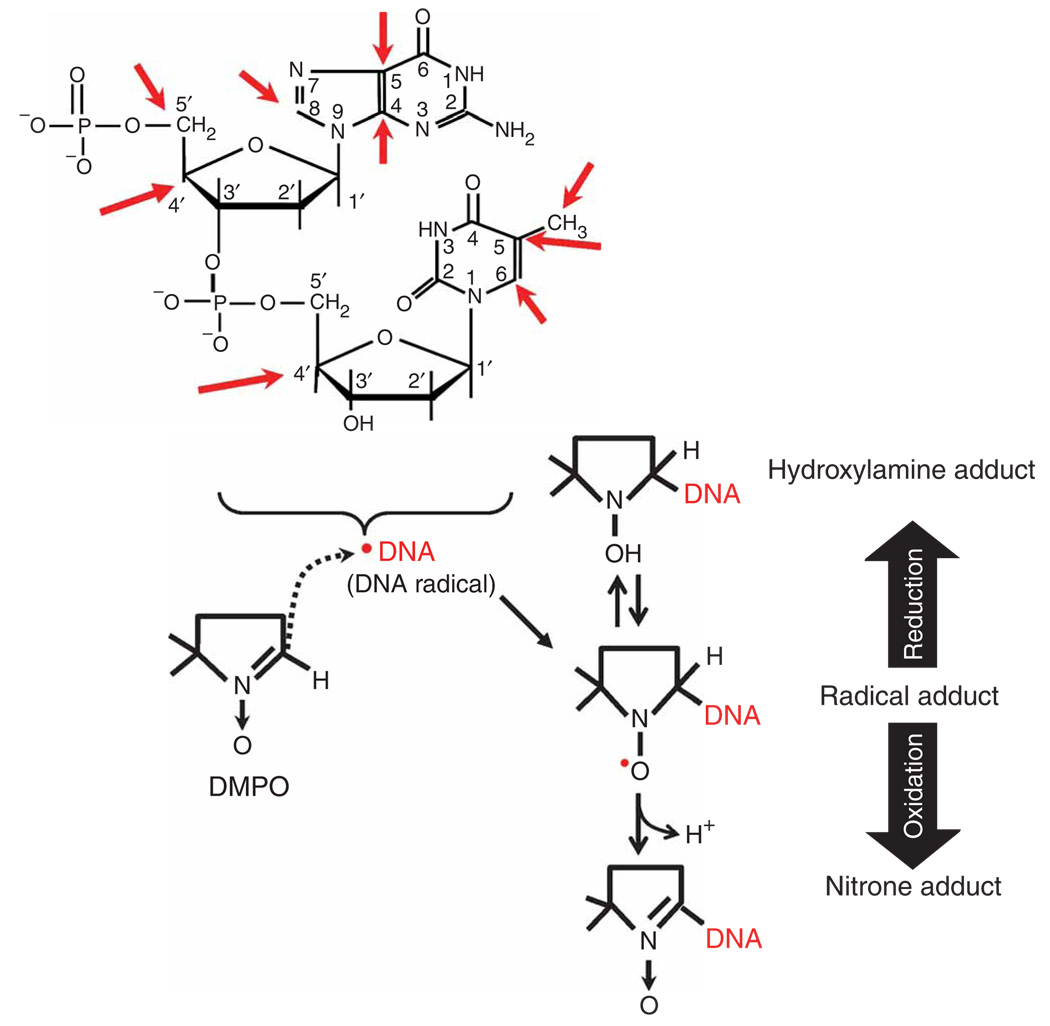
Oxidatively generated damage to DNA and detection of DNA Other oxidants radicals. One of the major pathways of DNA oxidation is via the hydroxyl radical, which can be produced by the reaction of ROS (e.g., H2O2 and the superoxide radical anion) with redox active transition metals (e.g., Cu1+/2+ and Fe2+/3+). Thus, the formation of DNA radicals can be prevented by the removal of any of these components. Because of their high reactivity, DNA radicals can react, depending on kinetics, with other biological components or with oxygen. The oxidation products depend on the localization of the radical. DNA radicals can be studied by ESR or electron paramagnetic resonance (EPR); however, because of their high reactivity, they can be detected for only a short time and under special conditions. Alternatively, DNA radicals can be trapped in situ and in real time with the cell-diffusible nitrone spin trap DMPO. Trapping DNA radicals with DMPO produces paramagnetic species known as DMPO-DNA radical adducts (referred to hereafter as radical adducts). Like the parent radical, radical adducts can be studied with ESR; however, most radical adducts decay in a matter of minutes to DNA-DMPO nitrone adducts (referred to hereafter as nitrone adducts). Nitrone adducts can be studied by heterogeneous immuno-spin trapping assays such as ELISAs (option I) or immuno-slot blots (option II). The red sphere indicates inhibition.
To increase DNA radical lifetime and facilitate radical detection, diamagnetic compounds called spin traps are used. Among these, the nitrone spin trap 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) is one of the least toxic to cells18,19 and animals20, and possesses convenient pharmacokinetics (uptake, distribution, metabolism and excretion) in biological systems21. The trapping of free-radical sites with the spin trap produces radical adducts that can be seen by ESR for minutes and occasionally hours. This technique is called ESR-spin trapping (Fig. 1). Spin trapping was an important advance in the technology for detecting free radicals in biological systems.
The analysis of DNA radicals by ESR or spin trapping is usually performed in chemical systems by exposing isolated DNA11,16 or its components (bases, sugars, nucleosides and nucleotides)22,23 to oxidizing conditions and analyzing the reaction mixture by ESR12. However, the ESR analysis of DNA radicals and radical adducts is difficult in biological systems due to the time required to isolate the DNA from the biological matrix relative to the decay of the parent radicals and/or radical adducts to ESR-silent species (Fig. 1, Fig. 2).
Figure 2.
Schematic representation of the generation and trapping of DNA radicals with the nitrone spin trap DMPO forming DNA nitrone adducts. DNA is oxidized in cells and tissues under oxidative stress with the intermediacy of DNA radicals. DNA radicals can be located on different components of the DNA (i.e., bases or sugar) and at different positions on these components (marked with red arrows). DMPO can trap, in situ, in real time and with high efficiency, many, if not all, of these radicals, preventing further oxidative consequences (e.g., 8-oxo-dG and fragmentation). The trapping of DNA radicals with DMPO involves the creation of a new covalent bond between the DNA molecule and the spin trap, forming paramagnetic radical adducts that quickly (in minutes) decay by reduction or oxidation to ESR-silent species, the hydroxylamine adduct or the nitrone adducts, respectively. Nitrone adducts are resistant to degradation during phenol/chloroform extraction. The nitrone adduct is recognized by the anti-DMPO antibody in the immunoassays (see PROCEDURE).
A DNA radical can be centered in the base and/or the sugar9. During the process of trapping free radicals with DMPO, a new covalent bond is formed between DMPO and the atom where the unpaired electron is most localized in the biomolecule radical (Fig. 2). Once a radical is formed, the highly oxidizing microenvironment necessary to form the radical site will also oxidize the radical adduct to the corresponding nitrone24, which is a facile reaction. With an octanol/water partition coefficient of 0.1 (ref. 25), DMPO is ten times more soluble in water than in octanol. Thus, although the spin trap crosses cell membranes21, it is not retained in the lipid bilayer and is easily removed during washing steps. Accordingly, DMPO is able to access the cell and can trap DNA radicals in the nucleus and mitochondria of cells exposed to an oxidizing environment. The adducts thus formed will remain stably bound, facilitating their extraction and analysis.
We have developed a new technology to detect protein26,27 and DNA radicals28, which is known as immuno-spin trapping29 (Fig. 1, Fig. 2). In immuno-spin trapping, protein and DNA radicals are trapped, in situ and in real time, with the nitrone spin trap DMPO to form DMPO–protein and DMPO–DNA nitrone adducts (hereafter referred to as DNA nitrone adducts; Fig. 2). After purification, the nitrone adducts are analyzed by heterogeneous immunoassays (e.g., ELISAs and immuno-slot blots)28 using an anti-DMPO Ab30 (Fig. 3). Immuno-spin trapping can be used to detect DNA radicals formed and trapped inside functioning cells, combining the specificity of free radical–spin trap and antigen–Ab interactions27. However, the use of heterogeneous immunoassays is essential because the anti-DMPO Abs recognize both free DMPO and DMPO covalently bound to the macromolecule27.
Figure 3.
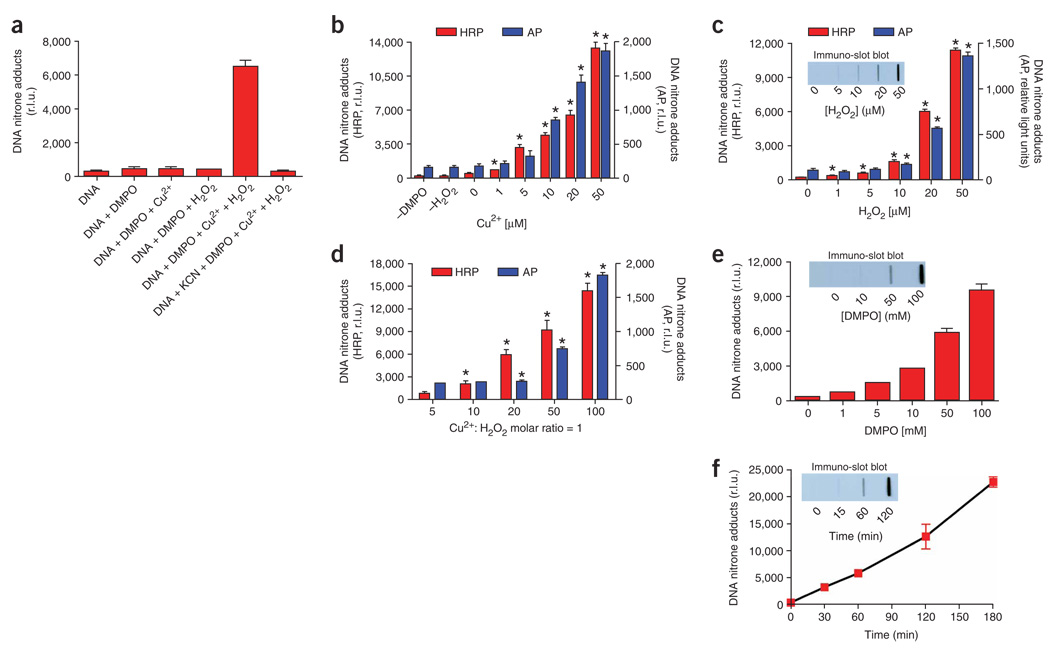
Immuno-spin trapping analysis of DNA radicals. (a) We mixed the following components: 20 µM DNA (as nucleotides), 20 µM Cu2+ (as chloride salt), 20 µM H2O2 and/or 50 mM DMPO in 100 mM chelexed PB (pH 7.4), in a total volume of 300 µl. We added a 10-X stock DNA solution (30 µl) and the other components from a 100-X stock solution (3 µl). After 1 h incubation, we stopped the reaction with 3 µl of 1 M KCN or 100 mM DTPA. Stopping the reaction with either reagent gave similar results. After stopping, we froze the samples until analysis. (b) The procedure was as described for (a), but we added different concentrations of Cu2+ or omitted one of the components in the reaction mixture. *P < 0.05 with respect to “zero” Cu2+. (c) The procedure was as described for (b), but we varied the final concentration of H2O2 in the reaction mixtures. *P <0.05 (with respect to “zero” H2O2. (d) We added 20 µM DNA, 50 mM DMPO, and equal concentrations of Cu2+ and H2O2 (e.g., “5” corresponds to 5 µM Cu2+ and 5 µM H2O2, and so on). *P < 0.05 with respect to “5”(e.g., 5 µM Cu2+:5 µM H2O2). (e) The procedure was as described for (c), but we added different final concentrations of DMPO. (f) We mixed 20 µM DNA, 20 µM Cu2+, 50 mM DMPO and 20 µM H2O2, and then added 3 µl of 1 M KCN to stop the reaction at different times after the addition of H2O2. For time “zero”, we added KCN just before H2O2. We performed the immuno-spin trapping analysis as described in the PROCEDURE. Insets show the immuno-slot blot corresponding to the samples analyzed by ELISA in the main graph. We compared AP (blue bars) with our improved developing system using HRP (red bars). We developed the immuno-slot blot using the HRP system. Data show the mean ± s.e.m. from three separate experiments in quadruplicate (n = 12).
A detailed protocol for determining protein radicals has been published elsewhere27 (Box 1). Accordingly, a method to separate DNA from other biomolecules without alteration of DMPO epitopes is needed to detect DNA nitrone adducts in cells and tissues.
BOX 1 | IMMUNO-SPIN TRAPPING ANALYSIS OF PROTEIN RADICALS.
Proteins are abundant components of biological systems, and are one of the preferred targets for ROS oxidation with the intermediacy of protein radicals. These protein radicals can be trapped with DMPO through carbon, nitrogen and sulfur centers, forming DMPO radical adducts that then decay to nitrone adducts26. Unlike DNA nitrone adducts, the detection of DMPO–protein nitrone adducts by immuno-spin trapping does not require further purification. In order to identify samples containing DMPO–protein nitrone adducts, tissues or cells are homogenized in a buffer containing detergents, DNase (to degrade nucleic acids) and protease inhibitors (to prevent enzymatic protein degradation), and then diluted and analyzed by ELISAs (screening). Protein nitrone adducts can be characterized after separation on 1D or 2D SDS–polyacrylamide gels, blotting to a nitrocellulose membrane, and visualized by Western blot27 (Fig. 1 option II, immuno-slot blot procedure). Visualized protein nitrone adducts (spots in the Western blot) are localized in a gel stained with an MS compatible stain, digested and analyzed by LC/MS. Finally, their identity is assigned by comparison with protein databank sequences.
Here we present two step-by-step protocols for improved immuno-spin trapping of DNA nitrone adducts produced in calf thymus DNA treated with a Fenton-like system (Cu2+/H2O2) and in iron- or copper-loaded RAW 264.7 macrophages treated with tert-butylhydroperoxide (tert-BOOH). Thus, depending on available supplies, researchers can choose between two options to detect purified DNA nitrone adducts: ELISA (Fig. 1, option I) or immuno dot/slot blot (Fig. 1, option II). Immuno-spin trapping allows the early, sensitive, reliable and economic detection of oxidatively generated damage to DNA in cells.
Experimental design
DNA radicals can be produced in numerous situations when ROS production overwhelms the antioxidant defenses in cells and animals. Immuno-spin trapping of DNA radicals requires that the nitrone spin trap DMPO be present at the site of radical formation at a sufficient concentration to out-compete oxygen and antioxidants. Immuno-spin trapping with DMPO can be applied to evaluate oxidatively generated damage to DNA in any biological system (e.g., mammalian cells and tissues, mitochondria, bacteria, fungi and plants).
Immuno-spin trapping analysis of DNA damage can be applied to investigate the genomic damage induced by ROS produced as a response to environmental or metabolic stress. For example, it can be used to investigate DNA oxidation (nuclear and mitochondrial) induced by exposure of cells or animals to toxicants, drugs, cytokines, radiation and so on, which enhance ROS production. In Figure 4 we present typical results for DNA radicals produced in cells exposed to tert-BOOH and metals. The range of applications of the analysis of DNA radicals is wide and diverse; therefore, experimental designs will differ depending on the stimuli, interest and systems (e.g., chemical systems, cells or animals). Box 2 describes a typical design for immuno-spin-trapping analysis of DNA radicals.
Figure 4.
Immuno-spin trapping of DNA radicals in macrophages. (a) We loaded RAW 264.7 macrophages (70% confluence) with 100 µM of either ferric citrate (yellow bars) or cupric chloride (blue bars) in complete medium (DMEM plus 10% (vol/vol) FCS) for 18 h in an incubator. After washing with HBSS− plus 1 mM DTPA (HBSS−/DTPA), we harvested and washed the cells with pre-warmed (37 °C) HBSS−/DTPA. We counted the cells and ensured that their viability was >90%. We then divided the cells (106 cells per ml) in clean, clear Eppendorf tubes in 1 ml HBSS−/DTPA and added 100 mM DMPO from a 10-M stock DMPO solution. As a control, we ran experiments in which we added, at the same time as DMPO, the cell-permeable iron and copper chelators 1 mM DP and 100 µM sodium diethyldithiocarbamate (DETC), respectively. We added different concentrations of tert-BOOH (as 100 X stock in HBSS−; <0.1% DMSO) and incubated the tubes at 37 °C for 1 h. Finally, we washed the cells with HBSS−/DTPA, counted them, and determined their viability (green bars) using hemocytometer counting and Trypan blue. We washed the cells twice with HBSS−/DTPA and pulled them down for DNA extraction. We loaded purified DNA samples onto two separate microtiter plates. After coating, one of the plates was washed with phosphate buffer and used to determine bound DNA using a CyQUANT cell proliferation assay kit (Molecular Probes, Invitrogen, cat. no. C7026) that uses λ DNA as standard. We washed the other plate with washing buffer to determine nitrone adducts using HRP as the detection system (see PROCEDURE). The results are the mean values of DNA nitrone adducts per mg DNA ± s.e.m. from three experiments performed in triplicate (n = 9). *P < 0.05 with respect to cells without added tert-BOOH. r.l.u., relative light units. (b) We analyzed the samples from (a) using the immuno-slot blot procedure (option II). Nitrone adducts from cells loaded with copper and treated with tert-BOOH, and immunocomplexes detected using HRP-conjugated secondary antibodies, are shown. 100# indicates macrophages pre-treated with DETC before treatment with tert-BOOH.
BOX 2 | BASIC DESIGN OF IMMUNO-SPIN TRAPPING EXPERIMENTS IN CELLS AND ANIMALS.
Add DMPO to cell or tissue culture.
Apply stressor (to induce ROS).
Harvest the cells and wash.
Isolate DNA.
Analyze DNA nitrone adducts.
In cells
If experiments involve long-lasting incubations or treatments (e.g., chronic exposures to drugs or toxicants), we recommend the addition of DMPO to the culture medium at least 1–4 h before the harvesting of the cells for DNA extraction. This is important to allow enough time to accumulate DNA nitrone adducts.
In animals
In animals suffering chronic inflammatory conditions, for example, DMPO must be administered 1–4 h before sacrifice and, if possible, in more than one administration, usually by intraperitoneal injection. However, the administration of DMPO depends on the main target tissue of interest (for example, intra-joint tissue for arthritis). We recommend a total of 2 g kg−1 in two or three dosages separated by 1 h intervals.
We use the well-known oxidizing system Cu2+/H2O2, which is known as a Fenton-like system, and calf-thymus DNA to produce positive controls (Box 3). Highly purified DNA (typically shows a 260 nm/280 nm ratio between 1.8 and 2.0) isolated from any tissue can be used instead of calf thymus DNA with similar results. The use of positive and negative controls is essential to assure that nitrone adducts are not the result of artifacts during the sample processing or immunoassays. We have not seen such artifacts in our studies. The selection of controls depends on the type of system under study (i.e., chemical system, cells or animals).
BOX 3 | CONTROLS IN IMMUNO-SPIN TRAPPING EXPERIMENTS.
Positive controls
Positive controls always include the target (DNA), oxidizing system or agent and DMPO. Treated cells or animals refer to cells treated with a compound or drug that induces oxidative stress or animals that are suffering with, for example, a chronic inflammatory disease.
-
Chemical systems
Calf-thymus DNA + Cu2+ + H2O2 + DMPO
Calf-thymus DNA + Fe2+ + H2O2 + DMPO
Calf-thymus DNA + oxidizing agent + DMPO
-
Cells
Cells + oxidizing agent + DMPO
Treated cells + DMPO
-
Animals
Treated animals + DMPO
Negative controls
Trial refers to cells or animals receiving a preventive therapy (e.g., to inhibit ROS production).
-
Chemical systems
Calf thymus DNA
Calf thymus DNA + DMPO
Calf thymus DNA + oxidizing agent
-
Cells
Cells + oxidizing agent
Cells + DMPO
Treated cells + trial + DMPO
-
Animals
Untreated or healthy animals + DMPO
Treated animals + trial + DMPO
Limitations
The main limitation of this protocol is that DMPO toxicity has not been assessed in humans, and so it cannot be administered to patients. Another limitation of immuno-spin trapping of DNA radicals is that there is no DNA nitrone standard to make the procedure quantitative. This is mainly due to the variety of sites in the DNA that can be targets of free-radical formation (Fig. 2); we assume that many different combinations are possible, all of which will be recognized and trapped by DMPO, and, therefore, recognized by anti-DMPO. However, we envision that the recent development of the powerful high-performance liquid chromatography (HPLC)/electrospray ionization (ESI)–MS/MS approach to the detection of nucleoside and RNA-DMPO nitrone adducts, providing structural information for nitrone adducts24, will assist immuno-spin trapping by developing a standard to quantify DNA nitrone adducts in biological systems.
Among possible sources of interference, the most important are protein nitrone adducts27. We added protein nitrone adducts to a DNA solution and processed it for DNA isolation; no nitrone adducts were detected, suggesting that the purity gained with our DNA-extraction method is enough to ensure reliable DNA nitrone adduct measurements.
Note regarding reagents
Not all the reagents and equipment described are needed for each variant of immuno-spin trapping assays. Before starting, read the entire protocol to check which reagents and equipment will be needed for a particular option.
MATERIALS
REAGENTS
Chelex 100 ion-exchange resin, analytical grade, 100–200 mesh (BioRad Laboratories, Inc., cat. no. 142-2832)
DMPO ultrapure (Alexis, cat. no. ALX-430-090-6001)
100 mM cupric chloride (Alfa Aesar, cat. no. 35673)
H2O2, 30% (wt/vol; Fluka, cat. no. H325-500)
1 M fresh KCN in distilled water; prepare fresh before use
 Highly toxic
Highly toxicComplete culture medium, DMEM (Invitrogen) plus 10% (vol/vol) heat-inactivated fetal calf serum (FCS, Invitrogen)
TrypLE Express (Invitrogen, cat. no. 12604-039)
Calcium-free and magnesium-free Hank’s balanced salt solution (HBSS−) without phenol red (Invitrogen, cat. no. 14175-103)
DMSO, anhydrous >99% (Sigma, cat. no. D-8779)
Digestion buffer: 1% SDS (wt/vol), 100 mM NaCl, 10 mM Tris–HCl (pH 8.0), 25 mM DTPA
Chloroform (Sigma)
 Harmful; handle and dispose of following institutional safety guidelines
Harmful; handle and dispose of following institutional safety guidelines3-methyl-1-butanol (isoamyl alcohol), ≥99% (Sigma, cat. no. 30,943-5)
 Harmful; handle and dispose of following institutional safety guidelines
Harmful; handle and dispose of following institutional safety guidelinesChloroform/isoamyl alcohol (49:1) mixture
 Harmful; handle and dispose of following institutional safety guidelines
Harmful; handle and dispose of following institutional safety guidelines  Prepare fresh before use
Prepare fresh before usePhenol:chloroform:isoamyl alcohol (25:24:1) ultrapure MB grade (USB Corp., cat. no. 75831)
 Harmful; handle and dispose of following institutional safety guidelines
Harmful; handle and dispose of following institutional safety guidelinesAbsolute ethanol
 Ethanol is flammable; handle and dispose of following institutional safety guidelines
Ethanol is flammable; handle and dispose of following institutional safety guidelinesEthanol (70%, vol/vol) in distilled water
9 M ammonium acetate
 Harmful; handle and dispose of following institutional safety guidelines
Harmful; handle and dispose of following institutional safety guidelinesTE buffer: 10 mM Tris-HCl (pH 8.0), 1 mM EDTA
Reacti-Bind DNA coating solution (Pierce, cat. no. 17250)
 Irritant; refer to instructions for proper use
Irritant; refer to instructions for proper useTween-20 (polyoxyethylene sorbitan monolaureate) ultrapure (USB Corp., cat. no. 20605)
Immunoblot blocking reagent (consisting of non-fat dry milk; Upstate, cat. no. 20-200)
Blocking buffer: 1 X CMF–PBS + 3% immunoblot blocking reagent
HyBond-ECL nitrocellulose membrane (Amersham Biosciences, cat. no. RPN2020D)
Neutralizing solution: 1 mM EDTA, 1.5 M NaCl, 0.5 M Tris-HCl (pH 7.2)
1 M NaOH freshly prepared
 Harmful; handle and dispose of following institutional safety guidelines
Harmful; handle and dispose of following institutional safety guidelines20 X sodium chloride/sodium citrate (SSC)
DNA sodium salt from calf thymus (Sigma, cat. no. D3664)
RAW 264.7 murine macrophage cell line (ATCC, cat. no. TIB-71)
Ferric citrate (Sigma, cat. no. F-6129)
2,2′-dipyridyl (DP; CAS no. 366-18-7), ≥99% (Aldrich, cat. no. D216305)
tert-BOOH (Sigma, cat. no. B2633)
DTPA (Sigma, cat. no. D-1133)
RNase A (Sigma, cat. no. R-5500)
20 mg ml−1 proteinase K (Sigma, cat. no. P2308)
Ultrapure buffer-saturated phenol (Invitrogen, cat. no. 15513-039)
Goat anti-rabbit IgGFc horseradish peroxidase (HRP) conjugated (Upstate, cat. no. 12-348)
Immuno-Pure goat anti-rabbit IgGFc alkaline phosphatase (AP) conjugated (Pierce; cat. no. 31341)
LumiGLO chemiluminescent substrate (Upstate, cat. no. 20-212)
EQUIPMENT
Eppendorf tubes (0.75 and 1.5 ml)
Thermomixer (Eppendorf)
Microcentrifuge (Eppendorf)
Automatic micropipettes (P10, P100 and P1000; Rainin)
Multichannel micropipettes (P20–P200; Eppendorf)
SpectraFluor Plus microplate reader (SpectraFluorPlus, Tecan)
Optional: microplate washer (Tecan)
Slot blot manifold Hoefer (Amersham Pharmacia Biotech, cat. no. PR648)
Microplate incubator (e.g., a plastic container with lid with wet paper towels in it, placed inside a cell-culture incubator)
Scanner or an Image Station 1000 (Kodak)
DU 640 UV–visible light spectrophotometer (Beckman Coulter, Inc.) or a NanoDrop
White Maxisorp FluoroNunc 96-well microtiter plates (PGC Scientifics, cat. no. 05-6109-00)
CL-Xposure Film (Pierce, cat. no. 34090)
10-kDa cut-off dialyzer cassette (Pierce)
Plastic policeman (Corning, Inc., Costar, cat. no. 3008)
Optional: GraphPad Prism package software (GraphPad Software Inc; http://www.graphpad.com) or equivalent
REAGENT SETUP
DMPO ultrapure
The molar concentration is ~ 9M(i.e., the concentration of a pure solution of DMPO as received from Alexis). ε228 = 7,800M−1 cm−1. Store at −80 °C in 50 µl aliquots in Eppendorf tubes after gently blowing a stream of argon at the surface of the solution.
100 mM cupric chloride
Freshly prepared in distilled water. To prepare a 1-mM working solution, dilute the stock in distilled water  Do not dilute stock of cupric solutions in phosphate because it will form insoluble phosphates.
Do not dilute stock of cupric solutions in phosphate because it will form insoluble phosphates.
H2O2, 30%(wt/vol)
The molar concentration is ~ 10M, ε240 = 43.6M−1 cm−1. Prepare fresh before use  Harmful; handle and dispose of following institutional safety guidelines.
Harmful; handle and dispose of following institutional safety guidelines.
20 X SSC
Comprises 3 M NaCl (175 g l−1) and 0.3 M Na2citrate · 2H2O (88 g l−1); adjust pH to 7.0 with 1 M HCl.
DNA sodium salt from calf thymus
To prepare a calf thymus DNA stock solution (1 mg ml−1), dissolve 2 mg lyophilized calf thymus DNA in 2 ml of 100 mM chelexed sodium phosphate buffer (PB; pH 7.4). Dialyze the DNA solution against 100 mM PB in a 10-kDa cut-off dialyzer cassette overnight with three changes of buffer. Collect the DNA solution and determine the DNA concentration in a DU 640 UV–visible light spectrophotometer or a NanoDrop by measuring the absorbance at 280 nm (1 unit of absorbance of double-stranded (ds) DNA is 50 µg dsDNA per ml, ~ 150 µM as nucleotides). Highly purified DNA isolated from animal tissues or plasmid DNA can also be used.
RAW 264.7 murine macrophage cell line
Passage 2–10 times at 70–80% confluence. The uptake of metals and DMPO by cells is independent of anchorage; therefore, this protocol can be applied to any kind of cell, whether in suspension or anchorage-dependent. DMPO toxicity is cell-dependent and should be one of the first control experiments (different concentrations and times of incubation).Our RAW264.7 cells do not significantly lose their viability after 24 h of exposure in a complete medium with 50 mM DMPO.
100 mM chelexed PB
Prepared by treating 100 mM PB with Chelex 100 ion-exchange resin following the instruction manual.
Ferric citrate
Prepare a fresh 100 mM stock solution by dissolving the appropriate amount of powder in distilled water pre-warmed to 65 °C. Allow the salt to dissolve at 65 °C for 1 h with occasional vortexing. Use fresh.
DP, ≥99%
Prepare a 1-M stock solution in DMSO and then prepare a 100-mM working solution (100 X) in distilled water.
tert-BOOH
Prepare a 100-mM stock solution in DMSO and then a 100 X working solution in HBSS−.  Harmful; handle and dispose of following institutional safety guidelines.
Harmful; handle and dispose of following institutional safety guidelines.
DTPA
Prepare a 100-mM solution in 1 M NaOH and adjust pH to 7.4 with concentrated HCl before adding to the reaction mixture or to HBBS− to prevent changes in buffer pH values.
DNase-free RNase A (20 mg ml−1)
Re-suspend 200 mg RNase A in 10 ml distilled water and add 3.3 µl of 3 M sodium acetate (pH 4.5). Boil the solution for 10 min to dissolve. Aliquot and store at −20 °C. Stable for 6 months at −20 °C.
Proteinase K solution
Comprises 50mM Tris–HCl (pH8.0), 1 mM CaCl2 and 20 mg ml−1 proteinase K. Because a cloudy suspension is formed during storage at 4 °C, the solution should be mixed well before use. Store at 4 °C for up to 6 months.
Ultrapure buffer-saturated phenol
Add 100 µl of 100 mM DTPA solution to the upper buffer phase.  Phenol is toxic; handle and dispose of following institutional safety guidelines.
Phenol is toxic; handle and dispose of following institutional safety guidelines.
10 X calcium-free and magnesium-free (CMF)–PBS
Comprises 80 g NaCl, 2 g KCl, 11.5 g Na2HPO4 · 7H2O and 2 g KH2PO4 dissolved in 800 ml distilled water. Adjust pH to 7.4 and sterilize by filtration.
Washing buffer 1 X
Comprises CMF–PBS + 0.05% (wt/vol) non fat-dry milk + 0.1% (vol/vol) Tween-20.
DMPO nitrone adduct polyclonal antiserum
Prepare before use by diluting the serum at 1:10,000 (1 µl per 10 ml) in washing buffer. The anti-DMPO antiserum has been licensed to the following companies: Abcam (cat. no. ab23702), Alexis Biochemicals (cat. no. ALX-210-530-R100), Cayman Chemicals (cat. no. 10006170-1) and Oxford Biomedical Research (cat. no. RT15).
Goat anti-rabbit IgGFc horseradish peroxidase (HRP) conjugated
Re-suspend the lyophilized powder as indicated by the manufacturer, aliquot and keep at −80 °C for up to 1 yr. Avoid freeze and thaw. Before use, dilute 1:10,000 in washing buffer. We have included the use of Immuno-Pure goat anti-rabbit IgGFc AP conjugated in this protocol. Follow the manufacturer’s instructions to prepare this Ab.
LumiGLO chemiluminescent substrate
Take one part of reagent A and one part of reagent B, and mix by vortexing. A 5-ml sample of themixture is enough for one 96-wellmicrotiter plate and 10ml is enough for one slot blot membrane. Let the reagents reach 15–25 °C and mix immediately before use.
PROCEDURE
Production of DNA nitrone adducts
-
1
Produce and purify DNA nitrone adducts using, for example, DNA from either a commercial source (Box 4) or cultured cells (continue to Step 2).
BOX 4 | DMPO SPIN TRAPPING OF DNA RADICALS PRODUCED IN A FENTON-LIKE SYSTEM  ~1.5 H.
~1.5 H.
Prepare the following working solutions: 200 µM DNA (calf thymus), 2 mM H2O2 and 5 M DMPO solution in 100 mM Chelex-treated PB (pH 7.4).
Use the working solutions to prepare DNA nitrone adducts. In a 0.75-ml Eppendorf tube, mix the following reagents: 261 µl 100 mM Chelex-treated phosphate buffer (pH 7.4), 30 µl DNA solution, 3 µl cupric chloride, 3 µl DMPO and start the reaction with 3 µl H2O2.
-
Incubate the mixture at 37 °C for 60 min in a thermomixer and stop the reaction with 3 µl of a 1-M KCN solution or 3 µl of a 100-mM DTPA solution.
 Samples can be stored at −20 °C or −80 °C for up to 6 months until analysis.
Samples can be stored at −20 °C or −80 °C for up to 6 months until analysis.
Production of DNA nitrone adducts in macrophages loaded with iron or copper and treated with tert-BOOH  ~ 36–48 h
~ 36–48 h
-
2
Grow RAW 264.7 macrophages (2–10 passages) until they reach a 70 or 80% confluence in 6 ml complete medium in a T-75 culture flask.
-
3
Replace the culture medium with fresh culture medium containing 100 µM ferric citrate or 100 µM cupric chloride and incubate the monolayers for 5–18 h in a cell-culture incubator (5% CO2/99% humidity).
-
4
Remove the culture medium and wash the monolayer with pre-warmed HBSS− containing 1 mM DTPA (HBSS−/DTPA).
 To prepare HBSS−/DTPA, adjust the pH of DTPA stock to 7.4 to avoid cell death. DTPA is a Cu2+ and Fe3+ chelator, and is added to remove metals adsorbed on the surface of the cells.
To prepare HBSS−/DTPA, adjust the pH of DTPA stock to 7.4 to avoid cell death. DTPA is a Cu2+ and Fe3+ chelator, and is added to remove metals adsorbed on the surface of the cells. -
5
Wash the monolayer with HBSS−/DTPA and harvest it with TrypLE Express (2–4 min). Help cell detachment with a plastic policeman as needed.
-
6
Collect the cells in a 15-ml Falcon tube and wash three times with HBSS−/DTPA (200g/5 min/4 °C).
-
7
Count the cells in a hemocytometer or coulter counter and determine viability by determining the exclusion of 0.4% (wt/vol) Trypan blue31.
 Cell viability should not be <85% to ensure reproducibility and biological significance. Always include controls with and without treatments.
Cell viability should not be <85% to ensure reproducibility and biological significance. Always include controls with and without treatments. -
8
Dilute the cells to 106 cells per ml. Put 1 ml of cells in a 1.5-ml Eppendorf tube and centrifuge at 11,700g for 20 s at 15–25 °C. Discard the supernatant.
-
9
Add 1 ml of 100 mM DMPO in pre-warmed HBSS−/DTPA and re-suspend the cells. Incubate the tubes for 30 min at 37 °C in a thermomixer at 200 r.p.m.
 Include controls with and without DMPO (Box 3).
Include controls with and without DMPO (Box 3). -
10
Add tert-BOOH and continue the incubation for 1 h (Fig. 3). Wash the cells twice with HBSS−/DTPA by centrifugation (11,700g for 20 s at 15–25 °C). tert-BOOH is a cell-permeable organic peroxide that depletes cellular reduced glutathione, and, when reacting with iron or copper, produces a powerful alkoxyl radical (tert-BO•) that can induce DNA radicals.
-
11
Re-suspend the pellet in 1 ml HBSS−/DTPA, determine viability (as described in Step 7) and centrifuge (as described in Step 8).
-
12
Remove the supernatant, and proceed with the isolation and analysis of DNA nitrone adducts from the cell pellet.
 Because DMPO–DNA nitrone adducts are stable, the cell pellet can be frozen at −80 °C until DNA extraction (up to 6 months).
Because DMPO–DNA nitrone adducts are stable, the cell pellet can be frozen at −80 °C until DNA extraction (up to 6 months).
Extraction of DNA nitrone adducts from cells  ~ 2–3 h
~ 2–3 h
-
13
Directly add 500 µl digestion buffer and 25 µl proteinase K solution to 106 washed and pelleted cells.
 Some of the substances and solutions used are hazardous. Wear a laboratory coat, appropriate gloves and safety glasses when necessary throughout the protocol. Work in a fume hood and follow institutional safety guidelines for proper disposal of discarded reagents.
Some of the substances and solutions used are hazardous. Wear a laboratory coat, appropriate gloves and safety glasses when necessary throughout the protocol. Work in a fume hood and follow institutional safety guidelines for proper disposal of discarded reagents. Although we have not seen variation of nitrone adducts with or without DTPA, 8-oxo-dG has been affected. DMPO is easily removed from cells by washing; therefore, no further nitrone adduct is possible. However, for the digestion of tissues we recommend including DTPA in the digestion buffer and organic reagents as a precaution against further nitrone adduct formation, especially in tissues.
Although we have not seen variation of nitrone adducts with or without DTPA, 8-oxo-dG has been affected. DMPO is easily removed from cells by washing; therefore, no further nitrone adduct is possible. However, for the digestion of tissues we recommend including DTPA in the digestion buffer and organic reagents as a precaution against further nitrone adduct formation, especially in tissues. -
14
Incubate for 1 h at 52 °C with occasional vortexing. The digest should be clear before proceeding with the next step.
-
15
Cool down the digest to 37 °C by lowering the temperature of the thermomixer. Add 10 µl RNase A solution, and continue the incubation at 37 °C for 1 h.
-
16
Let the sample cool to 15–25 °C.
-
17
Add 500 µl ultrapure phenol/DTPA. Mix gently by inversion.
 Take the lower phase (phenol) and avoid formation of emulsion.
Take the lower phase (phenol) and avoid formation of emulsion. -
18
Centrifuge at 11,700g in a microcentrifuge at 15–25 °C for 2 min. Remove 350 µl of the upper aqueous phase to a fresh tube.
-
19
Add 150 µl digestion buffer and 500 µl phenol:chloroform:isoamyl alcohol (25:24:1) to the aqueous phase. Mix gently by inversion.
-
20
Centrifuge and remove 350 µl of the upper aqueous phase as described in Step 18.
-
21
Complete the volume to 500 µl with digestion buffer and add 500 µl chloroform/isoamyl alcohol (24:1). Mix gently by inversion.
-
22
Centrifuge and remove 350 µl of the upper aqueous phase as described in Step 18.
-
23
Take 300 µl of the aqueous phase and add 35 µl of 9 M ammonium acetate (final concentration is ~ 300 mM) and 750 µl ice-cold ethanol to precipitate DNA. Mix gently by inversion. Incubate for 1 h or overnight at −20 °C to increase the recovery of DNA.
-
24
Pellet the DNA by centrifuging the tube for 5 min at 11,700g and 15–25 °C.
-
25
Wash the DNA pellet twice with 70% (vol/vol) ethanol.
-
26
Let the pellet air dry under a powder-free flow.
-
27
Re-suspend the DNA in 50–100 µl buffer TE.
-
28
Determine the DNA concentration and purity. Make a 1/100 dilution of the DNA preparation in TE buffer and read against a blank of TE buffer at room temperature (15–25 °C) at 260 and 280 nm. One absorbance unit at 260 nm (A260) of a solution of dsDNA is equal to 50 µg ml−1 of dsDNA or 150 µM as nucleotides. Consider the dilution factors. Pure DNA, with a low protein concentration, should exhibit an A260/A280 ratio between 1.8 and 2.0.
 Ensure that DNA purity is optimal because the anti-DMPO antiserum will also recognize protein nitrone adducts.
Ensure that DNA purity is optimal because the anti-DMPO antiserum will also recognize protein nitrone adducts. The DNA solution can be stored at 4 °C for up to 1 wk, or at −20 or −80 °C for up to 6 months without loss of DNA nitrone adducts.
The DNA solution can be stored at 4 °C for up to 1 wk, or at −20 or −80 °C for up to 6 months without loss of DNA nitrone adducts.
-
29
Analyze the DNA nitrone adducts by ELISA (option A) or immuno-slot blot (option B; Fig. 3, Fig. 4).
-
(A) Enzyme-linked immunosorbent assay
 ~ 4–8 h
~ 4–8 hDilute the purified DNA nitrone adducts to 5 µg ml−1 in 1 X PBS.
Mix 25 µl DNA solution and 25 µl Reacti-Bind DNA coating solution in each well, in duplicate or triplicate, of a 96-well microtiter plate. Cover the plate with an appropriate lid. Use an automatic micropipette and fresh tips. It is important to include positive and negative controls in each plate with samples (see EXPERIMENTAL DESIGN).
-
Incubate the microplate for 2–4 h at 37 °C.
 Overnight incubations at 4 °C with the Reacti-Bind DNA solution will produce loss of DNA nitrone adducts by an unknown process.
Overnight incubations at 4 °C with the Reacti-Bind DNA solution will produce loss of DNA nitrone adducts by an unknown process.
-
Wash each well with 300 µl washing buffer at 15–25 °C.
 We suggest the use of a microplate washer and multichannel micropipettes to increase reproducibility of the ELISA analysis.
We suggest the use of a microplate washer and multichannel micropipettes to increase reproducibility of the ELISA analysis. -
Add 120 µl blocking solution and incubate for 90–120 min at 37 °C.
 Alternatively, incubate the plate overnight at 4 °C. In this case, next morning let the plate and washing buffer reach 15–25 °C before proceeding with the next step.
Alternatively, incubate the plate overnight at 4 °C. In this case, next morning let the plate and washing buffer reach 15–25 °C before proceeding with the next step. -
Wash once with 300 µl washing buffer by incubating the plate for 5 min on an orbital shaker at 15–25 °C. Discard the washing buffer.
 Plates can be kept covered with film at −20 °C for up to 2 wk without any significant change in the immunoreactivity. Before continuing the analysis, add 200 µl washing buffer at 15–25 °C, and let the plate stand for at least 30 min before discarding the solution and continuing with the addition of the first Ab.
Plates can be kept covered with film at −20 °C for up to 2 wk without any significant change in the immunoreactivity. Before continuing the analysis, add 200 µl washing buffer at 15–25 °C, and let the plate stand for at least 30 min before discarding the solution and continuing with the addition of the first Ab. Add 100 µl anti-DMPO serum (1:10,000) in washing buffer and incubate for 60 min at 37 °C.
Wash three times as described in Step 29A(vi).
-
Add 100 µl secondary Ab conjugated to HRP (1:10,000) diluted in washing buffer.
 To increase sensitivity, anti-rabbit IgGFc conjugated to HRP must be used instead of the corresponding AP conjugate (Fig. 2).
To increase sensitivity, anti-rabbit IgGFc conjugated to HRP must be used instead of the corresponding AP conjugate (Fig. 2). Wash three times as described in Step 29A(vi).
-
Let the development reagent reach 15–25 °C before use. Add 50 µl development reagent, wait for 30 s and read the luminescence in a SpectraFluor Plus microplate reader or equivalent.
 Use the type of development reagent suggested in this protocol. Usually, researchers are tempted to use high-sensitivity development reagents, especially for HRP; however, some of these reagents give high backgrounds (~50,000 relative light units).
Use the type of development reagent suggested in this protocol. Usually, researchers are tempted to use high-sensitivity development reagents, especially for HRP; however, some of these reagents give high backgrounds (~50,000 relative light units). Analyze and plot the results using GraphPad Prism package software or equivalent.
-
(B) Immuno-slot blot
 ~6 h
~6 hPrepare slot blot manifold: wash the dot/slot blot manifold with 1% (wt/vol) SDS solution, then with distilled water prior to use. Let it dry under a powder-free hood.
Using gloves, cut a piece of Hybond-ECL nitrocellulose membrane and a piece of Whatman 3 MM filter paper to completely cover the surface of the manifold.
Soak both the nitrocellulose membrane and the filter paper in 2 X SSC for 5 min prior to use.
Place the Whatman paper into the manifold with the nitrocellulose membrane on top. Clamp the entire structure together following the instruction manual.
Prepare DNA samples and loading: dilute the DNA sample to 10 µg ml−1 (100 µl) in distilled water.
Heat samples at 95 °C for 10 min, then quickly chill on ice.
Add an equal volume of freshly made 1 N NaOH to the DNA sample.
Incubate the DNA solution at 15–25 °C for 20 min.
Apply DNA solutions to the manifold according to the manufacturer’s instructions.
Allow the DNA to stay in contact with the membrane for 60 min at 15–25 °C.
Apply a vacuum to the manifold to draw the DNA solution through the membrane.
After the DNA solution has been drawn through, remove the membrane from the manifold using forceps.
-
Incubate the membrane in 50 ml neutralizing solution for 30 min. Wash with distilled water.
 Membranes can be air dried and stored flat (e.g., between two pieces of filter paper inside a book) until analysis.
Membranes can be air dried and stored flat (e.g., between two pieces of filter paper inside a book) until analysis. -
Analyze DNA nitrone adducts. Rinse the membrane with water and incubate at 15–25 °C for 60 min with blocking buffer. Incubations can be performed in a square plastic dish taking care to completely cover the membrane with the solution.
 Cover the membrane with blocking buffer and incubate at 4 °C overnight. Next morning, let every reagent reach 15–25 °C before continuing with the next step.
Cover the membrane with blocking buffer and incubate at 4 °C overnight. Next morning, let every reagent reach 15–25 °C before continuing with the next step. Wash once with 50 ml washing buffer for 10 min in an orbital shaker at 200 r.p.m. at 15–25 °C.
-
Decant the washing buffer and add 25 ml of a solution of the anti-DMPO antiserum (1:10,000) in washing buffer. Incubate for 60 min at room temperature.
 This incubation can be done overnight in the refrigerator. Cover the membrane with enough solution to avoid dehydration.
This incubation can be done overnight in the refrigerator. Cover the membrane with enough solution to avoid dehydration. Discard the Ab solution. Wash three times as described in Step 29B(xv).
Add 25 ml anti-rabbit IgG–HRP conjugated at a 1:10,000 dilution in washing buffer. Incubate for 60 min at 15–25 °C.
Repeat Step 29B(xv). Wash the membrane with PBS. Blot the excess liquid in the membrane with filter paper, then put the membrane on a piece of plastic folder.
Cover the membrane with 5 ml developing solution and let it react for 5 min. Blot the excess liquid in the membrane with filter paper, then put the membrane inside a plastic folder (office) and expose the membrane using CL-Xposure film or observe in an Image Station 1000.
-
![]()
Preparation of calf thymus DNA nitrone adducts: ~90 min
Production of nitrone adducts in cells: ≤24 h
Extraction of DNA nitrone adduct from cells: ~2–3 h
ELISA analysis: ~4–8 h
Immuno-slot blot: ~6 h
![]()
Troubleshooting advice can be found in Table 1.
Table 1.
Troubleshooting table.
| Problem | Possible reason | Solution |
|---|---|---|
| No signal | Defective secondary Ab | Follow manufacturer’s instructions to rehydrate the secondary Ab. Mix 10 µl of a 1:10,000 dilution of the secondary Ab in washing buffer and 50 µl of the developing reagent in a microplate, and read the chemiluminescence |
| Not enough DNA loaded in each well |
Run positive controls and load different concentrations of the purified DNA nitrone adducts |
|
| No DNA nitrone adducts | Run a positive and a negative control in each plate. Do not incubate the nitrone adducts with Reacti-Bind DNA coating solution for >6 h |
|
| Treatments bleach DNA nitrone adducts* |
Extract the positive control under the same conditions used to extract DNA from samples |
|
| High background | Excessive amount of DNA loaded in each well |
Use 25 µl of a 5–10 µg µl−1 DNA solution. Use the type of development reagent suggested in this protocol |
| If purified DNA from blood or tissue, beware of hemopro- teins as contaminants |
Add 10 µl of DNA extracted from cells or tissues without any treatment and 50 µl of the development reagent. If hemoproteins are present, there will be a chemiluminescent signal. Re-purify DNA sample |
|
| Poor reproducibility | Operational failures | Use multichannel micropipettes and an automatic washer. Include a positive control sample |
The term bleaching refers here to an alteration of the epitope (the nitrone motif) by unknown chemical mechanisms with a consequent loss of Ab binding.
ANTICIPATED RESULTS
Previously, we have used AP-conjugated secondary Ab and CDP-star (AP, blue bars, Fig. 3) as development reagents in ELISAs to detect DNA nitrone adducts28. In order to improve the sensitivity of our immuno-spin trapping analysis of DNA nitrone adducts, we performed extensive crisscross analyses32 for control (one or more reagents omitted, Box 2) and complete systems (DNA + metal + H2O2 + DMPO), and determined the optimal immunoreagent concentration to use in this protocol. We have validated the following parameters: amount of DNA nitrone adducts to add to each well, blocking reagent, concentration of the anti-DMPO serum and the HRP-conjugated secondary antiserum, and the chemiluminescent substrate (see REAGENTS and PROCEDURE). Figure 3 shows typical results when evaluating the same sample with secondary Abs conjugated with AP (blue bars) and HRP (red bars). HRP secondary Abs allowed a greater sensitivity than AP-conjugated Abs in assessing DNA radicals.
We used the described protocols to explore the production of DNA radicals in RAW 264.7 macrophages pre-loaded with redox-active transition metals (Cu2+ or Fe3+) and treated with tert-BOOH (Fig. 4). Copper and iron diffuse inside the cell through cell membranes mostly by ion-exchange transporters. The compound tert-BOOH is a lipid-soluble peroxide; it diffuses easily through cell membranes and is degraded by glutathione peroxidase in a process that uses 2 mol reduced glutathione per 1 mol tert-BOOH degraded. It has been shown that iron and/or copper play a role both in the metabolic activation of tert-BOOH to tert-BO• and methyl radicals, and in DNA damage in the liver33 and isolated hepatocytes34 of rats. We were able to detect DNA nitrone adducts in whole cells treated with redox-active transition metals and tert-BOOH (Fig. 4). As observed in Figure 4, the treatments did not affect cell viability (P<0.05); therefore, the DNA nitrone adducts detected are formed and trapped inside functioning cells. Once nitrone adducts are formed, the free DMPO is washed away during the washing steps, ruling out the further formation of nitrone adducts during DNA extraction.
Finally, the DNA-extraction procedure described in this protocol can be used for any tissues and cells. The digestion step for some tissues (e.g., heart, skin and kidney) can require overnight incubation with digestion buffer. DNA nitrone adducts will remain stable even under such conditions. Using the protocol described, we have observed no significant loss of nitrone adducts or formation of further (artifactual) nitrone adducts in any of our experimental models; however, the chelator DTPA is added as a normal precaution against transition metal-catalyzed free-radical formation and/or damage to the already formed adducts.
ACKNOWLEDGMENTS
This work was supported by the National Institute of Environmental Health Sciences (NIEHS) Intramural Research Program. D.C.R. is the recipient of a National Institutes of Health Pathway for Independence Award (1 K99 ES015415-01). We acknowledge J. Corbett for her excellent technical assistance, and M. Ehrenshaft and M. Waalkes for their useful comments. We also thank M. Mason and A. Motten for helping in the editing of this manuscript.
Footnotes
COMPETING INTERESTS STATEMENT The authors declare that they have competing financial interests.
References
- 1.Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br. J. Pharmacol. 2004;142:231–255. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dröge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 3.Halliwell B, Gutteridge JM. Free Radicals in Biology and Medicine. New York, USA: Oxford Univ. Press Inc; 1985. The chemistry of oxygen radicals and other oxygen-derived species; pp. 20–66. [Google Scholar]
- 4.Gracy RW, Talent JM, Kong Y, Conrad CC. Reactive oxygen species: the unavoidable environmental insult? Mutat. Res. 1999;428:17–22. doi: 10.1016/s1383-5742(99)00027-7. [DOI] [PubMed] [Google Scholar]
- 5.Winyard PG, Moody CJ, Jacob C. Oxidative activation of antioxidant defence. Trends Biochem. Sci. 2005;30:453–461. doi: 10.1016/j.tibs.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 6.Imlay JA, Chin SM, Linn S. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science. 1988;240:640–642. doi: 10.1126/science.2834821. [DOI] [PubMed] [Google Scholar]
- 7.Cooke MS, Evans MD, Dizdaroglu M, Lunec J. Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J. 2003;17:1195–1214. doi: 10.1096/fj.02-0752rev. [DOI] [PubMed] [Google Scholar]
- 8.Cadet J, Douki T, Gasparutto D, Ravanat J-L. Oxidative damage to DNA: formation, measurement and biochemical features. Mutat. Res. 2003;531:5–23. doi: 10.1016/j.mrfmmm.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 9.von Sonntag C. The Chemical Basis of Radiation Biology. London, UK: Taylor & Francis Ltd; 1987. DNA model systems; pp. 116–294. [Google Scholar]
- 10.Hall DB, Holmlin RE, Barton JK. Oxidative DNA damage through long-range electron transfer. Nature. 1996;382:731–735. doi: 10.1038/382731a0. [DOI] [PubMed] [Google Scholar]
- 11.Purkayastha S, Milligan JR, Bernhard WA. Correlation of free radical yields with strand break yields produced in plasmid DNA by the direct effect of ionizing radiation. J. Phys. Chem. B. 2005;109:16967–16973. doi: 10.1021/jp0518409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Sonntag C. The chemistry of free-radical-mediated DNA damage. Basic Life Sci. 1991;58:287–321. doi: 10.1007/978-1-4684-7627-9_10. [DOI] [PubMed] [Google Scholar]
- 13.Collins AR, Cadet J, Möller L, Poulsen HE, Viña J. Are we sure we know how to measure 8-oxo-7,8-dihydroguanine in DNA from human cells? Arch. Biochem. Biophys. 2004;423:57–65. doi: 10.1016/j.abb.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 14.Fraga CG, Shigenaga MK, Park J-W, Degan P, Ames BN. Oxidative damage to DNA during aging: 8-hydroxy-2′-deoxyguanosine in rat organ DNA and urine. Proc. Natl. Acad. Sci. USA. 1990;87:4533–4537. doi: 10.1073/pnas.87.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.European Standards Committee on Oxidative DNA Damage (ESCODD) Measurement of DNA oxidation in human cells by chromatographic and enzymic methods. Free Radic. Biol. Med. 2003;34:1089–1099. doi: 10.1016/s0891-5849(03)00041-8. [DOI] [PubMed] [Google Scholar]
- 16.Hildenbrand K, Schulte-Frohlinde D. ESR spectra of radicals of single-stranded and double-stranded DNA in aqueous solution. Implications for •OH-induced strand breakage. Free Radic. Res. Commun. 1990;11:195–206. doi: 10.3109/10715769009088916. [DOI] [PubMed] [Google Scholar]
- 17.Cullis PM, Jones GDD, Symons MCR, Lea JS. Electron transfer from protein to DNA in irradiated chromatin. Nature. 1987;330:773–774. doi: 10.1038/330773a0. [DOI] [PubMed] [Google Scholar]
- 18.Khan N, et al. Spin traps: in vitro toxicity and stability of radical adducts. Free Radic. Biol. Med. 2003;34:1473–1481. doi: 10.1016/s0891-5849(03)00182-5. [DOI] [PubMed] [Google Scholar]
- 19.Haseloff RF, et al. Cytotoxicity of spin trapping compounds. FEBS Lett. 1997;418:73–75. doi: 10.1016/s0014-5793(97)01349-5. [DOI] [PubMed] [Google Scholar]
- 20.Schaefer CF, Janzen EG, West MS, Poyer JL, Kosanke SD. Blood chemistry changes in the rat induced by high doses of nitronyl free radical spin traps. Free Radic. Biol. Med. 1996;21:427–436. doi: 10.1016/0891-5849(96)00039-1. [DOI] [PubMed] [Google Scholar]
- 21.Anzai K, et al. ESR measurement of rapid penetration of DMPO and DEPMPO spin traps through lipid bilayer membranes. Arch. Biochem. Biophys. 2003;415:251–256. doi: 10.1016/s0003-9861(03)00260-1. [DOI] [PubMed] [Google Scholar]
- 22.Ho WF, Gilbert BC, Davies MJ. EPR spin-trapping studies of radicals generated from FeII-catalysed degradation of nucleobase, nucleoside, RNA and DNA hydroperoxides. J. Chem. Soc. Perkin Trans. 1997;2:2525–2531. [Google Scholar]
- 23.Kuwabara M, Ohshima H, Sato F, Ono A, Matsuda A. Spin-trapping detection of precursors of hydroxyl-radical-induced DNA damage: identification of precursor radicals of DNA strand breaks in oligo(dC)10 and oligo(dT)10. Biochemistry. 1993;32:10599–10606. doi: 10.1021/bi00091a009. [DOI] [PubMed] [Google Scholar]
- 24.Maurel V, Ravanat J-L, Gambarelli S. Detection of reactive free radicals derived from nucleosides by liquid chromatography coupled to tandem mass spectrometry of DMPO spin trapping adducts. Rapid Commun. Mass Spec. 2006;20:2235–2242. doi: 10.1002/rcm.2579. [DOI] [PubMed] [Google Scholar]
- 25.Janzen EG, West MS, Kotake Y, DuBose CM. Biological spin trapping methodology. III. Octanol-water partition coefficients of spin-trapping compounds. J. Biochem. Biophys. Methods. 1996;32:183–190. doi: 10.1016/0165-022x(96)00008-5. [DOI] [PubMed] [Google Scholar]
- 26.Mason RP. Using anti-5,5-dimethyl-1-pyrroline N-oxide (anti-DMPO) to detect protein radicals in time and space with immuno-spin trapping. Free Radic. Biol. Med. 2004;36:1214–1223. doi: 10.1016/j.freeradbiomed.2004.02.077. [DOI] [PubMed] [Google Scholar]
- 27.Ramirez DC, Mason RP. Immuno-spin trapping: detection of protein-centered radicals. In: Costa LG, Maines MD, Reed DJ, Sassa S, Sipes IG, editors. Current Protocols in Toxicology. Hoboken, New Jersey, USA: John Wiley & Sons; 2005. pp. 17.7.1–17.7.16. [DOI] [PubMed] [Google Scholar]
- 28.Ramirez DC, Gomez-Mejiba SE, Mason RP. Immuno-spin trapping of DNA radicals. Nat. Methods. 2006;3:123–127. doi: 10.1038/nmeth852. [DOI] [PubMed] [Google Scholar]
- 29.Ramirez DC, Chen Y-R, Mason RP. Immunochemical detection of hemoglobin-derived radicals formed by reaction with hydrogen peroxide: involvement of a protein-tyrosyl radical. Free Radic. Biol. Med. 2003;34:830–839. doi: 10.1016/s0891-5849(02)01437-5. [DOI] [PubMed] [Google Scholar]
- 30.Detweiler CD, et al. Immunological identification of the heart myoglobin radical formed by hydrogen peroxide. Free Radic. Biol. Med. 2002;33:364–369. doi: 10.1016/s0891-5849(02)00895-x. [DOI] [PubMed] [Google Scholar]
- 31.Strober W. Trypan blue exclusion test of cell viability. In: Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Strober W, editors. Current Protocols in Immunology. Hoboken, New Jersey, USA: John Wiley & Sons, Inc; 1997. pp. A3.B1–A3.B2. [Google Scholar]
- 32.Crowther JR. Methods in Molecular Biology: The ELISA Guidebook. Vol 149. Totowa, New Jersey, USA: Humana Press Inc; 2001. Titration of reagents; pp. 83–113. [Google Scholar]
- 33.Hix S, Kadiiska MB, Mason RP, Augusto O. In vivo metabolism of tert-butyl hydroperoxide to methyl radicals. EPR spin-trapping and DNA methylation studies. Chem. Res. Toxicol. 2000;13:1056–1064. doi: 10.1021/tx000130l. [DOI] [PubMed] [Google Scholar]
- 34.Latour I, Demoulin JB, Buc-Calderon P. Oxidative DNA damage by t-butyl hydroperoxide causes DNA single strand breaks which is not linked to cell lysis. A mechanistic study in freshly isolated rat hepatocytes. FEBS Lett. 1995;373:299–302. doi: 10.1016/0014-5793(95)01065-m. [DOI] [PubMed] [Google Scholar]