Abstract
Background
Lung ischemia-reperfusion (IR) injury leads to significant morbidity and mortality which remains a major obstacle after lung transplantation. However, the role of various subset(s) of lung cell populations in the pathogenesis of lung IR injury and the mechanisms of cellular protection remain to be elucidated. In the present study, we investigated the effects of adenosine A2A receptor (A2AAR) activation on resident lung cells after IR injury using an isolated, buffer-perfused murine lung model.
Methods
To assess the protective effects of A2AAR activation, three groups of C57BL/6J mice were studied: a sham group (perfused for 2 hr with no ischemia), an IR group (1 hr ischemia + 1 hr reperfusion) and an IR+ATL313 group where ATL313, a specific A2AAR agonist, was included in the reperfusion buffer after ischemia. Lung injury parameters and pulmonary function studies were also performed after IR injury in A2AAR knockout mice, with or without ATL313 pretreatment. Lung function was assessed using a buffer-perfused isolated lung system. Lung injury was measured by assessing lung edema, vascular permeability, cytokine/chemokine activation and myeloperoxidase levels in the bronchoalveolar fluid.
Results
After IR, lungs from C57BL/6J wild-type mice displayed significant dysfunction (increased airway resistance, pulmonary artery pressure and decreased pulmonary compliance) and significant injury (increased vascular permeability and edema). Lung injury and dysfunction after IR were significantly attenuated by ATL313 treatment. Significant induction of TNF-α, KC (CXCL1), MIP-2 (CXCL2) and RANTES (CCL5) occurred after IR which was also attenuated by ATL313 treatment. Lungs from A2AAR knockout mice also displayed significant dysfunction, injury and cytokine/chemokine production after IR, but ATL313 had no effect in these mice.
Conclusion
Specific activation of A2AARs provides potent protection against lung IR injury via attenuation of inflammation. This protection occurs in the absence of circulating blood thereby indicating a protective role of A2AAR activation on resident lung cells such as alveolar macrophages. Specific A2AAR activation may be a promising therapeutic target for the prevention or treatment of pulmonary graft dysfunction in transplant patients.
Background
Ischemia-reperfusion (IR)-induced lung injury remains the major cause of primary graft failure after lung transplantation [1,2]. IR injury causes significant mortality and morbidity in the early post-operative period and is reported to be an independent predictive factor for the development and progression of bronchiolitis obliterans syndrome, which is the most common cause of death after lung transplantation [1,3]. We have previously demonstrated that alveolar macrophage activation [4] and alveolar type II epithelial cell activation [5] are associated with the induction of lung IR injury. An event which follows macrophage and epithelial cell activation is neutrophil activation and infiltration into lung tissue which results in severe pulmonary dysfunction in the early post-transplant period [6-8]. Pulmonary IR injury also entails the induction of pro-inflammatory cytokines and chemokines [9,10], and the contribution of TNF-α, IL-1β, IL-6 and KC (CXCL1) in the genesis and progression of lung IR injury has been demonstrated [5,11,12].
One major anti-inflammatory mechanism after lung injury is mediated by the release of adenosine [13]. Adenosine receptors are found on various cell types, and the activation of these receptors often results in suppression of inflammatory function [14-17]. The A2A adenosine receptor (A2AAR) is one of four subtypes of the G protein-coupled adenosine receptor family which includes A1, A2A, A2B and A3. Adenosine receptor sub-classification has shown specifically that activation of A2AAR produces anti-inflammatory responses and prevents leukocyte adhesion [18,19]. Recent studies have shown that pharmacologic activation of A2AAR restores functional integrity in renal, cardiac, hepatic and spinal cord IR injury models [20-25]. A2AAR activation during reperfusion has also been shown to ameliorate lung IR injury while decreasing cellular and molecular inflammatory markers [26,27]. A2AARs are predominantly expressed on inflammatory cells including neutrophils, mast cells, macrophages, T cells, monocytes and platelets [28,29]. The attenuation of IR injury by A2AAR activation is postulated to involve a purinergic regulatory process whereby the A2AAR coupled to a stimulatory G protein leads to an increase in cyclic adenosine monophosphate (cAMP), thereby resulting in reduced cytokine release and inactivation of inflammatory cells [30,31].
This study focuses on the role of resident lung leukocytes in IR injury and the effects of A2AAR activation on these cells using an isolated, buffer-perfused mouse model of lung IR injury. This model allows the investigation of specific and direct effects of A2AAR activation on lung function independent of circulating platelets and neutrophils. The anti-inflammatory actions of selective A2AAR agonists have been attributed to circulating leukocytes in previous studies. However, in the present study, we hypothesize that specific activation of A2AAR on resident lung cells would attenuate pulmonary injury and dysfunction after IR despite the absence of circulating platelets and leukocytes from blood reperfusion.
Methods
Animals and study design
We utilized 8–10 week old wild-type (WT) C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME) and A2AAR knockout (KO) mice congenic to C57BL/6J [32]. Three groups of animals were studied (n = 6/group); a sham group, an IR group and an IR+ATL313 group. Lungs in the IR group were subjected to 1 hr ischemia followed by 1 hr reperfusion with Krebs-Henseleit buffer. As a control, Sham lungs received 2 hr reperfusion without ischemia, and the final 1 hr of perfusion in the sham lungs were compared with the 1 hr of reperfusion in the IR group. The IR+ATL313 group was identical to the IR group except that ATL313 (30 nM) was added to the perfusate buffer at the beginning of the reperfusion period. ATL313 (a gift from Adenosine Therapeutics, LLC, Charlottesville, VA) is a potent and highly specific activator of A2AAR [32]. The dose of ATL313 used in this study (30 nM) had no significant effects on pulmonary function or hemodynamics in lungs from sham animals (data not shown). A2AAR KO mice were also subjected to IR and IR+ATL313 as described above. This study was conducted under protocols approved by the University of Virginia's Institutional Animal Care and Use Committee. All animals received humane care in compliance with the "Principles of Laboratory Animal Care" formulated by the National Society for Medical Research, and "The Guide for the Care and Use of Laboratory Animals", prepared by the National Academy of Science and published by the National Institutes of Health.
Isolated, buffer-perfused lung IR model
For this study, we used an isolated, buffer-perfused mouse lung system (Hugo Sachs Elektronik, March-Huggstetten, Germany) as previously described by our laboratory [4]. Mice were anesthetized with ketamine and xylazine. A tracheotomy was performed, and animals were ventilated with room air at 100 breaths/min at a tidal volume of 7 μl/g body weight with a positive end expiratory pressure of 2 cm H2O using the MINIVENT mouse ventilator (Hugo Sachs Elektronik, March-Huggstetten, Germany). A midline abdominal incision was made, and the inferior vena cava was cannulated with a 30-gauge needle and injected with 500 units of heparin. Animals were exsanguinated by inferior vena caval transection. The subdiaphragmatic portion of the animal was excised and discarded. The anterior chest plate was removed, exposing the lungs and heart. A 4-0 silk suture was passed behind the pulmonary artery (PA) and the aortic root. A partial half-knot was created with the suture, leaving room for the cannula to be passed into the PA. A small curvilinear incision was made in the right ventricular outflow tract with the perfusate flowing at 0.2 ml/min, and the PA cannula was passed through the pulmonary valve into the PA. The partial half-knot was then tightened. The left ventricle was immediately vented with a small incision at the apex of the heart. The mitral apparatus was carefully dilated and the left atrial cannula was passed through the mitral valve into the left atrium. The placement of the left atrial and the PA cannulas were further confirmed by pressure tracings generated by the PULMODYN data acquisition system (Hugo Sachs Elektronik). The lungs were then perfused at a constant flow of 60 μl/g body wt/min with Krebs-Henseleit buffer (Sigma-Aldrich, St. Louis, MO) containing 0.1% glucose and 0.3% HEPES (335–340 mOsmol/kg H2O). The Krebs solution was prepared to mimic mixed venous blood using an oxygenator (Living Systems Instrumentation, Burlington, VT) with titrated gases generating a pH = 7.35–7.40, a pO2 = 60–70 mmHg, and a pCO2 = 50–60 mmHg. The buffered perfusate and isolated lungs were maintained at 37°C throughout the experiment by use of a circulating water bath.
Isolated lungs were allowed to equilibrate on the apparatus during a 15-min stabilization period. After equilibration, ventilation was decreased to 50 breaths/min, and the fraction of inspired oxygen was decreased to <1%. To initiate the ischemic period, hypoxic ventilation was maintained with 95% nitrogen and 5% carbon dioxide, and perfusion was arrested. After 60 min of ischemia and hypoxic ventilation, perfusion and room air ventilation were then resumed to initiate the reperfusion period. Hemodynamic and pulmonary function parameters were continuously recorded throughout the reperfusion period by the PULMODYN data acquisition system (Hugo Sachs Elektronik). Ventilation with hypoxic gas rather than stopping ventilation altogether during ischemia was performed to avoid atelectasis while still maintaining ischemia. Atelectasis and reexpansion has been shown to induce injury involving edema, free radical generation, and apoptosis [33,34]. This reexpansion-induced injury could obscure the effects of IR injury, an issue we wished to avoid.
Bronchoalveolar lavage (BAL) fluid collection
After perfusion, lungs were lavaged with 0.5 ml saline via tracheotomy. This procedure was performed three times, and the fluid was pooled together. An average of 1.2 ml total BAL fluid was collected from each mouse. BAL fluid was centrifuged at 4°C (1500 g for 15 min), and the supernatant was stored at -80°C.
Cytokine and chemokine protein analysis
Cytokine and chemokine protein content in BAL fluid was quantified using the Bioplex Bead Array technique using a mouse-specific multiplex cytokine panel assay (Bio-Rad Laboratories, Hercules, CA). The microplates were analyzed by the Bioplex array reader which is a fluorescent-based flow cytometer employing a specific bead-based multiplex technology, each of which is conjugated with a reactant specific for a different target cytokine. The array reader quantifies the magnitude of the bead fluorescence intensity associated with each target protein.
Lung wet/dry weight ratio
Lung wet/dry weight ratio was used as an indicator of pulmonary edema using separate groups of animals (n = 5/group). The lower lobe of the right lung from each animal was harvested, weighed and placed in a vacuum oven (at 54°C) until a stable, dry weight was achieved. The ratio of lung wet weight to dry weight was then calculated.
Vascular permeability assay
As another indicator of lung injury, lung vascular permeability was assessed using separate groups of animals (n = 5/group). At the completion of reperfusion, the perfusion buffer (Krebs Henseleit solution) was replaced with 30 mg/ml bovine serum albumin (BSA) solution (in PBS), and the lungs were perfused for an additional 5 min at the same flow rate as during reperfusion. After this, the BSA solution was changed back to Krebs Henseleit solution, and perfusion was continued for an additional 5 min to wash out the BSA solution from the lung vasculature. Using a BSA ELISA kit (Immunology Consultants Laboratory, OR), BSA concentration in BAL fluid was measured according to the manufacturer's instructions.
Myeloperoxidase (MPO) measurement
MPO, which is expressed in neutrophils, was measured in BAL fluid as an indicator of neutrophil infiltration into alveolar spaces. An MPO ELISA kit (Cell Sciences, Canton, MA) was utilized as instructed by the manufacturer.
Statistical analysis
Values are presented as the mean ± standard error of the mean (SEM). Analysis of variance (ANOVA) was used to determine if significant differences existed between groups. Bonferroni's HSD multiple comparison test was used to determine which groups were significantly different when the ANOVA results were significant. Data was considered significant when p < 0.05.
Results
Lung function after IR
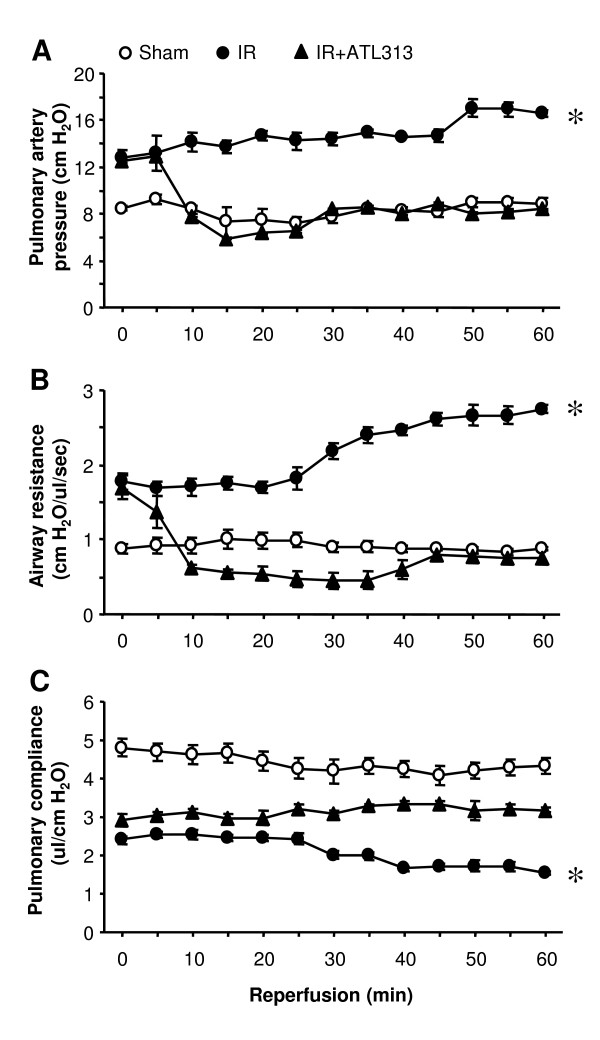
Using the isolated, buffer-perfused mouse model of IR, lung function in WT mice was measured during the 1 hr reperfusion period after ischemia and compared to Sham lungs. Significant lung dysfunction occurred after IR as shown in Figure 1. At the end of reperfusion, IR lungs exhibited significantly increased pulmonary artery pressure (16.5 ± 0.26 vs. 8.78 ± 0.56 cm H2O), increased airway resistance (2.74 ± 0.05 vs. 0.87 ± 0.02 cm H2O/μl/sec) and reduced pulmonary compliance (1.54 ± 0.05 vs. 4.34 ± 0.19 μl/cm H2O) compared to Sham (p < 0.01).
Figure 1.
Temporal changes in pulmonary function during reperfusion. Pulmonary artery pressure (A), airway resistance (B), and pulmonary compliance (C) were measured throughout 60 min reperfusion in WT sham and IR lungs. Lung function is significantly impaired after IR compared to sham, and ATL313 significantly attenuated lung dysfunction. Open circles, Sham lungs undergoing perfusion only; filled circles, IR lungs reperfused after 60 min ischemia; filled triangles, IR lungs treated with ATL313 (30 nM) during reperfusion. *p < 0.01 IR vs. all.
Lung dysfunction after IR is attenuated by A2AAR activation
To investigate the effects of A2AAR activation on lungs undergoing IR injury, ATL313, a specific A2AAR agonist, was administered during reperfusion. A significant improvement in lung function was observed in the ATL313-treated lungs compared to lungs undergoing IR alone (Figure 1). At the end of reperfusion, ATL313 significantly reduced pulmonary artery pressure (8.37 ± 0.55 vs. 16.5 ± 0.26 cm H2O), reduced airway resistance (0.75 ± 0.09 vs. 2.74 ± 0.05 cm H2O/μl/sec) and increased pulmonary compliance (3.18 ± 0.09 vs. 1.54 ± 0.05 μl/cm H2O) compared to IR alone (p < 0.01). In fact, lung function after IR in ATL313-treated lungs was comparable to Sham lungs.
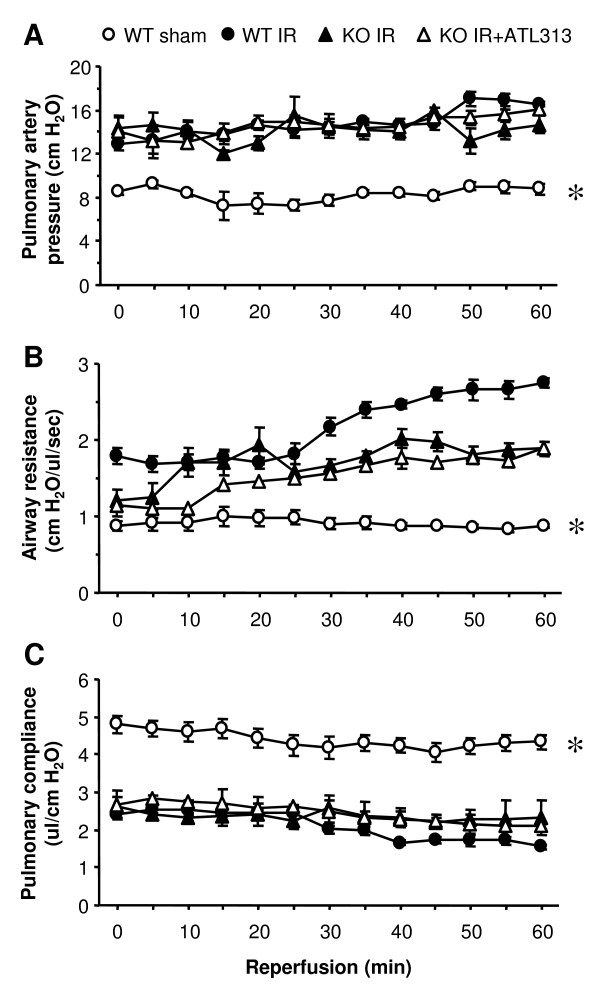
ATL313 specifically acts on A2AAR
To eliminate the possibility that ATL313 could have effects secondary to A2AAR activation, lung function was measured after IR in A2AAR KO mice with or without treatment with ATL313. Lung function was not different between sham A2AAR KO mice and sham WT mice (data not shown). Similar to WT mice, significant dysfunction occurred in lungs from A2AAR KO mice after IR (Figure 2). At the end of reperfusion, lungs from A2AAR KO mice displayed significantly increased pulmonary artery pressure (14.7 ± 0.83 vs. 8.78 ± 0.56 cm H2O), increased airway resistance (1.89 ± 0.09 vs. 0.87 ± 0.02 cm H2O/μl/sec) and decreased pulmonary compliance (2.32 ± 0.09 vs. 4.34 ± 0.19 μl/cm H2O) versus WT Sham (p < 0.01). Furthermore, treatment of A2AAR KO mice with ATL313 did not result in any improvement in lung function, and these mice displayed no significant difference in lung function compared to A2AAR KO mice undergoing IR alone (Figure 2).
Figure 2.
Pulmonary function during reperfusion in A2AAR KO mice. Pulmonary artery pressure (A), airway resistance (B), and pulmonary compliance (C) were measured throughout 60 min reperfusion in A2AAR KO lungs after IR with or without administration of ATL313. Lung function was significantly impaired in A2AAR KO mice, and ATL313 treatment offered no protection. Open circles, WT Sham lungs undergoing perfusion only; filled circles, WT IR lungs reperfused after 60 min ischemia; filled triangles, A2AAR KO IR lungs reperfused after 60 min ischemia; open triangles, A2AAR KO IR lungs treated with ATL313 (30 nM) during reperfusion. *p < 0.01 WT Sham vs. all.
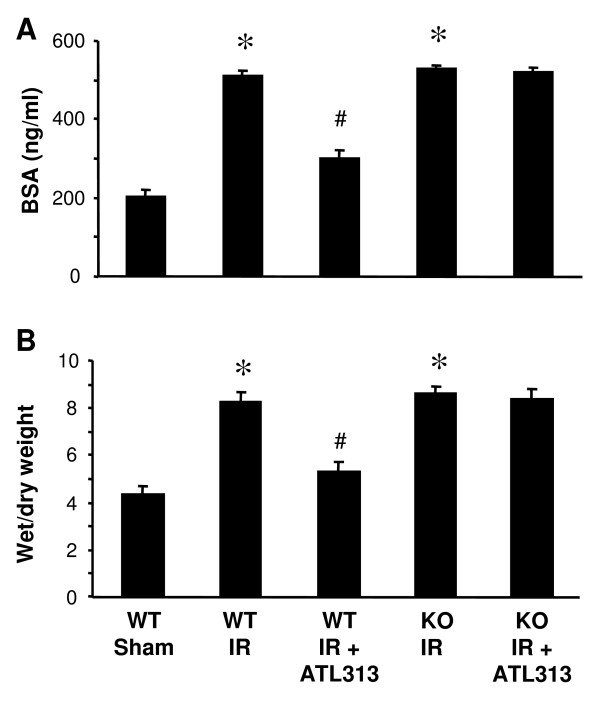
A2AAR activation inhibits lung IR injury
To assess lung injury after IR, vascular permeability (as measured by BSA content in BAL fluid) and pulmonary edema (as measured by wet/dry weight) were assessed at the end of the reperfusion period. A significant increase in vascular permeability (Figure 3A) and pulmonary edema (Figure 3B) occurred after IR in lungs from WT and A2AAR KO mice versus sham (p < 0.001). ATL313 significantly decreased vascular permeability and pulmonary edema in WT mice after IR (p < 0.01) but had no effect on lungs from A2AAR KO mice after IR (Figure 3).
Figure 3.
Lung IR injury is attenuated by A2AAR activation. Lung vascular permeability was assessed by measuring BSA concentration in BAL fluid (A). Lung edema was assessed by measuring wet/dry weight ratio (B). Significant lung injury (increased vascular permeability and edema) occurred after IR in WT mice which was attenuated by ATL313 treatment. Significant lung injury also occurred in A2AAR KO mice after IR, but ATL313 had no affect on A2AAR KO lungs. WT Sham, WT lungs undergoing perfusion only; WT IR, WT lungs undergoing IR; KO IR, A2AAR KO lungs undergoing IR; KO IR+ATL313, A2AAR KO IR lungs treated with ATL313 (30 nM) during reperfusion. *p < 0.001 IR vs. WT Sham; #p < 0.01 WT IR+ATL313 vs. WT IR.
A2AAR activation attenuates cytokine/chemokine expression
Pro-inflammatory cytokine/chemokine expression was measured in BAL fluid of WT and A2AAR KO mouse lungs after IR, with or without ATL313 treatment. A significant increase in the production of TNF-α, KC (CXCL1), MIP-2 (CXCL2) and RANTES (CCL5) occurred in both WT and A2AAR KO mice after IR (Figure 4, p < 0.01). Treatment with ATL313 significantly attenuated cytokine/chemokine activation after IR in WT mice (p < 0.001) but had no significant effect in A2AAR KO mice (Figure 4). In addition, there was no significant production of IL-6, MCP-1 or IFN-γ in WT or A2AAR KO mouse lungs after IR (data not shown).
Figure 4.
Cytokine/chemokine expression after lung IR. The expression of TNF-α, MIP-2 (CXCL2), RANTES (CCL5), and KC (CXCL1) in BAL fluid were significantly induced after IR in WT and A2AAR KO mice (*p < 0.01 vs. WT Sham). Cytokine/chemokine induction was significantly impaired by ATL313 treatment in WT mice but not in A2AAR KO mice (#p < 0.001, WT IR+ATL313 vs. WT IR). WT Sham, WT lungs undergoing perfusion only; WT IR, WT lungs undergoing IR; KO IR, A2AAR KO lungs undergoing IR; KO IR+ATL313, A2AAR KO IR lungs treated with ATL313 (30 nM) during reperfusion.
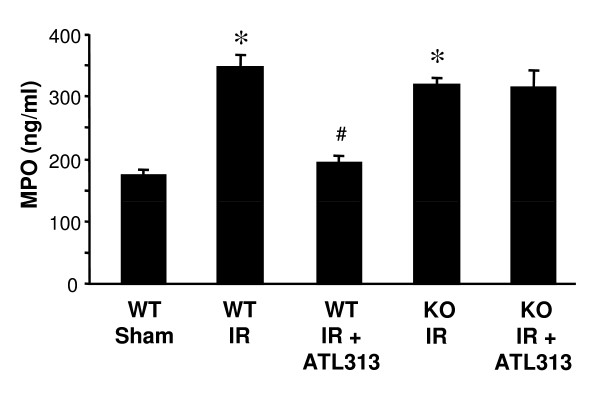
Neutrophil infiltration after lung IR is attenuated by A2AAR activation
MPO is abundantly present in azurophilic granules of polymorphonuclear neutrophils and its increased concentration in BAL fluid is an indicator of neutrophil activation and migration into alveolar airspaces. MPO content in BAL fluid was evaluated in WT and A2AAR KO lungs after IR with or without ATL313 treatment. MPO content was significantly increased in WT lungs after IR compared to Sham (Figure 5, p < 0.01). Treatment with ATL313 significantly reduced MPO content in WT lungs after IR (p < 0.001). In A2AAR KO mice, IR also resulted in significantly increased MPO content, however ATL313 treatment did not affect MPO content in these mice (Figure 5).
Figure 5.
Lung IR-induced alveolar MPO content is attenuated by A2AAR activation. MPO content in BAL fluid was significantly induced in lungs from WT and A2AAR KO mice after IR (*p < 0.01 vs. WT Sham). MPO content was significantly attenuated by ATL313 treatment in WT mice but not in A2AAR KO mice (#p < 0.001, WT IR+ATL313 vs. WT IR). WT Sham, WT lungs undergoing perfusion only; WT IR, WT lungs undergoing IR; KO IR, A2AAR KO lungs undergoing IR; KO IR+ATL313, A2AAR KO IR lungs treated with ATL313 (30 nM) during reperfusion.
Discussion
In this study, we investigated the role of A2AAR activation in mediating protection from lung IR injury utilizing a buffer-perfused mouse lung model. ATL313-mediated protection was illustrated by significant reductions in mean pulmonary artery pressure and airway resistance, and significant improvement in pulmonary compliance throughout the reperfusion period following ischemia. Lungs undergoing IR also displayed increased vascular permeability and pulmonary edema which were significantly attenuated by ATL313 treatment. We cannot discount the possibility that the attenuation in vascular permeability by ATL313 may be in part due to a decrease in intravascular pressure as well as to changes in endothelial permeability. In addition, A2AAR KO mice demonstrated significant lung injury and dysfunction, but ATL313 treatment failed to protect these lungs after IR, indicating the specificity of ATL313 for A2AAR. The induction of specific pro-inflammatory cytokines/chemokines (TNF-α, KC, MIP-2 and RANTES) and increased MPO content after IR were also significantly attenuated by A2AAR activation in WT lungs.
The results presented in this study support other reports of protection from IR injury by specific A2AAR agonists [16,22,27,35,36]. More importantly, while prior studies indicate that circulating cells such as platelets and neutrophils play a significant role in the anti-inflammatory effects of A2AAR activation, the present study describes A2AAR-mediated protection in the absence of circulating cells. These results suggest that the cellular targets for ATL313-mediated protection in buffer-perfused lungs are likely resident lung leukocyte populations including alveolar macrophages, neutrophils (including marginated neutrophils) or T cells. The source of the infiltrating neutrophils in alveolar airspaces after IR must be either interstitial or marginated neutrophils in this model. We have previously shown that a significant number of marginated neutrophils are retained in buffer-perfused lungs [4]. However, the contribution of other cells, such as epithelial or endothelial cells, to A2AAR-mediated protection cannot be eliminated. This study suggests that the specific activation of A2AAR improves pulmonary injury and function after IR in a non-blood perfused system, thereby indicating the importance of resident pulmonary cells in lung IR injury.
This study highlights an emerging therapeutic role for the use of A2AAR agonists, such as ATL313, as a logical extension of many intrinsic defense mechanisms inasmuch as adenosine is known to accumulate in response to inflammation or IR injury. Whereas the exact mechanisms of protection are complex and poorly understood, treatment with A2AAR agonists has been associated with inhibition of inflammatory cytokine release, reduction of apoptotic injury, and diminution of free radical production [37-39]. In this study, the markedly significant protection offered by ATL313 in WT mice suggests an important protective role of A2AARs in lung IR injury, and that specific activation of A2AAR, and not A1AR, A2BAR or A3AR, has broad anti-inflammatory effects that attenuate lung IR injury.
Although we anticipated that lung injury and dysfunction after IR would be worse in A2AAR KO mice, injury and function in A2AAR KO mice were comparable to WT mice. One possible explanation for this might be that the buffer-perfused model of IR injury, or the timeframe utilized (1 hr ischemia and 1 hr reperfusion), does not entail significant production of adenosine to result in enhanced anti-inflammatory effects in WT mice. A second possible explanation is that this model does not entail blood-perfusion whereby enhanced platelet activation and infiltration of circulating leukocytes may occur in A2AAR KO mice to exacerbate injury. However, using a blood-perfused mouse lung IR model involving left lung hilar clamp (1 hr ischemia followed by 2 hr reperfusion), we observed a similar pattern of injury in A2AAR KO mice as compared to WT mice (data not shown). A third possible explanation, and probably the most likely explanation, is that the A2AAR does not normally play a significant anti-inflammatory role during acute lung IR injury. It is plausible that activation of other adenosine receptors (A1, A2B and A3), which are all expressed in the lung [40], may have significant, anti-inflammatory roles in the pathogenesis of IR. For example, it is well documented that A1AR plays a role in protecting the heart [41] and kidney [42] against IR injury. However, A1AR antagonism has been shown to be beneficial in attenuating lung IR injury [43], results which add to the confusion regarding the role of A1AR in lung injury. A recent study showed that exposure to endotoxin results in augmented pro-inflammatory cytokine levels and increased leukocyte adhesion in A2BAR KO mice [44]. A study by Rivo et al. showed that activation of A3AR may protect the lung against IR injury [45]. Although it is possible that enhanced expression of other adenosine receptors could compensate for the lack of A2AAR in the KO mice, our data suggests that endogenous activation of A2AAR may not play a significant role in IR injury. The contribution of other adenosine receptors in the modulation of lung IR injury remains a subject for further investigation.
Previous studies from our group and others have demonstrated a significant role of alveolar macrophages in the initiation of lung IR injury [4,46,47]. We have also demonstrated that the intercellular interactions between alveolar macrophages and type II epithelial cells are important events in lung IR injury via activation of specific cytokines/chemokines such as TNF-α and KC, thereby leading to enhanced neutrophil chemotaxis [5]. The specific activation of A2AARs on macrophages has been shown to attenuate IR injury in other organ systems such as kidney, liver and heart [21,23,25,48]. However, the role of A2AAR activation specifically on resident lung cell populations after IR has not been previously explored. The pro-inflammatory cascade in lung IR injury involving TNF-α has also been previously documented by our group and others [12,49,50]. These findings highlight a complex cross-talk between various lung cell populations including alveolar macrophages, type II epithelial cells and neutrophils in the progression of IR injury. Our findings suggest that cytokines and chemokines, such as TNF-α, KC and MIP-2, released by alveolar macrophages and type II epithelial cells activate neutrophils, which serve as end effecters of lung IR injury. It is known that neutrophils progressively infiltrate the transplanted lung after reperfusion and contribute to injury by releasing oxygen free radicals [2]. We also observed that increased alveolar MPO content after IR was significantly attenuated by ATL313, indicative of the protective role of A2AARs on resident lung neutrophils. We have previously demonstrated the presence of marginated neutrophils in buffer-perfused mouse lungs [4]. A2AARs on these marginated neutrophils, in conjunction with resident lung leukocytes such as alveolar macrophages are likely key targets for ATL313-mediated protection against IR-induced lung injury.
This study focused on the acute, anti-inflammatory effects of A2AAR activation. It is plausible, however, that A2AAR-mediated protection is also a prolonged phenomenon attenuating the chronic fibroproliferative phase of lung IR injury. However, due to the inherent limitations of the acute ex-vivo, buffer-perfused model, the study of long term A2AAR-mediated protection is beyond the scope of the present study. Another limitation of the buffer-perfused IR model is the use of hypoxic ventilation to induce ischemia instead of clamping the lung in an inflated state, which is utilized in the human lung transplantation. However, the hypoxic ventilation was used to prevent atelectasis in the buffer-perfused IR model. Moreover, the in vivo hilar clamp IR model entails circulating blood and thus does not specifically address the role of resident lung cells in lung IR injury, which was the focus of this study.
Conclusion
In summary, our data suggests that attenuation of the inflammatory cascade by ATL313 activation of A2AARs on resident lung cells is sufficient in preventing lung injury and dysfunction after IR. Pulmonary IR injury entails complex signaling mechanisms involving the intercellular interactions of various lung cell populations via a pro-inflammatory cascade. The selective activation of A2AARs on resident lung leukocyte populations such as alveolar macrophages, marginated neutrophils, and possibly T lymphocytes by ATL313 is an anti-inflammatory intervention that inhibits neutrophil activation and subsequent infiltration and tissue injury. Thus, specific A2AAR activation may be a promising therapeutic target for the prevention or treatment of pulmonary graft dysfunction in transplant patients.
Competing interests
JL and ILK were shareholders in Adenosine Therapeutics, LLC, the corporation that provided ATL313, during the time of this study.
Authors' contributions
AKS conducted the research experiments, performed statistical analysis, and drafted the manuscript. JL and ILK helped with the analysis and interpretation of the data. VEL helped in conception and design of the experiments, analysis and interpretation of the data and drafting of the article. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
Funding for this project was provided by an R01 HL077301 award from the National Heart, Lung, and Blood Institute/NIH (VEL) and by a research grant award from the Roche Organ Transplant Research Foundation, Meggen, Switzerland (VEL). The authors are grateful to Dr. Jeffrey J. Lysiak for his assistance with the use of the Bioplex Bead Array System, and to Jayson Rieger of Adenosine Therapeutics, LLC, for providing ATL313.
Contributor Information
Ashish K Sharma, Email: aks2n@virginia.edu.
Joel Linden, Email: jl4v@virginia.edu.
Irving L Kron, Email: ilk@virginia.edu.
Victor E Laubach, Email: laubach@virginia.edu.
References
- Fiser SM, Tribble CG, Long SM, Kaza AK, Kern JA, Jones DR, Robbins MK, Kron IL. Ischemia-reperfusion injury after lung transplantation increases risk of late bronchiolitis obliterans syndrome. Ann Thorac Surg. 2002;73:1041–1047. doi: 10.1016/S0003-4975(01)03606-2. [DOI] [PubMed] [Google Scholar]
- de Perrot M, Liu M, Waddell TK, Keshavjee S. Ischemia-reperfusion-induced lung injury. Am J Respir Crit Care Med. 2003;167:490–511. doi: 10.1164/rccm.200207-670SO. [DOI] [PubMed] [Google Scholar]
- Boehler A, Estenne M. Post-transplant bronchiolitis obliterans. Eur Respir J. 2003;22:1007–1018. doi: 10.1183/09031936.03.00039103. [DOI] [PubMed] [Google Scholar]
- Zhao M, Fernandez LG, Doctor A, Sharma AK, Zarbock A, Tribble CG, Kron IL, Laubach VE. Alveolar macrophage activation is a key initiation signal for acute lung ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1018–1026. doi: 10.1152/ajplung.00086.2006. [DOI] [PubMed] [Google Scholar]
- Sharma AK, Fernandez LG, Awad AS, Kron IL, Laubach VE. Proinflammatory response of alveolar epithelial cells is enhanced by alveolar macrophage-produced TNF-alpha during pulmonary ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol. 2007;293:L105–113. doi: 10.1152/ajplung.00470.2006. [DOI] [PubMed] [Google Scholar]
- Ross SD, Tribble CG, Gaughen JR, Jr, Shockey KS, Parrino PE, Kron IL. Reduced neutrophil infiltration protects against lung reperfusion injury after transplantation. Ann Thorac Surg. 1999;67:1428–1433. doi: 10.1016/S0003-4975(99)00248-9. [DOI] [PubMed] [Google Scholar]
- Eppinger MJ, Deeb GM, Bolling SF, Ward PA. Mediators of ischemia-reperfusion injury of rat lung. Am J Pathol. 1997;150:1773–1784. [PMC free article] [PubMed] [Google Scholar]
- Eppinger MJ, Jones ML, Deeb GM, Bolling SF, Ward PA. Pattern of injury and the role of neutrophils in reperfusion injury of rat lung. J Surg Res. 1995;58:713–718. doi: 10.1006/jsre.1995.1112. [DOI] [PubMed] [Google Scholar]
- Bando K, Paradis IL, Similo S, Konishi H, Komatsu K, Zullo TG, Yousem SA, Close JM, Zeevi A, Duquesnoy RJ, et al. Obliterative bronchiolitis after lung and heart-lung transplantation. An analysis of risk factors and management. J Thorac Cardiovasc Surg. 1995;110:4–13. doi: 10.1016/S0022-5223(05)80003-0. [DOI] [PubMed] [Google Scholar]
- Snell GI, Esmore DS, Williams TJ. Cytolytic therapy for the bronchiolitis obliterans syndrome complicating lung transplantation. Chest. 1996;109:874–878. doi: 10.1378/chest.109.4.874. [DOI] [PubMed] [Google Scholar]
- Mal H, Dehoux M, Sleiman C, Boczkowski J, Leseche G, Pariente R, Fournier M. Early release of proinflammatory cytokines after lung transplantation. Chest. 1998;113:645–651. doi: 10.1378/chest.113.3.645. [DOI] [PubMed] [Google Scholar]
- Maxey TS, Enelow RI, Gaston B, Kron IL, Laubach VE, Doctor A. Tumor necrosis factor-alpha from resident lung cells is a key initiating factor in pulmonary ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2004;127:541–547. doi: 10.1016/j.jtcvs.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Novick RJ, Gehman KE, Ali IS, Lee J. Lung preservation: the importance of endothelial and alveolar type II cell integrity. Ann Thorac Surg. 1996;62:302–314. doi: 10.1016/0003-4975(96)00333-5. [DOI] [PubMed] [Google Scholar]
- Cronstein BN, Daguma L, Nichols D, Hutchison AJ, Williams M. The adenosine/neutrophil paradox resolved: human neutrophils possess both A1 and A2 receptors that promote chemotaxis and inhibit O2 generation, respectively. J Clin Invest. 1990;85:1150–1157. doi: 10.1172/JCI114547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein BN, Levin RI, Philips M, Hirschhorn R, Abramson SB, Weissmann G. Neutrophil adherence to endothelium is enhanced via adenosine A1 receptors and inhibited via adenosine A2 receptors. J Immunol. 1992;148:2201–2206. [PubMed] [Google Scholar]
- Nolte D, Lorenzen A, Lehr HA, Zimmer FJ, Klotz KN, Messmer K. Reduction of postischemic leukocyte-endothelium interaction by adenosine via A2 receptor. Naunyn Schmiedebergs Arch Pharmacol. 1992;346:234–237. doi: 10.1007/BF00165307. [DOI] [PubMed] [Google Scholar]
- Mullane K, Bullough D. Harnessing an endogenous cardioprotective mechanism: cellular sources and sites of action of adenosine. J Mol Cell Cardiol. 1995;27:1041–1054. doi: 10.1016/0022-2828(95)90073-X. [DOI] [PubMed] [Google Scholar]
- Murphree LJ, Sullivan GW, Marshall MA, Linden J. Lipopolysaccharide rapidly modifies adenosine receptor transcripts in murine and human macrophages: role of NF-kappaB in A(2A) adenosine receptor induction. Biochem J. 2005;391:575–580. doi: 10.1042/BJ20050888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein BN. Adenosine, an endogenous anti-inflammatory agent. J Appl Physiol. 1994;76:5–13. doi: 10.1152/jappl.1994.76.1.5. [DOI] [PubMed] [Google Scholar]
- Day YJ, Huang L, Ye H, Li L, Linden J, Okusa MD. Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: the role of CD4+ T cells and IFN-gamma. J Immunol. 2006;176:3108–3114. doi: 10.4049/jimmunol.176.5.3108. [DOI] [PubMed] [Google Scholar]
- Day YJ, Marshall MA, Huang L, McDuffie MJ, Okusa MD, Linden J. Protection from ischemic liver injury by activation of A2A adenosine receptors during reperfusion: inhibition of chemokine induction. Am J Physiol Gastrointest Liver Physiol. 2004;286:G285–293. doi: 10.1152/ajpgi.00348.2003. [DOI] [PubMed] [Google Scholar]
- Okusa MD, Linden J, Macdonald T, Huang L. Selective A2A adenosine receptor activation reduces ischemia-reperfusion injury in rat kidney. Am J Physiol. 1999;277:F404–412. doi: 10.1152/ajprenal.1999.277.3.F404. [DOI] [PubMed] [Google Scholar]
- Peart J, Flood A, Linden J, Matherne GP, Headrick JP. Adenosine-mediated cardioprotection in ischemic-reperfused mouse heart. J Cardiovasc Pharmacol. 2002;39:117–129. doi: 10.1097/00005344-200201000-00013. [DOI] [PubMed] [Google Scholar]
- Reece TB, Okonkwo DO, Ellman PI, Warren PS, Smith RL, Hawkins AS, Linden J, Kron IL, Tribble CG, Kern JA. The evolution of ischemic spinal cord injury in function, cytoarchitecture, and inflammation and the effects of adenosine A2A receptor activation. J Thorac Cardiovasc Surg. 2004;128:925–932. doi: 10.1016/j.jtcvs.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Bullough DA, Magill MJ, Firestein GS, Mullane KM. Adenosine activates A2 receptors to inhibit neutrophil adhesion and injury to isolated cardiac myocytes. J Immunol. 1995;155:2579–2586. [PubMed] [Google Scholar]
- Gazoni LM, Laubach VE, Mulloy DP, Bellizzi A, Unger EB, Linden J, Ellman PI, Lisle TC, Kron IL. Additive protection against lung ischemia-reperfusion injury by adenosine A2A receptor activation before procurement and during reperfusion. J Thorac Cardiovasc Surg. 2008;135:156–165. doi: 10.1016/j.jtcvs.2007.08.041. [DOI] [PubMed] [Google Scholar]
- Ross SD, Tribble CG, Linden J, Gangemi JJ, Lanpher BC, Wang AY, Kron IL. Selective adenosine-A2A activation reduces lung reperfusion injury following transplantation. J Heart Lung Transplant. 1999;18:994–1002. doi: 10.1016/S1053-2498(99)00066-2. [DOI] [PubMed] [Google Scholar]
- Sullivan GW, Linden J, Buster BL, Scheld WM. Neutrophil A2A adenosine receptor inhibits inflammation in a rat model of meningitis: synergy with the type IV phosphodiesterase inhibitor, rolipram. J Infect Dis. 1999;180:1550–1560. doi: 10.1086/315084. [DOI] [PubMed] [Google Scholar]
- Rieger JM, Brown ML, Sullivan GW, Linden J, Macdonald TL. Design, synthesis, and evaluation of novel A2A adenosine receptor agonists. J Med Chem. 2001;44:531–539. doi: 10.1021/jm0003642. [DOI] [PubMed] [Google Scholar]
- Linden J. Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annu Rev Pharmacol Toxicol. 2001;41:775–787. doi: 10.1146/annurev.pharmtox.41.1.775. [DOI] [PubMed] [Google Scholar]
- Link AA, Kino T, Worth JA, McGuire JL, Crane ML, Chrousos GP, Wilder RL, Elenkov IJ. Ligand-activation of the adenosine A2a receptors inhibits IL-12 production by human monocytes. J Immunol. 2000;164:436–442. doi: 10.4049/jimmunol.164.1.436. [DOI] [PubMed] [Google Scholar]
- Lappas CM, Rieger JM, Linden J. A2A adenosine receptor induction inhibits IFN-gamma production in murine CD4+ T cells. J Immunol. 2005;174:1073–1080. doi: 10.4049/jimmunol.174.2.1073. [DOI] [PubMed] [Google Scholar]
- Duggan M, Kavanagh BP. Pulmonary atelectasis: a pathogenic perioperative entity. Anesthesiology. 2005;102:838–854. doi: 10.1097/00000542-200504000-00021. [DOI] [PubMed] [Google Scholar]
- Saito S, Ogawa J, Minamiya Y. Pulmonary reexpansion causes xanthine oxidase-induced apoptosis in rat lung. Am J Physiol Lung Cell Mol Physiol. 2005;289:L400–406. doi: 10.1152/ajplung.00136.2005. [DOI] [PubMed] [Google Scholar]
- Cassada DC, Tribble CG, Long SM, Laubach VE, Kaza AK, Linden J, Nguyen BN, Rieger JM, Fiser SM, Kron IL, et al. Adenosine A2A analogue ATL-146e reduces systemic tumor necrosing factor-alpha and spinal cord capillary platelet-endothelial cell adhesion molecule-1 expression after spinal cord ischemia. J Vasc Surg. 2002;35:994–998. doi: 10.1067/mva.2002.123091. [DOI] [PubMed] [Google Scholar]
- Reece TB, Laubach VE, Tribble CG, Maxey TS, Ellman PI, Warren PS, Schulman AM, Linden J, Kern JA, Kron IL. Adenosine A2A receptor agonist improves cardiac dysfunction from pulmonary ischemia-reperfusion injury. Ann Thorac Surg. 2005;79:1189–1195. doi: 10.1016/j.athoracsur.2004.09.038. [DOI] [PubMed] [Google Scholar]
- Rivo J, Zeira E, Galun E, Einav S, Linden J, Matot I. Attenuation of reperfusion lung injury and apoptosis by A2A adenosine receptor activation is associated with modulation of Bcl-2 and Bax expression and activation of extracellular signal-regulated kinases. Shock. 2007;27:266–273. doi: 10.1097/01.shk.0000235137.13152.44. [DOI] [PubMed] [Google Scholar]
- Sullivan GW. Adenosine A2A receptor agonists as anti-inflammatory agents. Curr Opin Investig Drugs. 2003;4:1313–1319. [PubMed] [Google Scholar]
- Day YJ, Li Y, Rieger JM, Ramos SI, Okusa MD, Linden J. A2A adenosine receptors on bone marrow-derived cells protect liver from ischemia-reperfusion injury. J Immunol. 2005;174:5040–5046. doi: 10.4049/jimmunol.174.8.5040. [DOI] [PubMed] [Google Scholar]
- Chunn JL, Young HW, Banerjee SK, Colasurdo GN, Blackburn MR. Adenosine-dependent airway inflammation and hyperresponsiveness in partially adenosine deaminase-deficient mice. J Immunol. 2001;167:4676–4685. doi: 10.4049/jimmunol.167.8.4676. [DOI] [PubMed] [Google Scholar]
- Matherne GP, Linden J, Byford AM, Gauthier NS, Headrick JP. Transgenic A1 adenosine receptor overexpression increases myocardial resistance to ischemia. Proc Natl Acad Sci USA. 1997;94:6541–6546. doi: 10.1073/pnas.94.12.6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HT, Xu H, Nasr SH, Schnermann J, Emala CW. A1 adenosine receptor knockout mice exhibit increased renal injury following ischemia and reperfusion. Am J Physiol Renal Physiol. 2004;286:F298–306. doi: 10.1152/ajprenal.00185.2003. [DOI] [PubMed] [Google Scholar]
- Neely CF, Keith IM. A1 adenosine receptor antagonists block ischemia-reperfusion injury of the lung. Am J Physiol. 1995;268:L1036–1046. doi: 10.1152/ajplung.1995.268.6.L1036. [DOI] [PubMed] [Google Scholar]
- Yang D, Zhang Y, Nguyen HG, Koupenova M, Chauhan AK, Makitalo M, Jones MR, St Hilaire C, Seldin DC, Toselli P, et al. The A2B adenosine receptor protects against inflammation and excessive vascular adhesion. J Clin Invest. 2006;116:1913–1923. doi: 10.1172/JCI27933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivo J, Zeira E, Galun E, Matot I. Activation of A3 adenosine receptor provides lung protection against ischemia-reperfusion injury associated with reduction in apoptosis. Am J Transplant. 2004;4:1941–1948. doi: 10.1111/j.1600-6143.2004.00620.x. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Abu-Dahab R, Menger MD, Schafer U, Vollmar B, Wada H, Lehr CM, Schafers HJ. Depletion of alveolar macrophages by clodronate-liposomes aggravates ischemia-reperfusion injury of the lung. J Heart Lung Transplant. 2005;24:38–45. doi: 10.1016/j.healun.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Naidu BV, Krishnadasan B, Farivar AS, Woolley SM, Thomas R, Van Rooijen N, Verrier ED, Mulligan MS. Early activation of the alveolar macrophage is critical to the development of lung ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2003;126:200–207. doi: 10.1016/S0022-5223(03)00390-8. [DOI] [PubMed] [Google Scholar]
- Day YJ, Huang L, Ye H, Linden J, Okusa MD. Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: role of macrophages. Am J Physiol Renal Physiol. 2005;288:F722–731. doi: 10.1152/ajprenal.00378.2004. [DOI] [PubMed] [Google Scholar]
- Naidu BV, Woolley SM, Farivar AS, Thomas R, Fraga CH, Goss CH, Mulligan MS. Early tumor necrosis factor-alpha release from the pulmonary macrophage in lung ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2004;127:1502–1508. doi: 10.1016/j.jtcvs.2003.08.019. [DOI] [PubMed] [Google Scholar]
- Krishnadasan B, Naidu BV, Byrne K, Fraga C, Verrier ED, Mulligan MS. The role of proinflammatory cytokines in lung ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2003;125:261–272. doi: 10.1067/mtc.2003.16. [DOI] [PubMed] [Google Scholar]