Abstract
Introduction
Cigarette smoking during pregnancy is associated with poor maternal and child health outcomes. Effective interventions to increase smoking cessation rates are needed particularly for pregnant women unable to quit in their first trimester. Real-time ultrasound feedback focused on potential effects of smoking on the fetus may be an effective treatment adjunct, improving smoking outcomes.
Methods
A prospective randomized trial was conducted to evaluate the efficacy of a smoking cessation intervention consisting of personalized feedback during ultrasound plus motivational interviewing-based counseling sessions. Pregnant smokers (N = 360) between 16 and 26 weeks of gestation were randomly assigned to one of three groups: Best Practice (BP) only, Best Practice plus ultrasound feedback (BP+US), or Motivational Interviewing-based counseling plus ultrasound feedback (MI+US). Assessments were conducted at baseline and end of pregnancy (EOP).
Results
Analyses of cotinine-verified self-reported smoking status at EOP indicated that 10.8% of the BP group was not smoking at EOP; 14.2% in the BP+US condition and 18.3% who received MI+US were abstinent, but differences were not statistically significant. Intervention effects were found conditional upon level of baseline smoking, however. Nearly 34% of light smokers (≤10 cigarettes/day) in the MI+US condition were abstinent at EOP, followed by 25.8% and 15.6% in the BP+US and BP conditions, respectively. Heavy smokers (>10 cigarettes/day) were notably unaffected by the intervention.
Discussion
Future research should confirm benefit of motivational interviewing plus ultrasound feedback for pregnant light smokers and explore mechanisms of action. Innovative interventions for pregnant women smoking at high levels are sorely needed.
Introduction
Cigarette smoking during pregnancy is one of the leading preventable causes of low birth weight (U.S. Department of Health and Human Services, 2001) and is associated with multiple other adverse outcomes, such as placenta previa, premature birth, spontaneous abortions, stillbirth, and potential increased risk of neurodevelopmental disorders (Cnattingius, 2004). Despite risks to their babies and to themselves, approximately 11%–22% of U.S. women smoke through pregnancy (Substance Abuse and Mental Health Services Administration, 2000). Annual health care costs due to effects of prenatal smoking on neonatal outcomes are estimated to be $263–$366 million (Lightwood, Phibbs, & Glantz, 1999), and with the addition of first year of life increase to $593–$706 million (D. P. Miller, Villa, Hogue, & Sivapathasundaram, 2001). Novel behavioral interventions are needed to improve outcomes and reduce health care costs.
Estimates are that 20%–40% of women stop smoking during pregnancy, with the majority doing so in early pregnancy (Cnattingius, Lindmark, & Meirik, 1992; Fingerhut, Kleinman, & Kendrick, 1990; Wisborg, Henriksen, Hedegaard, & Secher, 1996). Women who are disadvantaged educationally and financially and those who are heavy smokers or more dependent on tobacco/nicotine are among the least likely to quit smoking during pregnancy (e.g., Cnattingius, 2004). A recent meta-analysis (Lumley, Oliver, Chamberlain, & Oakley, 2004) of 48 trials indicates a significant reduction in smoking for intervention groups compared with controls; however, absolute differences indicate only a 6% reduction in the number of women who continued to smoke throughout pregnancy. Clearly, more powerful interventions are needed.
Providing feedback, particularly about biological markers of risk or harm, may be useful to motivate or reinforce behavior change (W. Miller, Zweben, DiClemente, & Rychtarik, 1992; W. R. Miller & Rollnick, 2002). Objective, normative, and personalized feedback has been used as a primary intervention as well as an adjunct to behavioral treatments. In a review of randomized trials of feedback interventions for smokers, McClure (2004) concludes that there is growing evidence for the efficacy of biological feedback (e.g., carbon monoxide [CO], cotinine, genetic testing) as a motivational aid to increase quit attempts or abstinence from tobacco, particularly when combined with adjuvant treatments.
In pregnancy, innovative use of high technology physiological feedback methods, such as graphical real-time ultrasound, may enhance smoking cessation outcomes. Early research provides some support for this notion. Reading, Campbell, Cox, and Sledmere (1982) compared first-trimester “high feedback” with “low feedback” ultrasound and found that high-feedback women were more likely to report decreased smoking and drinking at 16 weeks of gestation, with a 2.35 (95% confidence interval (CI) = 1.08–5.15) relative risk of quitting. Another randomized trial (N = 5,000) evaluating the effects of 15-week routine ultrasound on pregnancy outcomes (vs. no ultrasound until after 19 weeks) found higher birth weights in the ultrasound group among mothers who reported smoking at the first prenatal visit (Waldenstrom et al., 1993). Although smoking status was not confirmed, infants of smokers receiving ultrasound weighed 75 g more than infants of smokers in the control group.
Two clinical trials using routine prenatal ultrasound alone found no effect on self-reported smoking rates. In the Routine Antenatal Diagnostic Imaging with Ultrasound Study, a substudy evaluated 1,368 women who reported smoking within 1 year of the pregnancy (LeFevre, Evans, & Ewigman, 1995). Birth records were reviewed for reports of continued maternal smoking. Rates of continued smoking were 54% in both experimental and control groups, with experimental women reporting slightly more cigarettes per day (14.5 vs. 13). The authors concluded that routine ultrasound might have falsely reassured smokers. Another study evaluating the effect of repeated routine ultrasounds (five vs. one) found no differences in smoking rates, with 27% of the each group reporting some level of smoking (Newnham, Evans, Michael, Stanley, & Landau, 1993). Smoking status, however, was not validated in either study.
These findings suggest that ultrasound alone is likely insufficient to change smoking behavior. However, it is conceivable that real-time ultrasound bolstered by smoking cessation counseling and feedback may have a significant impact, potentially motivating cessation attempts.
Motivational Interviewing (MI; W. R. Miller & Rollnick, 2002) may be an ideal counseling style for persistent late pregnancy smokers, as it is designed to increase problem recognition and the need for change in individuals ambivalent or resistant to change. Motivational interviewing uses a nonjudgmental style and has been found to be especially beneficial for individuals who are ambivalent about changing (Stotts, Schmitz, Rhoades, & Grabowski, 2001). While some studies have reported negative findings for the efficacy of MI in smoking populations (Burke, Arkowitz, & Menchola, 2003), others report reductions in tobacco use following motivational interventions with smokers, including one study of pregnant smokers (Colby et al., 1998; Soria, Legido, Escolano, Lopez Yeste, & Montoya, 2006; Stotts, Diclemente, & Dolan-Mullen, 2002).
Motivational interviewing may be an ideal platform on which to implement and deliver physiological feedback. This prospective randomized trial was conducted to evaluate the efficacy of a smoking cessation intervention consisting of personalized feedback during real-time ultrasound and subsequent MI session with a sample of mid- to late-pregnancy smokers. It was hypothesized that end-of-pregnancy (EOP) smoking rates would be lower for women in the experimental group compared with those who received Best Practice (BP; Windsor et al., 2000) or Best Practice plus ultrasound (BP+US).
Methods
Study design
Pregnant smokers in their second or third trimester who volunteered and completed a baseline assessment were randomly assigned to one of three groups: BP only, BP+US, or an MI-based intervention plus ultrasound feedback (MI+US). Assessments were conducted at baseline and EOP. The primary outcome was self-reported smoking status with saliva cotinine validation measured at EOP, that is, the eighth month of gestation. The study protocol was approved by the Committee for the Protection of Human Subjects, University of Texas–Houston Health Science Center’s Institutional Review Board. The study was conducted at the University Clinical Research Center (UCRC), located at Memorial Hermann Hospital.
Screening and recruitment
Pregnant smokers (N = 360) were recruited using two methods: (a) site-based screening in Houston and Harris County–area Women, Infants, and Children centers and the University of Texas–Houston Medical School obstetric clinics and (b) advertisement. Clinic staff routinely administered a screening form to all English-speaking clients. Completed forms were collected by study staff, and women who met eligibility criteria were contacted and invited to participate. A 2-inch ad addressing pregnant smokers in a widely distributed advertisement circular ran continuously throughout the study. Women who responded to the ad were screened via telephone and invited to participate when eligible. Eligibility criteria for this study included current smoking, that is, report of having smoked a cigarette in the past 7 days; age 16 years and older; gestational age between 16 and 26 weeks; and English speaking. Eligibility criteria were selected to recruit later-pregnancy continuing smokers who have had the most difficulty stopping smoking for the pregnancy (DiClemente, Dolan-Mullen, & Windsor, 2000a; R. Windsor, 2003; Windsor, Boyd, & Orleans, 1998). Ineligible smokers were offered the toll-free number to the American Cancer Society’s quit smoking hotline.
The Robert Wood Johnson Foundation’s Smoke-Free Families Core Screening Form was used to identify eligible smokers. This form includes a multicategorical response question specifically designed to increase reliability of self-reported smoking status among pregnant women, who are often reluctant to disclose the fact that they smoke. Such multicategorical response questions have been found to have higher agreement with biochemical measures of exposure to nicotine (Campbell, Sanson-Fisher, & Walsh, 2001; Mullen, Carbonari, Tabak, & Glenday, 1991).
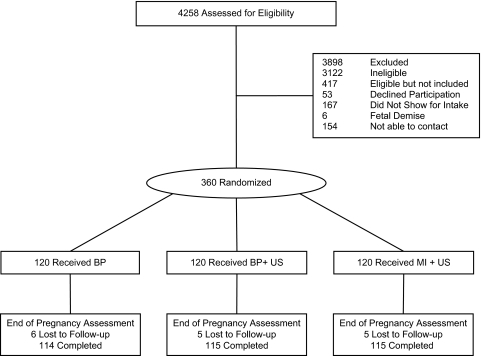
A total of 4,258 women were screened, of which 725 (17%) were found to be eligible, including the 360 (49.6%) who were randomly assigned to the three conditions, 120 per group (Figure 1). Eligible women who did not participate included those who declined enrollment, did not show for their appointments, or could not be contacted. Analyses of demographic information collected on screening forms indicated that eligible women who were not enrolled were similar to those who participated in the study with regard to age, race/ethnicity, gestational age at screening, and marital status.
Figure 1.
CONSORT diagram indicating randomization and retention.
Randomization
Eligible women who agreed to participate were scheduled for an appointment at the UCRC. A block randomization method, using blocks of six (two per condition), was used to generate 360 slots, 120 per intervention group (Graziano & Raulin, 1989). Women were not randomized to the study until they presented for the initial assessment. In the case of a missed intake appointment (see Figure 1), the randomized slot was filled by the next woman recruited.
Study procedures and measures
Women enrolled in the study completed baseline and EOP assessments. At the baseline visit, prior to the conduct of study activities, the women gave informed consent, submitted a saliva sample, and completed the intake questionnaire. After the assessment, women were escorted to their ultrasound appointment, with the exception of the BP-only group who went directly to the nurse for the BP session. Following the ultrasound, women then attended their MI or BP session. The EOP assessment was conducted in person in the UCRC during the eighth month of pregnancy and consisted of the self-report questionnaire and saliva sample.
In addition to demographic and smoking variables, other predictors of smoking cessation were included in the questionnaire. Stage of change was measured using an algorithm developed for pregnancy smoking cessation (Stotts et al., 2002). The algorithm separates individuals into precontemplation, contemplation, preparation, and action based on intentions and behaviors related to quitting smoking during this pregnancy. Depression was assessed using the Beck Depression Inventory, a widely used self-report measure of depression (Beck, 1967). The variable smoking networks was derived from one item on the baseline questionnaire: “How many family members and friends whom you see regularly are smokers?” Answers were on a Likert scale from 1 = none to 4 = most.
Self-reported smoking status was validated by saliva cotinine using a cutoff value of 20 ng/ml. The sample was collected using a cotton dental roll, placed in the mouth for 5–10 min until saturated. Saliva samples were stored at −70 °C until shipment to the laboratory. J-2 Laboratories, located in Tucson, AZ, conducted saliva cotinine measurement by method of gas chromatographic thermionic specific detector.
Intervention conditions
BP counseling.
“BP” counseling is based on the Agency for Healthcare Research Quality practice guidelines for identifying patients who smoke and intervening for smoking cessation (Katz, Muehlenbruch, Brown, Fiore, & Baker, 2004; Windsor et al., 2000). Best practices is a five-step strategy, referred to as the “5 A’s,” which involves: (a) asking all patients about smoking status, (b) advising patients to quit, (c) assessing readiness to quit, (d) assisting through counseling or referral, and (e) arranging for follow-up. Participants were also given American Cancer Society literature on prenatal smoking cessation and the toll-free number for the quit smoking hotline. Nurses at UCRC were trained by investigators in the use of this counseling strategy. All nurses attended two training sessions prior to study initiation and a refresher in-service midstudy. Nurses were instructed to keep counseling sessions to 10–15 min whenever possible to mimic what would likely occur in usual practice. All BP counseling sessions were tape-recorded and monitored by investigators.
Ultrasound feedback.
In addition to providing routine ultrasound results, the ultrasound session was designed to provide information regarding cigarette smoke’s adverse effects on the fetus using a motivational style. Four certified sonographers at the University of Texas Medical School Faculty Obstetrics and Gynecology clinic were trained to deliver risk messages related to smoking during the course of a comprehensive real-time fetal ultrasound with two initial 1-hr training sessions and a subsequent booster session. Sonographers were issued a laminated card containing major intervention messages to carry in their lab coat pockets for reference. They had the opportunity to practice and become comfortable with the delivery of the intervention messages via 30 pilot cases. Ultrasound sessions were tape-recorded for quality control, and sonographers were retrained as necessary.
Ultrasound sessions lasted approximately 30 min. Smoking risk messages were incorporated into discussion of anatomical features and included mention of the vasoconstricting effects of nicotine in the umbilical cord and placenta, reducing oxygen and nutrients to the fetus; accumulation of the poisonous gas CO in amniotic fluid and ingested by the fetus; possible premature separation of the placenta from the uterus; and smoking effects that lead to premature delivery and/or low birth weight. For the majority of ultrasounds in which no complications were discovered, sonographers summarized by confirming that the baby was okay or unaffected at the time, noting, however, that the third trimester was the most likely time for problems to develop. All messages were delivered using a motivational, non-alarming, nonjudgmental style. Concerns regarding potential distress caused by these procedures were alleviated in the initial pilot study of 30 women in which an anxiety self-report measure, the State-Trait Anxiety Inventory (Spielberger, Gorsuch, & Lushene, 1970), indicated no significant increase in anxiety postultrasound. In fact, there was a slight nonsignificant decrease in state anxiety immediately following the ultrasound (Groff et al., 2005). Investigators also met with a subset of these women and observed no significant distress postultrasound. Relief that their babies were healthy was most commonly noted.
MI intervention.
The MI intervention consisted of one 45- to 50-min, face-to-face, individual counseling session conducted immediately after the ultrasound; one personalized feedback letter mailed 1 week later; and one follow-up counseling session conducted via telephone 2 weeks subsequent to the initial session. Spacing was based, in part, on observations that typical 1-month intervals (corresponding to regularly scheduled prenatal visits) between intervention points may be too lengthy to have maximum impact (Secker-Walker, Solomon, Flynn, Skelly, & Mead, 1998).
Master’s-level counselors were trained to deliver the MI intervention designed to promote diminished smoking during pregnancy, emphasizing cessation. The intervention was based on a specific therapeutic style of MI (W. Miller & Rollnick, 1991), originally evaluated on individuals with alcohol problems (W. Miller et al., 1992) and extended to other problem behaviors (Colby et al., 1998; Saunders, Wilkinson, & Phillips, 1995). Elements of the Transtheoretical Model were also employed in this intervention (Prochaska, DiClemente, Velicer, & Rossi, 1993; Stotts et al., 2002).
More specifically, the first MI counseling session (face-to-face) consisted of the following components: (a) building rapport and gathering information, (b) assessing current motivation, (c) discussing attempts to quit smoking and the importance of doing so, (d) identifying barriers to change, and (e) eliciting a change goal. The second session was conducted by telephone and was 20–30 min in length. Session 2 focused primarily on the personalized feedback letter mailed to the woman within the previous 2 weeks. Feedback was delivered in a nonjudgmental objective manner consistent with an MI style. Women reviewed the feedback letter with the counselor, and reactions and comments were elicited. Reviewing progress and renegotiating the change plan also occurred in Session 2.
Personalized nonphysiological data based on the intake questionnaire were compiled for the feedback letter, including stage of change, pros and cons of quitting (decisional balance), cognitive/behavioral change strategies (processes of change), temptations to smoke, and confidence to abstain. Smoking in the household and social networks were also addressed. Similar feedback combined with an MI intervention was found to decrease smoking cessation by nearly 10% in a previous study of late-pregnancy smokers (Stotts et al., 2002).
Statistical analyses
Analyses were conducted using an intention-to-treat approach in SAS version 9.1.3, with missing observations treated as continued smoking. Evaluation of continuous and count data used linear and Poisson regression, respectively (SAS version 9.1.3; Proc GLM and Proc Genmod). Analysis of dichotomous outcomes included cross-tabulation and logistic regression (SAS version 9.1.3; Proc Freq and Proc Logistic).
Results
Subject characteristics
Inspection of demographic and smoking history variables (Table 1) suggested treatment group differences only for gestational age at baseline. Because inclusion of this variable in subsequent statistical models did not result in any alteration in substantive conclusions, and differences were not clinically meaningful (all three groups were in the fifth month of pregnancy), unadjusted results are reported.
Table 1.
Demographic and other characteristics
| BP only (n = 120) | BP+US (n = 120) | MI+US (n = 120) | |
| Age, M (SD) | 24.65 (5.69) | 25.45 (6.45) | 25.21 (6.01) |
| Education (years), M (SD) | 11.40 (1.99) | 11.37 (2.28) | 11.63 (1.72) |
| Number of cigarettes per day, M (SD) | 11.72 (8.73) | 11.78 (9.47) | 11.03 (8.14) |
| Age smoking regularly, M (SD) | 15.78 (3.15) | 16.02 (3.720) | 16.19 (4.35) |
| Number of births, M (SD) | 1.3 (1.4) | 1.2 (1.4) | 1.5 (1.5) |
| Gestational age (weeks)a, M (SD) | 23.63 (3.50) | 22.48 (3.64) | 21.12 (3.40) |
| Ethnicityb, n (%) | |||
| African American | 36 (31.30) | 46 (40.35) | 52 (44.44) |
| Caucasian | 75 (65.22) | 65 (57.02) | 58 (49.57) |
| Other | 4 (3.48) | 3 (2.63) | 7 (5.98) |
| Hispanic | 20 (16.67) | 25 (20.83) | 18 (28.57) |
| Marital status, n (%) | |||
| Married (living with partner) | 26 (21.67) | 18 (15.00) | 32 (26.67) |
| Not married (living with partner) | 39 (32.50) | 52 (43.33) | 45 (37.50) |
| Widowed/divorced/separated | 17 (14.17) | 11 (9.17) | 17 (14.17) |
| Never married (not living with a partner) | 38 (31.67) | 39 (32.50) | 26 (21.67) |
| Smoking partner, n (%) | 68 (68) | 82 (79.6) | 76 (72.4) |
| Income, n (%) | |||
| <$15,000 per year | 59 (49.58) | 67 (55.83) | 68 (56.67) |
| $15,000–$24,999 per year | 34 (28.57) | 28 (23.33) | 33 (27.50) |
| $25,000–$34,999 per year | 14 (11.76) | 15 (12.50) | 7 (5.83) |
| $35,000–$40,000 per year | 12 (10.08) | 10 (8.33) | 12 (10.00) |
| Ultrasound in current pregnancy, n (%) | 87 (72.5) | 89 (74.2) | 90 (75.0) |
| Stage for smoking cessation, n (%) | |||
| Precontemplation | 24 (20.0) | 9 (7.6) | 17 (13.9) |
| Contemplation | 36 (30.0) | 37 (31.1) | 33 (29.5) |
| Preparation | 60 (50.0) | 73 (61.3) | 69 (56.3) |
| Baseline cotinine (median) | 117.0 | 116.0 | 131.0 |
Note. BP, Best Practice; BP+US, Best Practice plus ultrasound feedback, MI+US, Motivational Interviewing-based intervention plus ultrasound feedback.
All groups differed from each other using Tukey’s correction for Type I error.
Fisher’s Exact Test (n = 346).
Smoking cessation
Logistic regression analysis indicated that treatment condition failed to demonstrate a significant effect on smoking cessation measured dichotomously at EOP. Results indicated that 10.8% of the BP group, 14.2% in the BP+US condition, and 18.3% who received MI+US were abstinent at EOP, χ2(2) = 2.39, p = .30. Comparison of all women who received an ultrasound (BP+US and MI+US) with those not receiving an ultrasound (BP) also did not result in a large enough difference in cessation to produce a significant effect, χ2(1) = 1.87, p = .17.
Exploratory analyses
An exploratory model including two variables hypothesized to interact with treatment examined responses to treatment of subgroups based on self-reported baseline smoking (i.e., number of cigarettes smoked per day) and stage of change. Covariates, chosen based on the existing smoking cessation literature, were also included to control for these potentially predictive factors: ethnicity (African American/non–African American; e.g., Hahn, Folsom, & Sprafka, 1989), depression (e.g., Ludman et al., 2000), and smoking networks (e.g., McBride, Pirie, & Curry, 1992). The main effect of treatment, baseline smoking, and stage of change as well as two-way interactions between treatment and baseline smoking and between treatment and stage of change were of particular interest, controlling for ethnicity, depression, and smoking networks. To obtain adequate cell sizes, the three stage categories for stopping smoking during pregnancy (precontemplation, contemplation, and preparation) were collapsed into two groups: precontemplation/contemplation (n = 186) and preparation (n = 169).
Logistic regression of cotinine-verified smoking status at EOP onto these variables and the specified interactions demonstrated that the model fit the data, Hosmer and Lemeshow goodness-of-fit χ2(8) = 6.59, p = .58, yielding the results displayed in Table 2. The interaction of treatment with baseline smoking predicted EOP smoking cessation.
Table 2.
Exploratory variables predicting smoking abstinence at the end of pregnancy
| df | χ2 | p value | |
| Smoking networks | 1 | 5.33 | .02 |
| Depression | 1 | 2.78 | .10 |
| Ethnicity | 1 | 0.07 | .79 |
| Baseline (BL) smoking | 1 | 24.4 | .00 |
| Stage of change | 1 | 0.29 | .59 |
| Treatment | 2 | 9.09 | .01 |
| Treatment × BL Smoking | 2 | 8.09 | .02 |
| Treatment × Stage/Change | 2 | 0.39 | .82 |
Examination of the simple effects revealed that within the BP condition, baseline smoking did not predict cessation, χ2(1) = 1.09, p = .30. However, baseline smoking did predict cessation in both the BP+US condition, χ2(1) = 9.58, p = .002, and the MI+US condition, χ2(1) = 11.19, p = .001. For every additional cigarette the participant smoked at baseline, odds of quitting decreased by a factor of 0.78 (95% CI = 0.66–0.91) and 0.73 (95% CI = 0.61–0.87) for BP+US and MI+US, respectively. For meaningful interpretation, baseline smoking was dichotomized into low and high levels. Dichotomization was based on the natural break in the distribution as well as on clinically relevant cutoffs where increased risks to the fetus have been found (e.g., Guzikowski & Pirogowicz, 2008; Poets et al., 1995): Low level of smoking was defined as ≤10 cigarettes/day (n = 217; median cotinine level = 80.0 ng/ml, 95% CI = 63.99–96.01) and high level of baseline smoking was defined as >10 cigarettes/day (n = 142; median cotinine levels = 205.0 ng/ml, 95% CI = 181.5–228.5). As Figure 2 suggests, lower levels of smoking at baseline were associated with higher rates of cessation at EOP, and this effect appears to be more pronounced for those receiving the ultrasound intervention.
Figure 2.
Percent smoking abstinent as a function of baseline level of smoking and treatment condition. Note: BP, Best Practice; BP+US, Best Practice plus ultrasound feedback, MI+US, Motivational Interviewing-based intervention plus ultrasound feedback. Light smoker, smoking ≤10 cigarettes/day at baseline (n = 217); heavy smoker, smoking >10 cigarettes/day at baseline (n = 142).
Discussion
An ultrasound feedback with or without an MI intervention delivered to women who continued to smoke in their second trimester did not significantly increase smoking cessation rates at EOP relative to a briefer BP intervention. However, intervention effects were moderated by amount of smoking, revealing significant effects for women smoking at lower levels. Specifically, almost 34% of women smoking 10 or fewer cigarettes per day who received the MI+US intervention were abstinent at EOP compared with about 26% and 16% who received BP+US and BP only, respectively. More than 60% of the sample was in the lighter smoking group, making this a notable finding in need of prospective replication.
Results of this study are consistent with the negative findings of a few less methodologically rigorous studies investigating the effects of routine ultrasound on smoking cessation and add to the mixed findings regarding the effects of biological feedback to promote smoking cessation (Bize, Burnand, Mueller, & Cornuz, 2005). Motivational interviewing interventions have also resulted in mixed findings for smoking cessation (Burke et al., 2003; Dunn, Deroo, & Rivara, 2001). However, results of this study should be viewed within the context of several relevant factors. First, most smokers who quit during pregnancy do so in their first trimester (Windsor et al., 1998). Most pregnancy smoking intervention studies begin in the first trimester and include women who may have quit smoking without intervention, thereby inflating smoking cessation rates attributed to the intervention. Rates of cessation in this study of second and third trimester smokers, who often are more resistant to change (DiClemente, Dolan-Mullen, & Windsor, 2000b; R. Windsor, 2003), are comparable to or better than studies that included first-trimester women. Second, the majority of participants reported having had a previous ultrasound during this pregnancy, perhaps dampening the impact of the ultrasound intervention. Future research should assess the effects of delivering the smoking intervention earlier during the woman’s first ultrasound of the pregnancy or with a naïve sample of later trimester pregnant smokers. Finally, our sample comprised woman with lower incomes and lower education levels who in previous research were much less likely to respond to smoking cessation interventions (Adams, Melvin, & Raskind-Hood, 2008), making the 34% cessation rate for light smokers in the MI+US group more salient.
The effects of the MI and ultrasound intervention were moderated by level of smoking at baseline, which can be interpreted as a marker of addiction level or dependence severity. Light smokers quit at significantly higher rates, particularly in the MI+US condition, implying that at lower levels of dependence, women are able to benefit more fully from risk messages and motivational enhancement strategies. This finding is consistent with several other studies of pregnant and nonpregnant smokers, suggesting that those who smoke fewer cigarettes per day are more responsive and likely to quit (Rigotti et al., 2006). Fortunately, in the pregnant smoker population, the majority smoke fewer than 10 cigarettes/day, indicating potential for developing and disseminating effective interventions for this group.
Perhaps even more striking are the extremely low cessation rates among the heavier smokers. Rigotti et al. (2006) also reported no effect of their telephone counseling intervention for pregnant women smoking 10 or more cigarettes per day. Among heavy smokers in our study, cessation rates were highest in the BP group at 7%, followed by BP+US (2%). None of the heavy smokers receiving the MI plus ultrasound intervention stopped smoking by EOP. Although not statistically significant and counterintuitive, it may be clinically meaningful that ultrasound conditions had poorer cessation rates with heavy smokers. As concluded in a previous study (LeFevre et al., 1995) and anecdotally from our observations, heavier smokers appeared notably relieved after receiving the ultrasound feedback, which most often indicated a healthy fetus. We realized that most ultrasounds would result in normal findings and scripted phrases such as “so far . . . ” and “smoking effects usually don’t show up until later in the third trimester.” Regardless, these heavy-smoking pregnant women are highly unlikely to achieve smoking abstinence during pregnancy. Often, they do reduce the number of cigarettes smoked and may believe that this will protect their baby from harm. The ultrasound may help confirm this belief.
Several study limitations should be considered. Conclusions regarding each element of the intervention (i.e., MI vs. ultrasound feedback) are limited by the lack of a fully factorial design. Although the design was intended to be additive, the MI+US condition best practice was not performed in exactly the manner as the other groups, making comparisons between the conditions less clear. Another weakness is the lack of infant outcome data. Tracking infant data would require significant resources because Medicaid-eligible pregnant women have options to receive care in a large number of practices in the area. Also, only half of the eligible participants were contacted and agreed to participate, limiting generalizability to the entire pregnant smoker population. Finally, subgroup analyses were not determined a priori, and therefore results could be capitalizing on chance variability. Prospective replication is recommended.
Given the differential effects found between lighter and heavier smokers, future research should investigate this distinction. The differential response may have been due to physical dependence, perhaps heavier smokers were less motivated/more resistant, or possibly the explanation may be social in nature, that is, smoking saturated environments. Undoubtedly, there is a complex interaction among nicotine dependence, motivation, and social factors. Research on such factors would be useful in directing treatment. Greater attention to the characteristics of treatment failures could lead to significant enhancements in current treatment strategies for women who continue to smoke while pregnant, with the ultimate goal of improving infant morbidity and mortality. Clearly, there is a continued need for development and testing of innovative smoking cessation interventions for pregnant women.
Funding
This study was funded by a grant from the Robert Wood Johnson Foundation. This study also received support from the General Clinical Research Center, funded by National Institutes of Health grant MO1RR02558.
Declaration of Interests
None declared.
Supplementary Material
Acknowledgments
We would like to acknowledge the ultrasonographers, nurses, and physicians of the University of Texas–Houston Obstetrics and Gynecology clinic as well as the General Clinical Research Center staff for their assistance with this project.
References
- Adams EK, Melvin CL, Raskind-Hood CL. Sociodemographic, insurance, and risk profiles of maternal smokers post the 1990s: How can we reach them? Nicotine & Tobacco Research. 2008;10:1121–1129. doi: 10.1080/14622200802123278. [DOI] [PubMed] [Google Scholar]
- Beck AT. Depression: Clinical, experimental, and theoretical aspects. New York: Harper & Row; 1967. Measure of depression: The depression inventory. [Google Scholar]
- Bize R, Burnand B, Mueller Y, Cornuz J. Biomedical risk assessment as an aid for smoking cessation. Cochrane Database Systematic Review. 2005;4 doi: 10.1002/14651858.CD004705.pub2. CD004705. [DOI] [PubMed] [Google Scholar]
- Burke BL, Arkowitz H, Menchola M. The efficacy of motivational interviewing: A meta-analysis of controlled clinical trials. Journal of Consulting Clinical Psychology. 2003;71:843–861. doi: 10.1037/0022-006X.71.5.843. [DOI] [PubMed] [Google Scholar]
- Campbell E, Sanson-Fisher R, Walsh R. Smoking status in pregnant women assessment of self-report against carbon monoxide (CO) Addictive Behaviors. 2001;26:1–9. doi: 10.1016/s0306-4603(00)00070-8. [DOI] [PubMed] [Google Scholar]
- Cnattingius S. The epidemiology of smoking during pregnancy: Smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine & Tobacco Research. 2004;6(Suppl. 2):S125–S140. doi: 10.1080/14622200410001669187. [DOI] [PubMed] [Google Scholar]
- Cnattingius S, Lindmark G, Meirik O. Who continues to smoke while pregnant? Journal of Epidemiology and Community Health. 1992;46:218–221. doi: 10.1136/jech.46.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby SM, Monti PM, Barnett NP, Rohsenow DJ, Weissman K, Spirito A, et al. Brief motivational interviewing in a hospital setting for adolescent smoking: A preliminary study. Journal of Consulting and Clinical Psychology. 1998;66:574–578. doi: 10.1037//0022-006x.66.3.574. [DOI] [PubMed] [Google Scholar]
- DiClemente CC, Dolan-Mullen P, Windsor RA. The process of pregnancy smoking cessation: Implications for interventions. Tobacco Control. 2000a;9(Suppl. 3):III16–III21. doi: 10.1136/tc.9.suppl_3.iii16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiClemente CC, Dolan-Mullen P, Windsor RA. The process of pregnancy smoking cessation: Implications for interventions. Tobacco Control. 2000b;9(Suppl. 3):III16–III21. doi: 10.1136/tc.9.suppl_3.iii16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn C, Deroo L, Rivara FP. The use of brief interventions adapted from motivational interviewing across behavioral domains: A systematic review. Addiction. 2001;96:1725–1742. doi: 10.1046/j.1360-0443.2001.961217253.x. [DOI] [PubMed] [Google Scholar]
- Fingerhut LA, Kleinman JC, Kendrick JS. Smoking before, during, and after pregnancy. American Journal of Public Health. 1990;80:541–544. doi: 10.2105/ajph.80.5.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano AM, Raulin ML. Research methods: A process of inquiry. New York: Harper & Row; 1989. [Google Scholar]
- Groff J, Stotts A, Velasquez M, Benjamin-Garner R, Green C, Mastrobattista J. Ultrasound and motivational enhancement for prenatal smoking cessation. 2005 Paper presented at the Annual meeting of the Society for Behavioral Medicine, Boston, MA. [Google Scholar]
- Guzikowski W, Pirogowicz I. Influence of tobacco smoking on newborn's birth weight–analysis of dates concerning births from Maternity Hospital named Dr S. Mossor's in Opole City. Przegl Lek. 2008;65:424–426. [PubMed] [Google Scholar]
- Hahn LP, Folsom AR, Sprafka JM. Cigarette smoking and cessation behaviors among urban blacks and whites. Public Health Reports. 1989;105:290–295. [PMC free article] [PubMed] [Google Scholar]
- Katz DA, Muehlenbruch DR, Brown RL, Fiore MC, Baker TB. Effectiveness of implementing the Agency for Healthcare Research and Quality smoking cessation clinical practice guideline: A randomized, controlled trial. Journal of the National Cancer Insitute. 2004;96:594–603. doi: 10.1093/jnci/djh103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeFevre ML, Evans JK, Ewigman B. Is smoking an indication for prenatal ultrasonography? RADIUS Study Group. Archives of Family Medicine. 1995;4:120–123. doi: 10.1001/archfami.4.2.120. [DOI] [PubMed] [Google Scholar]
- Lightwood JM, Phibbs CS, Glantz SA. Short-term health and economic benefits of smoking cessation: Low birth weight. Pediatrics. 1999;104:1312–1320. doi: 10.1542/peds.104.6.1312. [DOI] [PubMed] [Google Scholar]
- Ludman EJ, McBride CM, Nelson JC, Curry SJ, Grothaus LC, Lando HA, et al. Stress, depressive symptoms, and smoking cessation among pregnant women. Health Psychology. 2000;19:21–27. doi: 10.1037//0278-6133.19.1.21. [DOI] [PubMed] [Google Scholar]
- Lumley J, Oliver SS, Chamberlain C, Oakley L. Interventions for promoting smoking cessation during pregnancy. Cochrane Database of Systematic Reviews. 2004;4 doi: 10.1002/14651858.CD001055.pub2. CD001055. [DOI] [PubMed] [Google Scholar]
- McBride CM, Pirie PL, Curry SJ. Postpartum relapse to smoking: A prospective study. Health Education Research. 1992;7:381–390. doi: 10.1093/her/7.3.381. [DOI] [PubMed] [Google Scholar]
- McClure J. Motivating prepartum smoking cessation: A consideration of biomarker feedback. Nicotine & Tobacco Research. 2004;6(Suppl. 2):S153–S161. doi: 10.1080/14622200410001669222. [DOI] [PubMed] [Google Scholar]
- Miller DP, Villa KF, Hogue SL, Sivapathasundaram D. Birth and first-year costs for mothers and infants attributable to maternal smoking. Nicotine & Tobacco Research. 2001;3:25–35. doi: 10.1080/14622200020032079. [DOI] [PubMed] [Google Scholar]
- Miller W, Rollnick S. Motivational interviewing: Preparing people for change. New York: Guilford Press; 1991. [Google Scholar]
- Miller W, Zweben A, DiClemente CC, Rychtarik R. Motivational enhancement therapy manual. Rockville, MD: National Institute on Alcohol Abuse and Alcoholism; 1992. [Google Scholar]
- Miller WR, Rollnick S. Motivational interviewing: Preparing people for change. 2nd ed. New York: Guilford Press; 2002. [Google Scholar]
- Mullen PD, Carbonari JP, Tabak ER, Glenday MC. Improving disclosure of smoking by pregnant women. American Journal of Obstetrics Gynecology. 1991;165:409–413. doi: 10.1016/0002-9378(91)90105-z. [DOI] [PubMed] [Google Scholar]
- Newnham JP, Evans SF, Michael CA, Stanley FJ, Landau LI. Effects of frequent ultrasound during pregnancy: A randomised controlled trial. Lancet. 1993;342:887–891. doi: 10.1016/0140-6736(93)91944-h. [DOI] [PubMed] [Google Scholar]
- Poets CF, Schlaud M, Kleemann WJ, Rudolph A, Diekmann U, Sens B. Sudden infant death and maternal cigarette smoking: Results from the Lower Saxony Perinatal Working Group. European Journal of Pediatrics. 1995;154:326–329. doi: 10.1007/BF01957372. [DOI] [PubMed] [Google Scholar]
- Prochaska JO, DiClemente CC, Velicer WF, Rossi JS. Standardized, individualized, interactive, and personalized self-help programs for smoking cessation. Health Psychology. 1993;12:399–405. doi: 10.1037//0278-6133.12.5.399. [DOI] [PubMed] [Google Scholar]
- Reading AE, Campbell S, Cox DN, Sledmere CM. Health beliefs and health care behaviour in pregnancy. Psychological Medicine. 1982;12:379–383. doi: 10.1017/s0033291700046717. [DOI] [PubMed] [Google Scholar]
- Rigotti NA, Park ER, Regan S, Chang Y, Perry K, Loudin B, et al. Efficacy of telephone counseling for pregnant smokers: A randomized controlled trial. Obstetrics & Gynecology. 2006;108:83–92. doi: 10.1097/01.AOG.0000218100.05601.f8. [DOI] [PubMed] [Google Scholar]
- Saunders B, Wilkinson C, Phillips M. The impact of a brief motivational intervention with opiate users attending a methadone programme. Addiction. 1995;90:415–424. doi: 10.1046/j.1360-0443.1995.90341510.x. [DOI] [PubMed] [Google Scholar]
- Secker-Walker RH, Solomon LJ, Flynn BS, Skelly JM, Mead PB. Reducing smoking during pregnancy and postpartum: Physician's advice supported by individual counseling. Preventive Medicine. 1998;27:422–430. doi: 10.1006/pmed.1998.0287. [DOI] [PubMed] [Google Scholar]
- Soria R, Legido A, Escolano C, Lopez Yeste A, Montoya J. A randomised controlled trial of motivational interviewing for smoking cessation. British Journal of General Practice. 2006;56:768–774. [PMC free article] [PubMed] [Google Scholar]
- Spielberger C, Gorsuch R, Lushene R. State-trait anxiety inventory test manual. Palo Alto, CA: Consulting Psychologists; 1970. [Google Scholar]
- Stotts AL, Diclemente CC, Dolan-Mullen P. One-to-one: A motivational intervention for resistant pregnant smokers. Addictive Behaviors. 2002;27:275–292. doi: 10.1016/s0306-4603(01)00174-5. [DOI] [PubMed] [Google Scholar]
- Stotts AL, Schmitz JM, Rhoades HM, Grabowski J. Motivational interviewing with cocaine-dependent patients: A pilot study. Journal of Consulting & Clinical Psychology. 2001;69:858–862. doi: 10.1037//0022-006x.69.5.858. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Summary of findings from the 1999 National Household Survey on Drug Abuse. Rockville, MD: Department of Health and Human Services: 2000. [Google Scholar]
- U.S. Department of Health and Human Services. Health consequences of smoking among women. Rockville, MD: Centers for the Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office of Smoking and Health; 2001. [Google Scholar]
- Waldenstrom U, Axelsson O, Nilsson S, Eklund G, Fall O, Lindeberg S, et al. Effects of routine one-stage ultrasound screening in pregnancy: A randomized controlled trial. Lancet. 1993;342:887–891. [Google Scholar]
- Windsor R. Smoking cessation or reduction in pregnancy treatment methods: A meta-evaluation of the impact of dissemination. American Journal of Medical Sciences. 2003;326:216–222. doi: 10.1097/00000441-200310000-00013. [DOI] [PubMed] [Google Scholar]
- Windsor RA, Boyd NR, Orleans CT. A meta-evaluation of smoking cessation intervention research among pregnant women: Improving the science and art. Health Education Research. 1998;13:419–438. doi: 10.1093/her/13.3.419. [DOI] [PubMed] [Google Scholar]
- Windsor RA, Woodby LL, Miller TM, Hardin JM, Crawford MA, DiClemente CC. Effectiveness of Agency for Health Care Policy and Research clinical practice guideline and patient education methods for pregnant smokers in medicaid maternity care. American Journal of Obstetrics & Gynecology. 2000;182:68–75. doi: 10.1016/s0002-9378(00)70492-3. [DOI] [PubMed] [Google Scholar]
- Wisborg K, Henriksen TB, Hedegaard M, Secher NJ. Smoking during pregnancy and preterm birth. British Journal of Obstetrics & Gynaecology. 1996;103:800–805. doi: 10.1111/j.1471-0528.1996.tb09877.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.