Abstract
The cell surface receptor αvβ6 is epithelial-specific and its expression is tightly regulated; it is low or undetectable in adult tissues but has been shown to be increased in many different cancers, including pancreatic, cervical, lung, and colon cancer. Studies have described αvβ6 as prognostic biomarker linked to poor survival. We have recently demonstrated the feasibility of imaging αvβ6 in vivo by PET using the peptide [18F]FBA-A20FMDV2. Here we describe improved αvβ6 imaging agents and test their efficacy in a mouse model with endogenous αvβ6-expression. The modified compounds maintained high affinity for αvβ6 and >1000-fold selectivity over related integrins (by ELISA), and demonstrated significantly improved, αvβ6-dependent binding in cell-based assays (>60% binding vs <10% for [18F]FBA-A20FMDV2). In vivo studies using either a melanoma cell line (transduced αvβ6-expression) or the BxPC-3 human pancreatic carcinoma cell line (endogenous αvβ6-expression) revealed that the modified compounds showed significantly improved tumor retention. This, along with good clearance of nonspecifically bound activity, particularly for the new radiotracer [18F]FBA-PEG28-A20FMDV2, resulted in improved PET-imaging. Tumor/pancreas and tumor/blood biodistribution-ratios of >23:1 and >47:1, respectively, were achieved at 4 h. Significantly, [18F]FBA-PEG28-A20FMDV2 was superior to [18F]-fluorodeoxyglucose ([18F]FDG) in imaging the BxPC-3 tumors. Pancreatic ductal adenocarcinoma is highly metastatic and current preoperative evaluation of resectability using non-invasive imaging has limited success, with most patients having metastases at time of surgery. The fact that these tumors express αvβ6 suggests that this probe has significant potential for the in vivo detection of this malignancy, thus having important implications for patient care and therapy.
INTRODUCTION
In recent years, positron emission tomography (PET) imaging using 2-[18F]fluoro-2-deoxy-D-glucose ([18F]FDG), has become routine for detection of tumors and monitoring of treatment response (1-3). [18F]FDG, a radiolabeled analogue of glucose, is taken up and trapped primarily in cells with high metabolism. As glucose hypermetabolism is commonly found in inflammation as well as in many malignancies, distinguishing cancer from other hypermetabolic states remains a challenge (1, 4, 5). Although PET has been combined with other imaging modalities, most notably computed tomography (CT) which relies on subtle differences in soft tissue density to distinguish between normal and malignant tissues (5-7), [18F]FDG-PET/CT still leaves considerable room for improvement in diagnosis and clinical management of many cancers (8). The availability of more disease-specific imaging agents (7) could help to mitigate the limited specificity of [18F]FDG for malignant tissue. Rather than relying on slight changes in tissue density or metabolic rate alone, targeting disease-specific tissue markers could considerably aid in tumor-detection and localization. The feasibility of this approach has been demonstrated with [18F]galacto-RGD, a small radiolabeled peptide used for PET imaging of the cancer-related integrin αvβ3 (9). In contrast to αvβ3, other members of the integrin family have received relatively little attention. However, newly emerging evidence indicates that αvβ6 may also be an important target for diagnosis and treatment of cancer (10-14). The integrin αvβ6 was first identified in a human pancreatic carcinoma cell line (15). Subsequent studies established that although αvβ6 is epithelial-specific, its expression is low or undetectable in healthy adult tissues. Upregulation has been recognized during tissue remodeling, including inflammation and wound healing (16-18). Significantly, expression has also been shown to be increased in many different cancers (13, 19). Among gastroenteropancreatic adenocarcinomas αvβ6-expression was found to be strongest in pancreatic ductal adenocarcinomas (PDAC) (20). Moreover, αvβ6 has recently been described as prognostic indicator, with high levels of expression correlating with poor prognosis for cancer of the colon, cervix, lung, and stomach (10-12, 21). Antibody-blockade of αvβ6 was shown to inhibit tumor progression in vivo in animal models (13). Thus, mounting evidence implicates αvβ6 as an important and biologically relevant target for molecular imaging of cancer.
Recently, we demonstrated the ability to selectively image αvβ6-expressing tumors in vivo with microPET using the αvβ6-specific [18F]fluorobenzoyl labeled peptide [18F]FBA-A20FMDV2 (22). While targeted imaging was achieved, low uptake and poor retention in the target tissue limited its general utility. For this current study, we conjugated small, monodisperse poly(ethylene glycol) (PEG) polymers to A20FMDV2 to improve pharmacokinetics, generating the two new radiotracers [18F]FBA-PEG28-A20FMDV2 and [18F]FBA-(PEG28)2-A20FMDV2. We report that both PEGylated A20FMDV2 variants showed significantly improved retention in two different αvβ6-expressing human tumor xenograft models: DX3puroβ6, a human melanoma cell line transduced to express αvβ6, and BxPC-3, a human pancreatic carcinoma cell line that endogenously expresses αvβ6. In the BxPC-3 model, retention of [18F]FBA-PEG28-A20FMDV2 in tumors was 12-fold greater than retention of the non-PEGylated [18F]FBA-A20FMDV2. Tumor/pancreas and tumor/blood ratios of >23:1 and >47:1, respectively, were achieved with [18F]FBA-PEG28-A20FMDV2 at 4 h. The ability to non-invasively detect regional tumor invasion or occult metastatic disease using αvβ6 PET imaging would have a large impact on patient management, reducing the number of unnecessary surgeries of unresectable disease (23).
METHODS
Reagents and cell lines
Reagents, materials and DX3puro/DX3puroβ6 cell lines have been described previously (22). Pancreatic cancer cell lines were obtained from ATCC. Human latency associated peptide (TGFβ1-LAP) was purchased from Sigma. A Leica CM1850 cryostat (Leica Microsystems) was used for tissue sectioning.
Competitive binding ELISA
The in vitro efficacies of the peptides towards integrins αvβ6, αvβ3, αvβ5, α5β1, and αIIbβ3 were analyzed by competitive binding ELISA as described previously (22).
Flow cytometry
Integrin expression on the cell lines was determined as previously described (24).
Cell binding and internalization
A cell suspension (3.75 × 106 cells) was incubated with the radiotracer (0.2 μCi) in serum free medium (pH 7.2, 100 μL). For each radiotracer the cell lines were assayed concurrently (n = 4 samples/cell line/time point). The fraction of bound radioactivity was determined with a γ-counter (cell pellet vs. supernatant). To determine the fraction of internalized radioactivity, the cells were then treated with acidic wash buffer (0.2 mol/L sodium acetate, 0.5 mol/L sodium chloride, pH 2.5, 4°C) to release surface-bound activity (25). The internalized fraction was determined with a γ-counter (cell pellet vs. radioactivity released into supernatant).
Xenograft cell lines and animal model
All animal studies were carried out using male athymic nude mice (nu/nu; Charles River Laboratories) following approved protocols. DX3puroβ6 (αvβ6-positive) and DX3puro (control) cells were injected (n = 20 mice; 3 × 106 cells/cell line in 100 μL serum-free DMEM; Invitrogen) s.c. on opposite flanks in the shoulder region as described previously (22). BxPC-3 cells were injected (n = 30 mice; 1.5 × 106 cells in 100 μL 1:1 serum-free DMEM/MatriGel HC; BD Biosciences) s.c. in the left shoulder region. Studies commenced once tumors had reached a diameter of ∼0.5 cm.
Biodistribution studies
Radiotracers (∼15 to 30 μCi) in saline/PBS (150 to 200 μL) were administered and data analyzed as previously described (n = 3/time point) (22).
MicroPET imaging studies
The [18F]FBA-peptides or [18F]FDG (∼120 to 230 μCi) in saline/PBS (100 to 200 μL) were administered as previously described (n = 3/time point) (22). Two animals were imaged side-by-side in a feet-first prone position. For blocking studies the [19F]FBA-peptide (30 mg/kg as 10 mg/mL solution in saline) was administered 10 min before the radiotracer. Imaging data were collected and analyzed as previously described (22, 26).
Standard uptake values (SUVs) were computed by dividing the activity concentration in each voxel by the injected dose and then multiplying by the weight of the animal (26). Maximum intensity projection (MIP) images were created by collapsing across one dimension with the maximum value in each column of voxels.
Autoradiography studies
Frozen tissue slices (tumor and surrounding tissue, 50 μm) in freezing medium (Tissue-Tek, Sakura Finetek) were exposed to a storage phosphor-screen (GE Healthcare) over night. The screen was read at 50 μm resolution using a Storm 860 phosphorimager (GE Healthcare).
Statistical analysis
The data are reported as mean ± standard deviation (SD). Two-tailed Student’s t-tests were performed to evaluate statistical significance. P <0.05 was considered significant. In plots, the SD is smaller than the size of the symbol for some data points.
RESULTS
PEGylated peptide ligands for αvβ6
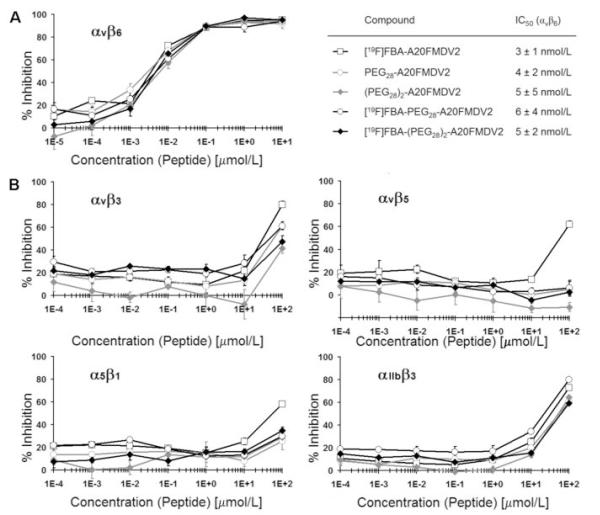
PEGylated A20FMDV2 variants were compared by ELISA to the established αvβ6 peptide-ligand FBA-A20FMDV2 (Fig. 1). They exhibited IC50 of 3 to 6 nmol/L, similar to FBA-A20FMDV2. Inhibition of binding by the natural ligands to integrins αvβ3, αvβ5, α5β1, and αIIbβ3 required at least >1000-fold higher concentrations of peptide (IC50 >10 μmol/L), indicating that introduction of the PEG moieties did not have a deleterious effect on αvβ6 affinity and selectivity of A20FMDV2.
Figure 1.
Use of ELISA to measure the effect of introducing PEG groups with and without [19F]FBA on the ability of A20FMDV2 to inhibit binding of biotinylated (Bt) natural ligands to immobilized integrins. Peptides and biotinylated ligands were mixed and allowed to compete during one hour for binding to integrin αvβ6 (A); αvβ3, αvβ5, α5β1, or αIIbβ3 (B). Ligands used were Bt-fibronectin (αvβ6, α5β1), Bt-vitronectin (αvβ3, αvβ5), or Bt-fibrinogen (αIIbβ3). Plots represent triplicate experiments at each concentration. Points, % inhibition; bars, SD. IC50 values were derived from graph A.
Integrin αvβ6 expression in human pancreatic cancer cell lines
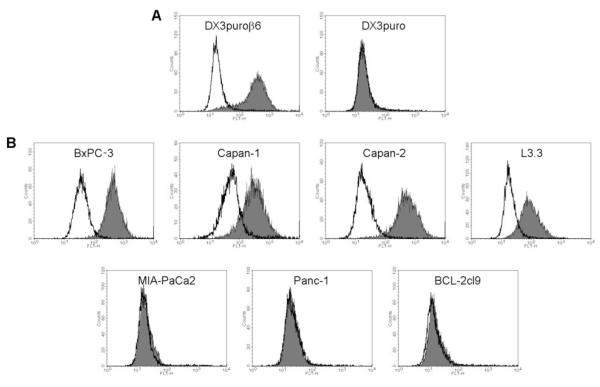
Seven cell lines were screened by flow cytometry for αvβ6-expression (Fig. 2). For reference, the melanoma cell lines DX3puroβ6, stably transduced to express αvβ6, and its paired control line DX3puro, lacking expression of αvβ6, were analyzed as positive and negative control, respectively; both of these cell lines express similar levels of other integrins, including αvβ3, αvβ5, and α5β1 (22). BxPC-3 and Capan-2 showed high αvβ6 expression levels, comparable to that of the positive control DX3puroβ6. Capan-1 and L3.3 showed intermediate expression of αvβ6, while MIA-PaCa2, Panc-1, and BCL-2cl9 demonstrated expression levels equivalent to that of the negative control DX3puro.
Figure 2.
Expression of integrin αvβ6 by cell lines used in this study. Flow cytometry histogram plots for an αvβ6-positive (DX3puroβ6) and an αvβ6-negative (DX3puro) cell line (A) and seven pancreatic cancer cell lines (B) were obtained. Levels of αvβ6 (gray histograms) were determined using the integrin αvβ6-specific antibody 10D5. Mouse IgG (MOPC 21; white histogram) was used as antibody control.
Cell binding and internalization
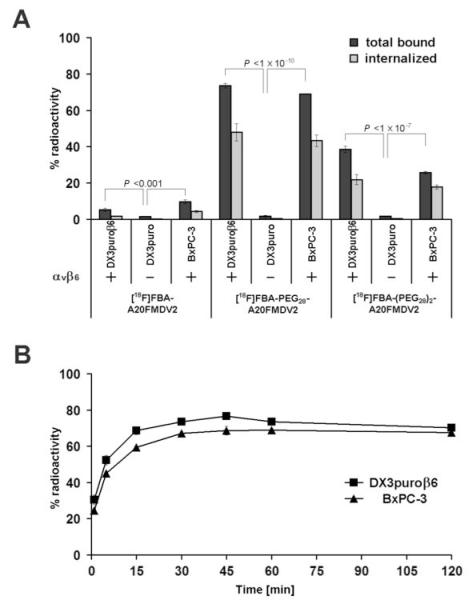
Binding of the radiotracers [18F]FBA-(PEG28)n-A20FMDV2 (n = 0, 1, 2; Supplementary Fig. S1, S2; (22)) to DX3puroβ6, DX3puro, and BxPC-3 cell lines was strongly dependent on the expression of αvβ6 by the cells and on the presence of a PEG unit in the radiotracer (Fig. 3). Whereas less than 2% of any radiotracer bound to the αvβ6-negative DX3puro within one hour (Fig 3A), 5.3 ± 0.8% and 9.7 ± 1.2% of [18F]FBA-A20FMDV2 bound to the αvβ6-expressing DX3puroβ6 and BxPC-3 cell lines, respectively. For [18F]FBA-PEG28-A20FMDV2 the binding levels increased significantly to 73.5 ± 1.3% and 68.9 ± 0.2%, respectively. Similarly, [18F]FBA-(PEG28)2-A20FMDV2 showed markedly elevated binding levels of 38.5 ± 1.7% and 25.8 ± 0.6%, respectively. Approximately two-thirds of the bound radioactivity was internalized in the αvβ6-expressing cell lines in the case of the two PEGylated radiotracers, while less than half of the bound [18F]FBA-A20FMDV2 was internalized. Incubation in the presence of excess non-radioactive [19F]FBA-PEG28A20FMDV2 (≥1μmol/L) completely abrogated binding of the radiotracer to DX3puroβ6 cells (Supplementary Fig S3).
Figure 3.
Binding and internalization of [18F]FBA-A20FMDV2, [18F]FBA-PEG28-A20FMDV2, and [18F]FBA-(PEG28)2-A20FMDV2 for DX3puroβ6, DX3puro, and BxPC-3 cell lines after a 1 hour incubation (A. Dark gray bars, total bound radioactivity; light gray bars, internalized) and binding kinetics of [18F]FBA-PEG28-A20FMDV2 for the αvβ6-positive cell lines DX3puroβ6 and BxPC-3 (B). Plots represent quadruplicate experiments with 3.75 × 106 cells at each condition or time point, respectively. Filled bars, points, % radioactivity in cell sample; thin bars, SD.
Figure 3B illustrates the rapid cell binding of radiotracer. Within one minute, approximately one quarter of [18F]FBA-PEG28-A20FMDV2 bound to DX3puroβ6 and BxPC-3 (30.5 ± 2.7% and 24.5 ± 1.2%, respectively). Binding levels approached their maximum within 15 to 30 minutes and then remained steady. When the two cell lines were incubated with [18F]FBA-PEG28-A20FMDV2 for 1 hour in the presence of latency associated peptide (LAP), the radiotracer was able to efficiently compete for binding: binding levels in presence of 5 nmol/L LAP remained at ≥75% of those observed under LAP-free conditions (Supplementary Fig. S4).
[18F]FBA-(PEG28)n-A20FMDV2 (n = 0, 1, 2) biodistribution in the DX3puro/DX3puroβ6 model
Initial in vivo evaluation of [18F]FBA-PEG28-A20FMDV2 and [18F]FBA-(PEG28)2-A20FMDV2 was performed using our previously described mouse model bearing the paired human xenografts of DX3puroβ6 (αvβ6-positive) and DX3puro (αvβ6-negative) (Supplementary Table S1). While uptake of both PEGylated radiotracers in the target tissue at 1 h post-injection (p.i.) was slightly below the level previously seen for the non-PEGylated [18F]FBA-A20FMDV (22), the introduction of the PEG unit(s) resulted in excellent retention of radioactivity in the αvβ6-positive tumor ([18F]FBA-PEG28-A20FMDV2, 1 h: 0.49 ± 0.12% ID/g, 4 h: 0.49 ± 0.04% ID/g; [18F]FBA-(PEG28)2-A20FMDV2, 1 h: 0.52 ± 0.09% ID/g, 4 h: 0.54 ± 0.08% ID/g; compared with [18F]FBA-A20FMDV2, 1 h: 0.66 ± 0.09% ID/g, 4 h: 0.06 ± 0.00% ID/g). A comparison of uptake in the two xenografts consistently showed preferential uptake in the αvβ6-positive xenograft over the αvβ6-negative xenograft, particularly for the PEGylated radiotracers. While [18F]FBA-A20FMDV resulted in a 4.3:1 maximal αvβ6-positive/-negative ratio, ratios of 8.0:1 and 9.2:1 were seen for [18F]FBA-PEG28-A20FMDV2 and [18F]FBA-(PEG28)2-A20FMDV2, respectively (P-values: see Supplementary Table S1).
[18F]FBA-(PEG28)n-A20FMDV2 (n = 0, 1, 2) biodistribution in the αvβ6-endogenous BxPC-3 model
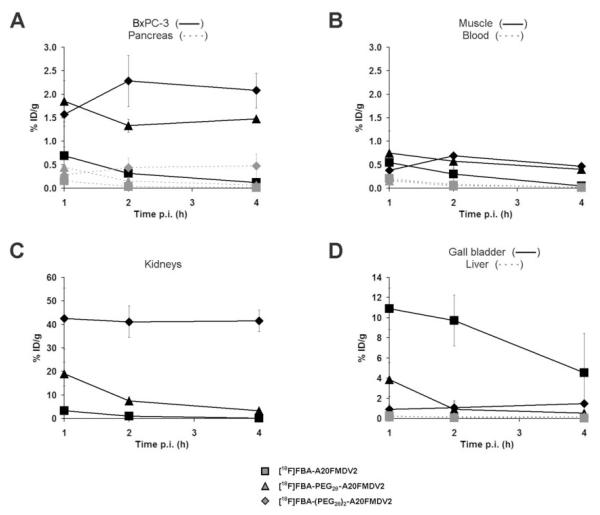
The feasibility of αvβ6-targeted pancreatic tumor detection in vivo was evaluated in the BxPC-3 mouse model (Fig. 4, Supplementary Table S1). For [18F]FBA-A20FMDV2 initial uptake in and washout from the BxPC-3 xenograft (1 h: 0.69 ± 0.19% ID/g, 4 h: 0.12 ± 0.03% ID/g) were similar to those seen in the DX3puroβ6 xenograft (1 h: 0.66 ± 0.09% ID/g, 4 h: 0.06 ± 0.00% ID/g). By contrast, for both of the PEGylated radiotracers uptake in the BxPC-3 xenograft 1 h after injection was three to four times higher than in the DX3puroβ6 xenograft, combined with excellent retention ([18F]FBA-PEG28-A20FMDV2, 1 h: 1.85 ± 0.44% ID/g, 4 h: 1.48 ± 0.04% ID/g; [18F]FBA-(PEG28)2-A20FMDV2, 1 h: 1.57 ± 0.25% ID/g, 4 h: 2.08 ± 0.37% ID/g). Autoradiography images obtained for the PEGylated radiotracers of BxPC-3 xenografts 1 h after injection also showed preferential, tumor specific uptake (Fig. 5).
Figure 4.
Evaluation of pharmacokinetics. Biodistribution data for [18F]FBA-A20FMDV2, [18F]FBA-PEG28-A20FMDV2, and [18F]FBA-(PEG28)2-A20FMDV2 in BxPC-3 tumors and healthy tissues obtained in male nude mice, expressed as decay corrected percent injected dose per gram of tissue (% ID/g ± SD). Points, % ID/g; bars, SD. Data were obtained 1, 2, and 4 hours after i.v. injection of the radiotracer (n = 3/time point/radiotracer) for the BxPC-3 tumor, pancreas (A); muscle, blood (B); kidneys (C); gall bladder, and liver (D). For all three radiotracers and time points, the corresponding differences in uptake in BxPC-3 vs pancreas were statistically significant (A; P-values: see Supplementary Table S1).
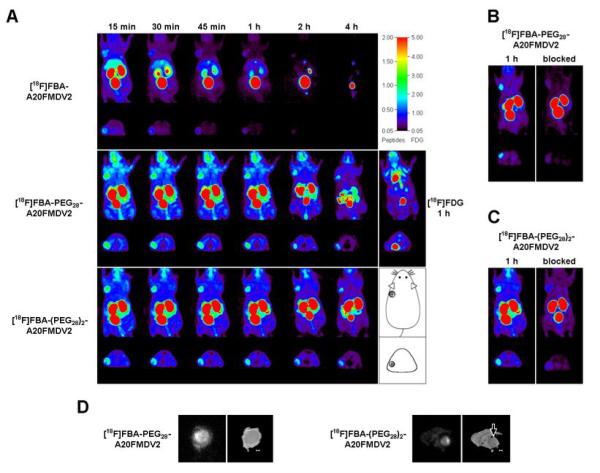
Figure 5.
In vivo evaluation of radiotracers in BxPC-3 xenograft bearing male nu/nu mice using microPET (A-C), as well as autoradiography (D). A, Representative coronal and transaxial maximum intensity projections (MIPs) obtained in the same animal after bolus injection of the radiotracers into the tail vein. For all [18F]FBA-peptides the first 4 panels were obtained as part of a dynamic 4 × 15 min scan started 15 min after injection. Subsequent panels depict 15 min scans at 2 h and 4 h after injection, respectively, with the animal having been awake between scans. The radioactivity was allowed to clear fully between injections of the different radiotracers. Panel A also depicts MIPs of an [18F]fluoro-2-deoxy-D-glucose ([18F]FDG) scan (middle row, right. Same animal). The location of the tumor is indicated in the diagram (lower right). B, Effect of prior administration of [19F]FBA-PEG28-A20FMDV2. Images obtained after administration of [18F]FBA-PEG28-A20FMDV2 only, left, and images obtained after first administering non-radioactive [19F]FBA-PEG-A20FMDV2 (i.v., 30 mg/kg) 10 min prior to [18F]FBA-PEG28-A20FMDV2, right, are shown. Both images were obtained 1 h after administration of the radiotracer. C, Same as (B) using [19F]FBA-(PEG28)2-A20FMDV2/[18F]FBA-(PEG28)2-A20FMDV2 in same animal as in (A). D, Autoradiography images of tumor slices obtained from animals sacrificed 1 h after injection of ∼500 μCi [18F]FBA-PEG28-A20FMDV2 or [18]FBA-(PEG28)2-A20FMDV2. Photographic images are shown for comparison (right). Bars, 0.1 cm; arrow, location of tumor.
Renal clearance was the major route of elimination for all three radiotracers (Fig. 4, and data not shown). HPLC analyses of urine samples showed three metabolites for [18F]FBA-A20FMDV2, and one major metabolite for each of the PEGylated radiotracers. Distribution of the radiotracers in healthy tissues generally depended on the number of PEG units present (Figure 4, Supplementary Table S1). Non-PEGylated [18F]FBA-A20FMDV2 was washed out efficiently from all tissues examined, with gall bladder and kidneys containing highest levels of radioactivity. By contrast, the diPEGylated [18F]FBA-(PEG28)2-A20FMDV2 was generally retained throughout, most notably in the kidneys (∼42% ID/g); only blood showed clearing of radioactivity. Pharmacokinetics of the monoPEGylated [18F]FBA-PEG28-A20FMDV2 in healthy tissues compared favorably to the diPEGylated radiotracer, resulting in a desirable clearing behavior resembling that observed for the non-PEGylated compound in healthy tissues; highest levels of radioactivity were detected in the kidneys and the gall bladder.
The BxPC-3/blood ratio remained relatively low for [18F]FBA-A20FMDV2 (1 h: 3.3:1, 4 h: 7.0:1) and the BxPC-3/muscle ratio only reached statistically significant levels at the 4 h time point (1 h: 1.3:1, P = 0.38; 4 h: 2.5:1, P = 0.02) because of more rapid washout from healthy tissues than from the tumor (Fig. 4, Supplementary Table S1). By comparison, improved BxPC-3/muscle ratios were seen for [18F]FBA-PEG28-A20FMDV2 (1 h: 2.5:1, 4 h: 3.6:1) and [18F]FBA-(PEG28)2-A20FMDV2 (1 h: 4.1:1, 4 h: 4.4:1), along with significantly higher BxPC-3/blood ratios ([18F]FBA-PEG28-A20FMDV2, 1 h: 11.8:1, 4 h: 47.9:1; [18F]FBA-(PEG28)2-A20FMDV2, 1 h: 9.0:1, 4 h: 150:1), reflecting the improved retention of these radiotracers in the BxPC-3 tumor.
Importantly, levels of radioactivity in the BxPC-3 tumor were at least 4-fold higher than those for the pancreas itself for all radiotracers at all time points measured (Supplementary Table S1). For [18F]FBA-A20FMDV2 the BxPC-3/pancreas ratio rose to 9.5:1 at 4 h p.i. due to more efficient washout from the pancreas than from the tumor. On the other hand, because of retention of [18F]FBA-(PEG28)2-A20FMDV2 in healthy pancreas, the BxPC-3/pancreas ratio for this radiotracer remained nearly unchanged throughout (1 h: 5.6:1, 4 h: 4.4:1). [18F]FBA-PEG28-A20FMDV2 exhibited good retention in the tumor together with efficient washout from the pancreas (and other healthy tissues), resulting in a steadily increasing BxPC-3/pancreas ratio (1 h: 4.2:1, 4 h: 23.6:1). In summary, the monoPEGylated [18F]FBA-PEG28-A20FMDV2 had the most favorable pharmacokinetic characteristics.
MicroPET imaging of the αvβ6-endogenous BxPC-3 pancreatic cancer model
Figure 5A depicts representative coronal and transaxial maximum intensity projections (MIPs) obtained with the three [18F]FBA-peptide tracers and with [18F]FDG (1 h p.i.). Tumor uptake was compared based on maximum standard uptake values (SUVmax; n = 3 animals).
The observations made for the peptide radiotracers during microPET imaging paralleled the results seen in the biodistribution study with preferential uptake noted in the tumor along with major uptake in kidneys, bladder (urine), and, for [18F]FBA-A20FMDV2, gall bladder. [18F]FBA-A20FMDV2 showed moderate initial tumor uptake and significant washout over time (SUVmax, 15min: 0.50 ± 0.22, 1 h: 0.22 ± 0.11; 4 h: < 0.05). [18F]FBA-(PEG28)2-A20FMDV2 showed improved uptake and retention in the tumor (SUVmax, 15min: 0.99 ± 0.18, 1 h: 0.79 ± 0.24, 4 h: 0.59 ± 0.20), along with significant renal retention. [18F]FBA-PEG28-A20FMDV2 combined favorable tumor uptake characteristics (SUVmax, 15min: 1.07 ± 0.29, 1 h: 0.91 ± 0.25, 4 h: 0.63 ± 0.12) with good renal clearance.
Administration of non-radioactive [19F]FBA-PEG28-A20FMDV2 (30 mg/kg) ten minutes prior to the corresponding [18F]FBA-peptide greatly reduced BxPC-3 tumor uptake (Δ(SUVmax) = -77%; Fig. 5B). Similar results were obtained for FBA-(PEG28)2-A20FMDV2 (30 mg/kg)/[18F]FBA-peptide (Δ(SUVmax) = -60%; Fig. 5C). Interestingly, [18F]FBA-PEG28-A20FMDV2 and [18F]FBA-(PEG28)2-A20FMDV2 were retained in the mouse oral cavity in scans without prior administration of non-radioactive FBA-peptide, suggesting that there may be limited expression of αvβ6 (27).
MicroPET scans obtained with [18F]FDG revealed an SUVmax of 0.62 ± 0.13 in the BxPC-3 tumor (1 h p.i.; n = 3; Fig. 5A). This level of tumor uptake is relatively low for [18F]FDG and it was within the range observed for the surrounding tissue (∼0.4 to 0.8). Thus, while the [18F]FBA-peptide tracers clearly revealed the BxPC-3 tumor in vivo, [18F]FDG did not.
DISCUSSION
The integrin αvβ6 has been identified as prognostic biomarker for cancer of the colon, cervix, lung, and stomach (10-12, 21). In addition, αvβ6 is reported to be expressed by many other types of carcinoma (13, 19) whereas corresponding normal tissue is weak or negative. Thus αvβ6 represents an excellent target for imaging and therapy of carcinoma. We previously demonstrated the ability to selectively image αvβ6-expressing tumors in vivo using the PET radiotracer [18F]FBA-A20FMDV2 (22). While targeted imaging was achieved, low uptake and poor retention in the target tissue together with metabolic instability limited its general utility, leading us to explore strategies to improve pharmacokinetics. As introduction of PEG-moieties (PEGylation) generally increases the biological half-life, metabolic stability, and tumor-uptake (28, 29) we generated two variants of A20FMDV2 that included PEG-moieties on the N-terminus of the peptide. These PEGylated variants of [18F]FBA-A20FMDV2 were examined and compared with the parent compound. Monodisperse PEG polymers of moderate molecular weight (Fw(PEG28) = 1.3 kDa) are short enough to be compatible with solid-phase chemistries, yet large enough to be expected to approach a range with some beneficial in vivo (pharmacokinetic) properties. Additionally, owing to the monodisperse nature of the polymer, the final product is obtained as a single compound, allowing for precise characterization and batch-to-batch reproducibility, in contrast to mixtures containing polymer-chains of different lengths encountered with the larger PEG products available.
As presented here, initial in vitro evaluation by ELISA indicated unchanged, high αvβ6-specificity for the PEGylated compounds (Fig. 1). Further binding experiments with the DX3puroβ6 and DX3puro cell lines demonstrated strong, αvβ6-specific and PEG-dependent binding to the receptor expressed on cells in the presence of other, related integrins (including αvβ3, αvβ5, and α5β1) (Fig. 3). In vivo studies also showed improved, αvβ6-targeted PEG-dependent tumor-retention in the DX3puroβ6/DX3puro model (Supplementary Table S1). Taken together, these data confirmed our initial expectations for the two new PEGylated radiotracers, [18F]FBA-PEG28-A20FMDV2 and [18F]FBA-(PEG28)2-A20FMDV2.
The DX3puroβ6/DX3puro melanoma cancer model was generated by viral transduction of human β6 into human melanoma cells. In order to examine a model more closely resembling the actual human disease, we sought a cancer where expression of αvβ6 was relevant to the disease and for which cell lines existed that had endogenous expression of αvβ6; we chose to investigate pancreatic cancer. Sipos et al (20) reported as part of a study of gastroenteropancreatic adenocarcinomas that αvβ6 expression was strongest in pancreatic ductal adenocarcinomas (PDAC). Thus of the 34 human PDAC samples analyzed 32 (94%) received the maximum score (the mean score was 2.8/3.0, with none of the other carcinomas receiving a score over 1.5). These observations make αvβ6 a very appealing target for selective in vivo detection of pancreatic cancer. The possibility of detecting primary tumors and metastases earlier by imaging αvβ6, and thus directing therapy sooner, may improve upon the very poor prognosis of this disease. In addition, recent studies associate strong expression of αvβ6 with poor prognosis (for colon, lung, cervical, and stomach cancer) (10-12, 21), so the relevance of our data is not restricted only to pancreatic cancer.
As demonstrated here by flow cytometry (Fig. 2), many common pancreatic carcinoma cell lines exhibit an αvβ6 profile that reflects expression seen for PDAC in the clinic (20). In vitro cell binding assays showed that [18F]FBA-PEG28-A20FMDV2 performed equally well with the endogenously αvβ6-expressing pancreatic BxPC-3 cell line and the transduced αvβ6-expressing DX3puroβ6 cell line, demonstrating rapid and high binding together with significant internalization (Fig. 3). Contrary to conditions in the in vivo environment, these assays were performed in the absence of natural ligands competing for binding to αvβ6. We therefore sought to further demonstrate their relevance as predictors for in vivo performance by carrying out a binding assay in the presence of a biologically relevant αvβ6 ligand. Latency associated peptide (LAP) was chosen as it binds to αvβ6 with high affinity and has significance in the context of tumor biology (13, 18, 30). In association with transforming growth factor β (TGFβ), particularly TGFβ1, LAP binds to αvβ6, thereby affecting signaling pathways understood to play significant roles in tumor development (18, 30). We found that even in the presence of 5 nmol/L LAP, the radiotracer, present at sub-picomolar levels, was still able to efficiently bind to the cells (at ≥75% of binding observed under LAP-free conditions. Supplementary Fig. S4). The feasibility of evaluating αvβ6-specific in vivo imaging radiotracers for pancreatic carcinoma was shown in a mouse model bearing BxPC-3 xenografts. In microPET images the BxPC-3 tumors were clearly identifiable throughout. These data confirm that selective in vivo imaging of αvβ6-expressing cancers is possible with cancers that endogenously express αvβ6 and not only those engineered to express it. Importantly for pancreatic imaging the best pharmacokinetic behavior was observed for [18F]FBA-PEG28-A20FMDV2, resulting in the highest tumor/pancreas ratio (>23:1; 4 h), making this radiotracer a promising lead compound for in vivo detection.
Close examination of the biodistribution data revealed that introduction of the second PEG unit was not beneficial. Simply modifying from [18F]FBA-PEG28-A20FMDV2 to [18F]FBA-(PEG28)2-A20FMDV2 resulted only in a slight increase in tumor uptake while, concurrently, washout of radioactivity from healthy tissue (except blood) was suppressed over the time span examined; particularly notable are the high and constant levels of radioactivity in the kidneys (Fig. 4). PET images also demonstrated that [18F]FBA-PEG28-A20FMDV2 outperformed [18F]FBA-(PEG28)2-A20FMDV2; it showed slightly higher uptake (SUVmax) together with better BxPC-3/background ratios, largely due to preferential clearing of nonspecifically bound activity (Fig. 5). Thus, in this study, [18F]FBA-PEG28-A20FMDV2 appeared to have the best balance of αvβ6-dependent tumor uptake and a suitable rate of clearance from healthy tissues, including the pancreas. The high retention of [18F]FBA-(PEG28)2-A20FMDV2 in healthy tissues, caused by introduction of the second PEG unit, had not been predicted. These observations appear to stand in contrast to the common belief that renal clearance remains highly efficient for compounds bearing small PEG units (under ∼30 kDa) (28, 29). Despite the comparatively small PEG units employed in this study, the PEGylation of A20FMDV2 appeared to result in an ‘enhanced permeation and retention’ (EPR) effect towards the BxPC-3 tumors along with some improved radiotracer stability (29). Positive effects of PEGylation on the in vivo stability of pharmaceuticals are generally recognized (28). Likewise, in our study only one major radioactive metabolite was found in HPLC samples of urine collected 1 h after injection of [18F]FBA-PEG28-A20FMDV2 or [18F]FBA-(PEG28)2-A20FMDV2, while the non-PEGylated analogue had yielded three equally large metabolite-signals (22). However, since the cell-based assays demonstrated very rapid binding (∼25% of [18F]FBA-PEG28-A20FMDV2 bound to BxPC-3 within one minute, Fig 3B) and significant, PEG-dependent internalization (approximately two-thirds of bound activity were internalized at 1 hour, Fig 3A), delivery efficiency to the target likely was of principal importance for these radiotracers, provided that radioactive metabolites were rapidly cleared from the body, as observed particularly for [18F]FBA-PEG28-A20FMDV2. Taken together, these data suggest that rapid binding and internalization of the PEGylated radiotracers in cells are key to increased uptake and retention in the tumors. That being said, a detailed characterization of metabolites and further modification of the radiotracers remain of interest. They include selective deletion of redundant amino acids and replacement with unnatural amino acids in conjunction with multimeric radiotracers and incorporation of branched PEG. These approaches have already been successful in improving the pharmacokinetics of tracers for integrin αvβ3 (28, 31, 32).
Paralleling the in vitro blocking studies with the αvβ6-expressing cell lines, in vivo blocking experiments with the non-radioactive analogues showed a significant reduction of uptake in the BxPC-3 tumors. Prior to the blocking experiments the animals were also evaluated by [18F]FDG-PET, the standard radiotracer for PET imaging. Significantly, [18F]FDG failed in all animals to detect the BxPC-3 tumors. In the clinical setting [18F]FDG is by far the most widely used PET radiotracer for the detection of many cancers, including pancreatic cancer, despite the risk of false positive (eg. pancreatitis) or false negative (eg. low GLUT-1 glucose transporter expression in tumor; hyperglycemic patient) diagnosis. Thus, our observation parallels findings from the clinical setting where [18F]FDG is estimated to miss over one third of all pancretic malignancies (and about half of the lesions smaller than 1 cm) (6, 8, 33).
Better understanding of the biology of pancreatic cancer (34) and improved early, more accurate diagnosis are sorely needed. By combining detailed anatomical information with functional metabolic information [18F]FDG-PET/CT has shown some promise (5, 6, 8, 33). However, the use of [18F]FDG as radiotracer does not take advantage of the molecular biological differences of tumors beyond their increased (glucose) metabolism. The integrin αvβ6 is expressed strongly by most human pancreatic cancers (20) and is likely to be a prognostic indicator for this cancer. Therefore, it is clear that targeted in vivo imaging of αvβ6 will be a major improvement for preoperative staging and monitoring response to treatment for this lethal malignancy in humans. Using a metabolic marker ([18F]FDG) in conjunction with receptor-specific radiotracers in PET/CT could provide crucial additional information, particularly for occult disease. In a step towards making this a clinical reality we have shown that [18F]FBA-PEG28-A20FMDV2 holds promise for αvβ6-specific tumor imaging.
Supplementary Material
ACKNOWLEDGEMENTS
We thank J. Choi, D. L. Kukis, L. Planutyte, C. D. Griesemer, J. Y. Fung, and S. V. Rendig for excellent technical support. NIH funding: R21 CA107792
REFERENCES
- 1.Alavi A, Kung JW, Zhuang HM. Implications of PET based molecular imaging on the current and future practice of medicine. Semin Nucl Med. 2004;34:56–69. doi: 10.1053/j.semnuclmed.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Keogan MT, Tyler D, Clark L, et al. Diagnosis of pancreatic carcinoma: Role of FDG PET. Am J Roentgenol. 1998;171:1565–70. doi: 10.2214/ajr.171.6.9843289. [DOI] [PubMed] [Google Scholar]
- 3.Rose DM, Delbeke D, Beauchamp RD, et al. 18Fluorodeoxyglucose positron emission tomography in the management of patients with suspected pancreatic cancer. Ann Surgery. 1999;229:729–38. doi: 10.1097/00000658-199905000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Czernin J, Allen-Auerbach M, Schelbert HR. Improvements in cancer staging with PET/CT: Literature-based evidence as of September 2006. J Nucl Med. 2007;48:78S–88S. [PubMed] [Google Scholar]
- 5.Heinrich S, Goerres GW, Schäfer M, et al. Positron emission tomography/computed tomography influences on the management of resectable pancreatic cancer and its cost-effectiveness. Ann Surgery. 2005;242:235–43. doi: 10.1097/01.sla.0000172095.97787.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schöder H, Gönen M. Screening for cancer with PET and PET/CT: Potential and limitations. J Nucl Med. 2007;48:4S–18S. [PubMed] [Google Scholar]
- 7.Blodgett TM, Meltzer CC, Townsend DW. PET/CT: Form and function. Radiology. 2007;242:360–85. doi: 10.1148/radiol.2422051113. [DOI] [PubMed] [Google Scholar]
- 8.Parsons CM, Sutcliffe JL, Bold RJ. Preoperative evaluation of pancreatic cancer. J Hepato-Biliary-Pancreatic Surgery. 2008;15:429–35. doi: 10.1007/s00534-007-1240-7. [DOI] [PubMed] [Google Scholar]
- 9.Beer AJ, Haubner R, Goebel M, et al. Biodistribution and pharmacokinetics of the αvβ3-selective tracer 18F-galacto-RGD in cancer patients. J Nucl Med. 2005;46:1333–41. [PubMed] [Google Scholar]
- 10.Bates RC, Bellovin DI, Brown C, et al. Transcriptional activation of integrin β6 during the epithelial-mesenchymal transition defines a novel prognostic indicator of aggressive colon carcinoma. J Clin Invest. 2005;115:339–47. doi: 10.1172/JCI23183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hazelbag S, Kenter GG, Gorter A, et al. Overexpression of the αvβ6 integrin in cervical squamous cell carcinoma is a prognostic factor for decreased survival. J Pathol. 2007;212:316–24. doi: 10.1002/path.2168. [DOI] [PubMed] [Google Scholar]
- 12.Elayadi AN, Samli KN, Prudkin L, et al. A peptide selected by biopanning identifies the integrin αvβ6 as a prognostic biomarker for nonsmall cell lung cancer. Cancer Res. 2007;67:5889–95. doi: 10.1158/0008-5472.CAN-07-0245. [DOI] [PubMed] [Google Scholar]
- 13.Van Aarsen LA Koopman, Leone DR, Ho S, et al. Antibody mediated blockade of integrin αvβ6 inhibits tumor progression in vivo by a transforming growth factor-β-regulated mechanism. Cancer Res. 2008;68:561–70. doi: 10.1158/0008-5472.CAN-07-2307. [DOI] [PubMed] [Google Scholar]
- 14.McCarty JH. αv Integrins lead the way for colorectal metastases. Clin Cancer Res. 2008;14:6351–3. doi: 10.1158/1078-0432.CCR-08-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheppard D, Rozzo C, Starr L, Quaranta V, Erle DJ, Pytela R. Complete amino acid sequence of a novel integrin β subunit (β6) identified in epithelial cells using the polymerase chain reaction. J Biol Chem. 1990;265:11502–7. [PubMed] [Google Scholar]
- 16.Breuss JM, Gallo J, Delisser HM, et al. Expression of the β6 integrin subunit in development, neoplasia and tissue-repair suggests a role in epithelial remodeling. J Cell Sci. 1995;108:2241–51. doi: 10.1242/jcs.108.6.2241. [DOI] [PubMed] [Google Scholar]
- 17.Breuss JM, Gillett N, Lu L, Sheppard D, Pytela R. Restricted distribution of integrin β6 messenger-RNA in primate epithelial tissues. J Histochem Cytochem. 1993;41:1521–7. doi: 10.1177/41.10.8245410. [DOI] [PubMed] [Google Scholar]
- 18.Thomas GJ, Nystrom ML, Marshall JF. αvβ6 integrin in wound healing and cancer of the oral cavity. J Oral Pathol Med. 2006;35:1–10. doi: 10.1111/j.1600-0714.2005.00374.x. [DOI] [PubMed] [Google Scholar]
- 19.Nemeth JA, Nakada MT, Trikha M, et al. Alpha-v integrins as therapeutic targets in oncology. Cancer Investigation. 2007;25:632–46. doi: 10.1080/07357900701522638. [DOI] [PubMed] [Google Scholar]
- 20.Sipos B, Hahn D, Carceller A, et al. Immunohistochemical screening for β6-integrin subunit expression in adenocarcinomas using a novel monoclonal antibody reveals strong up-regulation in pancreatic ductal adenocarcinomas in vivo and in vitro. Histopathology. 2004;45:226–36. doi: 10.1111/j.1365-2559.2004.01919.x. [DOI] [PubMed] [Google Scholar]
- 21.Zhang ZY, Xu KS, Wang JS, et al. Integrin αvβ6 acts as a prognostic indicator in gastric carcinoma. Clinical Oncology. 2008;20:61–6. doi: 10.1016/j.clon.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 22.Hausner SH, DiCara D, Marik J, Marshall JF, Sutcliffe JL. Use of a peptide derived from foot-and-mouth disease virus for the noninvasive imaging of human cancer: Generation and evaluation of 4-[18F]fluorobenzoyl A20FMDV2 for in vivo imaging of integrin αvβ6 expression with positron emission tomography. Cancer Res. 2007;67:7833–40. doi: 10.1158/0008-5472.CAN-07-1026. [DOI] [PubMed] [Google Scholar]
- 23.Chen EL, Prinz RA. Long-term survival after pancreatic cancer treatment. Am J Surgery. 2007;194:S127–S130. [Google Scholar]
- 24.Thomas GJ, Lewis MP, Hart IR, Marshall JF, Speight PM. αvβ6 integrin promotes invasion of squamous carcinoma cells through up-regulation of matrix metalloproteinase-9. Int J Cancer. 2001;92:641–50. doi: 10.1002/1097-0215(20010601)92:5<641::aid-ijc1243>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 25.Reilly RM, Kiarash R, Cameron RG, et al. 111In-labeled EGF is selectively radiotoxic to human breast cancer cells overexpressing EGFR. J Nucl Med. 2000;41:429–38. [PubMed] [Google Scholar]
- 26.Zasadny KR, Wahl RL. Standardized uptake values of normal tissues at PET with 2-[fluorine-18]-fluoro-2-deoxy-D-glucose - variations with body weight and a method for correction. Radiology. 1993;189:847–50. doi: 10.1148/radiology.189.3.8234714. [DOI] [PubMed] [Google Scholar]
- 27.Ghannad F, Nica D, Fulle MIG, et al. Absence of αvβ6 integrin is linked to initiation and progression of periodontal disease. Am J Pathology. 2008;172:1271–86. doi: 10.2353/ajpath.2008.071068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris JM, Chess RB. Effect of PEGylation on pharmaceuticals. Nat Rev Drug Discovery. 2003;2:214–21. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 29.Greenwald RB, Choe YH, McGuire J, Conover CD. Effective drug delivery by PEGylated drug conjugates. Adv Drug Delivery Rev. 2003;55:217–50. doi: 10.1016/s0169-409x(02)00180-1. [DOI] [PubMed] [Google Scholar]
- 30.Sheppard D. Integrin-mediated activation of latent transforming growth factor β. Cancer and Metastasis Reviews. 2005;24:395–402. doi: 10.1007/s10555-005-5131-6. [DOI] [PubMed] [Google Scholar]
- 31.Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discovery. 2003;2:347–60. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- 32.Liu S. Radiolabeled multimeric cyclic RGD peptides as integrin αvβ3 targeted radiotracers for tumor imaging. Mol Pharmaceuticals. 2006;3:472–87. doi: 10.1021/mp060049x. [DOI] [PubMed] [Google Scholar]
- 33.Delbeke D, Pinson CW. Pancreatic tumors: role of imaging in the diagnosis, staging, and treatment. J Hepato-Biliary-Pancreatic Surgery. 2004;11:4–10. doi: 10.1007/s00534-002-0775-x. [DOI] [PubMed] [Google Scholar]
- 34.Li CW, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–7. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.