Abstract
Background
IL-33, a recently discovered IL-1 family cytokine, is implicated in the development of Th2-type responses in vivo. However, the cellular target(s) for IL-33 are poorly understood.
Objective
We tested the hypotheses that dendritic cells (DCs) respond to IL-33 and that IL-33-activated DCs prime naïve CD4+ T cells to produce Th2-type cytokines.
Methods
DCs were derived from mouse bone marrow, and their expression of the IL-33 receptor, ST2, was examined by FACS and real-time RT-PCR. The DCs’ responses to IL-33 were examined by FACS (MHC II and CD86 expression) and by ELISA (IL-6 and IL-12 production). The ability of IL-33-activated DCs to prime naïve T cells was assessed by coculture with isolated CD4+ T cells and by measuring cytokines in the supernatants.
Results
ST2 mRNA was detectable in highly purified DCs. ST2 protein was abundant within DCs, but was barely detectable on their cell surface. Incubation of DCs with IL-33 increased their expression of MHC II and CD86 and production of IL-6, but IL-12 was not produced. Anti-ST2 antibody inhibited IL-6 production from IL-33-activated DCs by approximately 60%; anti-ST2 did not affect IL-6 production from LPS-activated DCs. When incubated with naïve CD4+ T cells alone, IL-33 failed to stimulate cytokine production. In contrast, naïve CD4+ T cells incubated with IL-33-activated DCs showed robust production of IL-5 and IL-13, but IL-4 and IFN-γ were undetectable.
Conclusions
DCs respond directly to IL-33 through ST2. The IL-33 and DC interaction may represent a new pathway to initiate Th2-type immune responses.
Keywords: IL-33, dendritic cells, Th2 cytokines, allergy, inflammation
Key Messages.
Dendritic cells respond directly to IL-33 through the receptor ST2.
IL-33-activated DCs trigger an atypical Th2-type response of IL-5 and IL-13, but not IL-4, from naïve T cells.
The IL-33 and DC interaction may represent a new pathway to initiate Th2-type immune responses.
Introduction
IL-33 is a new member of IL-1-family cytokines 1,2. In general, IL-1 family cytokines are expressed in hematopoietic cells 3. The expression pattern of human IL-33 appears restricted to epithelial cells from bronchus and small airways, fibroblasts, and smooth muscle cells 1, suggesting its potential involvement in mucosal immunity. Intraperitoneal administration of IL-33 to naive mice led to increased expression of Th2 cytokines, marked eosinophilia in various mucosal organs, increased serum levels of IgE and IgA, and pathological changes in the lungs and gastrointestinal tracts, such as mucus production and epithelial hyperplasia 1. Administration of IL-33 during a primary infection with the nematode, Trichuis muris, skewed the host’s immune response toward a Th2-type and enhanced expulsion of the parasites 4; however, in chronically infected animals, IL-33 failed to redirect the established Th1 immune response. Using a monoclonal (mAb) against the receptor for IL-33, ST2, or lack of the ST2 gene also attenuated production of Th2 cytokines and enhanced the Th1 response in several mouse models, including Th2 cell adoptive transfer 5, parasite infection 6,7, and OVA-induced allergic airway inflammation 8,9. Thus, IL-33 and ST2 likely play critical roles in inducing the Th2 response and suppressing the Th1 response, particularly during the early stages.
In spite of such profound and compelling biological activities for IL-33 in vivo, little is known regarding the mechanisms for IL-33 to induce Th2 responses. The IL-33 receptor, ST2, is expressed on mast cells, eosinophils, and differentiated Th2 cells; it is not expressed on naïve T cells or Th1 cells 5,8,10-12. Accumulating evidence suggests that IL-33 activates mast cells, basophils, and eosinophils11-16. However, previous studies on the effects of IL-33 on T cells were somewhat conflicting. For example, the absence of ST2 (i.e., ST2-deficient mice) did not affect Th2 cell development 6,17. IL-33 did not induce Th2 cytokine production from non-polarized naïve CD4+ T cells 18. In contrast, IL-33 or crosslinking of ST2 by mAb did enhance cytokine production by polarized Th2 cells; these cells were derived after several rounds of culture in Th2-driving conditions 18-20. Recently, IL-33 through the MAPK and NF-κB pathway enhanced Th2-like differentiation of naive CD4+ T cells stimulated with anti-CD3 Ab or antigen 21. Thus, major questions still remain. Does IL-33 directly induce Th2 differentiation of naïve CD4+ T cells? Does IL-33 expand committed Th2 cells? What are the roles for antigen presenting cells (APCs)?
Dendritic cells (DCs) integrate signals from tissue microenvironments and instruct naïve T cells 22. DCs also play important roles in determining the outcome of T cell differentiation, such as Th1, Th2, Th17 and regulatory T cells 23,24. Therefore, we hypothesized that IL-33 may influence DCs and that these IL-33-activated DCs may lead to Th2-type cytokine production from naïve T cells. Previously, DCs showed no detectable surface expression of ST2 5, and no studies have been reported that examined the effects of IL-33 on the functions of DCs. Herein, we found that IL-33 potently activates the expression of cell surface molecules on DCs and IL-6 production by DCs. More importantly, IL-33-activated DCs induced robust production of IL-5 and IL-13 from naïve CD4+ T cells; IL-4 and IFN-γ were undetectable. Thus, interaction between IL-33 and DC may represent a new pathway to initiate Th2-type immune responses.
Methods
Mice
BALB/cJ, C57BL/6J (B6), and C57BL/6-Tg(TcraTcrb)425Cbn/J (OT-II) mice were from Jackson Laboratory (Bar Harbor, ME) and housed in the Mayo Clinic animal house facility as per institutional guidelines. These studies were approved by the Mayo Clinic Institutional Animal Care and Use Committee.
Generation of mouse bone marrow (BM)-derived DCs
DCs were generated from mouse BM using an established protocol 25 with minor modifications 26. Briefly, mouse BM was obtained from the long bones of the hind legs. After erythrocyte lysis, BM cells were suspended at 1×106/ml in RPMI 1640 with 10% FBS, 10 ng/ml GM-CSF and 1 ng/ml IL-4. Cells were incubated at 37 °C for 2 days; the medium was changed and cells were incubated 4 days longer. The purity of CD11c+ DCs after a 6-day culture was >70%. In some experiments (e.g. real-time RT-PCR and cytokine production, vida infra), DCs were further purified by a magnetic cell separation system (MACS®; Miltenyi Biotech, Auburn, CA) and anti-CD11c immunomagnetic beads (Miltenyi Biotec); the purity of these purified DCs were >93%. Experiments used 6-day culture DCs unless stated otherwise.
Real-time RT-PCR
The expression of ST2 mRNA in purified DCs was detected by RT-PCR. Total RNA was isolated from MACS®-purified DCs with TRIzol and Invitrogen Pure Link Micro-to-Midi columns (Invitrogen, Carlsbad, CA). As a control, total RNA was also isolated from the cells in the flow-through population during the MACS® purification process; on average, 23% of these flow-through cells were CD11c+. RNA samples were treated with DNase I (Invitrogen), and cDNA was reverse-transcribed by using Bio-Rad iScript (Bio-Rad Laboratories, Hercules, CA). Mouse ST2 (IL1RL1) mRNA transcripts were quantified by real-time PCR with Taqman Gene Expression Array primer-probe sets (Applied Biosystems, Foster City, CA) and iCycler with iq5 Real-Time PCR Detection System (Bio-Rad Laboratories). Transcription was normalized to the 18S rRNA transcription in each sample. Amplified PCR products were observed by gel electrophoresis using 2% agarose.
Detection of ST2 by FACS
BM-derived DCs were pre-incubated with Fc-receptor blockers (anti-CD16/32, BD Pharmingen, San Diego, CA) for 30 minutes at 4 °C. To detect cell surface ST2, DCs were stained with PE-conjugated anti-CD11c (clone HL3, BD Pharmingen) and FITC-conjugated rat anti-mouse T1/ST2 (clone DJ8, MD Biosciences, St. Paul, MN) or FITC-conjugated rat IgG1 isotype control (MD Biosciences). To detect intracellular ST2, the cells, which had been stained with PE-conjugated anti-CD11c, were fixed and permeabilized by Cytofix/Cytoperm reagents (BD Pharmingen) and then stained with FITC-conjugated anti-mouse T1/ST2 or rat IgG1 isotype control. The cells were washed and suspended in PBS containing 1% BSA and 0.1% NaN3 and fixed in 1% paraformaldehyde. The cells were analyzed by a FACScan flow cytometer (BD Immunocytometry Systems, Mountain View, CA) by gating on a CD11c-positive forward scatter-high cell population.
Stimulation of DCs and detection of cell surface molecule expression and cytokine production
To examine the effects of IL-33 on DCs, BM-derived DCs were suspended at 1×106/ml in RPMI 1640 with 10% FBS, and stimulated with medium alone, recombinant murine IL-33 (final concentration 0.1-100 ng/ml, R&D Systems, Minneapolis, MN), or E. coli (O127:B8) LPS (1 μg/ml, Sigma-Aldrich, St. Louis, MO) for 24 h at 37 °C. In some experiments, ST2 blocking Ab (goat anti-mouse IL-1 R4, 3-30 μg/ml) or goat IgG control (both R&D Systems) was added to the culture.
The functional responses of DCs to IL-33 and LPS were assessed by expression of cell surface molecules and cytokine production by FACS and ELISA, respectively. For FACS analysis, DCs were preincubated with Fc-receptor blockers (anti-CD16/CD32) for 30 min at 4 °C and stained with PE-conjugated anti-CD11c (clone HL3) and FITC-conjugated anti-MHC Class II I-Ad (AMS-32.1), or anti-CD86 (GL1) for 30 min at 4 °C; FITC-conjugated mouse IgG2b and rat IgG2a were used as isotype controls (all from BD Pharmingen). After washing, DCs were resuspended, fixed, and analyzed by a FACScan flow cytometer as described above. For cytokine analysis, cell-free supernatants were collected after 24 h incubation. Concentrations of IL-6 and IL-12p70 were analyzed by ELISA kits (R&D Systems); sensitivities for IL-6 and IL-12p70 were 16 and 62 pg/ml, respectively.
DC/CD4+ T cell coculture
The effects of IL-33-activated DCs on T cell cytokine production were examined by culturing BM-derived DCs with CD4+ T cells isolated from naïve B6 mice, naïve TCR-transgenic OT-II mice (B6 background), or naïve BALB/c mice. OT-II mice express a transgenic TCR that recognizes an OVA peptide presented by MHC class II. CD4+ T cells were isolated from mouse spleens by using a mouse CD4+ T cell enrichment kit (EasySep®, Stem Cell Technologies, Vancouver, BC, CA). BM-derived DCs from the same strain of animal were seeded at 0.1×106 cells/ml in round-bottom 96-well tissue culture plates and cultured with isolated CD4+ T cells at a 1:10 DC: T cell ratio in the presence of medium alone, IL-33 (0.1, 1 or 10 ng/ml) or IL-1β (1 ng/ml) (both from R&D Systems) for 6 or 10 days. Wells with DCs alone or CD4+ T cells alone served as controls. In some experiments, DCs were cultured with CD4+ T cells isolated from OT-II mice in the presence of medium alone, IL-33 or IL-1β with or without endotoxin-free OVA (100 μg/ml). Endotoxin-free OVA was prepared from SPF chicken eggs (Charles River Laboratories, Wilmington, MA) under sterile conditions as previously described27. Culture supernatants were collected, and concentrations of IL-4, IL-13, and IFN-γ (all day 6) and IL-5 (day 10) were measured by ELISA (R&D Systems). Sensitivities for IL-4, IL-5, IL-13 and IFN-γ were 8, 16, 8, and 9 pg/ml, respectively. After 10 days in culture, the supernatants were also analyzed by a Bio-Plex mouse 23-cytokine assay kit and the Bio-Plex suspension array system (Bio-Rad Laboratories).
Statistical analysis
Results show the mean±SEM based on the number of experiments. The paired t-test was used to compare differences between groups with p<0.05 considered statistically significant. A statistical software package (Instat 3.0, GraphPad Sofware, La Jolla, CA) was used.
Results
Expression of the IL-33 receptor, ST2, in DCs
Previously, ST2 was not detected on the surface of DCs 5. Therefore, we first examined whether DCs express ST2 mRNA. By using the MACS® purification system, we obtained highly purified CD11c+ DCs (>93% CD11c+ cells) from BM-derived DCs to examine ST2 mRNA expression by real-time RT-PCR (n=3). As a control, we also examined the flow-through population from the CD11c+ DC MACS® purification step; this population contained on average 23% CD11c+ DCs (n=3). By using real-time RT-PCR and gel electrophoresis, we detected ST2 mRNA in the purified CD11c+ DCs (Figure 1A, 1B). By increasing the number of PCR cycles, ST2 mRNA was also detectable in the flow-through cell population. Using arbitrary units normalized to 18S, ST2 mRNA levels in the purified DC populations were about 6-fold higher than the levels in the flow-through populations (4.6±0.9 vs 0.7±0.2 units, Mean±SEM, n=3, p<0.05), suggesting that the amounts of ST2 mRNA roughly correlate with the purity of the CD11c+ DC. Second, by gating electronically on the CD11c+ forward scatter-high DC population, we used FACS to study both surface and intracellular ST2 protein expression. Consistent with a previous report 5, we found that DCs express no or minimal ST2 protein on their surface (Figure 1C). In contrast, intracellular staining showed that ST2 protein was expressed abundantly within DCs. Thus, ST2 is present in CD11c+ DCs at both the transcriptional and protein levels.
Figure 1.
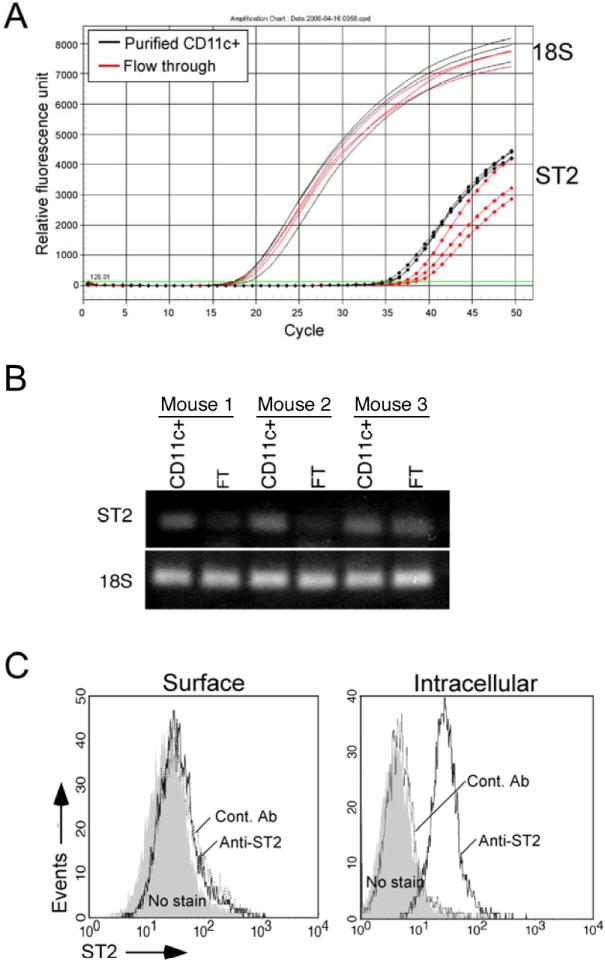
DCs express ST2 mRNA and ST2 protein. A, Total RNA was purified from MACS® purified DCs (>93% CD11c+ cells) (n=3) and from flow-through (23% CD11c+ cells) populations (n=3). ST2 mRNA was quantified by real-time RT-PCR. B, Gel electrophoresis comparison of amplified PCR products: CD11c+ denotes purified DCs, FT denotes flow-through DCs. C, FACS analyses of purified DCs show surface and intracellular expression of ST2 protein; control Ab is rat IgG1 isotype control (dashed line), no stain (shaded area), anti-ST2 (solid line).
IL-6, but not IL-12, is produced by IL-33-activated DCs
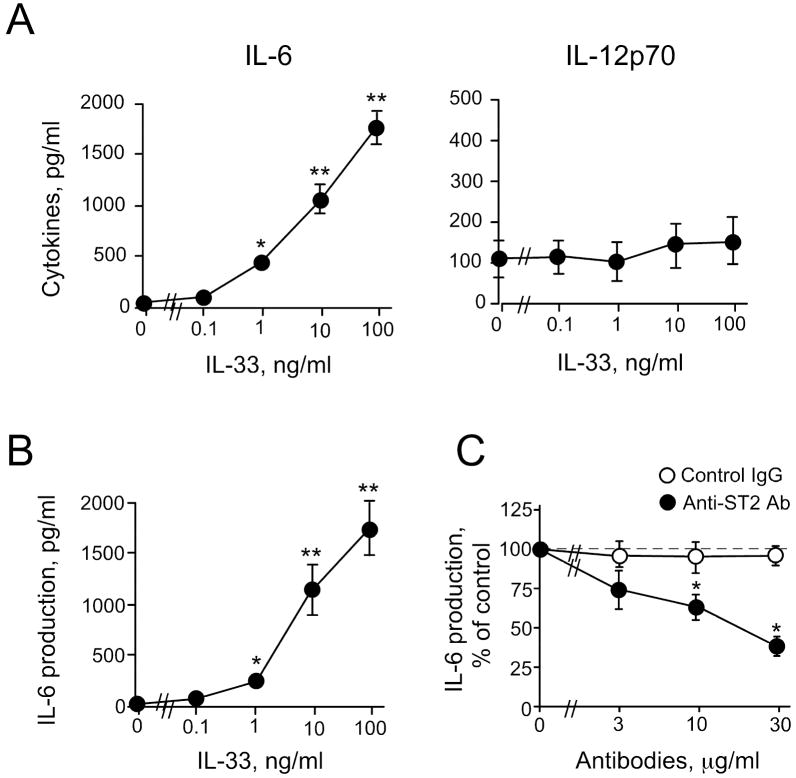
Previously, DCs were reported to produce IL-6 and IL-12, and these cytokines play important roles in regulating CD4+ T cell differentiation 11,23,28. Because ST2 protein was barely detectable on the DCs’ surface by FACS, we investigated whether IL-33 could induce any functional responses from DCs. After DCs were incubated with IL-33 or E. coli LPS (as a control) for 24 hours, the concentrations of released cytokines were measured in the cell-free supernatants. IL-33 induced robust IL-6 production from DCs in a concentration-dependent manner; significant effects were observed with IL-33 as low as 1 ng/ml (p<0.05) (Figure 2A). In contrast, ≤ 100 ng/ml IL-33 did not induce IL-12 from DCs; a positive control, 1 μg/ml LPS, induced abundant IL-12 from DCs, 13.4 × 103±3.4 × 103 pg/ml (mean±SEM, n=3). To verify that DCs are the major source of IL-6 in the IL-33-stimulated BM-derived DC populations, we used MACS® to purify further (>93%) the CD11c+ DCs. After stimulation with IL-33, these purified DCs showed a similar concentration-response curve (Figure 2B) to that from the non-purified DC populations (Figure 2A). Furthermore, IL-6 levels were roughly comparable between the purified DCs and non-purified DCs (i.e., ~1.6 × 103 pg/ml with 100 ng/ml IL-33). We also analyzed the IL-33-activated DC production of 23 mouse cytokines, including IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12 (p40), IL-12 (p70), IL-13, IL-17, eotaxin, G-CSF. GM-CSF, IFN-γ, KC, MCP-1, MIP-1α, MIP-1β, RANTES and TNF-α, by a Bio-Plex assay; IL-6 was the only cytokine produced in response to IL-33 (data not shown).
Figure 2.
IL-33-activated DCs produce IL-6, but not IL-12. DCs (>70% CD11c+) (A) or MACS® purified DCs (>93% CD11c+ cells) (B) were cultured with IL-33, and IL-6 and IL-12 levels were measured in the supernatants. C, DCs were cultured with IL-33 (1.0 ng/ml) and anti-ST2 blocking Ab or control (goat) IgG. *p<0.05, **p<0.01, compared to medium alone, n=5.
We next used a blocking Ab to examine whether ST2 mediates these DCs’ cytokine responses to IL-33. Anti-ST2 Ab inhibited IL-33-induced IL-6 production in a concentration-dependent manner; anti-ST2 Ab (30 μg/ml) reduced IL-6 production by ~60% (p<0.05, n=5) (Figure 2C). LPS-induced IL-6 production was unaffected by anti-ST2 (data not shown), and control goat IgG showed no effects on IL-33-induced IL-6 production from DCs. Altogether, IL-33 stimulates IL-6 production in vitro from DCs through ST2 without inducing IL-12 production.
IL-33-stimulated DCs show increased expression of cell surface molecules
The MHC class II and co-stimulatory molecules expressed by APCs play important roles in the proliferation and differentiation of CD4+ T cells 23. Therefore, we examined the expression of MHC class II and CD86 by DCs after a 24 h incubation with IL-33. The FACS histograms show that IL-33-activated DCs express MHC class II and CD86 (Figure 3A). Expression of both MHC class II and CD86 increased in a concentration-dependent manner and reached a plateau with 1 ng/ml IL-33 (Figure 3B). The CD86 expression levels induced by 100 ng/ml IL-33 were considerably lower than levels induced by 1 μg/ml LPS (i.e., 65.5±5.0 vs. 183.7±21.5 MFI, respectively). IL-33 did not increase DC expression of CD40 or CD80 (data not shown). Thus, although IL-33 is likely less potent than LPS, IL-33 increases the DCs’ expression of MHC class II and certain co-stimulatory molecule(s).
Figure 3.
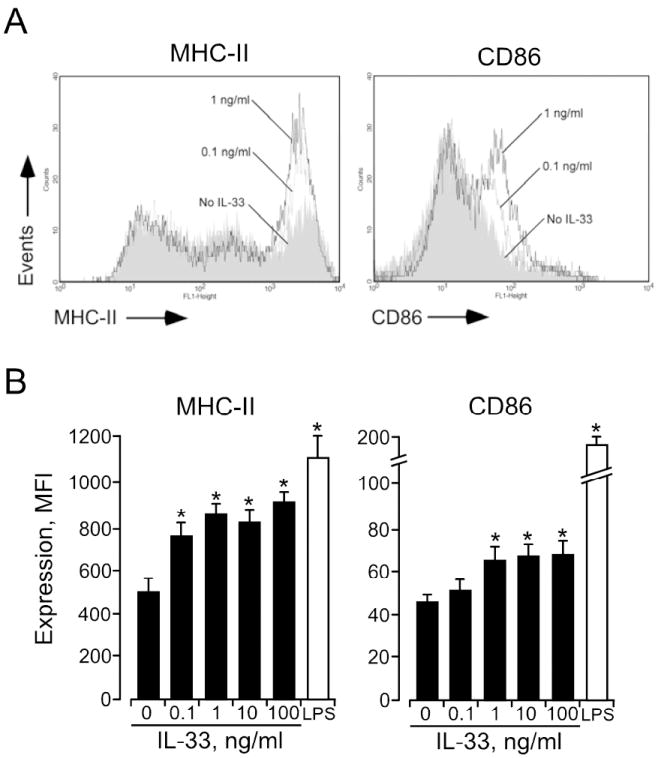
IL-33-activated DCs show increased expression of MHC II and CD86. A, After 24 h culture of DCs with medium (shaded area) or IL-33 (0.1 ng/ml (broken line) and 1.0 ng/ml (solid line)), MHC II and CD86 expression levels were examined by flow cytometry. B, After 24 h culture of DCs with IL-33 or LPS (1 μg/ml), MHC II and CD86 expression levels were examined. *p<0.05, compared to medium alone, n=5.
Coculture of DCs/CD4+ T cells with IL-33 induces IL-5 and IL-13 production
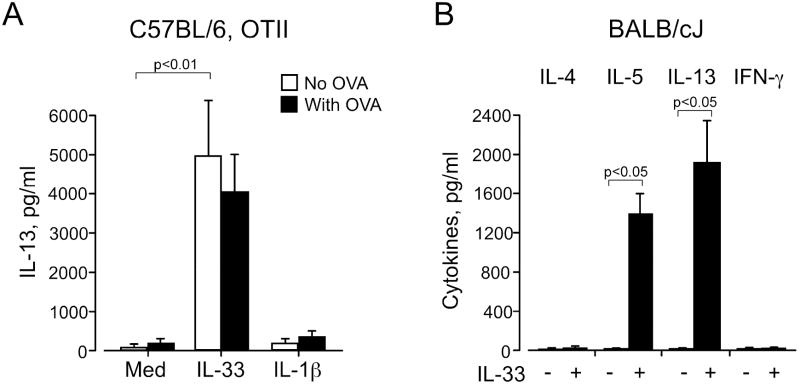
Previous studies showed that IL-33 enhances Th2-type cytokine production from Th2-polarized CD4+ T cells 18,19; however, IL-33 by itself or even with IL-2 failed to induce non-polarized naïve CD4+ T cells to produce Th2-type cytokines 18. Because IL-33 did enhance IL-6 production and increased expression of co-stimulatory molecule(s) by DCs, we hypothesized that IL-33-activated DCs might induce Th2-type cytokine production from naïve CD4+ T cells. We isolated CD4+ T cells from spleens of naïve B6 mice or naïve OT-II mice (B6 background) and incubated them with DCs in the presence of IL-33 without antigen. Because the responses of the CD4+ T cells from B6 mice and OT-II mice were identical, we pooled these data. When naïve CD4+ T cells alone were incubated with IL-33, no IL-4, IL-5, IL-13 or IFN-γ was detected (Figure 4), consistent with previous observations 18. Furthermore, when naïve CD4+ T cells were incubated with DCs in the absence of IL-33, none of these cytokines were produced. In contrast, when IL-33 (0.1 or 1 ng/ml) was added to the DC/CD4+ T cell coculture, robust production of IL-5 and IL-13 was observed (p<0.01, n=6). In contrast, no IL-4 or IFN-γ was detected in these coculture supernatants.
Figure 4.
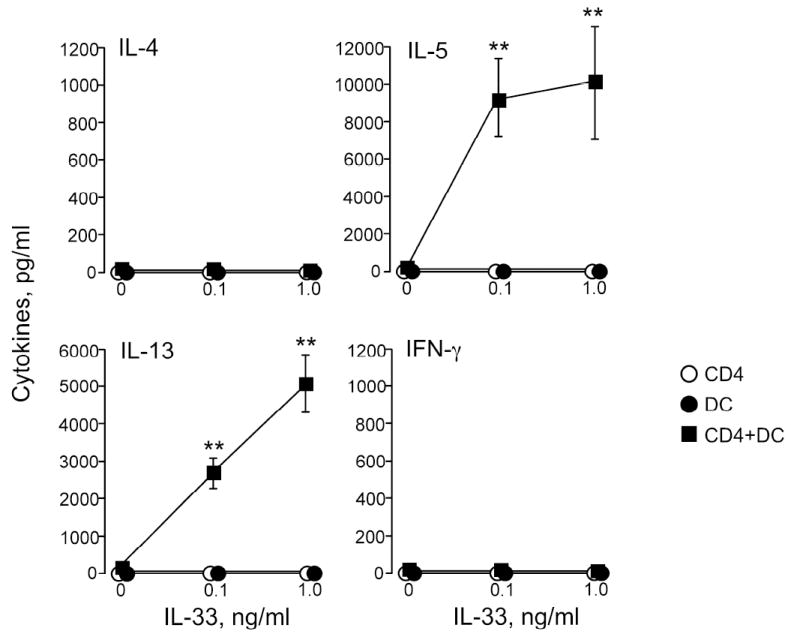
Naïve CD4+ T cells cultured with IL-33-activated DCs produce IL-5 and IL-13. CD4+ T cells isolated from naïve B6 or naïve OT-II mice were cultured with or without DCs and IL-33 for 6 or 10 days; no antigens were added. Supernatants were analyzed for cytokines on day 6 (IL-4, IL-13, and IFN-γ) and on day 10 (IL-5). *p<0.05, compared to medium alone, n=6.
These DC/CD4+ T cell coculture experiments did not use specific antigen. Thus, we next examined whether IL-33 can enhance antigen-specific responses by naïve CD4+ T cells, and we compared the enhancement effects of IL-33 to those of an authentic IL-1 family cytokine, IL-1β. CD4+ T cells from naïve OT-II mice, which recognize an OVA peptide presented by MHC class II, were cocultured with DCs in the presence or absence of 100 μg/ml OVA antigen, with or without 1 ng/ml IL-33 or IL-1β. Without any cytokines, OVA weakly induced IL-13 production from naïve OT-II CD4+ cells (Figure 5A). When cocultured with IL-33-activated DCs even in the absence of OVA, these OT-II CD4+ cells produced marked levels of IL-13; incubation in the presence of OVA also produced marked levels of IL-13. In addition, incubation with IL-1β did not significantly increase (compared to medium alone) IL-13 production by naive OT-II CD4+ cells with or without OVA. We also examined whether these observations are reproducible in mice with a different background. DCs and naive CD4+ T cells from BALB/cJ mice were incubated with or without IL-33 in the absence of antigens; the supernatants with IL-33-activated DCs contained abundant quantities of IL-5 and IL-13, but negligible quantities of IL-4 or IFN-γ (Figure 5B). Altogether, IL-33-activated DCs can induce production of IL-5 and IL-13, but not IL-4 or IFN-γ, from naïve CD4+ T cells in vitro. Antigen presentation is probably unnecessary for naïve T cells to produce cytokines in the presence of IL-33-activated DCs.
Figure 5.
Cytokine production by naïve CD4+ T cells cultured with IL-33-activated DCs is independent of antigen. A, CD4+ T cells from naïve OT-II mice were cultured with DCs with or without OVA antigen and with or without 1 ng/ml IL-33 or IL-1β for 6 days. n=3-6 B, CD4+ T cells and DCs from naïve BALB/cJ mice were cultured in the absence of antigen with or without 10 ng/ml IL-33 for 6 days. n=4.
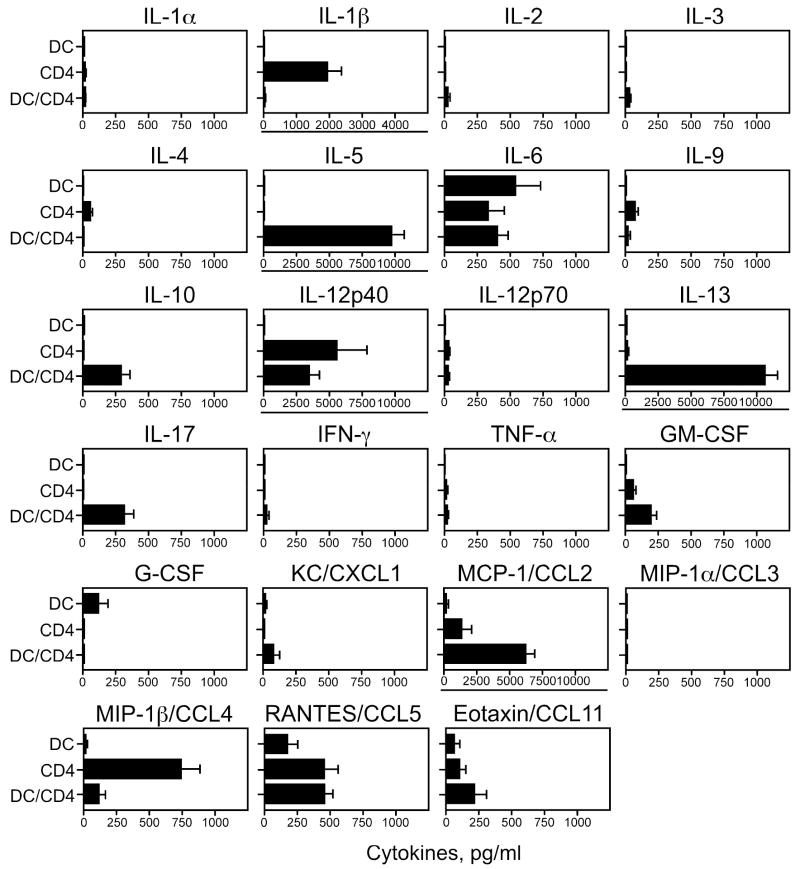
Finally, we used the Bio-Plex cytokine assay to screen among 23 possible cytokines to identify cytokines produced when CD4+ T cells are cocultured with DCs and IL-33. DCs and CD4+ T cells from naïve B6 mice were cultured for 10 days with or without 10 ng/ml IL-33 in the absence of antigen; cocultured cells incubated with IL-33 produced robust quantities of IL-5 and IL-13, but IL-4 and IFN-γ were minimal (Figure 6). Cocultured cells produced a large quantity of MCP-1/CCL2 and smaller quantities of IL-10 and IL-17. Interestingly, CD4+ T cells incubated with IL-33 produced IL-1β and MIP-1β/CCL4, but the production was inhibited when DCs were added to CD4+ T cells. Cocultured cells without IL-33 produced no or little (<50 pg/ml) cytokine, except for the following: IL-12p40 (150 pg/ml), MCP-1/CCL2 (662 pg/ml), and RANTES/CCL5 (545 pg/ml) (mean, n=4).
Figure 6.
IL-5 and IL-13 are produced by naïve CD4+ T cells cultured with IL-33-activated DCs. DCs or CD4+ T cells from naïve B6 mice or their combination were cultured with 10 ng/ml IL-33 for 10 days. Cytokines levels in the supernatants were measured by a Bio-Plex mouse 23 cytokine assay kit. Note that expanded scales (underlined) are used for IL-1β, IL-5, IL-12p40, IL-13, and MCP-1/CCL2. n=4.
Discussion
The in vivo biological activities of IL-33 and its receptor, ST2, are profound. In mucosal organs, administration of IL-33 increases expression of Th2 cytokines and induces marked eosinophilia and pathological changes1. Blockade of or deficiency in the ST2 gene attenuates Th2 cytokine production and enhances Th1 cytokine production in several mouse models 5-9. However, little is known regarding the mechanisms for how IL-33 induces Th2 responses and suppresses Th1 responses. Our study suggests that DCs constitutively express ST2 and are activated by IL-33 through ST2 (Figures 1, 2, and 3). In addition, in the presence of IL-33-activated DCs, naïve CD4+ T cells robustly produce Th2-type cytokines, IL-5 and IL-13 (Figures 4 and 6). A Th2-related chemokine, MCP-1/CCL2 29, was also produced (Figure 6). In contrast, naïve CD4+ T cells did not respond to IL-33 alone without DCs, suggesting that interactions among DCs, T cells, and IL-33 are required for robust Th2-type cytokine production by naïve CD4+ T cells. IL-33 appears to be expressed by epithelial cells from bronchus and small airways, fibroblasts, and smooth muscle cells 1. Thymic stromal lymphopoietin (TSLP) is also expressed by keratinocytes and airway epithelial cells 30,31, and TSLP-activated DCs prime naïve CD4+ T cells to differentiate into Th2 cells 32. Therefore, similarly to TSLP, IL-33 may be another tissue-derived factor that activates DCs and profoundly affects production of Th2-type cytokines or differentiation of CD4+ T cells.
Previously, when IL-33 was added to the culture, Th2-polarized CD4+ T cells expressed ST2 and increased their production of Th2 cytokines 18. In contrast, naïve CD4+ T cells and Th1 polarized CD4+ T cells did not express ST2 nor did they respond to exogenous IL-33 18. Recently, Kurowska-Stolarska et al reported that IL-33 polarizes naïve CD4+ T cells to produce IL-5 and IL-13, when cells are stimulated with anti-CD3 or APC plus antigen 21. Similarly, we found that naïve CD4+ T cells alone did not produce cytokines when incubated with IL-33 (Figure 4). In contrast, when cocultured with DCs and IL-33, naïve CD4+ T cells produced abundant IL-5 and IL-13, but not IL-4 (Figures 4 and 6). We also found that the TCR and MHC class II/peptide interaction is probably not involved or necessary in the robust production of these cytokines by CD4+ T cells (Figure 5). These observations suggest mechanistic possibilities for how IL-33-activated DCs stimulate naïve CD4+ T cells to produce Th2-type cytokines. Several scenarios, which are not mutually exclusive, can be considered. First, IL-33-activated DCs may produce known or unknown soluble factor(s) and cell surface molecule(s), which are implicated in the production of Th2-type cytokines by naïve CD4+ T cells and/or in the differentiation of naïve CD4+ T cells toward Th2 cells. Second, IL-33-activated DCs may promote naïve CD4+ cells to express ST2, allowing them to respond directly and robustly to IL-33. Indeed, we found that IL-33-activated DCs produce IL-6 without producing IL-12 (Figure 2). Previously, IL-6 produced by DCs has been implicated in T cell differentiation to a Th2-type 11,28. Other studies suggested that Th2 responses likely result from the absence of IL-12 33,34. The expression of OX40L by TSLP-activated DCs was implicated in their ability to drive Th2 cell differentiation 32; however, we did not detect increased expression of OX40L by IL-33-activated DCs (results not shown). Therefore, future studies, perhaps including gene microarray analysis, will be needed to elucidate the known or novel soluble factor(s) and cell surface molecule(s) produced by IL-33-activated DCs, which are involved in naïve CD4+ T cells producing Th2-type cytokines. These studies may clarify the similarities and differences between the effects of IL-33 and TSLP on DCs.
The unique pattern of Th2 cytokines produced by naïve CD4+ T cells cocultured with IL-33-activated DCs, namely both IL-5 and IL-13 but not IL-4 (Figure 4), needs to be noted. Other investigators have made similar observations. For example, in mice infected with helminths or RSV, blockade of ST2 or lack of the ST2 gene did not affect IL-4 levels 17,35 although the IL-5 levels decreased 35. In polarized Th2 cells or naïve CD4+ T cells, IL-33 also enhances production of IL-5 and IL-13, but not IL-4 1,18,21. Thus, IL-33 appears to affect IL-5 and IL-13 production in preference to IL-4 production. When injected with alum adjuvant, IL-4-deficient mice produce IL-5 36. Recently, IL-4 and STAT-6 were shown to be unnecessary for Th2 lymphocyte differentiation in vivo, suggesting several pathways leading to the production of Th2-type cytokines 37. In mice epicutaneously sensitized to antigen, the Th2-type response was IL-13-dependent, but not IL-4-dependent 38. In humans, subpopulations of Th2 cells produce different cytokine patterns (28% IL-4, IL-5, and IL-13, 40% IL-5 and IL-13) 39. Clinically, the tissue cytokine pattern of increased IL-5 and IL-13 (but not IL-4) has been noted in patients with chronic rhinosinusitis 40-44 and eosinophilic esophagitis 45-48. Therefore, future studies need to examine the different patterns of Th2-type cytokine expression in diseased tissues and in Th2-like lymphocytes and to elucidate the potential roles for conventional Th2-driving factors, such as IL-4, and for novel Th2-driving factors, such as IL-33, in the pathophysiology of allergic diseases.
The critical functions of IL-33 may involve the early stages, but not the late stages, in the development of Th2 responses. IL-33 is produced early in nematode infection 4. IL-33 administration produced a shift to a Th2 pattern (from a Th1 pattern) only when given early in the immune response during parasitic infection 4. We observed in vitro that naïve CD4+ T cells produce IL-5 and IL-13 independently of antigen presentation and recognition (Figure 5). Interestingly, IL-33 induced IL-5 and IL-13 production in anti-CD3-activated naïve CD4+ T cells even without robust expression of a classical Th2 transcription factor, GATA3 21. We found that naive CD4+ T cells cultured with DCs and IL-33 produce IL-10 and IL-17 although much less than IL-5 or IL-13 (Figure 6), suggesting that these T cells may maintain their capacity to differentiate into Th17 or regulatory T cells. Therefore, the IL-33/ST2 pathway may be critically involved in the early differentiation of Th2 cells, connecting the bridge between innate and adaptive immune responses. Importantly, ST2 inhibits TLR4 signaling by sequestrating the adaptor proteins, MyD88 and Mal 49, and IL-33 downregulates the TLR4 response to a Th1 adjuvant, endotoxin 49,50. Furthermore, an immunosuppressant, rapamycin, promotes ST2 expression by DCs and makes them tolerant to TLR agonists 51. We observed that IL-33-activated DCs do not produce IL-12, while LPS-activated DCs produce abundant IL-12 (Figure 2). Thus, the IL-33/ST2 pathway may counteract the LPS/TLR4 pathway and may modulate or maintain a balance of downstream Th2- and Th1-type cytokine production.
In conclusion, IL-33 activates DCs and these IL-33-activated DCs trigger an atypical Th2-type response of IL-5 and IL-13 production. Identifying the triggers that lead to the production and release of IL-33 and elucidating the molecular mechanisms leading to IL-33-induced Th2-type cytokine production will offer important insights into the mechanism of allergic inflammation and provide novel targets to disrupt these pathways.
Acknowledgments
The authors wish to thank Cheryl R. Adolphson for editorial assistance and LuRaye S. Eischens for secretarial help.
Supported by National Institutes of Health R01 AI071106 and the Mayo Foundation
Abbreviations
- DCs
Dendritic cells
- BM
Bone marrow
- OT-II
C57BL/6-Tg(TcraTcrb)425Cbn/J
- mAb
Monoclonal antibody
- TSLP
Thymic stromal lymphopoietin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–90. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 2.Sims JE, Nicklin MJ, Bazan JF, Barton JL, Busfield SJ, Ford JE, et al. A new nomenclature for IL-1-family genes. Trends Immunol. 2001;22:536–7. doi: 10.1016/s1471-4906(01)02040-3. [DOI] [PubMed] [Google Scholar]
- 3.Dinarello CA. Interleukin-1. Cytokine Growth Factor Rev. 1997;8:253–65. doi: 10.1016/s1359-6101(97)00023-3. [DOI] [PubMed] [Google Scholar]
- 4.Humphreys NE, Xu D, Hepworth MR, Liew FY, Grencis RK. IL-33, a potent inducer of adaptive immunity to intestinal nematodes. J Immunol. 2008;180:2443–9. doi: 10.4049/jimmunol.180.4.2443. [DOI] [PubMed] [Google Scholar]
- 5.Lohning M, Stroehmann A, Coyle AJ, Grogan JL, Lin S, Gutierrez-Ramos JC, et al. T1/ST2 is preferentially expressed on murine Th2 cells, independent of interleukin 4, interleukin 5, and interleukin 10, and important for Th2 effector function. Proc Natl Acad Sci U S A. 1998;95:6930–5. doi: 10.1073/pnas.95.12.6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Townsend MJ, Fallon PG, Matthews DJ, Jolin HE, McKenzie AN. T1/ST2-deficient mice demonstrate the importance of T1/ST2 in developing primary T helper cell type 2 responses. J Exp Med. 2000;191:1069–76. doi: 10.1084/jem.191.6.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu D, Chan WL, Leung BP, Huang F, Wheeler R, Piedrafita D, et al. Selective expression of a stable cell surface molecule on type 2 but not type 1 helper T cells. J Exp Med. 1998;187:787–94. doi: 10.1084/jem.187.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coyle AJ, Lloyd C, Tian J, Nguyen T, Erikkson C, Wang L, et al. Crucial role of the interleukin 1 receptor family member T1/ST2 in T helper cell type 2-mediated lung mucosal immune responses. J Exp Med. 1999;190:895–902. doi: 10.1084/jem.190.7.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayakawa H, Hayakawa M, Kume A, Tominaga S. Soluble ST2 blocks interleukin-33 signaling in allergic airway inflammation. J Biol Chem. 2007;282:26369–80. doi: 10.1074/jbc.M704916200. [DOI] [PubMed] [Google Scholar]
- 10.Moritz DR, Rodewald HR, Gheyselinck J, Klemenz R. The IL-1 receptor-related T1 antigen is expressed on immature and mature mast cells and on fetal blood mast cell progenitors. J Immunol. 1998;161:4866–74. [PubMed] [Google Scholar]
- 11.Allakhverdi Z, Smith DE, Comeau MR, Delespesse G. Cutting edge: The ST2 ligand IL-33 potently activates and drives maturation of human mast cells. J Immunol. 2007;179:2051–4. doi: 10.4049/jimmunol.179.4.2051. [DOI] [PubMed] [Google Scholar]
- 12.Cherry WB, Yoon J, Bartemes KR, Iijima K, Kita H. A novel IL-1 family cytokine, IL-33, potently activates human eosinophils. J Allergy Clin Immunol. 2008;121:1484–90. doi: 10.1016/j.jaci.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iikura M, Suto H, Kajiwara N, Oboki K, Ohno T, Okayama Y, et al. IL-33 can promote survival, adhesion and cytokine production in human mast cells. Lab Invest. 2007;87:971–8. doi: 10.1038/labinvest.3700663. [DOI] [PubMed] [Google Scholar]
- 14.Suzukawa M, Iikura M, Koketsu R, Nagase H, Tamura C, Komiya A, et al. An IL-1 cytokine member, IL-33, induces human basophil activation via its ST2 receptor. J Immunol. 2008;181:5981–9. doi: 10.4049/jimmunol.181.9.5981. [DOI] [PubMed] [Google Scholar]
- 15.Suzukawa M, Koketsu R, Iikura M, Nakae S, Matsumoto K, Nagase H, et al. Interleukin-33 enhances adhesion, CD11b expression and survival in human eosinophils. Lab Invest. 2008;88:1245–53. doi: 10.1038/labinvest.2008.82. [DOI] [PubMed] [Google Scholar]
- 16.Pecaric-Petkovic T, Didichenko SA, Kaempfer S, Spiegl N, Dahinden CA. Human basophils and eosinophils are the direct target leukocytes of the novel IL-1-family member IL-33. Blood. 2008 doi: 10.1182/blood-2008-05-157818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoshino K, Kashiwamura S, Kuribayashi K, Kodama T, Tsujimura T, Nakanishi K, et al. The absence of interleukin 1 receptor-related T1/ST2 does not affect T helper cell type 2 development and its effector function. J Exp Med. 1999;190:1541–8. doi: 10.1084/jem.190.10.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kondo Y, Yoshimoto T, Yasuda K, Futatsugi-Yumikura S, Morimoto M, Hayashi N, et al. Administration of IL-33 induces airway hyperresponsiveness and goblet cell hyperplasia in the lungs in the absence of adaptive immune system. Int Immunol. 2008;20:791–800. doi: 10.1093/intimm/dxn037. [DOI] [PubMed] [Google Scholar]
- 19.Smithgall MD, Comeau MR, Yoon BR, Kaufman D, Armitage R, Smith DE. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int Immunol. 2008;20:1019–30. doi: 10.1093/intimm/dxn060. [DOI] [PubMed] [Google Scholar]
- 20.Meisel C, Bonhagen K, Lohning M, Coyle AJ, Gutierrez-Ramos JC, Radbruch A, et al. Regulation and function of T1/ST2 expression on CD4+ T cells: induction of type 2 cytokine production by T1/ST2 cross-linking. J Immunol. 2001;166:3143–50. doi: 10.4049/jimmunol.166.5.3143. [DOI] [PubMed] [Google Scholar]
- 21.Kurowska-Stolarska M, Kewin P, Murphy G, Russo RC, Stolarski B, Garcia CC, et al. IL-33 induces antigen-specific IL-5+ T cells and promotes allergic-induced airway inflammation independent of IL-4. J Immunol. 2008;181:4780–90. doi: 10.4049/jimmunol.181.7.4780. [DOI] [PubMed] [Google Scholar]
- 22.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 23.Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3:984–93. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 24.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–48. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 25.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Radhakrishnan S, Nguyen LT, Ciric B, Ure DR, Zhou B, Tamada K, et al. Naturally occurring human IgM antibody that binds B7-DC and potentiates T cell stimulation by dendritic cells. J Immunol. 2003;170:1830–8. doi: 10.4049/jimmunol.170.4.1830. [DOI] [PubMed] [Google Scholar]
- 27.Brimnes MK, Bonifaz L, Steinman RM, Moran TM. Influenza virus-induced dendritic cell maturation is associated with the induction of strong T cell immunity to a coadministered, normally nonimmunogenic protein. J Exp Med. 2003;198:133–44. doi: 10.1084/jem.20030266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rincon M, Anguita J, Nakamura T, Fikrig E, Flavell RA. Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells. J Exp Med. 1997;185:461–9. doi: 10.1084/jem.185.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gu L, Tseng S, Horner RM, Tam C, Loda M, Rollins BJ. Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature. 2000;404:407–11. doi: 10.1038/35006097. [DOI] [PubMed] [Google Scholar]
- 30.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–80. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 31.Kato A, Favoreto S, Jr, Avila PC, Schleimer RP. TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J Immunol. 2007;179:1080–7. doi: 10.4049/jimmunol.179.2.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–23. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jankovic D, Kullberg MC, Noben-Trauth N, Caspar P, Paul WE, Sher A. Single cell analysis reveals that IL-4 receptor/Stat6 signaling is not required for the in vivo or in vitro development of CD4+ lymphocytes with a Th2 cytokine profile. J Immunol. 2000;164:3047–55. doi: 10.4049/jimmunol.164.6.3047. [DOI] [PubMed] [Google Scholar]
- 34.Kaisho T, Hoshino K, Iwabe T, Takeuchi O, Yasui T, Akira S. Endotoxin can induce MyD88-deficient dendritic cells to support T(h)2 cell differentiation. Int Immunol. 2002;14:695–700. doi: 10.1093/intimm/dxf039. [DOI] [PubMed] [Google Scholar]
- 35.Walzl G, Matthews S, Kendall S, Gutierrez-Ramos JC, Coyle AJ, Openshaw PJ, et al. Inhibition of T1/ST2 during respiratory syncytial virus infection prevents T helper cell type 2 (Th2)- but not Th1-driven immunopathology. J Exp Med. 2001;193:785–92. doi: 10.1084/jem.193.7.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brewer JM, Conacher M, Hunter CA, Mohrs M, Brombacher F, Alexander J. Aluminium hydroxide adjuvant initiates strong antigen-specific Th2 responses in the absence of IL-4- or IL-13-mediated signaling. J Immunol. 1999;163:6448–54. [PubMed] [Google Scholar]
- 37.van Panhuys N, Tang SC, Prout M, Camberis M, Scarlett D, Roberts J, et al. In vivo studies fail to reveal a role for IL-4 or STAT6 signaling in Th2 lymphocyte differentiation. Proc Natl Acad Sci U S A. 2008;105:12423–8. doi: 10.1073/pnas.0806372105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herrick CA, MacLeod H, Glusac E, Tigelaar RE, Bottomly K. Th2 responses induced by epicutaneous or inhalational protein exposure are differentially dependent on IL-4. J Clin Invest. 2000;105:765–75. doi: 10.1172/JCI8624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cousins DJ, Lee TH, Staynov DZ. Cytokine coexpression during human Th1/Th2 cell differentiation: direct evidence for coordinated expression of Th2 cytokines. J Immunol. 2002;169:2498–506. doi: 10.4049/jimmunol.169.5.2498. [DOI] [PubMed] [Google Scholar]
- 40.Shin SH, Ponikau JU, Sherris DA, Congdon D, Frigas E, Homburger HA, et al. Chronic rhinosinusitis: an enhanced immune response to ubiquitous airborne fungi. J Allergy Clin Immunol. 2004;114:1369–75. doi: 10.1016/j.jaci.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 41.Sauter A, Stern-Straeter J, Chang RC, Hormann K, Naim R. Influence of interleukin-13 on beta-catenin levels in eosinophilic chronic rhinosinusitis cell culture. Int J Mol Med. 2008;21:447–52. [PubMed] [Google Scholar]
- 42.Anand VK, Kacker A, Orjuela AF, Huang C, Manarey C, Xiang J. Inflammatory pathway gene expression in chronic rhinosinusitis. Am J Rhinol. 2006;20:471–6. doi: 10.2500/ajr.2006.20.2891. [DOI] [PubMed] [Google Scholar]
- 43.Riechelmann H, Deutschle T, Rozsasi A, Keck T, Polzehl D, Burner H. Nasal biomarker profiles in acute and chronic rhinosinusitis. Clin Exp Allergy. 2005;35:1186–91. doi: 10.1111/j.1365-2222.2005.02316.x. [DOI] [PubMed] [Google Scholar]
- 44.al Ghamdi K, Ghaffar O, Small P, Frenkiel S, Hamid Q. IL-4 and IL-13 expression in chronic sinusitis: relationship with cellular infiltrate and effect of topical corticosteroid treatment. J Otolaryngol. 1997;26:160–6. [PubMed] [Google Scholar]
- 45.Yamazaki K, Murray JA, Arora AS, Alexander JA, Smyrk TC, Butterfield JH, et al. Allergen-specific in vitro cytokine production in adult patients with eosinophilic esophagitis. Dig Dis Sci. 2006;51:1934–41. doi: 10.1007/s10620-005-9048-2. [DOI] [PubMed] [Google Scholar]
- 46.Mishra A, Rothenberg ME. Intratracheal IL-13 induces eosinophilic esophagitis by an IL-5, eotaxin-1, and STAT6-dependent mechanism. Gastroenterology. 2003;125:1419–27. doi: 10.1016/j.gastro.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 47.Bullock JZ, Villanueva JM, Blanchard C, Filipovich AH, Putnam PE, Collins MH, et al. Interplay of adaptive th2 immunity with eotaxin-3/c-C chemokine receptor 3 in eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2007;45:22–31. doi: 10.1097/MPG.0b013e318043c097. [DOI] [PubMed] [Google Scholar]
- 48.Gupta SK, Fitzgerald JF, Kondratyuk T, HogenEsch H. Cytokine expression in normal and inflamed esophageal mucosa: a study into the pathogenesis of allergic eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2006;42:22–6. doi: 10.1097/01.mpg.0000188740.38757.d2. [DOI] [PubMed] [Google Scholar]
- 49.Brint EK, Xu D, Liu H, Dunne A, McKenzie AN, O’Neill LA, et al. ST2 is an inhibitor of interleukin 1 receptor and Toll-like receptor 4 signaling and maintains endotoxin tolerance. Nat Immunol. 2004;5:373–9. doi: 10.1038/ni1050. [DOI] [PubMed] [Google Scholar]
- 50.Sweet MJ, Leung BP, Kang D, Sogaard M, Schulz K, Trajkovic V, et al. A novel pathway regulating lipopolysaccharide-induced shock by ST2/T1 via inhibition of Toll-like receptor 4 expression. J Immunol. 2001;166:6633–9. doi: 10.4049/jimmunol.166.11.6633. [DOI] [PubMed] [Google Scholar]
- 51.Turnquist HR, Sumpter TL, Tsung A, Zahorchak AF, Nakao A, Nau GJ, et al. IL-1beta-driven ST2L expression promotes maturation resistance in rapamycin-conditioned dendritic cells. J Immunol. 2008;181:62–72. doi: 10.4049/jimmunol.181.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]