Abstract
Introduction
Depressive symptoms negatively impact smoking abstinence. However, few interventions have been targeted to smokers with current depression. Exercise improves mood and may benefit depressed smokers. This pilot study investigated the feasibility of an exercise intervention for depressed female smokers (Center for Epidemiological Studies Depression Scale [CES-D] score ≥16).
Methods
Participants (M = 41 years, 98% White) were randomized to 10 weeks of individually delivered exercise counseling (n = 30) or a health education contact control condition (n = 30). All participants received nicotine patch therapy and behavioral counseling for smoking cessation.
Results
The intervention was feasible as indicated by ability to recruit participants, exercise counseling session attendance (M = 7.6 of 10 sessions attended), and significant increase in exercise frequency and stage of change from baseline to end of treatment (EOT) (Week 10). Participant attrition rate was 35% by Week 10 but did not differ significantly between groups. Smoking abstinence rates at Week 10, using intention-to-treat analysis, were 17% for exercise counseling participants and 23% for health education participants (p = .75).
Discussion
An exercise counseling intervention was found to be feasible for depressed women smokers. More intensive intervention may be needed to increase smoking abstinence rates, and methods should be refined to reduce participant burden and attrition.
Introduction
Depression may pose a particular challenge for women smokers. Studies indicate that 34%–48% of smokers enrolled in clinical trials are depressed (Kinnunen, Doherty, Militello, & Garvey, 1996; Lerman et al., 1996, 1998) and that depressive symptoms predict poorer smoking treatment outcomes and relapse following a period of abstinence (Berlin & Covey, 2006; Niaura et al., 2001; Rausch, Nichinson, Lamke, & Matloff, 1990). Because women have higher rates of depression than men (Kessler et al., 1994), are more likely to use smoking as a coping strategy for managing negative affect (NA; Abrams et al., 1987), and are less likely to be successful in maintaining smoking abstinence (Bjornson et al., 1995; Wetter et al., 1999), some have recommended tailoring smoking cessation programs to depressed women (Borrelli, Bock, King, Pinto, & Marcus, 1996).
Exercise is an effective treatment for depression (e.g., Blumenthal et al., 1999, 2007; Dunn, Trivedi, Kampert, Clark, & Chambliss, 2005) and therefore may benefit depressed smokers. However, as a treatment for smoking cessation, the support for exercise is mixed (for a review, see Ussher, 2005). Marcus et al. (1999) randomly assigned female smokers to a 12-week cognitive behavioral intervention for smoking cessation plus exercise or the same cognitive behavioral intervention plus a contact control (i.e., health education). Participants in the exercise intervention arm completed three supervised gym-based vigorous intensity exercise sessions per week. The exercise arm achieved significantly higher levels of continuous abstinence than the health education group at the EOT (19.4% vs. 10.2%) and at 3 (16.4% vs. 8.2%) and 12 months (11.9% vs. 5.4%) following treatment. Additional studies have found higher cessation rates among exercise conditions compared with control conditions (Marcus, Albrecht, Niaura, Abrams, & Thompson, 1991; Marcus et al., 1995) and that levels of exercise adherence predicted long-term smoking abstinence (Marcus et al., 2005). Several other studies have indicated no effect of an exercise intervention on smoking cessation, but these studies are limited by small samples or by exercise interventions that are probably not intensive enough to aid smoking cessation (Ussher, 2005).
There is some evidence that exercise reduces NA during smoking abstinence. For example, Ussher, West, McEwen, Taylor, and Steptoe (2003) showed that, compared with an equal contact control condition, men and women receiving physical activity counseling reported less tension, anxiety, stress, irritability, and restlessness during the first weeks of smoking cessation. In addition, there is evidence to suggest an acute reduction in depressed mood following a bout of exercise during smoking abstinence (Taylor, Ussher, & Faulkner, 2007). Finally, survey results suggested that many individuals with depression are receptive to the idea of increasing physical activity as an aid to stopping smoking (Faulkner, Taylor, Munro, Selby, & Gee, 2007).
We are aware of no previous study that has examined the feasibility or efficacy of an exercise intervention among depressed female smokers. The purpose of this pilot study was to evaluate the feasibility of an individualized exercise counseling intervention for depressed smokers as indicated by recruitment and retention rates, exercise session attendance, and increases in exercise frequency and stage of change. This pilot study sample size is insufficient for statistical comparisons that would allow for determination of treatment efficacy. However, an additional aim was to obtain an estimate of the treatment effect size for the exercise intervention compared with a health education contact control group on smoking abstinence rates at the EOT (Week 10) and at follow-up (Week 24).
Methods
The study was approved by the Mayo Institutional Review Board prior to recruitment and enrollment of participants.
Participants
The target sample of 60 participants was based on the primary aim of feasibility. Participants were recruited over a 17-month period by news releases and advertisements in local print and electronic media that briefly described study details (i.e., study compares a women’s health education program with a women’s physical activity program, includes nicotine patch treatment, and involves 10 weekly sessions). Study staff also provided local mental health professionals with study details and study information sheets to be shared with patients. Recruitment materials were written in an attempt to appeal to depressed women smokers through the use of empathizing statements about the challenges depressed women face when quitting smoking. Interested individuals completed a telephone screening interview. We considered providing additional study information with exclusion criteria in a recorded message or mailed information sheet. Because depression is associated with low adherence (Wing, Phelan, & Tate, 2002), we considered the telephone screen an opportunity to build rapport and personally answer questions, perhaps enhancing motivation toward further study assessment.
Participants were 60 depressed women 18–65 years of age who were regular smokers (10 or more cigarettes per day during the previous 6 months). Depression was defined as a score of 16 or higher on the Center for Epidemiological Studies Depression Scale (CES-D; Radloff, 1977). Participants were sedentary, defined as exercising less than 20 min/day on fewer than 3 days/week. Other inclusion criteria included provision of written informed consent, the ability to participate in an exercise program (determined by study physician based on results from exercise treadmill test and physician examination), considered in good general health by a study physician, a negative pregnancy test, and a body mass index ≤40. Exclusion criteria included current (past 3 months) alcohol dependence as assessed by the self-administered alcohol screening test (Swenson & Morse, 1975), current (past 3 months) nonnicotine drug dependence as assessed by the Drug Abuse Screening Test-20 (Skinner, 1982), and the following criteria as determined through semistructured clinical interview and physical examination conducted by a study physician: current use (past 30 days) of nicotine containing medication or tobacco products other than cigarettes; current use (past 30 days) of any other smoking cessation treatment involving either behavioral or pharmacological interventions; any medical condition precluding the use of nicotine patch therapy; recent history (past 30 days) of myocardial infarction or stroke, history of severe skin allergies, or evidence of severe chronic dermatoses; past or current history of bipolar disorder, schizophrenia or psychotic symptoms including psychotic features within the context of a depressive episode (assessed with DSM-IV criteria); use of bupropion or nortriptyline within the past 30 days; and/or current suicidal intent or plan. All other antidepressant medications were acceptable if the participant had been on a steady dose (no dosage or medication changes) for the past 3 months.
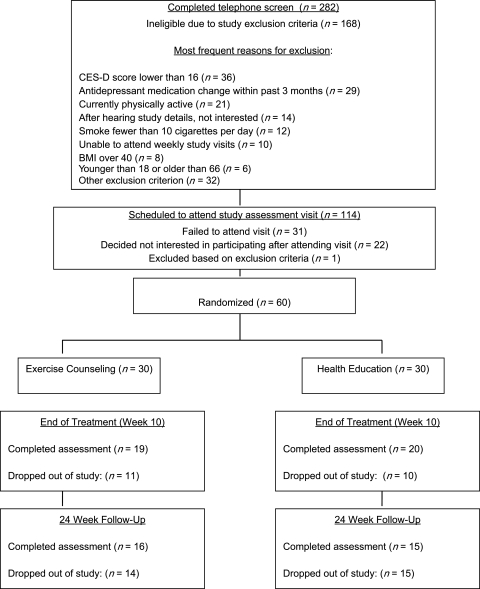
A total of 282 potential participants completed initial telephone screening, and 168 of these were deemed ineligible due to study exclusion criteria (Figure 1). The primary recruitment sources identified by potential participants as motivating them to call included information from medical/mental health professional (21%), posted flyer (21%), radio advertisement (19%), and newspaper advertisement (14%). A total of 114 women were scheduled to complete a more comprehensive in-person assessment of study eligibility and 31 of these canceled or failed to show up despite study staff making frequent attempts to reschedule and accommodate any scheduling concerns. Of the 83 women who did attend the assessment visit, 22 determined that they were not interested in participating for a wide variety of reasons (e.g., schedule, perceived burden of study assessment and visits, had already quit smoking, a desire to focus on psychiatric problems, current psychosocial stressors, ambivalence about attempting to quit smoking). One woman was excluded following physician examination due to psychiatric problems. The remaining 60 women were enrolled and randomized.
Figure 1.
Participant recruitment and loss to follow-up rates. BMI, body mass index.
Procedure
After completion of the baseline assessment, participants were randomly assigned to either exercise counseling (n = 30) or a health education contact control condition (n = 30). The randomization process involved a study assistant opening the next consecutively numbered sealed envelope containing study assignment previously prepared by a study statistician using a random number generating program. Randomization was not stratified on depression level, antidepressant treatment, or any other variable. Each condition consisted of 10 weekly individually tailored sessions. The two conditions were equal for counselor contact time (30 min/session). All participants received individual brief behavioral smoking cessation counseling delivered concurrently with the exercise counseling or health education interventions. Additionally, participants began nicotine patch therapy (21 mg/day) on the target quit date (Week 4) and continued using the nicotine patch through Week 10. All participants completed assessments at the EOT (Week 10) and at Week 24. To assist with study retention, research assistants were as accommodating as possible (while adhering to study protocol) in scheduling and rescheduling assessment and treatment sessions. Participants received $25 remuneration at the EOT and again after follow-up assessment.
Exercise counseling.
Participants received weekly individually tailored exercise counseling sessions designed to motivate increased regular physical activity and short bouts of exercise in response to NA and urges to smoke. The goal of this intervention was to increase activity level to that recommended by the Centers for Disease Control and Prevention and the American College of Sports Medicine, which, at the time of this study, was 30 min/day for at least 5 days/week (Pate et al., 1995). This intervention was based on social cognitive theory (Bandura, 1977, 1992) and incorporated cognitive behavioral exercise intervention strategies (Marcus & Forsyth, 2008) such as discussion of personal benefits of exercise, collaborative goal setting, reinforcement for steps toward increased exercise, problem solving around barriers to exercise, and handling lapses in activity without excessive frustration and shame. No supervised exercise was completed as part of the intervention. Instead, participants were encouraged to use environments that were most convenient, enjoyable, and feasible for exercise completion. By the end of each session, participants had worked toward identifying personally relevant action steps for exercise (short-term goals that participant rated high for confidence in likelihood of successful completion) that could be completed within the week. Counselors recorded participant progress in applying cognitive behavioral skills toward exercise. Participants recorded daily physical activity for discussion, reinforcement, and problem solving in session.
Health education.
Participants in the health education condition received information on a variety of health topics including sleep hygiene, nutrition, and health screening tests for women. Participants were also asked to read handouts on health education topics covered during the session. Participants were asked to record reading each day and bring records to each session.
Smoking cessation counseling.
All participants received brief smoking cessation counseling that closely followed weekly visit handouts and were given the National Cancer Institute “Clearing the Air” brochure. The sessions emphasized preparing for the target quit date, coping with urges to smoke and withdrawal symptoms, and appropriate use of nicotine patches. This component of the intervention required approximately 10 min at each visit.
Counselors and counselor training.
The exercise counseling and health education interventions were delivered by patient education specialists, with educational background in nursing or counseling, each with more than 5 years of clinical education experience delivering education sessions to medical patients. The behavioral smoking cessation counselors had educational background in nursing with prior experience in delivering protocol-based brief smoking cessation counseling. Intervention counselors were trained to deliver either health education or exercise counseling (no crossover). Training sessions and materials were developed and delivered by a clinical health psychologist (K.S.V.) with research and clinical experience in depression, behavior change, and patient education. The session-by-session treatment manual developed for this project included an instructor’s guide and handouts for participants. The trainer videotaped herself delivering each exercise counseling session to demonstrate application of the cognitive behavioral intervention strategies. Training sessions took place in small groups (separated by intervention type) for approximately 10 hr over 4 weeks and involved discussing and rehearsing session scripts using role-play with feedback. During the trial, the trainer met with interventionists weekly and additional training sessions were scheduled to rehearse strategies to redirect participants away from in-depth discussion of psychosocial stressors and to support participants following a smoking lapse or nonadherence to exercise goal.
Treatment fidelity.
Counselors were trained on all aspects of intervention delivery by K.S.V., who had developed the treatment manuals. Mock sessions were used to compare intervention delivery to treatment manual both before and during the trial. Ten percent of the actual intervention sessions were observed (K.S.V.) and evaluated using a treatment fidelity checklist for assessing presence of specific content and counselor behavior in delivery of the intervention (e.g., affect, collaborative approach, positive reinforcement of steps toward change). Observation feedback was shared with counselors along with additional training if needed. After each study visit, participants completed a checklist of intervention content received. Counselors also completed a form for each visit assessing minutes in length, content covered, problems encountered, perceived deviations from protocol, and areas for additional training.
Measures
Demographic information and tobacco use history (e.g., cigarettes per day, years of smoking, and number of quit attempts) were assessed at baseline. Participants were also weighed at baseline and at Weeks 10 and 24 using a calibrated electronic scale.
Smoking status.
At each visit, a research assistant (aware of group assignment but not involved in intervention delivery and trained in a scripted, minimal interaction) recorded the participant’s smoking status. The primary endpoint was 7-day point prevalence smoking abstinence defined as self-reported smoking of no cigarettes (not even a puff) for the previous 7 days at the EOT (Week 10) and at Week 24. Expired-air carbon monoxide concentrations of <8 parts per million were used as biochemical confirmation of self-reported smoking abstinence at the EOT. Urine cotinine was assessed at Week 24 follow-up if participants were no longer using the nicotine patch. Participants without biochemical verification of smoking status due to dropping out of study were classified as smokers.
Hamilton Rating Scale for Depression.
The Hamilton Rating Scale for Depression (HRSD; Hamilton, 1960), a 24-item structured clinical interview with documented psychometric properties, was administered to assess severity of depressive symptoms at baseline and at Weeks 10 and 24. Scores range from 0 to 74. A cutoff score of ≥20 suggests clinical depression, and 10 or below is considered within the normal range (Cleary & Guy, 1987). The HRSD is designed for repeated use in clinical trials. A recent review article (Bagby, Ryder, Schuller, & Marshall, 2004) summarizes the numerous studies that have examined psychometric properties of the HRSD (e.g., internal reliability estimates have ranged from 0.46 to 0.97, retest reliability has ranged from 0.81 to 0.98).
Center for Epidemiological Studies Depression Scale.
The CES-D (Radloff, 1977), a 20-item Likert-style scale, was administered by telephone to screen for depressive symptoms. The CES-D has high internal consistency (r = .85–.90) and test–retest reliability (r = .57 for 2–8 weeks) and correlates with clinical ratings of depression severity (Radloff, 1977). Scores range from 0 to 60, with higher scores indicating greater depression. The standard cutoff score of 16 was used to categorize participants as depressed (Radloff & Locke, 1986) as part of the study eligibility criteria. A higher cutoff score of 24 was considered, as was a maximum score cutoff, but the wide allowable range of 16–60 for study inclusion was ultimately decided upon for this feasibility study to allow for observation of level of depressive symptoms present among women responding to recruitment materials.
Fagerström Test for Nicotine Dependence.
The six-item Fagerström Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerström, 1991) is an abbreviated version of the Fagerström Tolerance Questionnaire (Fagerström, 1978). In several studies, the FTND predicted smoking abstinence and correlated with biochemical measures of nicotine dependence (e.g., Kozlowski, Porter, Orleans, Pope, & Heatherton, 1994). FTND scores range from 0 to 10, with higher scores indicating greater levels of nicotine dependence. In a psychometric study of the FTND among psychiatric patients (Buckley et al., 2005), the measure demonstrated stable test–retest reliability and was correlated at statistically significant levels with biological and psychological measures of dependence.
Weight Concerns Scale.
The Weight Concerns scale (Borrelli & Mermelstein, 1998) is a six-item measure assessing smoking for weight control and concerns about weight gain. This scale has high internal consistency (coefficient α = .87), and weight concern is predictive of weight gain following smoking cessation (Borrelli & Mermelstein, 1998).
Stage of Change for Exercise.
This four-item measure (Marcus, Rossi, Selby, Niaura, & Abrams, 1992) utilizes a yes/no format. Reliability (Κ = .78; Marcus et al., 1992) and concurrent validity (Marcus & Simkin, 1993) have been established. Respondents are categorized into stages of change for exercise based on their pattern of responding.
Positive and Negative Affect Scales.
The Positive and Negative Affect Scales (PANAS; Watson, Clark, & Tellegan, 1988) were administered weekly during the treatment phase to assess mood changes. The PANAS has yielded positive affect and negative affect scales with high internal consistency reliabilities and low intercorrelations between the scales. The NA scale is sensitive to the effects of smoking abstinence (Brandon, Copeland, & Saper, 1995).
Estimated maximal oxygen consumption (VO2 max) exercise test.
We assessed VO2 max using a treadmill exercise test at baseline and at the EOT (Week 10) to determine objective changes in fitness level. Similar measurement was used in previous exercise interventions for smokers (Marcus et al., 1999) to assess training effect of exercise condition and could assist in determining whether exercise aids in smoking cessation independent of change in fitness level (Ussher, Taylor, West, & McEwen, 2000). Patients were instrumented to measure heart rate electrocardiogram, gas exchange (mouthpiece and nose clip), and oxygen saturation (pulse oximetry). The initial treadmill speed and grade (2.0 mph and 0%, respectively) were adjusted every 2 min to increase the workload by approximately 2 metabolic equivalent units up to volitional fatigue. Gas exchange measurements were obtained using a calibrated breath-by-breath metabolic cart interfaced with a mass spectrometer. Patients were encouraged to reach a maximal effort by monitoring the respiratory exchange ratio (>1.15) and a perceived exertion of more than 17 on the Borg 6–20 scale.
Blair 7-day Physical Activity Recall Interview.
The Blair 7-day physical activity recall (PAR) interview (Blair et al., 1985) was administered by trained research study assistants at baseline and at Weeks 10 and 24 to assess self-reported levels of physical activity. The PAR has also been extensively validated using a variety of objective measures (Jacobs, Ainsworth, Hartman, & Leon, 1993). The PAR has been frequently utilized in exercise intervention trials (e.g., Dunn et al., 1999) and in exercise interventions for smoking cessation (Marcus et al., 1999, 2005). The 7-day total of minutes of moderate, hard, and very hard activity was calculated and used in our analyses.
Statistical analysis
Baseline demographics were compared between treatment groups using the chi-square test for categorical variables and the two-sample rank sum test for continuous variables. Fisher’s exact test was used to compare smoking abstinence rates at Weeks 10 and 24 between treatment groups. Treatment differences at baseline, Week 10, and Week 24 for exercise stage of change were compared between treatment groups using the two-sample rank sum test. Treatment differences at baseline, Week 10, and Week 24 for the HRSD, the PANAS, PAR, and participant weight were evaluated using the two-sample rank sum test. In all cases, two-tailed p values ≤ .05 were considered statistically significant. Missing data were handled with complete-case analysis, with the exception of smoking status, where participants with missing data were classified as smoking.
Results
Participants
Figure 1 summarizes participant recruitment and completion information. Table 1 presents the baseline characteristics by treatment group. The treatment conditions were similar on baseline characteristics (p > .05 for all cases).
Table 1.
Baseline characteristics
| Health education (n = 30) |
Exercise counseling (n = 30) |
|||
| Characteristics | M (SD) | n (%) | M (SD) | n (%) |
| Age, years | 41.8 (12.1) | 40.9 (11.8) | ||
| Race | ||||
| White | 30 (100) | 29 (97) | ||
| African American | 1 (3) | |||
| Marital status | ||||
| Never married | 6 (20) | 6 (20) | ||
| Married | 14 (47) | 10 (33) | ||
| Divorced/separated/widowed | 10 (33) | 14 (47) | ||
| Education | ||||
| High school | 9 (30) | 6 (20) | ||
| Post high school/college graduate | 19 (63) | 20 (67) | ||
| Other | 2 (7) | 4 (13) | ||
| Number of cigarettes smoked per day | 21.6 (7.3) | 20.0 (7.8) | ||
| 1–19 | 10 (33) | 13 (43) | ||
| 20–39 | 19 (63) | 16 (53) | ||
| ≥40 | 1 (3) | 1 (3) | ||
| Number of years of cigarette smoking | 21.6 (11.1) | 21.1(12.4) | ||
| Number of serious attempts to stop smoking | ||||
| 0 | 1 (3) | 3 (10) | ||
| 1 | 5 (17) | 4 (13) | ||
| 2–5 | 18 (62) | 18 (60) | ||
| ≥6 | 5 (17) | 5 (17) | ||
| Missing | 1 | |||
| Longest time without cigarettes | ||||
| <1 day | 1 (3) | 2 (7) | ||
| 1–30 days | 9 (30) | 8 (27) | ||
| 1–12 months | 15 (50) | 13 (43) | ||
| >1 year | 5 (17) | 7 (23) | ||
| FTND score | 6.2 (2.3) | 5.7 (2.4) | ||
| <6 | 10 (33) | 14 (47) | ||
| ≥6 | 20 (67) | 16 (53) | ||
| Weight Concerns Scale score | 6.6 (2.1) | 5.8 (2.2) | ||
| CES-D score | 32.4 (9.6) | 29.8 (9.3) | ||
| Treatment for depression | ||||
| Current psychotherapy | 11 (37) | 7 (23) | ||
| Current pharmacotherapy | 19 (63) | 16 (53) | ||
Note. FTND, Fagerström Test for Nicotine Dependence. CES-D, Center for Epidemiological Studies Depression Scale.
Intervention participation and quality
Ten of the 19 (53%) exercise counseling participants at Week 10 and 11 of the 20 (57%) health education participants at Week 10 had attended all 10 intervention sessions (p = 1.0). Exercise counseling participants attended an average (SD) of 7.6 (3.5) sessions during the intervention phase, while health education participants attended an average (SD) of 8.2 (2.7) sessions (p = .59). Participants in the exercise counseling condition reported nicotine patch use on 36% of the days during the nicotine patch phase compared with 31% for health education participants (p = .48). Exercise counseling participants exercised on 49% of the days during the intervention phase, while health education participants read assigned health education material on 21% of those days (p = .003).
Ten percent of completed sessions were observed, and feedback was given to interventionists. In more than 90% of observed sessions, the major content areas designated in the treatment protocol were delivered. Quantitative and qualitative data (observer comments) from session observations revealed that all counselors used positive and appropriate skills (empathy and positive reinforcement) and handled participant resistance, disclosure of frustration, stressors, and other challenges very well. Exercise counselors appeared somewhat more rushed than education counselors, especially when participants were encountering barriers to exercise behavior change. Ratings from participants and counselors following each session were very similar regarding content delivered. According to counselors, the primary threat to deviation from the protocol was the participant wanting to spend more time with the counselors than allotted and discussing personal topics unrelated to session content. Counselors completed additional training focused on strategies that protect rapport but emphasize a time-limited intervention session.
Smoking cessation rates.
There were no significant group differences on the 7-day point prevalence abstinence rate at Week 10 (EOT) or Week 24. Specifically, at Week 10, 3 of the 19 (17%) women in the exercise counseling condition and 4 of the 20 (23%) women in the health education condition were abstinent from smoking (p = .75). At Week 24, the 7-day point prevalence smoking abstinence rates were less than 7% (1 of 16 [6.3%] exercise participants, 1 of 15 [6.70%] health education participants; p = 1.0).
Mood, exercise, and weight at EOT and follow-up.
Table 2 displays the data for each treatment group at baseline, Week 10, and Week 24 for the HRSD, the PANAS, the PAR interview, and participant weight in kilograms. The change from baseline at Weeks 10 and 24 is also shown for each variable. Health education participants had a significantly greater reduction in depression scores at Week 10 on the HRSD than the exercise counseling participants. Exercise counseling participants significantly improved in change from baseline for the PANAS at Week 24. Exercise counseling participants significantly increased their physical activity at Weeks 10 and 24 and also had significant weight gains at these two timepoints.
Table 2.
Between- and within-group differences at baseline, EOT, and follow-up
| Health education |
Exercise counseling |
||||||
| Change from baseline | Change from baseline | ||||||
| Measure | Week | n | M (SD) | M (SD) | n | M (SD) | M (SD) |
| Hamilton Rating Scale | 0 | 30 | 15.4 (9.3) | 30 | 12.8 (6.0) | ||
| 10 | 20 | 12.0 (7.8) | −3.9 (11.1)† | 19 | 12.9 (7.5) | 2.1 (7.9)† | |
| 24 | 15 | 13.1 (9.4) | −2.3 (8.3) | 16 | 7.4 (4.6) | −2.6 (4.8) | |
| PANAS positive | 0 | 30 | 22.3 (5.8) | 30 | 22.7 (7.6) | ||
| 10 | 19 | 23.5 (8.0) | 2.0 (8.8) | 19 | 22.8 (8.7) | −0.1 (11.1) | |
| 24 | 15 | 24.7 (8.9) | 1.4 (6.3) | 16 | 27.6 (7.3) | 4.4 (9.2) | |
| PANAS negative | 0 | 30 | 23.5 (8.3) | 30 | 24.1 (7.3) | ||
| 10 | 19 | 19.5 (6.4) | −3.2 (11.2) | 19 | 20.6 (9.2) | −0.5 (12.3) | |
| 24 | 15 | 19.9 (9.2) | −2.6 (6.3) | 16 | 17.3 (4.1) | −4.1 (5.1)** | |
| Physical activity recall | 0 | 30 | 159.5 (173.1) | 30 | 108.0 (121.6) | ||
| 10 | 20 | 170.0 (193.2) | 10.5 (237.4)† | 19 | 213.9 (149.3) | 106.6 (191.8)†** | |
| 24 | 15 | 190.7 (140.2) | 37.7 (193.2) | 16 | 261.6 (233.8) | 142.8 (237.5)* | |
| Weight (kilograms) | 0 | 29 | 73.6 (15.7) | 30 | 76.0 (15.1) | ||
| 10 | 19 | 76.8 (15.3) | 0.7 (2.4) | 19 | 81.2 (14.0) | 1.9 (2.4)** | |
| 24 | 15 | 79.8 (17.5) | 1.1 (3.9) | 16 | 82.3 (13.9) | 2.2 (3.7)* | |
| VO2 test | 0 | 30 | 25.5 (5.4) | 30 | 25.0 (4.4) | ||
| 10 | 18 | 24.8 (4.8) | 0.2 (2.2) | 17 | 24.4 (4.3) | 0.7 (2.3) | |
Note. Please compare the ns for each timepoint in comparison to ns in Figure 1 to identify where data were missing. When data were missing for variables above, complete-case analysis was completed. Physical Activity Recall variable was total minutes of moderate, hard, and very hard activity. PANAS, Positive and Negative Affect Scales; VO2, maximum oxygen consumption.
.05 < p < .10 from two-sample rank sum test comparing the health education group to the exercise counseling group.
.01 < p ≤ .05 from one-sample signed-rank test comparing the change from baseline to 0 within the specific group.
.001 < p ≤ .01 from one-sample signed-rank test comparing the change from baseline to 0 within the specific group.
Over the course of the study, 30% of participants made a change in their antidepressant treatment (e.g., change dosage, initiate medication, change medication) and 20% made a change in psychotherapy (e.g., initiated or changed frequency of therapy). There were no significant differences between groups on change in depression treatments during course of study.
Exercise stage of change.
At baseline, there was no difference (p = .72) in exercise stage of change between groups (see table 3). At Week 10, 74% of exercise counseling participants were in the action stage of change versus 40% in the health education group (p = .01).
Table 3.
Exercise stage of change
| Health education, n (%) | Exercise counseling, n (%) | p valuea | |
| Baseline | .72 | ||
| Precontemplation | 2 (7) | 3 (10) | |
| Contemplation | 24 (80) | 23 (77) | |
| Preparation | 1 (3) | 3 (10) | |
| Action | 1 (3) | 1 (3) | |
| Maintenance | 2 (7) | 0 (0) | |
| Week 10 | .01 | ||
| Precontemplation | 1 (5) | 0 (0) | |
| Contemplation | 9 (45) | 3 (16) | |
| Preparation | 2 (10) | 1 (5) | |
| Action | 8 (40) | 14 (74) | |
| Maintenance | 0 (0) | 1 (5) | |
| Missing | 10 | 11 | |
| Week 24 | .45 | ||
| Precontemplation | 0 (0) | 0 (0) | |
| Contemplation | 5 (33) | 5 (33) | |
| Preparation | 6 (40) | 2 (13) | |
| Action | 1 (7) | 3 (20) | |
| Maintenance | 3 (20) | 5 (33) | |
| Missing | 15 | 15 |
Note. Percentages may not add to 100 due to rounding.
Two-sample rank sum test.
Discussion
This study supports the feasibility of engaging depressed women in an exercise intervention for smoking cessation. Despite study demands (e.g., numerous assessments including treadmill test, time-intensive clinical interviews, weekly meetings, and multiple behavior changes including use of nicotine patch and exercise) and the additional challenges of study participation while depressed, 65% of participants completed the 10 weeks of treatment (20 of 30 health education participants; 19 of 30 exercise counseling participants). This intervention adherence rate approximates that of studies examining exercise intervention session participation in nondepressed populations (e.g., 72.1% of smoking cessation sessions and 70.5% of weekly onsite supervised exercise sessions; Marcus et al., 2005). Our participant adherence to treatment rate is fairly impressive given that participants enrolled in this study were quite depressed, with depression scores in the range of clinical depression, and most receiving active treatment (medication and/or therapy) for their depression. Previous research has shown that adherence to health behavior is affected by depression (Wing et al., 2002) and that higher depression scores are associated with lower self-efficacy for exercise and more tendency to get off track and feel like a failure (Vickers, Nies, Patten, Dierkhising, & Smith, 2006). Despite the additional challenges of behavior change for depressed people, exercise counseling participants in this study were able to increase significantly their exercise frequency and exercise stage of change while attempting to quit smoking.
The exercise intervention was not associated with improved smoking abstinence rates compared with the contact control condition. The smoking abstinence rate at follow-up was low (7% using intention-to-treat analysis with 48% missing data), despite the fact that the participants had received a state-of-the-art treatment for smoking cessation (pharmacotherapy + behavioral counseling) (Fiore et al., 2008). However, results of a recent meta-analysis of controlled nicotine patch trials indicated that the rates of quitting smoking due to nicotine patch versus placebo are lower in women than in men (Perkins & Scott, 2008). Thus, future studies may benefit from supplementing nicotine patch treatment or using an alternative smoking cessation medication. Further, Ussher et al. (2003) found that an exercise counseling intervention similar to that used in the present study did not increase rates of smoking cessation compared with a control condition, and it was concluded that the exercise counseling did not increase levels of physical activity sufficiently. A higher intensity of exercise may have improved the smoking abstinence rates. Marcus et al. (1999, 2005) previously found that vigorous intensity but not moderate intensity exercise was superior to a health education contact control condition for treating smokers. Further, lower rates of exercise adherence were found for moderate intensity than for vigorous intensity exercise. In the vigorous intensity trial, participants completed a majority of their exercise at a supervised gym-based setting, whereas in the moderate intensity trial, the majority of exercise was completed at home. Further research is needed to evaluate the efficacy of vigorous intensity versus moderate intensity exercise and gym versus home-based exercise formats for depressed women attempting smoking cessation.
Future studies with depressed women smokers should limit participant burden and increase within-treatment social support. Depressive symptoms impacted almost every aspect of participation in this study. Many participants reported feeling burdened by study paperwork and assessments, and many participants wished to talk about psychosocial stressors and other depression-related content during intervention sessions. Exercise counseling participants often focused on the barriers to exercise and were quite self-critical when not meeting their exercise goals. Consequently, study staff must be trained to balance the empathy and support necessary to help depressed participants complete a demanding research study with adherence to assessments and intervention protocol. Future studies should carefully plan for and monitor treatment fidelity (Bellg et al., 2004; Waltz, Addis, Koerner, & Jacobson, 1993)
The majority of study participants across the two groups reported regret that the study was ending, as they so valued the social support associated with the weekly study visits. For instance, a group walking program or other exercise intervention that involves support may be particularly appealing to depressed women. Qualitative research methods may be useful in elucidating exercise and smoking cessation intervention preferences among depressed women who smoke.
There are several limitations to this pilot feasibility study. The study was sufficiently large for a feasibility or pilot study (Lancaster, Dodd, & Williamson, 2004), but it was relatively small compared with previous exercise intervention trials for smokers (e.g., Marcus et al., 2005). The sample was predominately White women with postsecondary education, which limits generalizability to the general population of smokers. Although we assessed changes in depression treatment (medication/therapy), we did not assess the impact of this on study outcomes, which would be necessary in future efficacy trials. Alternatively, future studies could attempt to recruit depressed smokers not currently on antidepressant medication or standardize medication as part of the trial. Most participants in our study had depression scores in the moderate to severe range, and exercise interventions may be more effective for women with milder depression. Many women decided not to participate in the study and, of those enrolled and randomized, nearly half dropped out by Week 24 follow-up, despite study staff's best efforts to be accommodating with schedule conflicts, to call and encourage those who missed a session, and to be supportive following lapses. The demands of study assessments (treadmill test, structured interviews, and daily activity records), psychosocial stressors, and ambivalence about quitting smoking all contributed to nonparticipation and dropout. Consequently, those that remained in the study likely represent a more motivated group and do not fully represent the general population of depressed smokers. Because attrition results in missing data, larger studies with depressed smokers should utilize best methods for handling missing data (Fielding, Maclennan, Cook, & Ramsay, 2008; Wood, White, & Thompson, 2004).
Women with depression represent a difficult-to-treat subgroup of smokers. Our participants had clinical levels of depression and a significant smoking history with previous quit attempts. Our study suggests that an exercise intervention is feasible among depressed female smokers and is associated with increased exercise. Additional research is needed to identify effective strategies for assisting depressed women in quitting smoking and maintaining their abstinence, perhaps with higher intensity exercise, supervised exercise, group-based support, and relapse prevention strategies for both exercise and smoking behavior.
Funding
This study was supported by grant CA94760 from the National Cancer Institute and M01-RR00585, General Clinical Research Centers Program.
Declaration of Interests
None declared.
Supplementary Material
Acknowledgments
The authors thank Betty Wirt, Donna Rasmussen, Carla Morrey, Julie Larson, Virginia Caspersen, and all our colleagues at the Mayo Clinic Nicotine Research Center and Mayo Clinic Section of Patient Education, who assisted with this study.
References
- Abrams DB, Monti PM, Pinto B, Elder JP, Brown RA, Jacobus SI. Psychosocial stress and coping in smokers who relapse or quit. Health Psychology. 1987;6:289–303. doi: 10.1037//0278-6133.6.4.289. [DOI] [PubMed] [Google Scholar]
- Bagby RM, Ryder AG, Schuller DR, Marshall MB. The Hamilton Depression Rating Scale: Has the gold standard become a lead weight? American Journal of Psychiatry. 2004;161:2163–2177. doi: 10.1176/appi.ajp.161.12.2163. Review. [DOI] [PubMed] [Google Scholar]
- Bandura A. Self-efficacy: Toward a unifying theory of behavioral change. Psychological Review. 1977;84:191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- Bandura A. Exercise of personal agency through the self-efficacy mechanism. In: Schwarzer R, editor. Self-efficacy: Thought control of action. Washington, DC: Hemisphere Publishing Corporation; 1992. pp. 3–38. [Google Scholar]
- Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci DS, Ory M, et al. Treatment Fidelity Workgroup of the NIH Behavior Change Consortium. Enhancing treatment fidelity in health behavior change studies: Best practices and recommendations from the NIH Behavior Change Consortium. Health Psychology. 2004;23:443–451. doi: 10.1037/0278-6133.23.5.443. [DOI] [PubMed] [Google Scholar]
- Berlin I, Covey LS. Pre-cessation depressive mood predicts failure to quit smoking: The role of coping and personality traits. Addiction. 2006;101:1814–1821. doi: 10.1111/j.1360-0443.2006.01616.x. [DOI] [PubMed] [Google Scholar]
- Bjornson W, Rand C, Connett JE, Lindgren P, Nides M, Pope F, et al. Gender differences in smoking cessation after 3 years in the Lung Health Study: Trials and interventions. American Journal of Public Health. 1995;85:223–230. doi: 10.2105/ajph.85.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair SN, Haskell WL, Ho P, Paffenbarger RS, Jr, Vranizan KM, Farquhar JW, et al. Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. American Journal of Epidemiology. 1985;122:794–804. doi: 10.1093/oxfordjournals.aje.a114163. [DOI] [PubMed] [Google Scholar]
- Blumenthal JA, Babyak MA, Doraiswamy PM, Watkins L, Hoffman BM, Barbour KA, et al. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosomatic Medicine. 2007;69:587–596. doi: 10.1097/PSY.0b013e318148c19a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal JA, Babyak MA, Moore KA, Craighead WE, Herman S, Khatri P, et al. Effects of exercise training on older adults with major depression. Archives of Internal Medicine. 1999;159:2349–2356. doi: 10.1001/archinte.159.19.2349. [DOI] [PubMed] [Google Scholar]
- Borrelli B, Bock B, King T, Pinto B, Marcus BH. The impact of depression on smoking cessation in women. American Journal of Preventive Medicine. 1996;12:378–387. [PubMed] [Google Scholar]
- Borrelli B, Mermelstein R. The role of weight concern and self-efficacy in smoking cessation and weight gain among smokers in a clinic-based cessation program. Addictive Behaviors. 1998;23:609–622. doi: 10.1016/s0306-4603(98)00014-8. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Copeland AL, Saper ZL. Programmed therapeutic messages as a smoking treatment adjunct: Reducing the impact of negative affect. Health Psychology. 1995;14:41–47. doi: 10.1037//0278-6133.14.1.41. [DOI] [PubMed] [Google Scholar]
- Buckley TC, Mozley SL, Holohan DR, Walsh K, Beckham JC, Kassel JD. A psychometric evaluation of the Fagerström Test for Nicotine Dependence in PTSD smokers. Addictive Behaviors. 2005;30:1029–1033. doi: 10.1016/j.addbeh.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Cleary R, Guy W. Factor analysis of the Hamilton Depression Scale. Drug Experimental & Clinical Research. 1987;1:115–120. [Google Scholar]
- Dunn AL, Marcus BH, Kampert JB, Garcia ME, Kohl HW, Blair SN. Comparison of lifestyle and structured interventions to increase physical activity and cardiorespiratory fitness: A randomized trial. Journal of the American Medical Association. 1999;281:327–334. doi: 10.1001/jama.281.4.327. [DOI] [PubMed] [Google Scholar]
- Dunn AL, Trivedi MH, Kampert JB, Clark CG, Chambliss HO. Exercise treatment for depression: Efficacy and dose response. American Journal of Preventive Medicine. 2005;28:1–8. doi: 10.1016/j.amepre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Fagerstrom KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addictive Behaviors. 1978;3:235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- Faulkner G, Taylor A, Munro S, Selby P, Gee C. The acceptability of physical activity programming within a smoking cessation service for individuals with severe mental illness. Patient Education and Counseling. 2007;66:123–126. doi: 10.1016/j.pec.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Fielding S, Maclennan G, Cook JA, Ramsay CR. A review of RCTs in four medical journals to assess the use of imputation to overcome missing data in quality of life outcomes. Trials. 2008;9:51. doi: 10.1186/1745-6215-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Treating tobacco use and dependence: 2008 update. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service; 2008. [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Jacobs DR, Jr, Ainsworth BE, Hartman TJ, Leon AS. A simultaneous evaluation of 10 commonly used physical activity questionnaires. Medicine and Science in Sports and Exercise. 1993;25:81–91. doi: 10.1249/00005768-199301000-00012. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, et al. Lifetime and 12-month prevalence of DSM-III-R Psychiatric Disorders in the United States: Results from the National Comorbidity Survey. Archives of General Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Kinnunen T, Doherty K, Militello FS, Garvey AJ. Depression and smoking cessation: Characteristics of depressed smokers and effects of nicotine replacement. Journal of Consulting and Clinical Psychology. 1996;64:791–798. doi: 10.1037//0022-006x.64.4.791. [DOI] [PubMed] [Google Scholar]
- Kozlowski LT, Porter CQ, Orleans CT, Pope MA, Heatherton T. Predicting smoking cessation with self-reported measures of nicotine dependence: FTQ, FTND, and HSI. Drug and Alcohol Dependence. 1994;34:211–216. doi: 10.1016/0376-8716(94)90158-9. [DOI] [PubMed] [Google Scholar]
- Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: Recommendations for good practice. Journal of Evaluation in Clinical Practice. 2004;10:307–312. doi: 10.1111/j..2002.384.doc.x. [DOI] [PubMed] [Google Scholar]
- Lerman C, Audrain J, Orleans CT, Boyd R, Gold K, Main D, et al. Investigation of mechanisms linking depressed mood to nicotine dependence. Addictive Behaviors. 1996;21:9–19. doi: 10.1016/0306-4603(95)00032-1. [DOI] [PubMed] [Google Scholar]
- Lerman C, Caporaso N, Main D, Audrain J, Boyd NR, Bowman ED, et al. Depression and self-medication with nicotine: The modifying influence of the dopamine D4 receptor gene. Health Psychology. 1998;17:56–62. doi: 10.1037//0278-6133.17.1.56. [DOI] [PubMed] [Google Scholar]
- Marcus BH, Albrecht AE, King TK, Parisi AF, Pinto BM, Roberts M, et al. The efficacy of exercise as an aid for smoking cessation in women. Archives of Internal Medicine. 1999;159:1229–1234. doi: 10.1001/archinte.159.11.1229. [DOI] [PubMed] [Google Scholar]
- Marcus BH, Albrecht AE, Niaura RS, Abrams DB, Thompson PD. Usefulness of physical exercise for maintaining smoking cessation in women. American Journal of Cardiology. 1991;68:406–407. doi: 10.1016/0002-9149(91)90843-a. [DOI] [PubMed] [Google Scholar]
- Marcus BH, Albrecht AE, Niaura RS, Taylor ER, Simkin LR, Feder SI, et al. Exercise enhances the maintenance of smoking cessation in women. Addictive Behaviors. 1995;20:87–92. doi: 10.1016/0306-4603(94)00048-4. [DOI] [PubMed] [Google Scholar]
- Marcus BH, Forsyth LAH. Motivating people to be physically active. 2nd ed. 2008. ISBN-10:0-7360-7247-0. Human Kinetics Publishers. [Google Scholar]
- Marcus BH, Lewis BA, Hogan J, King TK, Albrecht AE, Bock B, et al. The efficacy of moderate-intensity exercise as an aid for smoking cessation in women: A randomized controlled trial. Nicotine & Tobacco Research. 2005;7:871–880. doi: 10.1080/14622200500266056. [DOI] [PubMed] [Google Scholar]
- Marcus BH, Rossi JS, Selby VC, Niaura RS, Abrams DB. The stages and processes of exercise adoption and maintenance in a worksite sample. Health Psychology. 1992;11:386–395. doi: 10.1037//0278-6133.11.6.386. [DOI] [PubMed] [Google Scholar]
- Marcus BH, Simkin LR. The stages of exercise behavior. Journal of Sports Medicine & Physical Fitness. 1993;33:83–88. [PubMed] [Google Scholar]
- Niaura R, Britt DM, Shadel WG, Goldstein M, Abrams D, Brown R. Symptoms of depression and survival experience among three samples of smokers trying to quit. Psychology of Addictive Behaviors. 2001;15:13–17. doi: 10.1037/0893-164x.15.1.13. [DOI] [PubMed] [Google Scholar]
- Pate RR, Pratt M, Blair SN, Haskell WL, Macera CA, Bouchard C, et al. Physical activity and public health: A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. Journal of the American Medical Association. 1995;273:402–407. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Scott J. Sex differences in long-term smoking cessation rates due to nicotine patch. Nicotine & Tobacco Research. 2008;10:1245–1250. doi: 10.1080/14622200802097506. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depressive scale for research in the general population. Journal of Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Radloff LS, Locke BZ. The Community Mental Health Assessment Survey and the CES-D scale. In: Weissman MM, Myers JK, Ross CE, editors. Community Surveys of Psychiatric Disorders. New Brunswick, NJ: Rutgers University Press; 1986. pp. 177–189. [Google Scholar]
- Rausch JL, Nichinson B, Lamke C, Matloff J. Influence of negative affect on smoking cessation treatment outcome: A pilot study. British Journal of Addiction. 1990;85:929–933. doi: 10.1111/j.1360-0443.1990.tb03723.x. [DOI] [PubMed] [Google Scholar]
- Skinner HA. The Drug Abuse Screening Test. Addictive Behaviors. 1982;7:363–371. doi: 10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- Swenson WM, Morse RM. The use of a self-administered alcoholism screening test (SAAST) in a medical center. Mayo Clinic Proceedings. 1975;127:89–94. [PubMed] [Google Scholar]
- Taylor AH, Ussher MH, Faulkner G. The acute effects of exercise on cigarette cravings, withdrawal symptoms, affect and smoking behaviour: A systematic review. Addiction. 2007;102:534–543. doi: 10.1111/j.1360-0443.2006.01739.x. [DOI] [PubMed] [Google Scholar]
- Ussher M. Tobacco Addiction Module of the Cochrane Database of Systematic Reviews, Issue 1. Oxford: The Cochrane Collaboration; 2005. Exercise interventions in smoking cessation. Update Software, CD002295. [Google Scholar]
- Ussher MH, Taylor AH, West R, McEwen A. Does exercise aid smoking cessation? A systematic review. Addiction. 2000;95:199–208. doi: 10.1046/j.1360-0443.2000.9521996.x. [DOI] [PubMed] [Google Scholar]
- Ussher MH, West R, McEwen A, Taylor A, Steptoe A. Efficacy of exercise counseling as an aid to smoking cessation: A randomized controlled trial. Addiction. 2003;98:523–532. doi: 10.1046/j.1360-0443.2003.00346.x. [DOI] [PubMed] [Google Scholar]
- Vickers KS, Nies MA, Patten CA, Dierkhising R, Smith SA. Patients with diabetes and depression may need additional support for exercise. American Journal of Health Behavior. 2006;30:353–362. doi: 10.5555/ajhb.2006.30.4.353. [DOI] [PubMed] [Google Scholar]
- Waltz J, Addis ME, Koerner K, Jacobson NS. Testing the integrity of a psychotherapy protocol: Assessment of adherence and competence. Journal of Consulting and Clinical Psychology. 1993;61:620–630. doi: 10.1037//0022-006x.61.4.620. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS Scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wetter DW, Kenford SL, Smith SS, Fiore MC, Jorenby DE, Baker TB. Gender differences in smoking cessation. Journal of Consulting and Clinical Psychology. 1999;67:555–562. doi: 10.1037//0022-006x.67.4.555. [DOI] [PubMed] [Google Scholar]
- Wing RR, Phelan S, Tate D. The role of adherence in mediating the relationship between depression and health outcomes. Journal of Psychosomatic Research. 2002;53:877–881. doi: 10.1016/s0022-3999(02)00315-x. [DOI] [PubMed] [Google Scholar]
- Wood AM, White IR, Thompson SG. Are missing outcome data adequately handled? A review of published randomized controlled trials in major medical journals. Clinical Trials. 2004;1:368–376. doi: 10.1191/1740774504cn032oa. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.