Abstract
Pancreatic cancer is the fourth leading cause of cancer death in the United States. Prognostic biomarkers are lacking, and treatment has limited effect on survival. Tissues from Surveillance, Epidemiology, and End Results registries (Iowa, Hawaii, and Los Angeles) were used to build a tissue microarray of 161 pancreatic tumors (113 resections and 48 biopsies). Proportional hazard models adjusted for age, race, sex, stage, time-period of diagnosis, and treatment. Associations were examined between markers (MUC1, MUC2, MUC5AC, synaptophysin, chromogranin, neuron specific enolase, epidermal growth factor receptor, HER2, CD5, CD138, CK5/6, CK19, CK20, and p53) and survival time from diagnosis. After adjusting for covariates, borderline statistically significant associations were seen between expression of each of the three mucins (MUC1, MUC2, and MUC5AC) and shorter survival time. The associations strengthened for 154 (96%) adenocarcinomas, particularly the 120 (75%) well-differentiated to moderately differentiated ductal adenocarcinomas, a tumor type that occurred more often in the cohort among White cases than cases of other racial origin (P < 0.01). For differentiated ductal adenocarcinomas, associations with shorter survival time were seen for expression of all three mucins combined versus other mucin expression patterns (adjusted hazard ratio, 1.8; 95% confidence interval, 1.2–2.6) and for MUC2(+) versus MUC2(−) expression (adjusted hazard ratio, 1.6; 95% confidence interval, 1.1–2.4). Mucin gene expression, particularly MUC2 expression, may have prognostic value for differentiated adenocarcinomas. Tumor histologies differed in this and Japanese cohorts. The tissue microarray is available to evaluate other biomarkers. Tissue-based surveillance can be used to monitor tumor histology in populations and facilitate applied research.
Introduction
Pancreatic cancer is one of the most lethal cancers worldwide (1). In the United States, the estimated 5-year survival rate for this malignancy is 4.9%, which is the lowest survival rate among the common primary cancer sites.8 Thus, pancreatic cancer is one of the leading causes of cancer death in the United States (2). The poor prognosis for this cancer stems from the ability of pancreatic tumors to invade locally and metastasize to other organs and lymph nodes during early stages, without clinical signs (3). Although surgery is used with some success, recurrence is common (4). Because the behaviors of pancreatic tumors vary, there is a need to define the tumors that are most responsible for the burden of death. Researchers are therefore working to identify reliable biomarkers to predict pancreatic tumor prognosis (5) and focus clinical and etiologic research on the tumors based on prognosis. The lack of tissues with detailed patient information has been a barrier to this type of research.
Our objective was to develop a cohort for investigating diagnostic and prognostic markers in pancreatic cancer. Toward this goal, we investigated a panel of 14 potential diagnostic and prognostic markers. We assembled invasive tumor tissues from 161 pancreatic cancer cases in three cancer registries that are part of the Surveillance, Epidemiology, and End-Results Program (SEER) of the National Cancer Institute (NCI). Population-based tissue banks have the advantage of providing an unbiased sampling frame for evaluating new molecular classification schemes of cancer that will augment the pathomorphologic approach used presently (6). The resulting tissue microarray (TMA) is among the largest collection of pancreatic carcinomas currently available for study and is available to qualified investigators for additional studies.
Materials and Methods
Case identification and selection
A total of 13,650 pancreatic cancer cases were diagnosed in Hawaii, Iowa, and Los Angeles between 1983 and 2000. These registries, which participate in the NCI's SEER Residual Tissue Repository, retrieve tumor tissue from pathology laboratories that would otherwise be discarded. Only a fraction of cases are available within the discard repository, of which all were obtained and screened for inclusion within the TMA. These tissues are linked to cases from the registries, for which there are additional clinical and demographic data. Thus, cases with tissues in the SEER Residual Discard Repository can be compared with all cases in the population for representativeness and potential biases.
The present report included 113 excised primary pancreatic carcinomas and 48 biopsy specimens. The only selection criteria was epithelial tumor in the pancreas, with sufficient material for construction of the TMA. Residual pancreatic tissue from these cases was assembled and forwarded to the Tissue Array Research Program Laboratory at the NCI for preparation of H&E-stained sections. The 161 cases with sufficient material of epithelial tumors arising in the pancreas (n = 100) or consistent with pancreatic origin at metastatic sites (n = 61) were included in the TMA. Appropriate ethical and transfer of material approvals were obtained from originating sites, as well as the NCI.
TMA construction
A TMA of four replicate blocks was built by obtaining targeted tissue core sample of each region as previously described (7), using 1.0-mm needles with a TMArrayer (Pathology Devices). Fifteen cores of normal tissue from the matching pancreatic tumors as well as a selection of 15 adjacent benign tissues at other sites were included in the design. H&E-stained slides were made of every 50th section of the TMA. These slides were reviewed for presence of tumor and histologic assignment.
Histology and immunohistochemistry
The histology of each tumor was blindly reviewed twice by a pathologist (MT & BA) Tissue Array Research Program Laboratory, according to a published classification scheme (8). When different results were obtained from the two pathology reviews, they were reconciled via consultation with a second pathologist (SH). In the analyses presented in this report, the tumors were classified into four histologies:
(a) “differentiated ductal adenocarcinomas,” including both well- and moderately differentiated ductal adenocarcinoma; (b) “poorly differentiated ductal adenocarcinoma,” (c) “specialized adenocarcinoma,” which included cystadenocarcinoma, mucinous and papillary adenocarcinoma, and clear cell carcinomas; and (d) “neuroendocrine tumors.”
Immunohistochemical assays were performed according to manufacturer's protocols. Antibodies for mucin markers were purchased from Novocastra Laboratories. Antibodies for the neuron-specific enolase immunohistochemical assay were obtained from Vector Laboratories. Chromogranin, synaptophysin, HER2, EGFR, CD5, CD138, CK5/6, CK19, CK20, and p53 antibodies were obtained from Dako. Immunohistochemistry was performed on a Dako Autostainer following manufacturer's recommendations, with antigen retrieval performed with steam heating in a rice cooker.
Immunohistochemical assessment
The immunohistochemical scoring was performed blindly by pathologists (MT and BA). Signals were considered positive when reaction products localized in the expected cellular component. Markers were categorized by staining intensity and percent of cells stained. Intensity was scored as 0 (no signal), 1 (weak), 2 (moderate), and 3 (marked); and percent stained was scored as 0 (0%), 1 (1–25%), 2 (26–50%), 3 (51–75%), or 4 (76–100%). Mucin expression was recorded as negative, weak, moderate, or marked. Other markers were classified based on the product of staining and intensity scores (range, 0–12), dichotomized as negative to weak (0 or 1) versus moderate to marked signal (≥2).
Statistical models
The association of tumor markers with survival time was evaluated by Cox proportional hazard regression models (PROC PHREG SAS v.9; ref. 9). Survival time was defined as the interval in months from diagnosis to death. Data were censored for seven cases with pancreatic adenocarcinomas that were alive at last follow-up and one case with a neuroendocrine tumor that was lost to follow-up. Beginning with unadjusted and full models, stepwise forward and backward model selection was performed to develop a final model that adjusted for gender, age (<75 versus 75+ y), race (Asian versus non-Asian), stage of disease (local and regional versus distant and unstaged), time period of diagnosis (1983–1991 versus 1992–2000), tumor location (pancreatic versus metastatic site), and ever versus never surgical or radiation treatment for pancreatic cancer. Registry location had negligible effect when combined with these variables and was, therefore, not included in the final model. The covariates used for adjusting were kept constant with single markers added to estimate the adjusted hazard ratio. χ2 tests were performed to evaluate the statistical significance of associations between specific tumor histologies and racial subgroups in the TMA (PROC FREQ SAS v.9; ref. 10). Log-Rank Tests were performed to compare Kaplan-Meier survival distribution (11) for tumors by biomarker status (PROC LIFETEST SAS v.9).
Results
Representativeness of the TMA
The TMA (Fig. 1A) included tissue from 161 of 13,650 incident pancreatic cancer cases (1.2%) reported by the three registries (Table 1). Nearly half of cases in the TMA were White (48%), followed by Asians and Pacific Islanders (40%), and Blacks (12%). Hispanic ethnicity was reported by 4% of TMA cases. Overall, about half of cases in both the TMA cohort and reference population were male. A higher proportion of TMA cohort than reference cases were younger than age 75 years. The median survival time from diagnosis was longer for TMA cohort than reference cases. The years of diagnosis for TMA cohort cases extended from 1983 to 2000, with the majority of TMA cohort cases diagnosed in the years from 1992 to 1997. A higher proportion of TMA cohort than reference cases were diagnosed at a local or regional stage. In addition, TMA cohort cases were more likely than reference cases to have received surgery or radiation therapy. Differences between TMA cases and the reference population with respect to median survival time from diagnosis, stage of disease at time of diagnosis, and frequency of surgical or radiation treatment were more pronounced for cases from the Hawaii registry than from the Los Angeles or Iowa registries.
Figure 1.
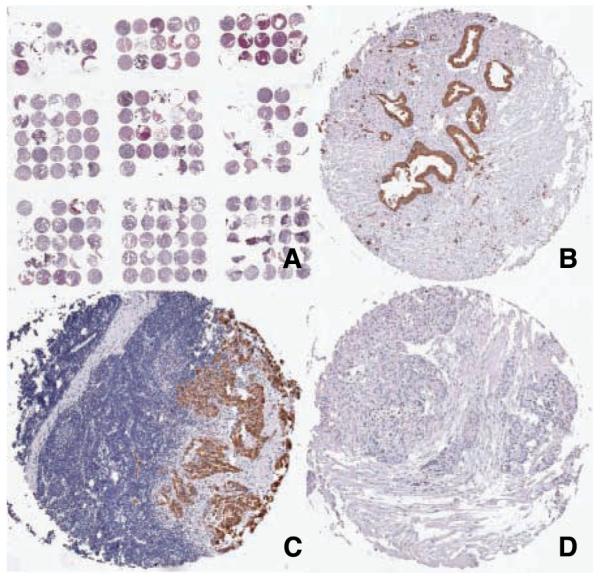
A, low power H&E image of the pancreatic cancer TMA. B to D, immunohistochemistry for MUC2. B, positive staining for MUC2 pancreatic adencarcinoma; C, positive staining for MUC2 in a pancreatic adenocarcinoma metastatic to a lymph node; D, a pancreatic neuroendocrine tumor negative for MUC2 expression.
Table 1.
Attributes of cases in the SEER Residual Tissue Repository pancreatic cancer TMA and all cases in the registries
| Iowa |
Hawaii |
Los Angeles |
Overall |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TMA | % | Registry | % | TMA | % | Registry | % | TMA | % | Registry | % | TMA | % | Registries | % | ||
| Race | |||||||||||||||||
| White | 35 | 97% | 3389 | 98% | 25 | 30% | 450 | 25% | 18 | 44% | 6574 | 78% | 78 | 48% | 10413 | 76% | |
| API | 0 | 0% | 9 | 0% | 56 | 67% | 1309 | 74% | 8 | 20% | 605 | 7% | 64 | 40% | 1923 | 14% | |
| Black | 1 | 3% | 60 | 2% | 3 | 4% | 8 | 0% | 15 | 37% | 1201 | 14% | 19 | 12% | 1269 | 9% | |
| AI/AN0 | 0% | 2 | 0% | 0 | 0% | 1 | 0% | 0 | 0% | 5 | 0% | 0 | 0% | 8 | 0% | ||
| Unknown | 0 | 0% | 0 | 0% | 0 | 0% | 1 | 0% | 0 | 0% | 36 | 0% | 0 | 0% | 37 | 0% | |
| Ethnicity | |||||||||||||||||
| Hispanic* | 0 | 0% | 9 | 0% | 0 | 0% | 8 | 0% | 6 | 13% | 1164 | 12% | 6 | 4% | 1181 | 9% | |
| Gender | |||||||||||||||||
| Male | 17 | 47% | 1696 | 49% | 45 | 54% | 960 | 54% | 19 | 46% | 4064 | 48% | 81 | 50% | 6720 | 49% | |
| Female | 19 | 53% | 1764 | 51% | 39 | 46% | 809 | 46% | 22 | 54% | 4357 | 52% | 80 | 50% | 6930 | 51% | |
| Diagnosis age | |||||||||||||||||
| <55 | 5 | 14% | 312 | 9% | 14 | 17% | 190 | 11% | 8 | 20% | 973 | 12% | 27 | 17% | 1475 | 11% | |
| 55-64 | 7 | 19% | 518 | 15% | 22 | 26% | 307 | 17% | 13 | 32% | 1509 | 18% | 42 | 26% | 2334 | 17% | |
| 65-74 | 13 | 36% | 1000 | 29% | 33 | 39% | 583 | 33% | 6 | 15% | 2650 | 31% | 52 | 32% | 4233 | 31% | |
| 75-84 | 9 | 25% | 1067 | 31% | 14 | 17% | 512 | 29% | 13 | 32% | 2357 | 28% | 36 | 22% | 3936 | 29% | |
| 85+ | 2 | 6% | 563 | 16% | 1 | 1% | 177 | 10% | 1 | 2% | 932 | 11% | 4 | 2% | 1672 | 12% | |
| Median Survival (mo)† | 5 | 3 | 13 | 4 | 3 | 3 | 6 | 3 | |||||||||
| Diagnosis year | |||||||||||||||||
| 1983-1985 | 2 | 6% | 342 | 10% | 3 | 4% | 265 | 15% | 0 | 0% | 0 | 0% | 5 | 3% | 607 | 4% | |
| 1986-1988 | 3 | 8% | 671 | 19% | 3 | 4% | 94 | 5% | 20 | 49% | 2275 | 27% | 26 | 16% | 3040 | 22% | |
| 1989-1991 | 0 | 0% | 0 | 0% | 6 | 7% | 342 | 19% | 10 | 24% | 2288 | 27% | 16 | 10% | 2630 | 19% | |
| 1992-1994 | 14 | 39% | 304 | 9% | 24 | 29% | 376 | 21% | 9 | 22% | 2264 | 27% | 47 | 29% | 2944 | 22% | |
| 1995-1997 | 14 | 39% | 1085 | 31% | 29 | 35% | 406 | 23% | 1 | 2% | 791 | 9% | 44 | 27% | 2282 | 17% | |
| 1998-2000 | 3 | 8% | 1058 | 31% | 19 | 23% | 286 | 16% | 1 | 2% | 803 | 10% | 23 | 14% | 2147 | 16% | |
| Stage at diagnosis | |||||||||||||||||
| Local/regional | 19 | 53% | 1012 | 29% | 59 | 70% | 623 | 35% | 3 | 7% | 1101 | 13% | 81 | 50% | 2736 | 20% | |
| Distant | 17 | 47% | 1757 | 51% | 24 | 29% | 900 | 51% | 8 | 20% | 1883 | 22% | 49 | 30% | 4540 | 33% | |
| Missing | 0 | 0% | 691 | 20% | 1 | 1% | 246 | 14% | 30 | 73% | 5437 | 65% | 31 | 19% | 6374 | 47% | |
| Radiation or surgery | |||||||||||||||||
| Ever | 16 | 44% | 847 | 24% | 61 | 73% | 550 | 31% | 19 | 46% | 1762 | 21% | 96 | 60% | 3159 | 23% | |
| Never | 20 | 56% | 2613 | 76% | 23 | 27% | 1219 | 69% | 22 | 54% | 6659 | 79% | 65 | 40% | 10491 | 77% | |
| Histology‡ | |||||||||||||||||
| AC | 35 | 97% | — | — | 79 | 94% | — | — | 40 | 98% | — | — | 154 | 96% | — | — | |
| Differentiated ductal AC | 27 | 75% | — | — | 62 | 74% | — | — | 31 | 76% | — | — | 120 | 75% | — | — | |
| Poorly differentiated ductal AC | 4 | 11% | — | — | 12 | 14% | — | — | 8 | 20% | — | — | 24 | 15% | — | — | |
| Specialized AC | 4 | 11% | — | — | 5 | 6% | — | — | 1 | 2% | — | — | 10 | 6% | — | — | |
| Neuroendocrine | 1 | 3% | — | — | 5 | 6% | — | — | 1 | 2% | — | — | 7 | 4% | — | — | |
| Total no.cases (% in TMA) | 36 | of | 3460 | 1.0% | 84 | of | 1769 | 4.7% | 41 | of | 8421 | 0.5% | 161 | of | 13650 | 1.2% | |
Abbreviations: API, Asians and Pacific Islanders; AI/AN, American Indians and Alaska Natives; AC, adenocarcinoma.
People reporting Hispanic ethnicity could also report white or black race.
Survival time from diagnosis excludes the 8 cases in the TMA and 308 cases in the registries that were alive at last follow up.
Histologic classification performed by NCI Tissue Array Research Program Pathologists (MT, SH), specialized adenocarcinomas: four cystadenocarcinomas, three mucinous adenocarcinomas, two papillary adenocarcinomas, and a clear cell carcinoma.
Histology
Although the TMA included various tumor type including 120 differentiated ductal adenocarcinomas, 24 poorly differentiated ductal adenocarcinomas, 10 specialized adenocarcinomas, and 7 neurorendocrine tumors, the vast majority of the tumors were adenocarcinomas (96%; Table 1). The 120 differentiated adenocarcinomas (75%) occurred significantly more frequently among White than non-White cases (P = 0.01; data not shown).
Survival analyses
For all 161 tumors in the TMA, after adjusting for covariates, borderline statistically significant associations were seen between mucin expression (MUC1, MUC2, and MUC5AC) and shorter survival time (Table 2). No other statistically significant associations between biomarker expression and survival time were observed.
Table 2.
Coxproportional hazard ratio and 95% confidence interval, time from diagnosis to death by biomarker status for all pancreatic tumors in the TMA
| Family | Marker | Signal | No* | Median survival† |
Unadjusted HR (95% CI) |
Adjusted‡ HR (95% CI) |
||
|---|---|---|---|---|---|---|---|---|
| Mucins | MUC1 | Positive | 143 | 6 | 2.6 | (1.5-4.5) | 1.7 | (1.0-3.1) |
| Negative | 18 | 27 | 1.0 | Reference | 1.0 | Reference | ||
| MUC2 | Positive | 89 | 5 | 1.5 | (1.1-2.1) | 1.4 | (1.0-2.1) | |
| Negative | 72 | 11 | 1.0 | Reference | 1.0 | Reference | ||
| MUC5AC | Positive | 99 | 5 | 1.3 | (0.9-1.8) | 1.4 | (1.0-2.0) | |
| Negative | 62 | 9.5 | 1.0 | Reference | 1.0 | Reference | ||
| Neuro- endocrine markers | Synaptophysin | Moderate/marked | 55 | 8 | 0.9 | (0.6-1.3) | 1.0 | (0.7-1.4) |
| None/weak | 101 | 6 | 1.0 | Reference | 1.0 | Reference | ||
| Chromogranin | Moderate/marked | 24 | 13 | 0.6 | (0.4-0.9) | 0.9 | (0.6-1.5) | |
| None/weak | 130 | 6 | 1.0 | Reference | 1.0 | Reference | ||
| Neuron-specific enolase | Moderate/marked | 52 | 5 | 1.3 | (0.9-1.9) | 1.1 | (0.8-1.7) | |
| None/weak | 99 | 10 | 1.0 | Reference | 1.0 | Reference | ||
| Growth factor receptors | EGFR | Moderate/marked | 40 | 5 | 1.2 | (0.8-1.8) | 1.2 | (0.8-1.8) |
| None/weak | 114 | 9.5 | 1.0 | Reference | 1.0 | Reference | ||
| HER2 | Moderate/marked | 96 | 6.5 | 1.2 | (0.9-1.7) | 0.9 | (0.6-1.3) | |
| None/weak | 57 | 8 | 1.0 | Reference | 1.0 | Reference | ||
| T cell marker | CD5 | Moderate/marked | 25 | 9 | 0.9 | (0.6-1.4) | 0.9 | (0.5-1.3) |
| None/weak | 131 | 7 | 1.0 | Reference | 1.0 | Reference | ||
| CD138 | Moderate/marked | 109 | 6 | 1.1 | (0.8-1.6) | 1.1 | (0.7-1.5) | |
| None/weak | 47 | 10 | 1.0 | Reference | 1.0 | Reference | ||
| Cytokeratins | CK20 | Moderate/marked | 70 | 6.5 | 1.0 | (0.7-1.5) | 1.1 | (0.8-1.6) |
| None/weak | 76 | 9.5 | 1.0 | Reference | 1.0 | Reference | ||
| CK5/6 | Moderate/marked | 74 | 9 | 0.8 | (0.5-1.0) | 0.9 | (0.6-1.2) | |
| None/weak | 80 | 6 | 1.0 | Reference | 1.0 | Reference | ||
| CK19 | Moderate/marked | 139 | 6 | 1.2 | (0.8-2.0) | 1.3 | (0.8-2.1) | |
| None/weak | 22 | 8.5 | 1.0 | Reference | 1.0 | Reference | ||
| Tumor protein | p53 | Moderate/strong | 119 | 6 | 1.2 | (0.8-1.8) | 1.0 | (0.6-1.5) |
| Negative/weak | 31 | 8 | 1.0 | Reference | 1.0 | Reference | ||
Abbreviation: HR, hazard ratio, CI, confidence interval; NSE, Neuron-specific enolase.
Number of cases with data available varied by marker.
Survival time in months from diagnosis to death.
Adjusted for gender, age (<75 versus 75+ y), race (Asian versus non-Asian), stage of disease (local and regional versus distant and unstaged), pancreatic origin versus metastatic site, and ever versus never surgical or radiation treatment for pancreatic cancer.
The associations between mucin expression and shorter survival time persisted for all 154 adenocarcinomas combined (96% of TMA tumors; Table 3), and further strengthened for 120 moderate to well-differentiated adenocarcinomas (75% of tumors in the cohort).
Table 3.
Cox proportional hazard ratios and 95% confidence intervals for time from diagnosis to death, all adenocarcinomas and moderately to well-differentiated adenocarcinoma, SEER Residual Tissue Repository TMA, by mucin expression status
| Histology | Mucin | No | Expression status |
Median survival* | Unadjusted model HR (95% CI) |
Adjusted model† HR (95% CI) |
||
|---|---|---|---|---|---|---|---|---|
| All ACs | All three mucins combined | 81 | Positive | 5.0 | 1.6 | (1.2-2.3) | 1.5 | (1.1-2.2) |
| 73 | Negative | 11.0 | 1.0 | Reference | 1.0 | Reference | ||
| (96% of tumors in the TMA) | MUC1 | 139 | Positive | 20.0 | 2.4 | (1.3-4.4) | 1.6 | (0.9-3.0) |
| 15 | Negative | 6.0 | 1.0 | Reference | 1.0 | Reference | ||
| MUC2 | 89 | Positive | 11.0 | 1.5 | (1.1-2.1) | 1.5 | (1.0-2.1) | |
| 65 | Negative | 5.0 | 1.0 | Reference | 1.0 | Reference | ||
| MUC5AC | 99 | Positive | 10.0 | 1.3 | (0.9-1.8) | 1.4 | (1.0-2.1) | |
| 55 | Negative | 5.0 | 1.0 | Reference | 1.0 | Reference | ||
| Well- and moderately differentiated ductal AC | All three mucins combined | 68 | Positive | 5.0 | 1.8 | (1.2-2.6) | 1.8 | (1.2-2.6) |
| 52 | Negative | 12.5 | 1.0 | Reference | 1.0 | Reference | ||
| (75% of tumors in the TMA) | MUC1 | 112 | Positive | 6.0 | 2.7 | (1.2-6.2) | 2.5 | (1.1-5.7) |
| 8 | Negative | 25.0 | 1.0 | Reference | 1.0 | Reference | ||
| MUC2 | 75 | Positive | 5.0 | 1.6 | (1.1-2.3) | 1.6 | (1.1-2.4) | |
| 45 | Negative | 13.0 | 1.0 | Reference | 1.0 | Reference | ||
| MUC5AC | 82 | Positive | 5.0 | 1.3 | (0.9-2.0) | 1.5 | (1.0-2.4) | |
| 38 | Negative | 12.0 | 1.0 | Reference | 1.0 | Reference | ||
NOTE: Associations not shown for 24 poorly differentiated ductal AC, 7 neuroendocrine, and 10 specialized adenocarcinomas.
Survival time in months from diagnosis to death.
Adjusted for gender, time period, age (<75 versus 75+ y), race (Asian versus non-Asian), stage (local and regional versus distant and unstaged), pancreatic versus metastatic tissue, and ever versus never surgical or radiation treatment for pancreatic cancer.
For moderate to well-differentiated adenocarcinomas, two noteworthy associations were found. Expression of all three mucins combined was associated with significantly shorter survival time from diagnosis (adjusted hazard ratio, 1.8; 95% confidence interval, 1.2–2.6). Similar results were found for negative and weak MUC2 expression versus moderate to strong MUC2 expression (adjusted hazard ratio, 1.6; 95% confidence interval, 1.1–2.4). The Log-Rank test for the survival distribution of these 2 groups of tumors was statistically significant (P = 0.02). Among cases with moderate to well-differentiated pancreatic adenocarcinomas, the association between MUC2 and survival time further strengthened when MUC2 negative tumors were compared with tumors with weak to strong MUC2 expression (P = 0.006, Log-Rank test; Table 4; Fig. 2). Adjustment for covariates had limited effect on the association between MUC2 and survival time. Figure 1B and C show primary and metastatic adenocarcinomas that expressed MUC2.
Table 4.
Cox-Proportional hazard ratios, time from diagnosis to death in months for 120 well-differentiated adenocarcinomas (75% of tumors in cohort) by MUC2 expression (negative versus weak, moderate, and strong)
| Histology | % of all tumors |
No | MUC2 expression |
Median survival* |
Unadjusted Model HR (95% CI) | Adjusted Model† HR (95% CI) |
||
|---|---|---|---|---|---|---|---|---|
| Differentiated ductal AC | 75% | 94 | Signal | 5.0 | 1.9 | (1.2, 2.9) | 1.9 | (1.2, 3.2) |
| 26 | No Signal | 17.5 | 1.0 | Reference | 1.0 | Reference | ||
NOTE: Associations not shown for 24 poorly differentiated ductal AC, 7 neuroendocrine, and 10 specialized adenocarcinomas.
Survival time in months from diagnosis to death.
Adjusted for gender, time period, age (<75 versus 75+ y), race (Asian versus non-Asian), stage (local and regional versus distant and unstaged), pancreatic versus metastatic tissue, and ever versus never surgical or radiation treatment for pancreatic cancer.
Figure 2.
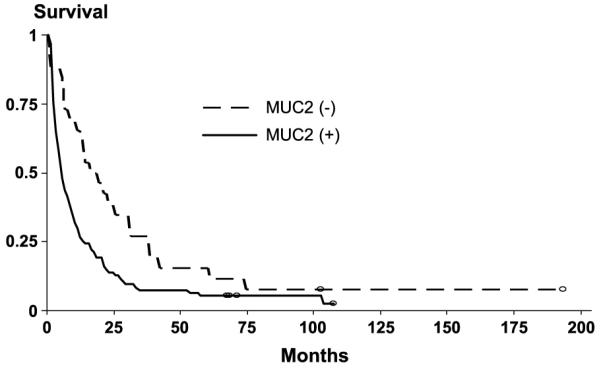
Kaplan-Meier survival curve, time from diagnosis to death in months for 120 well-differentiated adenocarcinomas (75% of tumors in cohort) by MUC2 expression (negative versus weak, moderate and strong).
Mucin expression was not associated with survival times for poorly differentiated ductal adenocarcinomas and there were too few specialized adenocarcinomas to provide informative results. All seven neuroendocrine tumors (Fig. 1D) in the TMA were negative for MUC2 expression (data not shown). The three tumors that expressed MUC5AC only were specialized adenocarcinomas occurring among Asian and Pacific Islanders. Two of these cases survived over 40 months from diagnosis (data not shown).
Discussion
Results of this study suggest that mucin gene expression, in particular, MUC2 expression, is a useful complement to histologic classification for predicting prognosis of cases of moderate to well-differentiated ductal adenocarcinoma, which predominated as tumor specimens available for analysis in this study. Among cases with these tumors, expression of all three mucins combined (MUC1, MUC2, and MUC5AC), as well as MUC2 alone, were associated with shorter survival time compared with tumors with other expression patterns. Moderate to well-differentiated ductal adenocarcinomas accounted for 75% of tumors in the cohort and occurred significantly more often among Whites than other races. The common histologies and mucin expression patterns reported in a Japanese study (12) were rare in the present study, occurring in three Asian and Pacific Islanders with specialized adenocarcinomas. Although the representativeness of the cases in the Japanese study compared with the population is unknown, the differences suggest that the distribution of pancreatic tumors may vary across populations.
The failure of the majority of markers to predict prognosis was unexpected. The selection of the biomarkers was based on those markers that we identified as potentially useful at classification of epithelial neoplasms within the pancreas. With the advent of molecular classification, it is common to define a prognostic significance to a marker or panel of markers that define a common group of tumors that cannot be accurately recognized by cytomorphology. For example, synaptophysin and chromogranin performed poorly as diagnostic markers for neuroendocrine tumors. Although synaptophysin and chromagranin did stain the neuroendocrine tumors of the pancreas, in agreement with the pathology literature (13), they also stained a fraction of adenocarcinomas, which reduced their diagnostic specificity. We did note an improvement in overall survival in those patients diagnosed in more recent years than those diagnosed in the early years of patient accrual.
In the present study, one family of markers did show potential prognostic value—the mucins, and particularly MUC2. Because multiple comparisons were performed in this exploratory study, additional analyses are recommended to assess whether these findings can be replicated. Mucins are high molecular weight glycoproteins that are produced by epithelial cells and contribute to protection, renewal, and cell signaling. Various expression patterns of mucins have been reported to predict prognosis for gastric (14), ovarian (15), and pancreatic carcinomas (16). Unlike the present study, in a Japanese study of 47 pancreatic tumors from a hospital-based sample (12), none of the 36 intraductal carcinomas expressed MUC2. Furthermore, patients in the Japanese study with MUC2-negative intraductal carcinomas had worse outcomes than patients with intraductal papillary-mucinous tumors, which generally expressed MUC2. Another Japanese study examined 50 intraductal papillary-mucinous tumors, a rare histology in the current study. In the Japanese study, MUC2 expression was associated with less favorable outcome (17). Because of differences in mucin expression and potential for malignancy, Nakamura and colleagues (17) speculated that prognostic interpretation of biomarkers vary by pancreatic tumors lineages. Differences in histology and expression in this and the Japanese studies also suggest that pancreatic tumor types differ across populations.
This study supports the utility of tissue-based cancer surveillance to test newly developed prognostic markers that may enhance emerging cancer classification systems, and facilitate etiologic research and help to prioritize tumor-specific cancer treatment protocols. For example, if the findings of this study can be replicated through ongoing tissue-based surveillance, moderately to well-differentiated pancreatic ductal adenocarcinomas with the MUC2 (+) phenotype could serve as a case definition for etiologic studies of a dominant pancreatic tumor among White cases that has an unfavorable prognosis. The MUC2 phenotype might also serve as an entry criterion for clinical trials or selection of candidates for surgery that are most likely to have favorable outcomes.
Some limitations of this study were that the TMA did not include enough tissues from Blacks, American Indians and Alaska Natives, and Hispanics to provide robust inferences on the distribution of histologies and prognostic markers of pancreatic tumors for these racial/ethnic groups. In addition, the small numbers of specimens for individual years hindered the ability to ascertain whether tumor patterns were changing over time.
Despite these limitations, the pancreatic cancer TMA assembled for this study is among the largest population-based collections available for evaluating prognostic markers within a well-characterized and racially diverse group of subjects. This resource was developed with the goal that it would be made available to researchers for testing additional biomarkers.9 It is also our hope that it will continue to be enriched with new case material from these and other registries (6).
Acknowledgments
Grant support: Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. The National Cancer Institute contracts for the participating SEER registries were N01-PC-35143 (Iowa), N01-PC-35137 (Hawaii), and N01-PC-35139 (Los Angeles).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Sahmoun AE, D'Agostino RA, Jr., Bell RA, Schwenke DC. International variation in pancreatic cancer mortality for the period 1955–1998. Eur J Epidemiol. 2003;18:801–16. doi: 10.1023/a:1025317410568. [DOI] [PubMed] [Google Scholar]
- 2.Espey DK, Wu XC, Swan J, Wiggins C, et al. Annual report to the nation on the status of cancer, 1975–2004, featuring cancer in American Indians and Alaska Natives. Cancer. 2007;110:2119–52. doi: 10.1002/cncr.23044. [DOI] [PubMed] [Google Scholar]
- 3.Singh M, Maitra A. Precursor lesions of pancreatic cancer: molecular pathology and clinical implications. Pancreatology. 2007;7:9–19. doi: 10.1159/000101873. [DOI] [PubMed] [Google Scholar]
- 4.Kure S, Kaneko T, Takeda S, Inoue S, Nakao A. Analysis of long-term survivors after surgical resection for invasive pancreatic cancer. HPB Oxford. 2005;7:129–34. doi: 10.1080/13651820510003744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tonini G, Pantano F, Vincenzi B, Gabbrielli A, Coppola R, Santini D. Molecular prognostic factors in patients with pancreatic cancer. Expert Opin Ther Targets. 2007;11:1553–69. doi: 10.1517/14728222.11.12.1553. [DOI] [PubMed] [Google Scholar]
- 6.Goodman MT, Hernandez BY, Hewitt S, et al. Tissues from population-based cancer registries: a novel approach to increasing research potential. Hum Pathol. 2005;36:812–20. doi: 10.1016/j.humpath.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 7.Kononen J, Bubendorf L, Kallioniemi A, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844–7. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 8.Yonezawa S, Nakamura A, Horinouchi M, Sato E. The expression of several types of mucin is related to the biological behavior of pancreatic neoplasms. J Hepatobiliary Pancreat Surg. 2002;9:328–41. doi: 10.1007/s005340200037. [DOI] [PubMed] [Google Scholar]
- 9.Cox DR. Regression models and life tables (with discussion) J R Stat Soc Series B. 1972;34:187–202. [Google Scholar]
- 10.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48. [PubMed] [Google Scholar]
- 11.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 12.Osako M, Yonezawa S, Siddiki B, et al. Immunohistochemical study of mucin carbohydrates and core proteins in human pancreatic tumors. Cancer. 1993;71:2191–9. doi: 10.1002/1097-0142(19930401)71:7<2191::aid-cncr2820710705>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 13.Rosai J. Ackerman's Surgical Pathology. 9th ed. Mosby; Baltimore: 2004. pp. 1083–5. [Google Scholar]
- 14.Utsunomiya T, Yonezawa S, Sakamoto H, et al. Expression of MUC1 and MUC2 mucins in gastric carcinomas: its relationship with the prognosis of the patients. Clin Cancer Res. 1998;4:2605–14. [PubMed] [Google Scholar]
- 15.Giuntoli RL, II, Rodriguez GC, Whitaker RS, Dodge R, Voynow JA. Mucin gene expression in ovarian cancers. Cancer Res. 1998;58:5546–50. [PubMed] [Google Scholar]
- 16.Terada T, Ohta T, Sasaki M, Nakanuma Y, Kim YS. Expression of MUC apomucins in normal pancreas and pancreatic tumours. J Pathol. 1996;180:160–5. doi: 10.1002/(SICI)1096-9896(199610)180:2<160::AID-PATH625>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura A, Horinouchi M, Goto M, et al. New classification of pancreatic intraductal papillarymucinous tumour by mucin expression: its relationship with potential for malignancy. J Pathol. 2002;197:201–10. doi: 10.1002/path.1109. [DOI] [PubMed] [Google Scholar]


