Summary
Neurotrophin 3 (NT-3) can support the survival of some embryonic sympathetic neuroblasts before they become nerve growth factor dependent. We show that NT-3 is produced in vivo by nonneuronal cells neighboring embryonic sympathetic ganglia. NT-3 mRNA is produced by these nonneuronal cells in vitro and is up-regulated by platelet-derived growth factor, ciliary neurotrophic factor, and glial growth factor 2 (a neuregulin). Nonneuronal cell–conditioned medium promotes survival and induces TrkA expression in isolated sympathetic neuroblasts, and this activity is blocked by anti-NT-3 antibody. Neuroblasts also enhance NT-3 production by nonneuronal cells. Neuroblasts synthesize several forms of neuregulin, and antibodies to neuregulin attenuate the effect of the neuroblasts on the nonneuronal cells. These data suggest a reciprocal cell–cell interaction, in which neuroblast-derived neuregulins promote NT-3 production by neighboring nonneuronal cells, which in turn promotes neuroblast survival and further differentiation.
Introduction
Growing evidence indicates that neurotrophins act not only as target-derived neuronal survival factors, but also can regulate mitotic activity and promote differentiation of neuronal progenitor cells, their survival, or both, in both the PNS (Sieber-Blum, 1991; Kalcheim et al., 1992; Wright et al., 1992) and CNS (Cattaneo and McKay, 1990; Collazo et al., 1992; Segal et al., 1992; Ghosh and Greenberg, 1995; Vicario-Abejón et al., 1995). For example, embryonic rat sympathetic neuroblasts can be supported by neurotrophin 3 (NT-3) before they become nerve growth factor (NGF) dependent (Birren et al., 1993; Dechant et al., 1993; DiCicco-Bloom et al., 1993), suggesting that NT-3 may act as an survival factor for these neuronal precursors (Figure 1A). Similar switches in neurotrophin-responsiveness have been documented for peripheral sensory neurons as well (Buchman and Davies, 1993; Buj-Bello et al., 1994; Davies, 1994).
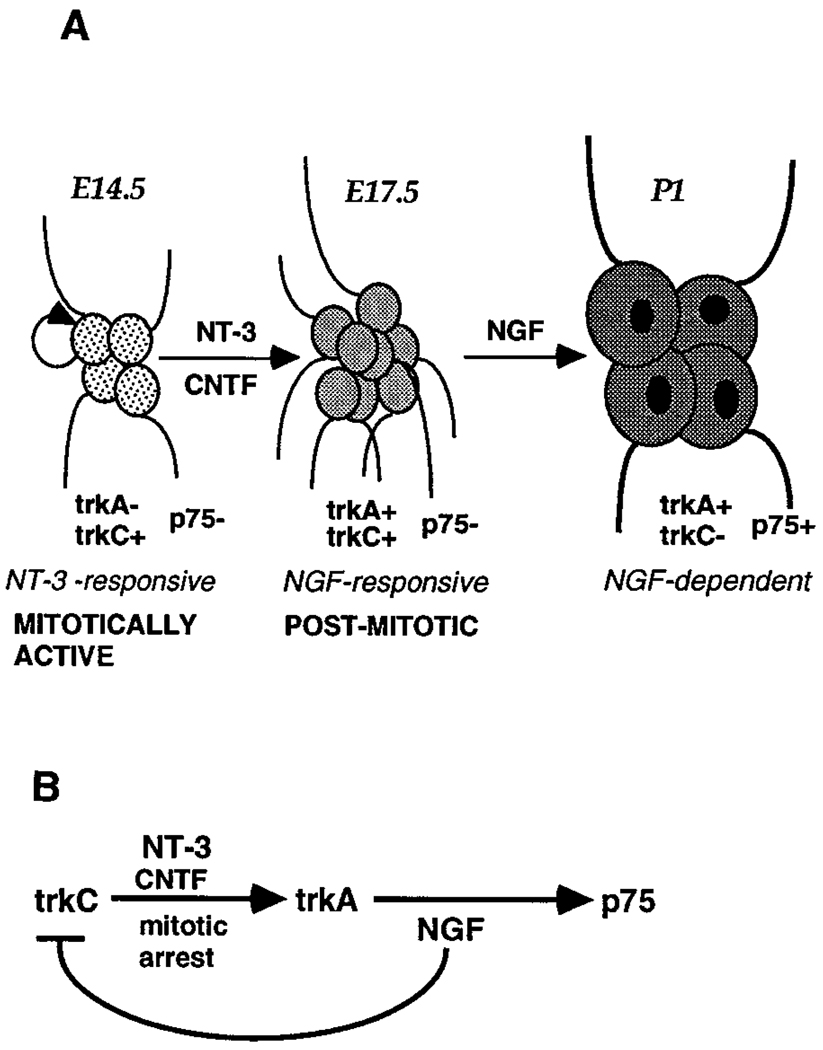
Figure 1.
Schematics Showing the Switch in Neurotrophin Responsiveness by Embryonic Rat Sympathetic Neuroblasts, and the Regulatory Circuits Underlying the Switch
(A) The switch in neurotrophin responsiveness by embryonic rat sympathetic neuroblasts (Birren et al., 1993; DiCicco-Bloom et al., 1993).
(B) The regulatory circuits underlying the switch.
The induction of TrkA expression in neuroblasts exposed to NT-3 or CNTF appears to be primarily a consequence of mitotic arrest (Verdi and Anderson, 1994). In vitro, exposure of TrkA-expressing neuroblasts to NGF results in both an induction of p75 expression (Wyatt and Davies, 1993; Verdi and Anderson, 1994) and a down-regulation of TrkC expression (Verdi et al., 1994b).
In cultured sympathetic neuroblasts, NT-3 not only supports survival; at higher doses it can also promote cell cycle arrest, leading to an induction of tyrosine receptor kinase A (TrkA) and the appearance of NGF responsiveness in embryonic day (E) 14.5 rat sympathetic neuroblasts (Figure 1B) (Verdi and Anderson, 1994). NT-3 also promotes cell cycle withdrawal in cortical neuroepithelial precursors (Ghosh and Greenberg, 1995). However, NT-3 is not unique in this action; ciliary neurotrophic factor (CNTF) has a similar effect on sympathetic neuroblasts as well (Ernsberger et al., 1989b; Verdi and Anderson, 1994). Indeed, antimitotic agents such as aphidicolin and mitomycin C induce TrkA even more efficiently than high doses of NT-3 and CNTF, suggesting that expression of this neurotrophin receptor is primarily a consequence of mitotic arrest (Figure 1B) (Verdi and Anderson, 1994).
Once TrkA is expressed, NGF in turn is able to upregulate expression of the low affinity NGF receptor p75 (Figure 1B) (Wyatt and Davies, 1993; Verdi and Anderson, 1994). A likely consequence of such increased p75 expression is an enhanced sensitivity to NGF (Davies et al., 1993; Barker and Shooter, 1994; Hantzopoulos et al., 1994; Lee et al., 1994; Verdi et al., 1994a). NGF can also down-regulate TrkC expression in the neuroblasts (Figure 1B) (Verdi et al., 1994b) and in this way may contribute to the switch from NT-3 dependence to NGF dependence. These data illustrate the way in which a relay or cascade of neurotrophins and neuropoietic cytokines can regulate sequential steps in the survival, early differentiation, and cell cycle arrest of primary sympathetic neuroblasts in vitro. Similar conclusions have been drawn from studies of immortalized sympathoadrenal progenitors (Ip et al., 1994).
Targeted inactivation of the NT-3 gene by homologous recombination in mice leads to a 50% reduction in neuronal number in superior cervical sympathetic ganglia (Ernfors et al., 1994; Fariñas et al., 1994). By contrast, no such effect on sympathetic development was observed in mice bearing a null mutation in the CNTF gene (Masu et al., 1993) or in the leukemia inhibitory factor (LIF) gene (Rao et al., 1993), which encodes a cytokine that interacts with a related receptor (Davis et al., 1993). In addition, CNTF expression is low or undetectable in early embryogenesis (Stöckli et al., 1989, 1991). These data provide evidence that NT-3 is important for the development of at least some sympathetic neurons in vivo, although they do not exclude a role for a CNTF-related molecule (Leung et al., 1992; Ip et al., 1993). The demonstration that NT-3 is essential in sympathetic neurogenesis in vivo means that it is important to define its source and the regulation of its production. Previous work indicated that NT-3 mRNA can be detected in forming sympathetic ganglia at E14.5 (Schecterson and Bothwell, 1992); however, these studies did not identify the cell type(s) that produce NT-3.
Here we demonstrate that NT-3 is produced by nonneuronal (nn) cells immediately surrounding sympathetic ganglia, among which are glial progenitors. In vitro, NT-3 mRNA expression in these nn cells can be strongly up-regulated by glial growth factor 2 (GGF2, a neuregulin), platelet-derived growth factor (PDGF), and CNTF. The induction of NT-3 mRNA in these nn cells is paralleled by an increased secretion of survival-promoting and TrkA-inducing activity for sympathetic neuroblasts; both such biological activities are inhibited by blocking anti-NT-3 antibodies. Addition of isolated sympathetic neuroblasts to nn cells also up-regulates NT-3 mRNA and enhances secretion of neuroblast survival– promoting activity, which is blocked by anti-NT-3 antibody. Freshly isolated sympathetic neuroblasts contain various forms of neuregulin mRNA. The effect of the neuroblasts on nonneuronal cells is attenuated by anti-GGF/neuregulin antibodies. Taken together, these data suggest that NT-3 is provided to embryonic sympathetic neuroblasts by surrounding nn cells and that its production in these cells may in turn be regulated by neuroblast-derived signals including neuregulins. In this way, a reciprocal cell–cell interaction mediated by neurotrophins and neuregulins may control the early survival and development of sympathetic neuroblasts.
Results
NT-3 Is Expressed in nn Cells Surrounding Sympathetic Ganglia
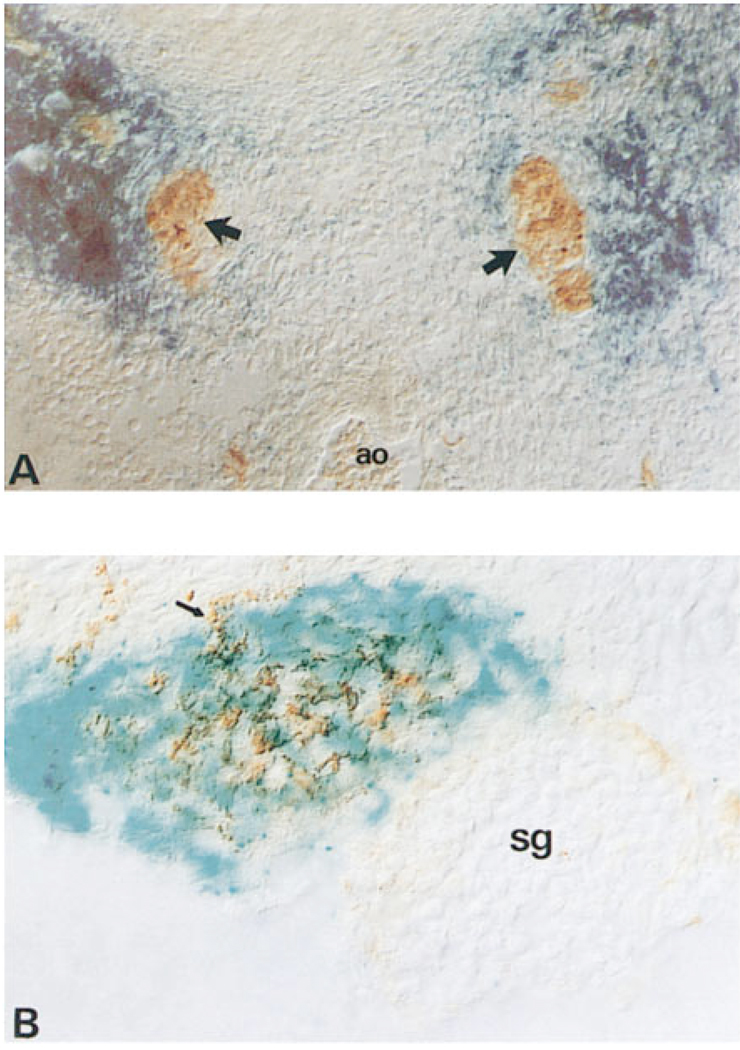
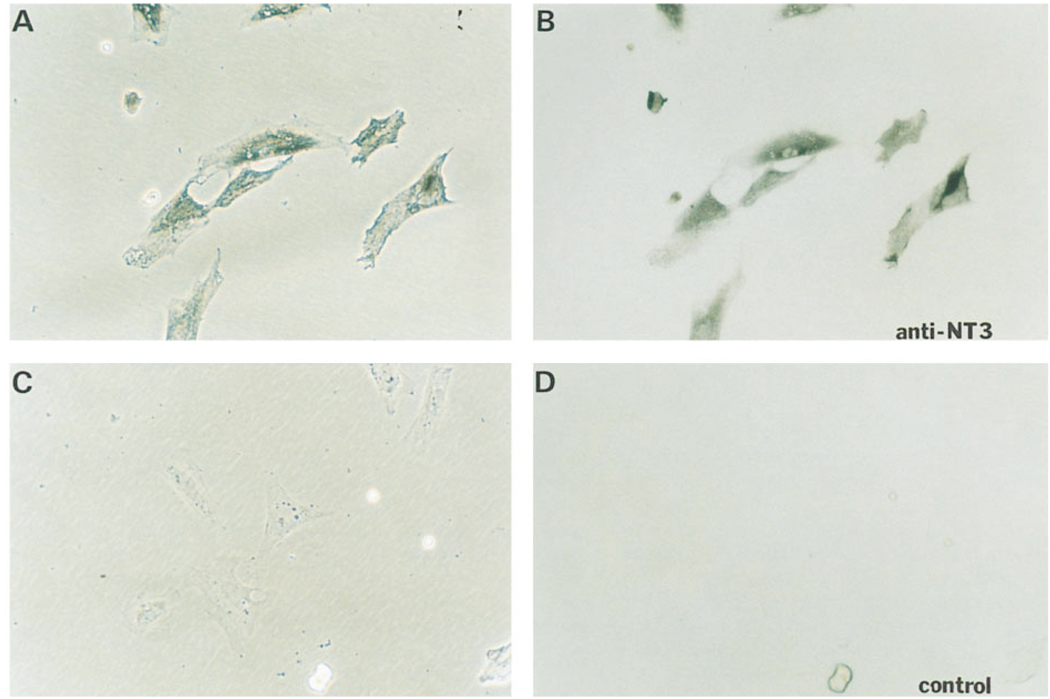
To determine the site of NT-3 expression relative to sympathetic ganglia in vivo, we examined sections of fetal mice harboring a lacZ gene inserted into the NT-3 locus by homologous recombination (Fariñas et al., 1994). The expression pattern of β-galactosidase in these mice reflects the expression of endogenous NT-3 (I. F., unpublished data). Strikingly, β-galactosidase-positive cells in these mice surrounded but excluded sympathetic ganglia primordia as revealed by counter-staining with antibody to peripherin (Figure 2A, arrows). The distribution of the β-galactosidase positive cells at E13.5 overlapped that of cells expressing p75LNGFR, a marker of peripheral glia and their neural crest progenitors (Stemple and Anderson, 1992) (Figure 2B, arrow). p75+ nn cells isolated from rat E14.5 sympathetic ganglia by preparative flow cytometry were labeled by an antibody to NT-3 (see Experimental Procedures; Figure 3A and 3B). The expression of NT-3 mRNA in these cells was independently confirmed by reverse transcription–polymerase chain reaction (RT–PCR) analysis, which also indicated that NT-3 mRNA was undetectable in neuroblasts isolated with monoclonal antibody B2 (see below; data not shown). Thus, both the antibody staining and the mRNA analysis confirm that the pattern of β-galactosidase expression seen in sections likely reflects that of endogenous NT-3 and demonstrates that this neurotrophin is not expressed by ganglionic neuroblasts at this stage, but rather by surrounding nn cells, at least some of which are p75+.
Figure 2.
Expression of NT-3 in Sympathetic Ganglionic Nonneuronal Cells In Vivo
Transverse sections through the thoracic region of E13.5 mouse embryos containing two copies of the lacZ gene inserted into the NT-3 locus by homologous recombination. In (A), the sections are counterstained with antibody to peripherin; arrows indicate the sympathetic ganglia. In (B), the sections are counterstained with antibody to p75, which reveals nonneuronal cells adjacent to the sympathetic ganglion. Ao, dorsal aorta; sg, sympathetic ganglion.
Figure 3.
Expression of NT-3 in Isolated p75+ Ganglionic Nonneuronal Cells
Rat E14.5 thoracic sympathetic ganglia were dissociated and sorted by use of anti-p75 monoclonal antibody. The isolated p75+ cells were plated overnight, fixed, and stained with rabbit anti-NT-3 antibody (see Experimental Procedures) at a 1:1000 dilution (A and B) or preimmune serum at the same dilution as a control (C and D). (A) and (C) are phase contrast micrographs of the bright-field images in (B) and (D), respectively.
Growth Factors Enhance NT-3 mRNA Expression and NT-3-Mediated Bioactivities in Cultured Nonneuronal Cells
We next examined the regulation of NT-3 production in nn cells isolated from ganglia by negative selection with monoclonal antibody (MAb) B2 (Birren et al., 1993). The B2− cell population excludes the sympathetic neuroblasts and contains the p75+ population as well as other nonneuronal cells (see Experimental Procedures). Because of the larger cell numbers obtained, it was more convenient to work with B2− cells than p75+ cells as a source of immunoisolated nn cells. These nonneuronal cells were cultured in an insulin-free, serum-free defined medium in the presence of various growth factors, including epidermal growth factor (EGF), insulin-like growth factor (IGF)-1 and IGF-2, fibroblast growth factor (FGF), NGF, brain-derived neurotrophic factor (BDNF), retinoic acid, and members of the transforming growth factor-β (TGFβ) superfamily. Of these factors, CNTF, rhGGF2, and PDGF all up-regulated of NT-3 mRNA by greater than 5-fold after 24 hr (Table 1). No significant effect was observed with IGF-1, NGF (Table 1), or the other factors tested (data not shown). By 48 hr, NT-3 mRNA levels were up-regulated over 40-fold (normalized to actin) in rhGGF2- or CNTF-treated cultures (Table 1). Cell number was also dramatically increased in rhGGF2-treated cultures (not shown); however, the normalization to actin indicates that there is a significant induction of NT-3 on a per cell basis, independent of proliferation.
Table 1.
Relative Levels of NT-3 mRNA in Cultures of B2− Ganglionic Nonneuronal Cells Treated with Various Factors
| Factor | 24 hr | 48 hr |
|---|---|---|
| Control | 1.0 ± 0.0 | 1.4 ± 0.3 |
| IGF-1 | 1.2 ± 0.3 | 0.8 ± 0.2 |
| NGF | 1.5 ± 0.3 | 1.9 ± 0.2 |
| CNTF | 5.2 ± 0.4 | 47 ± 2.4 |
| rhGGF2 | 6.4 ± 1.2 | 43 ± 3.8 |
| PDGF | 4.8 ± 1.6 | 12 ± 1.8 |
| B2+ neuroblasts | 7.1 ± 0.8 | 13 ± 1.4 |
Presented is the mean ± SEM from two experiments derived from independent cell isolations. Values represent the levels of NT-3 mRNA as assayed by a quantitative RT–PCR procedure. The values are normalized to the 24 hr control determinations for easier comparison; however, the control cultures contained easily detectable amounts of NT-3 mRNA.
We next asked whether the increase in NT-3 mRNA caused by growth factor treatment was correlated with an enhanced secretion of functional NT-3, as measured by bioassays using conditioned medium (CM) from the nn cells. It has been demonstrated previously that recombinant NT-3 has at least two biological activities on isolated B2+ sympathetic neuroblasts: at low concentrations (0.3–1.0 ng/ml), it can support their survival; at higher concentrations (30–50 ng/ml), it can promote mitotic arrest and consequent induction of TrkA (Verdi and Anderson, 1994). We therefore asked whether these bioactivities were present in nn cell CM and if so whether they could be inhibited by function-blocking antibodies to NT-3 (design illustrated in Figure 4A and 4B).
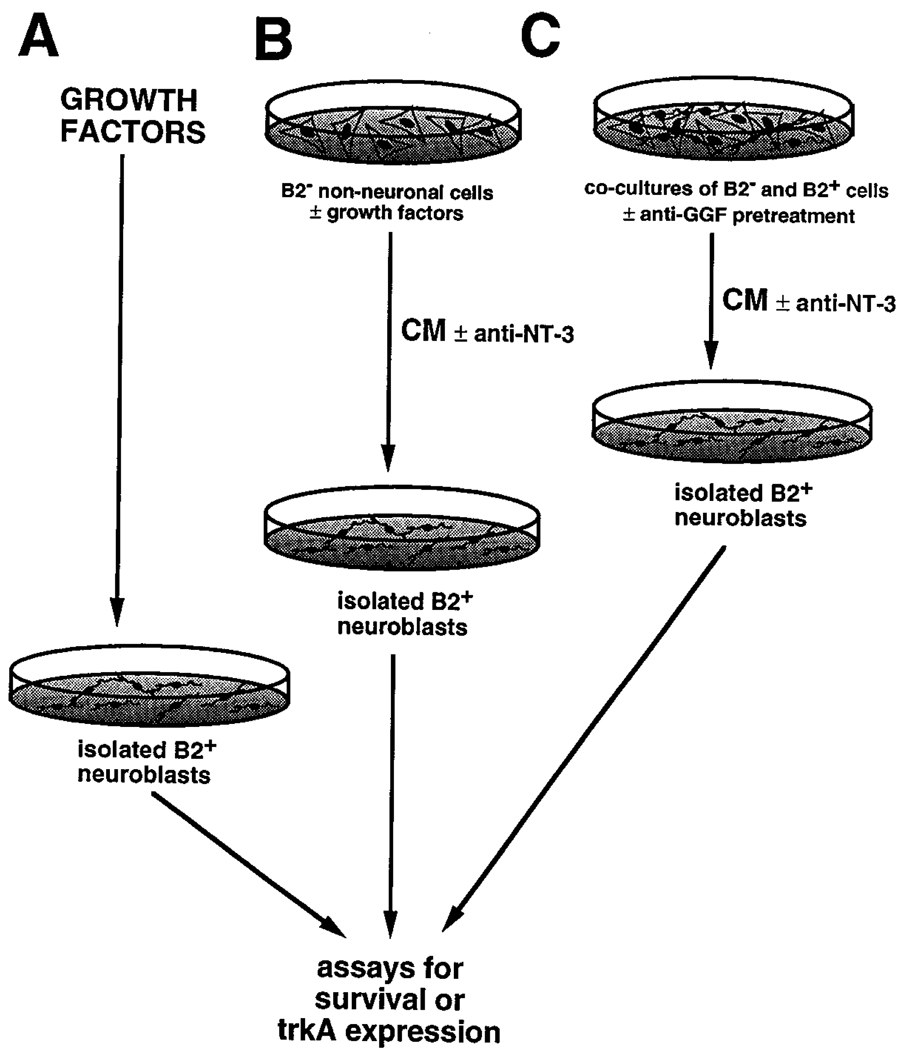
Figure 4.
Schematic Diagram Illustrating Design of Conditioned Medium Experiments
Survival or TrkA expression by isolated B2+ neuroblasts is measured after culture in the presence of specific growth factors (A), or in conditioned media (CM) derived from B2– nonneuronal cells cultured either alone (B) or in the presence of neuroblasts (C). The CMs are prepared in the presence or absence of various growth factors, or in the presence or absence of blocking antibodies to GGF/neuregulin (C). The CMs are applied to the isolated neuroblasts in the presence or absence of blocking antibodies to NT-3. The survival or expression of TrkA is measured in the isolated neuroblast preparations.
We first assayed survival in cultures of isolated E14.5 B2+ cells. CM from untreated B2− nn cells enhanced neuroblast survival 2-fold (Table 2, top, no addition, versus Table 2, bottom, CM [untreated B2− cells]). By 48 hr, the percentage of surviving neurons in the presence of CM was at least double that seen in controls (Table 2; see Table 5). This survival-enhancing effect was abolished by adding a function-blocking anti-NT-3 antibody (Ghosh et al., 1994; Ghosh and Greenberg, 1995) to the CM (compare Table 2, bottom, CM [B2− cells] plus anti-NT-3 with Table 2, top, no addition). The anti-NT-3 antibody had no effect on neuroblast survival in basal medium, nor did it reduce neuroblast survival in the presence of insulin (Table 2, top), another survival factor for sympathetic neuroblasts (DiCicco-Bloom and Black, 1988; DiCicco-Bloom et al., 1993; Zackenfels et al., 1995). These biological controls indicated that the anti-NT-3 antibody does not inhibit neuroblast survival nonspecifically. These data imply that nn cells constitutively secrete some NT-3, which is able to enhance neuroblast survival. This conclusion is consistent with the results of the RT–PCR and immunostaining analyses, in which NT-3 mRNA was clearly detectable in untreated nn cells (Figure 3; see Table 1).
Table 2.
Neuroblast Survival at 48 hr Promoted by Growth Factors and nn Cell–Conditioned Media
| Condition | Percentage of Neurons Recovered |
||
|---|---|---|---|
| Experiment I | Experiment II | Experiment III | |
| No addition | 22 ± 4.4 | 25 ± 1.4 | 15 ± 3.5 |
| Anti-NT-3 | 25 ± 1.8 | ND | ND |
| NT-3 | 66 ± 7.0 | 80 ± 4.2 | 75 ± 6.2 |
| Anti-NT-3 plus insulin | 70 ± 2.7 | ND | ND |
| rhGGF2 | 24 ± 1.9 | 28 ± 1.9 | 14 ± 0.9 |
| PDGF | ND | 27 ± 0.9 | 12 ± 1.3 |
| CNTF | ND | 25 ± 1.1 | 16 ± 6 |
| CM (untreated B2− cells) | 47 ± 0.6 | 69 ± 4.7 | 31 ± 4.2 |
| CM (B2− cells) plus anti-NT-3a | 22 ± 2.1 | 25 ± 4.6 | 13 ± 3.2 |
| CM (B2− cells plus GGF) | 67 ± 4.4 | 78 ± 1.6 | 59 ± 3.2 |
| CM (B2− cells plus GGF) plus anti-NT-3a | 19 ± 2.4 | 24 ± 3.9 | 10 ± 5.2 |
| CM (B2− cells plus CNTF) | 66 ± 3.2 | 70 ± 2.4 | 49 ± 1.6 |
| CM (B2− cells plus PDGF) | ND | 69 ± 3.3 | 43 ± 1.8 |
The percentage of surviving neuroblasts at 48 hr is indicated. Numbers represent the mean ± SEM of triplicate determinations of 100–200 cells/well, for each of three independent experiments. B2+ neuroblast survival was assayed in presence of purified factors (top), or in conditioned medium (CM) from B2− nonneuronal cells (bottom). Bottom, conditions in brackets indicate factors present during preculture to produce CM. The conditions shown at the bottom were tested in parallel with those at the top for each of three independent experiments; therefore the values at the bottom for each individual experiment should be compared with the control values (no addition) at the top for the corresponding experiment. ND, not done.
Anti-NT-3 was added at the time of assay of the CM on the isolated neuroblast cultures. Anti-NT-3 also blocked the activity of CM from PDGF- and CNTF-treated B2− cells (data not shown). This table shows first of all that CM enhances the survival of neuroblasts; second, that anti-NT-3 blocks the effect of CM; and third, that GGF, CNTF, and PDGF increase this activity of CM but have no direct effect on neuroblasts on their own.
Table 5.
Neuroblast Enhancement of Secretion of NT-3-Mediated Neurotrophic Activity from Nonneuronal Cells Is Dependent upon Endogenous Neuregulins
| Condition | Percentage of Neurons Recovered |
||
|---|---|---|---|
| Experiment I (%) | Experiment II (%) | Experiment III (%) | |
| No addition | 22 ± 4.4 | 25 ± 1.4 | 15 ± 3.5 |
| CM (untreated B2− cells) | 47 ± 0.6 (113.6) | 69 ± 4.7 (176) | 31 ± 4.2 (138) |
| CM (B2− plus B2+ cells, 10:1) | 56 ± 0.2 (154) | 70 ± 2.8 (180) | 44 ± 4.2 (193) |
| CM (B2− plus B2+) plus anti-NT-3 | 21 ± 1.8 (0) | 25 ± 3.7 (0) | 13 ± 1.9 (0) |
| CM (B2− plus B2+ plus anti-GGF) | 47 ± 2.2 (113.6) | 36 ± 4.5 (44) | 35 ± 3.5 (133) |
| CM (B2− plus B2+ plus anti-GGF plus GGF) | 72 ± 2.5 (188) | 56 ± 2.8 (273) | |
The percentage of surviving neuroblasts at 48 hr is indicated. B2+ neuroblast survival was assayed in control medium (no addition), or in conditioned medium (CM) from B2− nonneuronal cells precultured alone (untreated B2− cells) or together with B2+ neuroblasts in a 10:1 ratio (B2− /B2+). The data in the first two rows are taken from Table 2. Row 4, anti-NT-3 was added at the time of assay of the CM on the isolated neuroblast cultures. Rows 5 and 6, anti-GGF indicates that anti-GGF2 antibody was added to the cocultures at the time of plating (see Figure 4C). Numbers in parentheses indicate the percent increase over control (no addition) observed in each CM condition. This is calculated as ([% survival in CM – % survival in no addition] ÷ % survival in No Addition) × 100. All percent increases were statistically significant (p ≥.034) as determined by a 2-tailed Student’s t test. Note that while CM from cultures of B2− cells alone enhances survival (by 113.6%–176%), CM from cocultures of B2− and B2+ cells is more efficacious (by 154%–193%) (except in Experiment II, in which a higher basal level of survival activity in B2− CM was obtained). This greater efficacy is eliminated by inclusion of anti-GGF antibody during the preparation of CM, and the antibody effect is reversed by inclusion of excess recombinant human GGF. Anti-GGF antibody had no effect on CM from nonneuronal cells grown in the absence of neuroblasts (data not shown).
rhGGF2, CNTF, and PDGF all enhanced the neuroblast survival-promoting activity of B2− nn cell–CM (prepared from 24 hr cultures) by, on average, ∼50% (Table 2, bottom). Importantly, these factors had no neuroblast survival–promoting activity on their own (Table 2, top). In general, rhGGF2 was most effective, consistent with the RT–PCR data indicating that this growth factor produced the strongest up-regulation of NT-3 mRNA in the nn cells (see Table 1). Importantly, all the survival-enhancing activity of these growth factor–treated CMs could be blocked by the anti-NT-3 antibody (Table 2, bottom, CM [B2− cells plus GGF] plus anti-NT-3; data not shown). These data suggest that rhGGF2, PDGF, and CNTF all enhance neuroblast survival indirectly, by increasing the secretion of NT-3 from nn cells. The apparent difference between the extent of NT-3 mRNA induction by GGF at 24 hr (about 6-fold; Table 1), and the extent of the increase in survival-promoting activity with GGF-treated CM (50%) likely reflects the fact that the amount of additional NT-3 in the CM is saturating for the survival assay, as indicated by the fact that equivalent survival was obtained with this CM as with saturating amounts of recombinant NT-3 (Table 2). Alternatively, it could reflect posttranscriptional regulation of NT-3 synthesis or secretion.
In these experiments, we consistently observed a population of neuroblasts that survived independently of NT-3. In two experiments, this basal NT-3-independent survival was approximately 50% at 24 hr and 23% at 48 hr (Table 2, top, experiments I and II, no addition; data not shown). In a third experiment, in which the neuroblast plating density was reduced by a factor of 3, basal survival was 32% at 24 hr and 15% at 48 hr (Table 2, top, experiment III, no addition; data not shown). These data are consistent with the observation of a population of NT-3-independent sympathetic neurons in NT-3 −/− mice (Ernfors et al., 1994; Fariñas et al., 1994). The size of this NT-3-independent population in the superior cervical ganglion in vivo is approximately 50% of the total ganglionic neuronal population. These data suggest that in vitro as in vivo, some embryonic sympathetic neuroblasts do not require NT-3 for survival; however, the size of the NT-3-independent population may still be affected by environmental factors or cell–cell interactions.
We next determined whether nn cell CM could induce TrkA expression in isolated neuroblasts. CM from B2− nn cells pretreated with rhGGF2 or PDGF up-regulated trkA mRNA expression in isolated B2+ neuroblasts (Figure 5, right; compare GGF, PDGF, with no add). This CM activity was attenuated by a function-blocking anti-NT-3 antibody (Figure 5, GGF plus NT-3 AB, PDGF plus NT-3 AB). The anti-NT-3 antibody did not affect the TrkA-inducing activity of CM from cells exposed to CNTF (which has TrkA-inducing activity on its own [Verdi and Anderson, 1994]); this demonstrates that the antibody does not interfere nonspecifically with TrkA induction. As in the survival assay, rhGGF2 had no effect on TrkA expression when added directly to isolated neuroblasts (Figure 5, left, GGF). These data suggest that rhGGF2 and PDGF promote secretion of sufficient NT-3 from nonneuronal cells to induce trkA expression in neuroblasts. In contrast, CM from nn cells grown without these growth factors had little measurable effect on TrkA expression (Figure 5, right, compare no add with no add plus NT-3 AB). The enhancement of neuroblast survival (Table 2), but not TrkA expression, by untreated nn cell CM most likely reflects the fact that higher levels of NT-3 are required to induce TrkA expression than to promote survival (Verdi and Anderson, 1994).
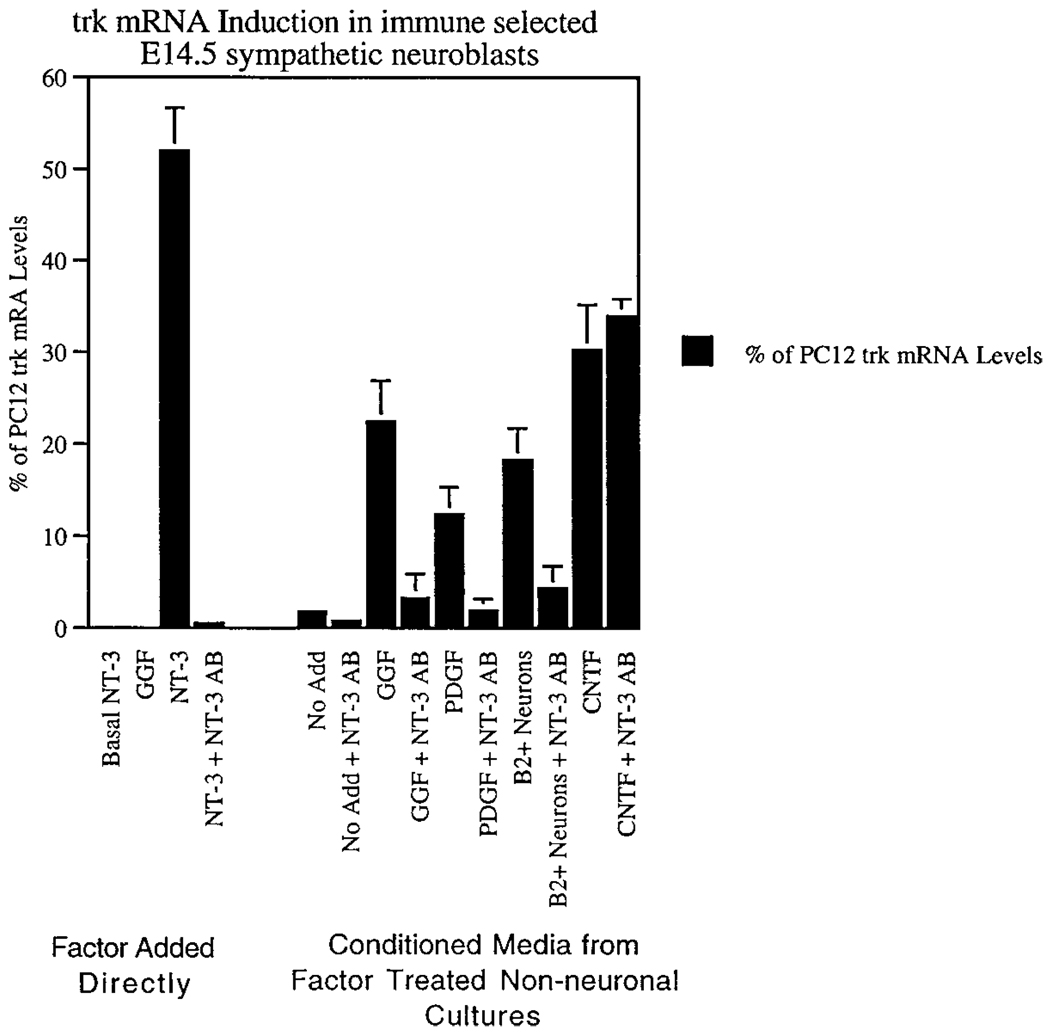
Figure 5.
Conditioned Medium from B2− Nonneuronal Cell Cultures Induces TrkA Expression in Cultures of Isolated Sympathetic Neuroblasts, via NT-3
The left of the graph (factor added directly) shows a series of controls in which factors or antibodies were added directly to isolated B2+ neuroblasts. Note that NT-3 induces trkA mRNA, and that the neutralizing anti-NT-3 antibody blocks this effect. In the right of the graph, expression of TrkA was monitored in cultures of isolated B2+ neuroblasts grown for 24 hr in various conditioned media from nonneuronal cultures. No add means that the conditioned medium was prepared from nn cultures grown with no added factors. Note that GGF strongly enhances the TrkA-inducing activity of nn cell CM, although by itself, GGF has no direct effect on TrkA expression in isolated neuroblasts (left side of the graph). The TrkA-inducing activity of the GGF-treated CM is strongly attenuated by anti-NT-3 antibody. A similar result is obtained with PDGF. The effect of CNTF is, as expected, not blocked by anti-NT-3, because CNTF itself has strong TrkA-inducing activity (Verdi and Anderson, 1994). B2+ neurons indicates that conditioned medium was prepared from a coculture of B2− nonneuronal cells and B2+ neurons recombined in a 10:1 ratio (see Figure 4C). Note that this CM has much higher TrkA-inducing activity than control CM (no add) and that this activity is again attenuated by the blocking anti-NT-3 antibody. The relative levels of trkA mRNA were determined by RT–PCR and are presented as the percentage of trkA mRNA detected in PC12 cell mRNA samples run in parallel; the numbers are the mean ± SEM of replicate samples. Similar results were obtained in two different experiments from separate isolations.
Sympathetic Neuroblasts Contain mRNA for Various Neuregulin Isoforms
The observation that growth factors such as GGF2/neuregulin and PDGF can up-regulate NT-3 mRNA expression and associated bioactivities in ganglionic nn cells raised the question of whether sympathetic neuroblasts could be a source of such NT-3-inducing factors. It has previously been demonstrated that a number of different CNS and PNS neuronal populations, including embryonic sympathetic neurons, make PDGF (Yeh et al., 1991). Neuregulins have also been demonstrated to be expressed in the developing nervous system (Marchionni et al., 1993; Meyer and Birchmeier, 1994; Corfas et al., 1995), but their expression in sympathetic neuroblasts at these stages has not specifically been examined.
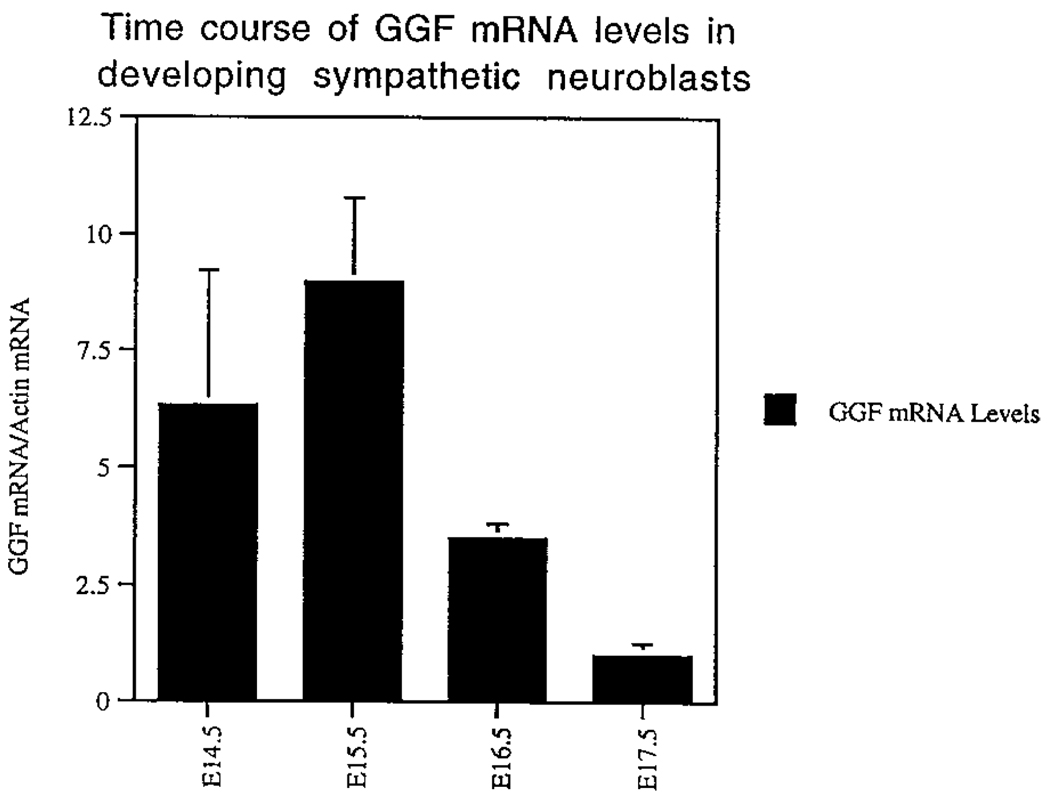
We therefore assayed neuregulin mRNA production in isolated E14.5 B2+ neuroblasts by RT–PCR. Using exon I-specific primers that identify mRNA encoding GGF2 (the form used in the cell culture experiments), strong signals were detected in E14.5 and E15.5 B2+ cells, but were lower in E16.5 and E17.5 cells (Figure 6). To determine whether other neuregulin isoforms besides GGF2 were expressed in these cells, we performed RT–PCR with several different primer combinations on mRNA isolated from E14.5 neuroblasts. Signals were detected by use of primers from the immunoglobulin and EGF domains, as well as from the EGF and cytoplasmic domains (Table 3). These data, although qualitative, indicate that various forms of neuregulins including membrane-bound forms are synthesized by embryonic sympathetic neuroblasts. Importantly, little or no neuregulin mRNA was amplified from E14.5 B2− nn cell cDNA (data not shown).
Figure 6.
Relative Levels of GGF2/Neuregulin in Freshly Isolated B2+ Sympathetic Neuroblasts
cDNA prepared from cells isolated at the indicated ages was amplified with exon 1–specific primers that detect the kringle domain of the GGF2 isoform (see Table 3). The values represent the mean ± SEM of two independent experiments, relative to the level detected at E17.5 after normalization to actin. Levels of GGF2 mRNA at E19.5 were below the detection limit of the assay. Note that GGF2 mRNA was not detectable in samples of B2− nonneuronal cell cDNA run in parallel. Qualitative results obtained with other neuregulin primer sets are presented in Table 3.
Table 3.
Expression of Neuregulin Isoforms by Isolated E14.5 B2+ Sympathetic Neuroblasts
| Primer Set | Exon Pairs | Expression Detected |
|---|---|---|
| 5′ kringle–3′ kringle | 1–1 | ++ |
| 5′ Ig–3′ EGF | 4–6 | + |
| 5′ Ig–3′ Cyt | 4–11 | − |
| 5′ Ig–3′ Sec | 4–10 | − |
| 5′ kringle–3′ Sec | 1–10 | +/− |
| 5′ EGF–3′ Cyt | 6–11 | +++ |
| 5′ EGF–3′ Sec | 6–10 | − |
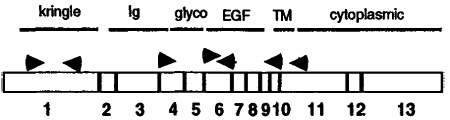 | ||
RT–PCR was performed on cDNA prepared from E14.5 B2+ neuroblasts, using the indicated primer pairs. The results of the amplification were visualized by agarose gel electrophoresis with ethidium bromide staining. Band intensities are approximated by plus and minus symbols. The strongest signal was obtained for a membrane-bound form containing the EGF-like domain (5′ EGF-3′ Cyt). Locations and orientations of the primers used are indicated in the diagram; 5′ primers point rightwards, 3′ primers leftwards. Exon numbers are indicated below the boxes in the diagram, and domains are overlined.
Neuregulin domain abbreviations: kringle, exon 1 (specific to rhGGF2); Ig, immunoglobulin-like domain, exon 4; EGF, EGF repeat domain, exon 6; Cyt, cytoplasmic domain, exon 11 (transmembrane form); Sec, putative secreted form with stop codon before transmembrane segment, exon 10.
Endogenous Neuregulins Enhance Neuroblast Survival in Cocultures with Nonneuronal Cells
The observations that sympathetic neuroblasts are a potential source of neuregulins, and that neuregulins enhance NT-3 production in nonneuronal cells, suggested that neuroblast-derived neuregulins might enhance neuroblast survival in cocultures with nonneuronal cells. To test this idea, we recombined isolated B2+ neuroblasts together with B2− nonneuronal cells in a ratio (1:10) reflecting their relative proportions in freshly sorted cell suspensions, and monitored neuroblast survival. To exclude possible contributions of de novo neurogenesis to overall neuronal number, cohorts of individual neuroblasts were identified several hours after plating and their survival followed with time.
Overall neuroblast survival in the cocultures at 24 hr was 77% ± 4.9% (Table 4), much better than the 45%–50% observed when B2+ neuroblasts are cultured in isolation at the same density (see above). Inclusion of anti-NT-3 antibodies in the cocultures inhibited survival of a portion of the neuroblasts (Table 4, anti-NT-3). Importantly, function-blocking antibodies to GGF2/neuregulin (see Experimental Procedures) also inhibited neuroblast survival (Table 4). However, at 48 hr, the reduction in survival with anti-GGF was not as great as with anti-NT-3. This finding is consistent with the observation that nn cells make some NT-3 even in the absence of sources of GGF/neuregulin (see above).
Table 4.
Survival of Serially Observed Cohorts of B2+ Cells in Coculture with B2− Nonneural Cells under Various Conditions
| Condition | Percentage of Neurons Surviving |
|
|---|---|---|
| 24 hr | 48 hr | |
| Control | 77 ± 4.9 | 63 ± 9.2 |
| rhGGF2 | 82 ± 3.8 | 78 ± 1.6 |
| Anti-GGF | 59 ± 7.4 | 51 ± 11.2 |
| Anti-NT-3 | 52 ± 8.2 | 30 ± 2.4 |
| Anti-GGF plus rhGGF2 | 79 ± 3.8 | 73 ± 1.4 |
| Anti-GGF plus insulin | 93 ± 0.7 | 86 ± 0.8 |
| Anti-NT-3 plus insulin | 81 ± 13.5 | 88 ± 2.8 |
For each of the above conditions, 100–150 neuroblasts were identified in each of three plates (for a total of 300–450 neurons per condition), at 2 hr after plating. The survival of these identified cells was monitored at 24 hr and at 48 hr. The percent survival relative to 2 hr after plating was calculated for each plate; the mean ± SEM of these percentages is indicated in the table. At 48 hr, the data are from duplicate rather than triplicate plates.
These data suggested that endogenous sources of neuregulin can enhance neuroblast survival in cocultures containing nonneuronal cells. We therefore next asked whether neuroblasts enhance NT-3 synthesis in nonneuronal cells via neuregulins.
Neuroblasts Up-regulate NT-3 mRNA Expression in Nonneuronal Cells via Neuregulins
B2+ neuroblasts were recombined with B2− nonneuronal cells in a 1:10 ratio, and the expression of NT-3 mRNA in these cocultures was subsequently measured by RT–PCR. After 48 hr, NT-3 mRNA levels were 13-fold higher in such cocultures than in sister cultures of nn cells grown alone (see Table 1, B2+ neuroblasts). To determine whether the NT-3 mRNA produced in these cocultures was derived from the nn cells, the neuroblasts were removed from the nn cells by use of EDTA (see Experimental Procedures), and both cell populations were assayed for NT-3 mRNA by RT–PCR. A strong NT-3 signal was detected in the nn cell cDNA, whereas no signal was detected in similar amounts of neuroblast cDNA amplified for the same number of cycles (data not shown). These data are consistent with our finding that NT-3 mRNA is detectable by RT–PCR in freshly isolated B2− but not B2+ cells from E14.5 ganglia (see above). Thus, these coculture experiments suggest that B2+ neuroblasts, as well as rhGGF2, PDGF, and CNTF, can up-regulate NT-3 mRNA production by B2− nn cells.
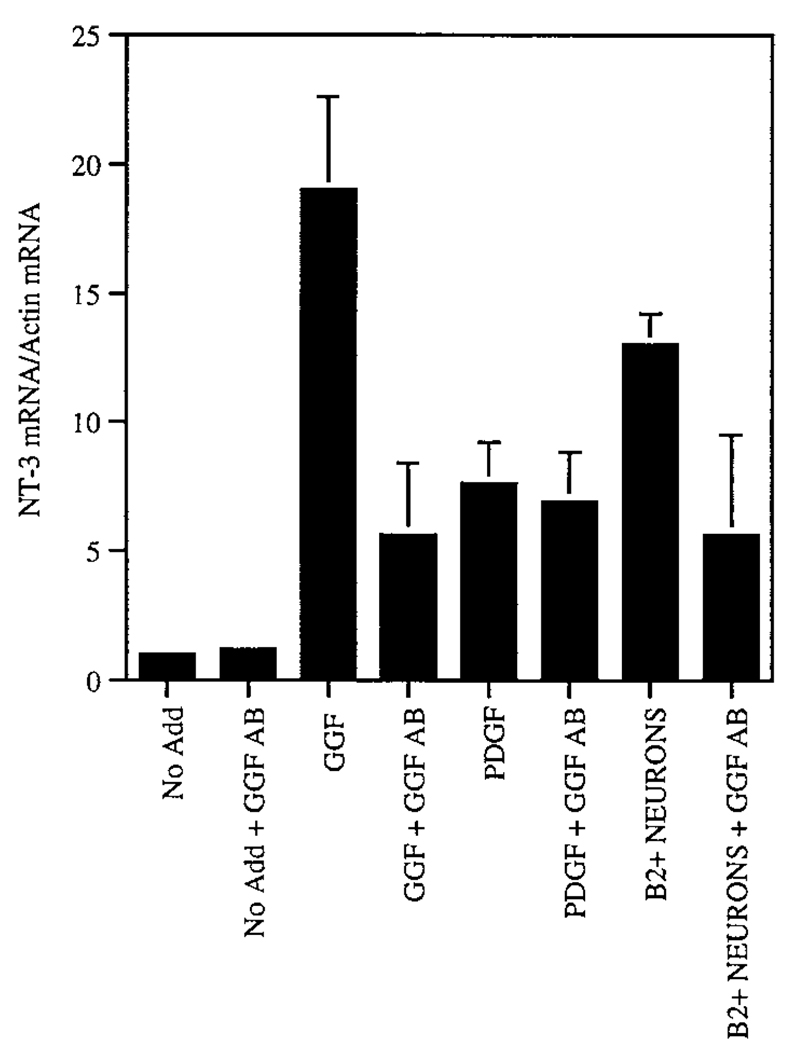
To determine whether the effect of B2+ neuroblasts in up-regulating NT-3 mRNA production in nn cells was mediated by endogenous neuregulins, we examined NT-3 mRNA levels in cocultures grown with or without the function-blocking anti-GGF2/neuregulin antibody. In control experiments, the antibody strongly attenuated the up-regulation of NT-3 mRNA by rhGGF2, but had no effect on NT-3 mRNA up-regulation by PDGF (Figure 7), demonstrating the specificity of the inhibition. Anti-GGF2/neuregulin also attenuated the up-regulation of NT-3 mRNA caused by coculture with E14.5 B2+ neuroblasts (Figure 7, compare B2+ neurons with B2+ neurons plus GGF AB). This indicated that the neuroblast enhancement of NT-3 mRNA production in nonneuronal cells is, at least in part, mediated by neuregulins. The failure of the antibody to produce a quantitative inhibition of either the neuroblast- or rhGGF2-mediated effect (Figure 7) suggests that the antibody may not have been saturating, or that not all neuregulin isoforms are blocked; alternatively, the neuroblasts may produce other NT-3-inducing signals (e.g., PDGF) not inhibited by the antibody.
Figure 7.
Induction of NT-3 mRNA in Nonneuronal Cells by Coculture with Neuroblasts Is Attenuated by Neutralizing Anti-GGF Antibody
Relative NT-3 mRNA levels in cultures of B2− nonneuronal cells grown under different conditions for 24 hr were determined by RT–PCR (see Table 1) and normalized to actin mRNA levels. rhGGF2 strongly up-regulates NT-3 (see also Table 1), and this effect is attenuated by anti-GGF antibody. By contrast, the up-regulation of NT-3 obtained with PDGF is unaffected by the anti-GGF antibody. NT-3 mRNA levels are also up-regulated by coculture with B2+ neuroblasts, and this effect is also attenuated by the anti-GGF antibody. The data represent the average ± SEM of two independent experiments.
Neuroblast-Derived Neuregulins Promote Up-regulation of NT-3-Mediated Bioactivities Secreted by Nonneuronal Cells
We next sought to determine whether the neuroblast enhancement of NT-3 mRNA expression in nn cells was accompanied by an increased secretion of NT-3-dependent bioactivities. Therefore, both TrkA induction and neuroblast survival assays were performed on neuroblasts cultured in CMs derived from untreated B2− nn cells, or from cocultures of B2− nn cells and neuroblasts (design illustrated in Figure 4B and 4C). As shown in Figure 5, conditioned medium from cocultures up-regulated trkA expression in isolated B2+ neuroblasts, whereas little effect on TrkA expression was observed with CM from nn cells alone (see Figure 5, compare B2+ neurons with no add). The effect of the coculture CM was attenuated by anti-NT-3 antibody (see Figure 5, B2+ neurons plus NT-3 AB). This suggests that most or all of the TrkA-inducing activity in CM derived from cocultures of neuroblasts and nonneuronal cells is due to NT-3.
CM from cocultures also enhanced neuroblast survival more effectively than did CM from untreated nn cells (Table 5, CM [untreated B2− cells] versus CM [B2− plus B2+ cells, 10:1]; experiments I and III). In one of three independent experiments, coculture and untreated nn cell CM enhanced survival to a similar extent (Table 5, experiment II), but the nn cell CM had a higher level of survival-promoting activity to begin with than it did in the other experiments (Table 5, row 2). Importantly, all the survival-promoting activity of the coculture CM was inhibited by the anti-NT-3 antibody (Table 5, CM [B2− plus B2+] plus anti-NT-3). Taken together, these data indicate that neuroblasts not only enhance the expression of NT-3 mRNA in nn cells, but also promote the secretion of NT-3-dependent bioactivities by these cells.
Finally, we asked whether the anti-rhGGF2/neuregulin antibody eliminated the enhanced secretion of survival-promoting activity by cocultures of neuroblasts and nonneuronal cells. For this experiment, cocultures of B2− and B2+ cells were allowed to condition their medium in the presence or absence of anti-rhGGF2 antibody; the survival activity of CM from these cultures was then compared with that of CM from untreated nn cell cultures (design illustrated in Figure 4C). In three independent experiments, pretreatment with the anti-GGF/neuregulin antibody attenuated the survival-promoting activity of the coculture-derived CM (Table 5, compare CM [B2− plus B2+ plus anti-GGF] with CM [B2− plus B2+ cells, 10:1]). Moreover, in two of three experiments, the anti-GGF antibody reduced the survival-promoting activity of the coculture CM to almost exactly that seen with CM from untreated nn cell cultures (Table 5, experiments I and III; compare CM [untreated B2− cells] with CM [B2− plus B2+ plus anti-GGF]). Control experiments indicated that the antibody effect was overcome by addition of excess rhGGF2 (Table 5, CM [B2− plus B2+ plus anti-GGF plus GGF]). Additional controls showed that the anti-GGF2/neuregulin antibody had no effect on neuroblasts grown in basal medium, or in medium containing insulin (provided to enhance survival by an NT-3-independent means) (data not shown). Taken together, these data indicated that neuroblast-derived neuregulin not only promotes NT-3 mRNA synthesis in nn cells, but also increases secretion of NT-3-dependent neuroblast survival activity from the nn cells.
Discussion
Recent studies have established that developing rat sympathetic neuroblasts undergo a switch in neurotrophin responsiveness (Birren et al., 1993; DiCicco-Bloom et al., 1993). At E14.5–E15.5, neuroblasts in both thoraco-lumbar and superior cervical ganglia express TrkC and respond to NT-3, but do not express TrkA. Beginning at about E16.5, these cells begin to express TrkA and acquire NGF responsiveness as measured by 24 hr survival assays. Over the same period of time, TrkC expression by these cells declines, and they become relatively less sensitive to NT-3 in similar survival assays (Figure 1). Similar switches in neurotrophin receptor expression have been documented in developing sensory neurons as well (Buchman and Davies, 1993; Buj-Bello et al., 1994; Davies, 1994). The observation that immature neurons or neuroblasts are transiently responsive to neurotrophins that differ from their ultimate target-derived survival factors raises the question of the source and regulation of production of these interim trophic factors.
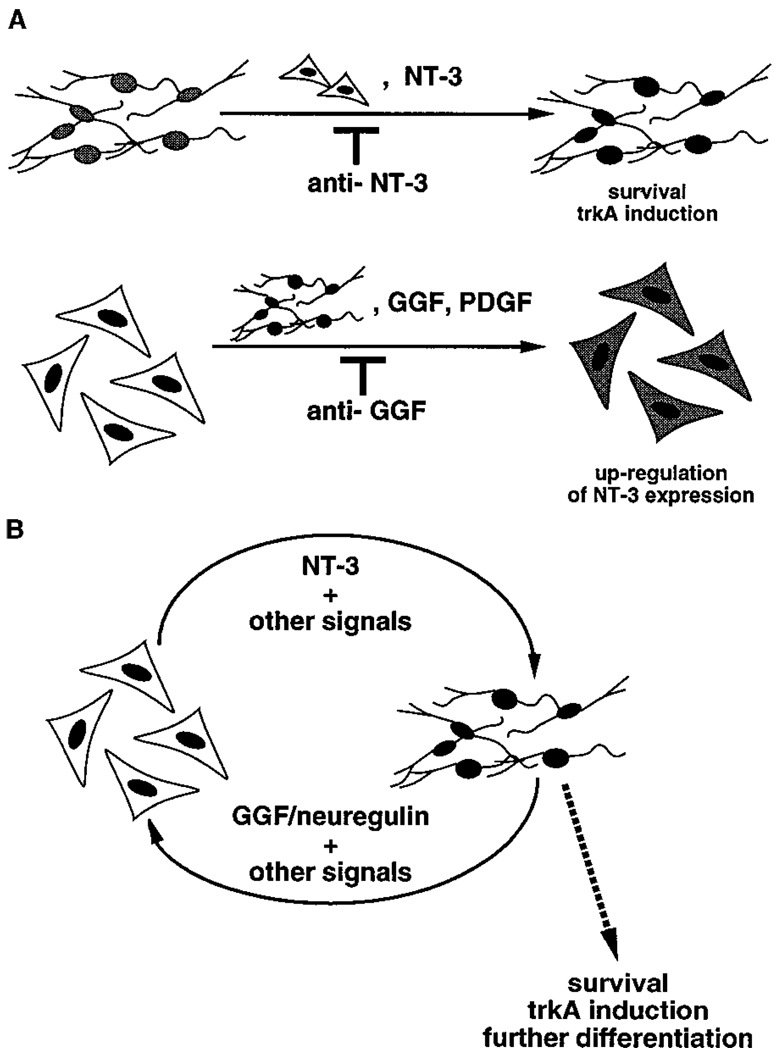
Here we show that NT-3 is synthesized by nn cells neighboring sympathetic ganglia, and that in vitro, these cells can provide sufficient NT-3 to promote survival and TrkA induction in isolated neuroblasts (Figure 8A, top). In turn, the neuroblasts can enhance NT-3 production in the nn cells in vitro, via neuregulins and perhaps other factors, such as PDGF (Figure 8A, bottom). These cell culture experiments thus demonstrate how a reciprocal cell–cell interaction can coordinate the development of embryonic sympathetic neuroblasts and their neighboring nonneuronal cells (Figure 8B). The appropriate spatial and temporal expression of NT-3, neuregulins, and PDGF (Yeh et al., 1991) suggests that this interaction reconstituted in vitro may reflect normal developmental events that occur in vivo. Bidirectional interactions between neuroblasts and glia have also been observed in studies of cerebellar development (Hatten, 1987, 1988), although the molecules involved on both sides of the interaction have not been fully identified. Such reciprocal cell–cell interactions in the developing nervous system may provide general examples of how the development of tissues containing several distinct but functionally interrelated cell types is coordinated.
Figure 8.
Summary of Experimental Observations and Model for Reciprocal Cell–Cell Interactions Controlling Early Stages in Sympathetic Gangliogenesis
(A, top) Illustration of the data indicating first of all that NT-3 promotes neuroblast survival and mitotic arrest/TrkA expression (see also Verdi and Anderson, 1994); second, that B2− ganglionic nonneuronal cells also promote neuroblast survival and TrkA expression (and express NT-3); and third, that blocking anti-NT-3 antibodies inhibit the survival-promoting and TrkA-inducing activities in conditioned medium from nonneuronal cells.
(A, bottom) Illustration of the data indicating first of all that GGF and PDGF (as well as CNTF) up-regulate NT-3 expression in nonneuronal cells; second, that neuroblasts also up-regulate NT-3 expression in non-neuronal cells (and themselves express forms of GGF/neuregulin as well as PDGF [Yeh et al., 1991]); and third, that blocking anti-GGF antibodies inhibit the ability of neuroblasts to up-regulate NT-3 expression in nonneuronal cells.
(B) Reciprocal cell–cell interactions that occur in vitro between neuroblasts and nonneuronal cells as suggested by the data.
Ganglionic Nonneuronal Cells Produce Interim Neurotrophins for Embryonic Sympathetic Neuroblasts
The insertion of a lacZ gene into the NT-3 locus by homologous recombination has allowed us to visualize the pattern of NT-3 expression in tissues neighboring the developing thoraco-lumbar sympathetic ganglia. The domain of lacZ-expressing cells excluded the ganglionic neuroblasts and overlapped that of cells expressing p75, a marker of peripheral glia and their neural crest precursors (Stemple and Anderson, 1992; Shah et al., 1994). Analysis of fluorescence-activated cell sorter (FACS)-isolated p75+ cells confirmed that these cells express NT-3 protein and mRNA. Freshly isolated p75+ cells are glial fibrillary acidic protein–negative (GFAP−), but can express GFAP when cultured in rhGGF2 plus serum (data not shown). This suggests that these cells are glial precursors, neural crest cells or both. Because p75+ cells constitute only 10%–15% of B2− cells, we cannot say that they are the only source of NT-3 in this cell population. However, RT–PCR measurements indicated that NT-3 transcripts are enriched at least 10-fold in isolated p75+ cells, compared with levels in B2− cells (after normalization to actin; data not shown). These observations are consistent with the idea that p75+ cells are the major if not the only source of NT-3 in the B2− fraction. Taken together, these data support the idea that at least some of the NT-3-expressing nn cells are glia or their precursors. However, it is possible that some nonglial mesenchymal cells in the vicinity of sympathetic ganglia express NT-3 as well and contribute to the local support of neuroblast survival.
It is well documented that neurotrophins are produced by peripheral targets of sympathetic and sensory innervation, where they function to support the survival of adequate numbers of neurons during the period of programmed cell death. The above mentioned data indicate that neurotrophins are also synthesized by nonneuronal cells located near the cell bodies of developing sympathetic neuroblasts. This supports our earlier suggestion that NT-3 may function to provide local, interim trophic support for embryonic sympathetic neuroblasts before these cells express functional NGF receptors and gain access to target-derived NGF (Birren et al., 1993). This view is consistent with the idea that most or all cells in the body require trophic support at multiple stages of development as well as in the adult (Raff, 1992). The acquisition of NGF dependence would then occur, in part, by a substitution of a target-derived neurotrophin—NGF—for the interim neurotrophin—NT-3—provided to the neuroblast cell bodies. In addition, NGF is likely to be provided by glial cells lining the pathway taken by sympathetic axons to their peripheral targets (Johnson et al., 1988). Such a model does not exclude the possibility that sympathetic neuroblasts are neurotrophin independent at yet earlier stages of development; evidence for this has been provided in the chick system (Ernsberger et al., 1989a). Evidence for a neurotrophin-independent stage in sensory neurogenesis has been provided as well (Vogel and Davies, 1991).
Regulation of NT-3 Production in nn Cells
NT-3 mRNA expression and the secretion of NT-3-dependent biologic activities in nn cells can be strongly up-regulated by environmental signals, including GGF2, PDGF, and CNTF. Earlier data as well as the present results suggest that in vivo, PDGF and GGF/neuregulin may be produced by sympathetic neurons neighboring the nonneuronal cells. PDGF is well known to be synthesized by a large number of neurons, including embryonic sympathetic neurons (Sasahara et al., 1991; Yeh et al., 1991). Previous work has shown that embryonic sympathetic ganglia contain at least some forms of neuregulin (Marchionni et al., 1993; Meyer and Birchmeier, 1994). Here we have demonstrated that sympathetic neuroblasts synthesize several forms of neuregulin, including GGF2, although a quantitative analysis of neuregulin isoforms in these cells was not undertaken. The fact that CM from B2+ neuroblasts did not induce NT-3 mRNA, whereas direct contact between these neuroblasts and the nn cells did, suggests that at least some of the NT-3-inducing activity provided by the neuroblasts may be membrane associated. In keeping with this, neuregulins are alternatively spliced to generate several membrane-associated isoforms, and we observe expression of the cytoplasmic domain–containing exon (i.e., a transmembrane form) in the neuroblasts by RT–PCR. However, it is possible that sympathetic neuroblasts also make some secreted forms of neuregulins, which did not accumulate to sufficient levels in conditioned medium from the low-density cultures, to influence NT-3 expression in nonneuronal cells.
Sympathetic neuroblasts are not the only neuronal cells that could provide a source of GGF/neuregulin for NT-3-expressing nn cells in the vicinity of the sympathetic ganglia. The preganglionic afferent nerves that grow to these ganglia contain the axons of motorneurons, which have also been shown to express various neuregulin isoforms, including ARIA, an acetylcholine receptor-inducing activity for skeletal muscle (Falls et al., 1993; Corfas et al., 1995). These preganglionic fibers pass through the area containing lacZ+ NT-3-expressing cells and could therefore be an additional source of NT-3-inducing signals. Motor neurons also contain PDGF mRNA (Sasahara et al., 1991; Yeh et al., 1991). The multiplicity of signals that can induce NT-3 in vitro makes it difficult to predict which, if any, of these signals will prove to be essential for NT-3 expression in vivo as determined by single-gene mutational analysis.
Function of NT-3 during Sympathetic Neurogenesis In Vivo
Previously, we demonstrated that NT-3 can exert a dual influence on developing sympathoblasts in vitro: it can support their survival and in addition can induce the expression of the NGF receptor TrkA, as a consequence of promoting cell cycle withdrawal (Verdi and Anderson, 1994). Previous analyses of NT-3−/− mice have provided evidence that NT-3 is indeed required by at least a subset of sympathetic neurons in vivo, although the precise stage of development at which this neurotrophin is needed has not yet been determined (Ernfors et al., 1994; Fariñas et al., 1994). On the other hand, in preliminary experiments we have observed that TrkA is expressed in the sympathetic ganglia of NT-3−/− mice (I. F., unpublished data). This may indicate that NT-3 is only one of several functionally redundant factors that can induce TrkA expression in sympathetic neuroblasts; indeed, we have shown that at least one other factor, CNTF, induces TrkA expression in vitro as efficiently as NT-3 (Verdi and Anderson, 1994). Moreover, both CNTF and NT-3 promoted mitotic arrest of sympathetic neuroblasts, and mitotic inhibitors such as aphidicolin and mitomycin C were also effective inducers of TrkA (Verdi and Anderson, 1994). These data suggested that the key event promoting the appearance of TrkA in sympathetic neuroblasts is cell cycle withdrawal. While NT-3 may accelerate this process in vitro, the timing of mitotic arrest in vivo may be determined by other environmental signals or by a cell-autonomous mechanism (e.g., see Hart et al., 1989).
In summary, our results demonstrate that a neurotrophic factor that acts on immature neuroblasts is produced by nonneuronal cells neighboring these neuroblasts, and that its production in these cells can in turn be regulated by neuroblast derived signals in vitro. While the experiments identify some of the signals that are necessary and sufficient to mediate this interaction in culture, the situation in vivo may be considerably more complex. Nevertheless, the results illustrate the kind of reciprocal cell–cell interactions that can occur between neuronal and glial precursors in developing peripheral ganglia. They also shed further light on how neurotrophins may regulate neuronal survival and differentiation in the period before these cells gain access to classical target-derived trophic factors.
Experimental Procedures
Cell Isolation and Culture
Sympathetic neuroblasts and associated ganglionic nonneuronal cells were isolated from E14.5 thoraco-lumbar sympathetic ganglia using MAb B2 for both positive and negative selection, as previously described (Birren et al., 1993; Verdi and Anderson, 1994). In some experiments, p75+ cells were isolated by preparative FACS using monoclonal antibody 192 Ig. No neurons were ever observed in p75-sorted cells; therefore, 100% of the p75+ cells must be contained within the B2− nonneuronal fraction. RT–PCR determinations indicated that NT-3 transcripts are enriched at least 10-fold (relative to actin transcripts) in the p75+ fraction compared with the B2− fraction. Since p75+ cells constitute about 7%–12% of the B2− fraction, this suggests that most if not all of the NT-3 synthesized by the B2− fraction is synthesized by p75+ cells within this fraction. However, because of the larger cell numbers obtained, it was more convenient to work with B2− cells than p75+ cells as a source of immunoisolated nonneuronal cells.
For assays of TrkA expression and survival in isolated neuroblasts, typically 3,000 cells were plated per well of a 96-well plate on a poly-D-lysine/laminin substrate, in 100 µl of defined, serum-free medium (L15–CO2 containing transferrin, selenium, putrescine, progesterone, and bovine serum albumin). Determinations of neuronal survival were made by manually counting the number of neurons at 24 and 48 hr and expressing the results as a percentage relative to the number of cells that attached 2–3 hr after plating. Duplicate or triplicate determinations were made for each experiment. The results are presented as the mean ± SEM.
In some experiments, neuroblasts and nonneuronal cells were reseparated following coculture according to the following procedure. Cultures were treated with Hanks’ balanced salt solution (HBSS; GIBCO) lacking phenol red and Mg2+, in the presence of 1 mM EDTA for 1 min. Following this incubation, the medium was removed, the plates tapped gently against the hood, and the dislodged neuroblasts removed in 1 ml of HBSS–EDTA. This procedure removed >90% of neuroblasts and none of the nonneuronal cells. The neurons were centrifuged and lysed in RNA extraction buffer, while the nonneuronal cells remaining on the plate were removed by incubation for 3 min in trypsin–EDTA (GIBCO) prewarmed to 37° C, spun down, and lysed.
Preparation of nn Cell CM
B2− nn cells (20,000–30,000) were plated with or without growth factors in a volume of 100 µl in wells of 96-well or 48-well plates. CM was removed after 48 hr for TrkA induction experiments, and after 24 hr for survival experiments. The medium was removed and mixed with 100 µl of fresh medium. This medium was then used to culture immunoisolated E14.5 sympathetic B2+ neuroblasts for 24 hr. In some experiments, similar numbers of nn cells were cocultured with a 10-fold lower number of B2+ neuroblasts and conditioned medium taken from these cocultures after 48 hr.
Neutralizing Antibodies
Turkey anti-NT-3 antibody was a gift from Amgen, Incorporated and has been previously demonstrated not to cross-react with other neurotrophins (Ghosh et al., 1994; Ghosh and Greenberg, 1995). This antibody was used at a final concentration of 580 ng/ml, which was 10-fold higher than the amount needed to block the survival-promoting activity of 40 ng/ml NT-3. In the experiment of Figure 3, a polyclonal rabbit anti-NT-3 antibody (the gift of D. Kaplan) was used for immunostaining at a dilution of 1:1000. This antibody is monospecific for NT-3 in Western blots (D. Kaplan, personal communication). Anti-GGF was a cocktail of two antibodies: CN16, an affinity-purified polyclonal antiserum raised against intact rhGGF2 and used at a final concentration of 20 µg/ml; and 2861, an affinity-purified polyclonal antibody raised against an exon I–specific peptide, used at 12 µg/ml. This amount of antibody was sufficient to block completely the activity of 10–20 ng/ml rhGGF2 in a Schwann cell DNA synthesis assay. A detailed characterization of these antibodies will be reported elsewhere (N. Ratner et al., submitted). Anti-GGF antibody was added to cocultures of neuroblasts plus nn cells and was preincubated with the neuroblasts prior to plating to facilitate binding to cell surface–associated forms of GGF/neuregulin.
RT–PCR
Conditions for RNA extraction, cDNA synthesis, and RT–PCR for trkA and actin were as described previously (Verdi and Anderson, 1994). RT–PCR for NT-3 was performed with the following primers and amplification conditions. Forward primer, AGG TGA TGT CCA TCT TGT TTT; reverse primer, GCC TCT CCC TGC TCT GGT TC. Conditions employed a hot start, 1 min at 97°C, 1 min at 52°C, 1 min at 72°C for 35 cycles. For all quantitative measurements, input cDNA levels were normalized with actin primers (Verdi and Anderson, 1994). RT–PCR for various isoforms of GGF/neuregulin was performed under the conditions described previously (Shah et al., 1994). Exact primer sequences used are available on request.
Growth Factors
Recombinant NT-3 and CNTF were obtained from Amgen, Incorporated and Regeneron, Incorporated, respectively, and were used at final concentrations of 25 and 2 ng/ml, respectively. rhGGF2 was obtained from Cambridge NeuroScience, Incorporated and was used at concentrations that gave maximal activity in a Schwann cell DNA synthesis assay (typically 10 nM). Recombinant PDGF was from Sigma and was used at a final concentration of 20 ng/ml.
Analysis of NT-3−/− Mice
Mice containing the lacZ gene inserted into the NT-3 locus by homologous recombination have been described previously (Fariñas et al., 1994). Embryos were genotyped by DNA blot analysis and processed by cryostat sectioning for detection of β-galactosidase activity with the X-Gal reagent, followed by immunostaining for peripherin (antibody from Sigma, Incorporated) or p75 (antibody Rex; Weskamp and Reichardt, 1991).
Acknowledgments
Correspondence should be addressed to D. J. A. We thank George Yancopoulos and Regeneron, Incorporated for their gifts of NT-3 and CNTF; Amgen, Incorporated for NGF, FGF, NT-3, IGF, CNTF, and the gift of anti-NT-3 neutralizing antibody; and David Kaplan for his gift of rabbit anti-NT-3 antibody. We thank Rochelle Diamond for help with cell sorting, Denis Bouchard for technical assistance, and Nirao Shah for advice and helpful discussion. We also thank Pat White for assistance with dissections at early stages in this project. This work was supported in part by a grant from the National Institutes of Health. J. M. V. is an Assistant Investigator of the Amgen Institute. D. J. A. is an Associate Investigator of the Howard Hughes Medical Institute.
References
- Barker PA, Shooter EM. Disruption of NGF binding to the low affinity neurotrophin receptor p75LNTR reduces NGF binding to trkA on PC12 cells. Neuron. 1994;13:203–215. doi: 10.1016/0896-6273(94)90470-7. [DOI] [PubMed] [Google Scholar]
- Birren SJ, Lo LC, Anderson DJ. Sympathetic neurons undergo a developmental switch in trophic dependence. Development. 1993;119:597–610. doi: 10.1242/dev.119.3.597. [DOI] [PubMed] [Google Scholar]
- Buchman VL, Davies AM. Different neurotrophins are expressed and act in a developmental sequence to promote the survival of embryonic sensory neurons. Development. 1993;118:989–1001. doi: 10.1242/dev.118.3.989. [DOI] [PubMed] [Google Scholar]
- Buj-Bello A, Pinon LGP, Davies AM. The survival of NGF-dependent but not BDNF-dependent cranial sensory neurons is promoted by several different neurotrophins early in their development. Development. 1994;120:1573–1580. doi: 10.1242/dev.120.6.1573. [DOI] [PubMed] [Google Scholar]
- Cattaneo E, McKay RDG. Proliferation and differentiation of neuronal stem cells regulated by nerve growth factor. Nature. 1990;347:762–765. doi: 10.1038/347762a0. [DOI] [PubMed] [Google Scholar]
- Collazo D, Takahashi H, McKay RDG. Cellular targets and trophic functions of neurotrophin-3 in the developing rat hippocampus. Neuron. 1992;9:643–656. doi: 10.1016/0896-6273(92)90028-c. [DOI] [PubMed] [Google Scholar]
- Corfas G, Rosen KM, Aratake H, Krauss R, Fischbach GD. Differential expression of ARIA isoforms in the rat brain. Neuron. 1995;14:103–115. doi: 10.1016/0896-6273(95)90244-9. [DOI] [PubMed] [Google Scholar]
- Davies AM. Switching neurotrophin dependence. Curr. Biol. 1994;4:273–276. doi: 10.1016/s0960-9822(00)00064-6. [DOI] [PubMed] [Google Scholar]
- Davies AM, Lee K-F, Jaenisch R. p75-deficient trigeminal sensory neurons have an altered response to NGF but not to other neurotrophins. Neuron. 1993;11:565–574. doi: 10.1016/0896-6273(93)90069-4. [DOI] [PubMed] [Google Scholar]
- Davis S, Aldrich TH, Stahl N, Pan L, Taga T, Kishimoto T, Ip NY, Yancopoulos GD. The tripartite CNTF receptor includes components of the bipartite LIF receptor as heterodimerizing signal transducers. Science. 1993;260:1805–1808. doi: 10.1126/science.8390097. [DOI] [PubMed] [Google Scholar]
- Dechant G, Rodríguez-Tébar A, Kolbeck R, Barde Y-A. Specific high-affinity receptors for neurotrophin-3 on sympathetic neurons. J. Neurosci. 1993;13:2610–2616. doi: 10.1523/JNEUROSCI.13-06-02610.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCicco-Bloom E, Black IB. Insulin growth factors regulate the mitotic cycle in cultured rat sympathetic neuroblasts. Proc. Natl. Acad. Sci. USA. 1988;85:4066–4070. doi: 10.1073/pnas.85.11.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCicco-Bloom E, Friedman WJ, Black IB. NT-3 stimulates sympathetic neuroblast proliferation by promoting precursor survival. Neuron. 1993;11:1101–1111. doi: 10.1016/0896-6273(93)90223-e. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Lee K-F, Kucera J, Jaenisch R. Lack of neurotrophin-3 leads to deficiencies in the peripheral nervous system and loss of limb proprioceptive afferents. Cell. 1994;77:503–512. doi: 10.1016/0092-8674(94)90213-5. [DOI] [PubMed] [Google Scholar]
- Ernsberger U, Edgar D, Rohrer H. The survival of early chick sympathetic neurons in vitro is dependent on a suitable substrate but independent of NGF. Dev. Biol. 1989a;135:250–262. doi: 10.1016/0012-1606(89)90177-2. [DOI] [PubMed] [Google Scholar]
- Ernsberger V, Sendtner M, Rohrer H. Proliferation and differentiation of embryonic chick sympathetic neurons: effects of ciliary neurotrophic factor. Neuron. 1989b;2:1275–1284. doi: 10.1016/0896-6273(89)90312-7. [DOI] [PubMed] [Google Scholar]
- Falls DL, Rosen KM, Corfas G, Lane WS, Fischbach GD. ARIA, a protein that stimulates acetylcholine receptor synthesis, is a member of the Neu ligand family. Cell. 1993;72:801–815. doi: 10.1016/0092-8674(93)90407-h. [DOI] [PubMed] [Google Scholar]
- Fariñas I, Jones KR, Backus C, Wang X-Y, Reichardt LF. Severe sensory and sympathetic deficits in mice lacking neurotrophin-3. Nature. 1994;369:658–661. doi: 10.1038/369658a0. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Carnahan J, Greenberg ME. Requirement f or BDNF in activity-dependent survival of cortical neurons. Science. 1994;263:1618–1623. doi: 10.1126/science.7907431. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Greenberg ME. Distinct roles for bFGF and NT-3 in the regulation of cortical neurogenesis. Neuron. 1995;15:89–103. doi: 10.1016/0896-6273(95)90067-5. [DOI] [PubMed] [Google Scholar]
- Hantzopoulos PA, Suri C, Glass DJ, Goldfard MP, Yancopoulos GD. The low affinity NGF receptor, p75, can collaborate with each of the trks to potentiate functional responses to the neurotrophins. Neuron. 1994;13:187–201. doi: 10.1016/0896-6273(94)90469-3. [DOI] [PubMed] [Google Scholar]
- Hart IK, Richardson WD, Bolsover SR, Raff MC. PDGF and intracellular signaling in the timing of oligodendrocyte differentiation. J. Cell Biol. 1989;109:3411–3417. doi: 10.1083/jcb.109.6.3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatten ME. Neuronal inhibition of astroglial cell migration is membrane-mediated. J. Cell Biol. 1987;104:1353–1360. doi: 10.1083/jcb.104.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatten ME, Lynch M, Rydel RE, Sanchez J, Joseph-Silverstein J, Moscatelli D, Rifkin DB. In vitro neurite extension by granule neurons is dependent upon astroglial-derived fibroblast growth factor. Dev. Biol. 1988;125:280–289. doi: 10.1016/0012-1606(88)90211-4. [DOI] [PubMed] [Google Scholar]
- Ip NY, Boulton TG, Li Y, Verdi JM, Birren SJ, Anderson DJ, Yancopoulos GD. CNTF, FGF, and NGF collaborate to drive the terminal differentiation of MAH cells into post-mitotic neurons. Neuron. 1994;13:443–455. doi: 10.1016/0896-6273(94)90359-x. [DOI] [PubMed] [Google Scholar]
- Ip NY, McClain J, Barrezueta NX, Aldrich TH, Pan L, Li Y, Wiegand SJ, Friedman B, Davis S, Yancopoulos GD. The αcomponent of the CNTF receptor is required for signaling and defines potential CNTF targets in the adult and during development. Neuron. 1993;10:89–102. doi: 10.1016/0896-6273(93)90245-m. [DOI] [PubMed] [Google Scholar]
- Johnson EM, Jr, Taniuchi M, DiStefano PS. Expression and possible function of nerve growth factor receptors on Schwann cells. Trends Neurosci. 1988;11:299–304. doi: 10.1016/0166-2236(88)90090-2. [DOI] [PubMed] [Google Scholar]
- Kalcheim C, Carmeli C, Rosenthal A. Neurotrophin 3 is a mitogen for cultured neural crest cells. Proc. Natl. Acad. Sci. USA. 1992;89:1661–1665. doi: 10.1073/pnas.89.5.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K-F, Davies AM, Jaenisch R. p75-deficient embryonic dorsal root sensory and neonatal sympathetic neurons display a decreased sensitivity to NGF. Development. 1994;120:1027–1033. doi: 10.1242/dev.120.4.1027. [DOI] [PubMed] [Google Scholar]
- Leung DW, Parent AS, Cachianes G, Esch F, Coulombe JN, Nikolics K, Eckenstein FP, Nishi R. Cloning, expression during development, and evidence for release of a trophic factor for ciliary ganglion neurons. Neuron. 1992;8:1045–1053. doi: 10.1016/0896-6273(92)90126-x. [DOI] [PubMed] [Google Scholar]
- Marchionni MA, Goodearl ADJ, Chen MS, Bermingham-McDonogh O, Kirk C, Hendricks M, Danehy F, Misumi D, Sudhalter J, Kobayashi K, Wroblewski D, Lynch C, Baldassare M, Hiles I, Davis JB, Hsuan JJ, Totty NF, Otsu M, McBurney RN, Waterfield MD, Stroobant P, Gwynne D. Glial growth factors are alternatively spliced erbB2 ligands expressed in the nervous system. Nature. 1993;362:312–318. doi: 10.1038/362312a0. [DOI] [PubMed] [Google Scholar]
- Masu Y, Wolf E, Holtmann B, Sendtner M, Brem G, Thoenen H. Disruption of the CNTF gene results in motor neuron degeneration. Nature. 1993;365:27–32. doi: 10.1038/365027a0. [DOI] [PubMed] [Google Scholar]
- Meyer D, Birchmeier C. Distinct isoforms of neuregulin are expressed in mesenchymal and neuronal cells during mouse development. Proc. Natl. Acad. Sci. USA. 1994;91:1064–1068. doi: 10.1073/pnas.91.3.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff MC. Social controls on cell survival and cell death. Nature. 1992;356:397–400. doi: 10.1038/356397a0. [DOI] [PubMed] [Google Scholar]
- Rao MS, Sun Y, Escary JL, Perreau J, Tresser S, Patterson PH, Zigmond RH, Brulet P, Landis SC. Leukemia Inhibitory factor mediates an injury response but not a target-directed developmental transmitter switch in sympathetic neurons. Neuron. 1993;11:1175–1185. doi: 10.1016/0896-6273(93)90229-k. [DOI] [PubMed] [Google Scholar]
- Sasahara M, Fries JWU, Raines EW, Gown AM, Westrum LE, Frosch MP, Bonthron DT, Ross R, Collins T. PDGF β-chain in neurons of the central nervous system, posterior pituitary and in a transgenic model. Cell. 1991;64:217–227. doi: 10.1016/0092-8674(91)90223-l. [DOI] [PubMed] [Google Scholar]
- Schecterson LC, Bothwell M. Novel roles for neurotrophins are suggested by BDNF and NT-3 mRNA expression in developing neurons. Neuron. 1992;9:449–463. doi: 10.1016/0896-6273(92)90183-e. [DOI] [PubMed] [Google Scholar]
- Segal RA, Takahashi H, McKay RDG. Changes in neurotrophin responsiveness during the development of cerebellar granule neurons. Neuron. 1992;9:1041–1052. doi: 10.1016/0896-6273(92)90064-k. [DOI] [PubMed] [Google Scholar]
- Shah NM, Marchionni MA, Isaacs I, Stroobant PW, Anderson DJ. Glial growth factor restricts mammalian neural crest stem cells to a glial fate. Cell. 1994;77:349–360. doi: 10.1016/0092-8674(94)90150-3. [DOI] [PubMed] [Google Scholar]
- Sieber-Blum M. Role of the neurotrophic factors BDNF and NGF in the commitment of pluripotent neural crest cells. Neuron. 1991;6:949–955. doi: 10.1016/0896-6273(91)90235-r. [DOI] [PubMed] [Google Scholar]
- Stemple DL, Anderson DJ. Isolation of a stem cell for neurons and glia from the mammalian neural crest. Cell. 1992;71:973–985. doi: 10.1016/0092-8674(92)90393-q. [DOI] [PubMed] [Google Scholar]
- Stöckli KA, Lottspeich F, Sendtner M, Masiakowski P, Carroll P, Gotz R, Lindholm D, Thoenen H. Molecular cloning, expression and regional distribution of rat ciliary neurotrophic factor. Nature. 1989;342:920–923. doi: 10.1038/342920a0. [DOI] [PubMed] [Google Scholar]
- Stöckli KA, Lillien LE, Naher-Noe M, Breitfeld G, Hughes RA, Raff MC, Thoenen H, Sendtner M. Regional distribution, developmental changes, and cellular localization of CNTF-mRNA and protein in the rat brain. J. Cell Biol. 1991;115:447–459. doi: 10.1083/jcb.115.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdi JM, Anderson DJ. Neurotrophins regulate sequential changes in neurotrophin receptor expression by sympathetic neuroblasts. Neuron. 1994;13:1359–1372. doi: 10.1016/0896-6273(94)90421-9. [DOI] [PubMed] [Google Scholar]
- Verdi JM, Birren SJ, Ibáñez CF, Persson H, Kaplan DR, Benedetti M, Chao MV, Anderson DJ. p75LNGFR regulates trk signal transduction and NGF-induced neuronal differentiation in MAH cells. Neuron. 1994a;12:733–745. doi: 10.1016/0896-6273(94)90327-1. [DOI] [PubMed] [Google Scholar]
- Vicario-Abejón C, Johe KK, Hazel TG, Collazo D, McKay RDG. Functions of basic fibroblast growth factor and neurotrophins in the differentiation of hippocampal neurons. Neuron. 1995;15:105–114. doi: 10.1016/0896-6273(95)90068-3. [DOI] [PubMed] [Google Scholar]
- Vogel KS, Davies AM. The duration of neurotrophic factor independence in early sensory neurons is matched to the time course of target field innervation. Neuron. 1991;7:819–830. doi: 10.1016/0896-6273(91)90284-7. [DOI] [PubMed] [Google Scholar]
- Weskamp G, Reichardt LF. Evidence that biological activity of NGF is mediated through a novel subclass of high affinity receptors. Neuron. 1991;6:649–663. doi: 10.1016/0896-6273(91)90067-a. [DOI] [PubMed] [Google Scholar]
- Wright EM, Vogel KS, Davies AM. Neurotrophic factors promote the maturation of developing sensory neruons before they become dependent on these factors for survival. Neuron. 1992;9:1–20. doi: 10.1016/0896-6273(92)90229-7. [DOI] [PubMed] [Google Scholar]
- Wyatt S, Davies AM. Regulation of expression of mRNAs encoding the nerve growth factor receptors p75 and trkA in developing sensory neurons. Development. 1993;119:635–647. doi: 10.1242/dev.119.3.635. [DOI] [PubMed] [Google Scholar]
- Yeh H-J, Ruit KG, Wang Y-X, Parks WC, Sinder WD, Deuel TF. PDGF α-chain gene is expressed by mammalian neurons during development and in maturity. Cell. 1991;64:209–216. doi: 10.1016/0092-8674(91)90222-k. [DOI] [PubMed] [Google Scholar]
- Zackenfels K, Oppenheim RW, Rohrer H. Evidence for an important role of IGF-I and IGF-II for the early development of chick sympathetic neurons. Neuron. 1995;14:731–741. doi: 10.1016/0896-6273(95)90217-1. [DOI] [PubMed] [Google Scholar]