Abstract
ABCG2 is a member of the ATP-binding cassette (ABC) family of transporters, the overexpression of which is associated with tumor resistance to a variety of chemotherapeutic agents. Accordingly, combining ABCG2 inhibitor(s) with chemotherapy has the potential to improve treatment outcome. To search for clinically useful ABCG2 inhibitors, a bioluminescence imaging (BLI)-based assay was developed to allow high-throughput compound screening. This assay exploits our finding that D-luciferin, the substrate of firefly luciferase (fLuc), is a specific substrate of ABCG2, and ABCG2 inhibitors block the export of D-luciferin and enhance bioluminescence signal by increasing intracellular D-luciferin concentrations. HEK293 cells, engineered to express ABCG2 and fLuc, were used to screen the Hopkins Drug Library that includes drugs approved by the US Food and Drug Administration (FDA) as well as drug candidates that have entered phase II clinical trials. Forty seven compounds demonstrated BLI enhancement, a measure of anti-ABCG2 activity, of five-fold or greater, the majority of which were not previously known as ABCG2 inhibitors. The assay was validated by its identification of known ABCG2 inhibitors and by confirming previously unknown ABCG2 inhibitors using established in vitro assays (e.g. mitoxantrone resensitization and BODIPY-prazosin assays). Glafenine, a potent new inhibitor, also inhibited ABCG2 activity in vivo. The BLI-based assay is an efficient method to identify new inhibitors of ABCG2. As they were derived from an FDA-approved compound library, many of the inhibitors uncovered in this study are ready for clinical testing.
Keywords: multidrug resistance, ABC transporter, cancer chemotherapy, BCRP, drug screen
Introduction
ABCG2 is a recently described member of the ATP-binding cassette (ABC) transporters, a family of proteins that use the energy of ATP hydrolysis to transport certain chemicals out of cells (1, 2). The overexpression of ABC transporters has been associated with multidrug resistance (MDR), a major impediment to successful cancer chemotherapy. ABCG2 confers resistance to several chemotherapeutic agents such as mitoxantrone (MTX), daunorubicin, doxorubicin, bisantrene, topotecan and flavopiridol (3). In addition, ABCG2 has been found to affect drug transport across the gastrointestinal epithelium and the blood-brain barrier (BBB) (4). Many believe that judiciously combining ABCG2 inhibitor(s) with standard cancer chemotherapy will nullify the protection tumor cells receive, preventing cancer survival and metastasis (2), (4), (5). However, that idea remains to be tested, largely due to the lack of suitable ABCG2 inhibitors, despite significant efforts at discovering them.
We recently demonstrated that D-luciferin, the substrate of firefly luciferase (fLuc), is a specific substrate for ABCG2 (6). fLuc is the most commonly used reporter for imaging transgene expression in vivo. fLuc catalyzes the oxidation of D-luciferin, releasing photons that can be quantified through bioluminescence imaging (BLI) (7). In cells that express both ABCG2 and fLuc, ABCG2 inhibitors, such as fumitremorgin C (FTC) (8), block the export of D-luciferin and increase the effective intracellular concentration of D-luciferin that is accessible to fLuc, causing enhanced light output. BLI is an increasingly widespread imaging technique, provides an extremely high signal-to-background ratio, and is easy to perform (9).
This study describes the development of a BLI-based high-throughput assay to screen for ABCG2 inhibitors. This assay was applied to screen a clinically relevant compound library established at Johns Hopkins University, namely, the Hopkins Drug Library (HDL) (10, 11). We identified 47 compounds causing a greater than five-fold increase in bioluminescence, consistent with marked ABCG2 inhibition. Some are known ABCG2 inhibitors. Many of those not previously known were confirmed using established methods.
Materials and Methods
Reagents
D-Luciferin sodium salt was obtained from Gold Biotechnology, Inc. (St. Louis, MO). Verapamil (VP), colchicine (Col), and MTX were purchased from Sigma Chemical Company (St Louis, MO). BODIPY-prazosin was obtained from Invitrogen (Carlsbad, CA). Glafenine, flavoxate hydrochloride and doxazosin mesylate were obtained from Sigma Chemical Company (St. Louis, MO). Fumitremorgin C (FTC) was a kind gift of Dr. S. Bates (National Cancer Institute). All compounds were prepared in dimethylsulfoxide (DMSO) for in vitro experiments. For in vivo experiments, ABCG2 inhibitor was dissolved in ethanol/cremophor EL/saline (1:1:6).
Cell lines
The establishment of ABCG2-overexpressing HEK293 cells stably transfected with CMV-luc2CP/Hygro (referred to here as HEK293/ABCG2/fLuc) has been described previously (12). Control empty vector-transfected HEK293 cells were stably transfected with CMV-luc2CP/Hygro in the same way and are referred to here as HEK293/empty/fLuc. Cells were cultured in MEM (Invitrogen) supplemented with 10% FBS, penicillin and streptomycin. HEK293 cells stably transfected with the ABCG2-expressing construct were maintained in medium containing 1 mg/mL of G418 and 100 μg/mL of hygromycin B. ABCG2-overexpressing NCI-H460 human non-small cell lung carcinoma cells (National Cancer Institute, Frederick, MD) were established and characterized as described previously (13). They were maintained in RPMI 1640 medium supplemented with 10% FBS, penicillin and streptomycin. All cultures were maintained at 37°C in a humidified 5% CO2/95% air incubator.
BLI assay
HEK293/ABCG2/fLuc cells were plated into 96-well plates at a density of 4 × 104 cells/100 μL per well and were allowed to attach overnight. The following day, 10 μL of each compound or the control solvent was transferred from a compound library in a 96-well, high-throughput format into the wells using a multichannel pipette. The final concentration of each compound was approximately 17 μM. 5 μL of D-luciferin (1.2 mg/mL in PBS) were then added to achieve a final concentration of ~ 50 μg/mL. The plates were gently tapped to assure that all solutions were well mixed, and imaging commenced immediately. Images were taken every 5 min for ~ 1 h. Light output from each well was quantified at the 40 min time point after initiation of imaging, and the signal-to-background (S/B) ratio of the light output from each compound divided by that from the control well was calculated. This S/B ratio serves as an indicator of the potency of ABCG2 inhibition, the mechanism by which BLI signal is enhanced.
Assay performance
Signal-to-noise (S/N) ratio, S/B and Z′ values, which indicate the robustness of the assay, were calculated as described previously (14). Background was defined as the light output from cells incubated with D-luciferin and the solvent only.
Resensitization assay
The ABC transporter-inhibiting ability of the potential inhibitors identified were further tested by evaluating their ability to resensitize ABCG2-overexpressing, human non-small cell lung carcinoma (NCI-H460/MX20) cells to MTX, or MDCKII cells overexpressing Pgp or MRP1, to Col. Cells were plated in 96-well plates at 1 × 104 per well and allowed to attach. MTX was added to 15 μM or 30 μM, with or without a putative ABCG2 inhibitor. Colchicine was added at 1 μM for MDCKII/Pgp cells and 0.3 μM for MDCKII/MRP1 cells. After two days of incubation cell viability was assessed using the XTT assay as described previously (12). All results were normalized to a percentage of absorbance obtained in controls.
BODIPY-prazosin uptake assay
HEK293/ABCG2 cells were plated in 6-well plates at a density of 1.1 × 106 cells per well and allowed to attach. Cells were then changed into medium containing 0.25 μM BODIPY-prazosin (15), and compound to be tested was added to a final concentration of 20 μM, followed by incubation at 37°C for 1 h. Cells were then harvested, washed with ice-cold PBS once, resuspended in cold PBS, and analyzed with flow cytometry. Analyses were performed with FACScan (Becton Dickinson, Fullerton, CA) with an excitation wavelength of 488 nm and an emission wavelength of 530 nm. Ten thousand events were counted per sample. The resultant histograms were analyzed with CellQuest software (Becton Dickinson).
In vivo bioluminescence imaging
Animal protocols were approved by the Johns Hopkins University Animal Care and Use Committee. Both HEK293/ABCG2/fLuc and HEK293/empty/ABCG2 cells were implanted subcutaneously into 6-week-old female nude mice at 1 × 106 cells at each site. The IVIS 200 small animal imaging system (Xenogen Corp., Alameda, CA) was used for BLI and 2.5% isoflurane was used for anesthesia. D-luciferin was injected intraperitoneally (i.p.) into mice at 150 mg/kg, and imaging was performed every few minutes for more than 1 h. ABCG2 inhibitor was administered via tail vein injection as a bolus during imaging, with imaging continued thereafter.
Data analysis
LivingImage (Xenogen Corp.) and IGOR (Wavemetrics, Lake Oswego, OR) image analysis software were used to superimpose and analyze the corresponding gray scale photographs and false color BLI images. Light intensities of regions of interest (ROIs) were expressed as total flux (photons/sec). The IC50 values of identified ABCG2 inhibitors were calculated using GraphPad Prism version 4.0 for Windows (GraphPad Software, San Diego, CA) using variable-slope logistic nonlinear regression analysis. Data are presented as mean ± SEM, n = 3.
Results
Statistical evaluation of the BLI-based assay
A HEK293/ABCG2/fLuc stable clone was chosen for this BLI-based high-throughput screen because FTC, a known specific inhibitor of ABCG2, enhances its BLI signal output significantly (8). The screen was performed in a 96-well format. HEK293/ABCG2/fLuc cells were plated from 1 – 8 × 104/well and treated with solvent only or with FTC. D-luciferin concentrations varied from 20 – 100 μg/mL, and imaging data were acquired every 5 min for 1 h. The quality of the BLI based high-throughput screen assay was evaluated statistically as described previously (14). Z′ values were calculated for each combination of parameters. An ideal assay is expected to produce Z′ = 1, with 1 > Z′ ≥ 0.5 reflecting a robust assay. The Z′-values obtained from this assay ranged from 0.5 to 0.9.
Screening of the HDL using the BLI assay
The HDL is composed primarily of drugs approved by the US Food and Drug Administration (FDA) and is the most complete library of clinically-approved drugs (10), (11).
Images were taken every 5 min for ~ 1 h, and light output from each well at the 40 min time point was chosen for quantification. The S/B of the light output from each compound divided by that from the control well was calculated. This ratio was used as an indicator of the potency of ABCG2 inhibition, the mechanism by which BLI signal is enhanced.
The result of the full screen is presented in Supplementary Table 1. Two hundred and nineteen candidate ABCG2 inhibitors were identified from 3,273 compounds screened. Candidate inhibitors are defined as compounds producing at least two-fold signal enhancement over control values. About 150 weaker inhibitors were also identified. Among the 219 potent (> two-fold signal enhancement) inhibitors, 88 (~ 40%) had not been previously reported as an inhibitor or substrate of any ABC transporter. The majority (~ 84%) of the ~ 150 weak inhibitors had not been previously reported as either inhibitors or substrates of ABC transporters. Forty seven compounds demonstrated signal enhancement of ≥ five-fold (Table 1). Of those, 10 are known ABCG2 inhibitors or substrates (Table 1A), validating the assay. The identification of many previously reported ABCG2 inhibitors, including both potent and weak ones, such as gefitinib (16), reserpine (17), dipyridamole (18), and curcumin (19), suggests that the assay is sensitive. The most potent of the novel inhibitors, glafenine, enhanced the BLI signal by ~ 20-fold (Table 1B).
Table 1.
Compounds with five-fold or greater BLI enhancement
| A) Previously known ABCG2 inhibitors | ||
|---|---|---|
| Compound | Fold | Known therapeutic effect |
| gefitinib (39) | 19.0 | antineoplastic |
| harmine (40) | 8.9 | n/a* |
| prazosin (41) | 8.4 | antihypertensive |
| dipyridamole (18) | 7.1 | antithrombotic |
| curcumin (42) | 6.3 | nutrient |
| nelfinavir mesylate (37) | 6.1 | antiviral |
| niguldipine (43) | 5.9 | antihypertensive |
| riboflavin (44) | 5.4 | antispasmodic |
| reserpine (4) | 5.4 | antihypertensive |
| hesperetin (45) | 5.0 | antispasmodic |
| B) Previously unknown as ABCG2 inhibitors | ||
| Compound | Fold | Known therapeutic Effect |
| glafenine | 20.6 | analgesic |
| tracazolate | 20 | sedative |
| calcimycin (A23187) | 16.9 | calcium ionophore |
| doxazosin mesylate salt | 15.9 | antihypertensive |
| verteporfin | 11.3 | ophthalmic |
| flavoxate hydrochloride | 11.2 | antispasmodic |
| Brij 30 | 11.1 | n/a* |
| quinacrine | 10.6 | anthelmintic |
| grapefruit oil | 10.6 | n/a* |
| dihydroergotamine mesylate | 9.6 | vasoconstrictor, specific in migraine |
| harmaline | 8.8 | CNS stimulant, antiparkinsonian |
| clebopride maleate | 7.9 | antiemetic, antispasmodic |
| silver nitrate | 7.7 | antibacterial |
| isorhamnetin | 7.0 | n/a* |
| gramicidin A | 6.9 | antibacterial |
| clebopride | 6.7 | antiemetic |
| rotenone | 6.7 | acaricide, ectoparasiticide, inhibits NADH2 oxidation to NAD |
| clomiphene citrate | 6.7 | gonad stimulating principle |
| aromatic cascara fluid extract | 6.6 | therapeutic plant |
| sildenafil | 6.6 | impotency therapy |
| emodin | 6.4 | antimicrobial, anticancer, cathartic |
| flubendazole | 6.3 | anthelminthic |
| metyrapone (2-methyl-1,2-di- | 6.3 | diagnostic aid |
| periciazine (propericiazine) | 6.3 | antipsychotic |
| isoreserpine | 6.2 | antihypertensive |
| acepromazine | 6.2 | sedative |
| flutamide | 6.1 | antineoplastic |
| podophyllum resin | 6.1 | dermatologic |
| gambogic acid | 6.0 | antibacterial, inhibit Hela cell growth in vitro |
| piperacetazine | 5.8 | antipsychotic |
| digitoxin | 5.7 | cardiotonic, cardiotoxic; inhibits Na+/K+ ATPase |
| acetophenazine maleate | 5.6 | antipsychotic |
| eupatorin | 5.6 | emetic ex Eupatorium spp and other Compositae |
| estrone hemisuccinate | 5.4 | estrogen |
| raloxifene hydrochloride | 5.4 | bone resorption |
| o-dianisidine | 5.0 | not approved |
| oligomycin | 5.0 | antibiotic, antifungal |
No therapeutic effect available
Confirmation of candidate ABCG2 inhibitors using established assays
We used two established assays, the MTX resensitization assay (1) and the BODIPY-prazosin fluorescent dye uptake assay (15), to confirm that compounds identified in the BLI screen were indeed ABCG2 inhibitors.
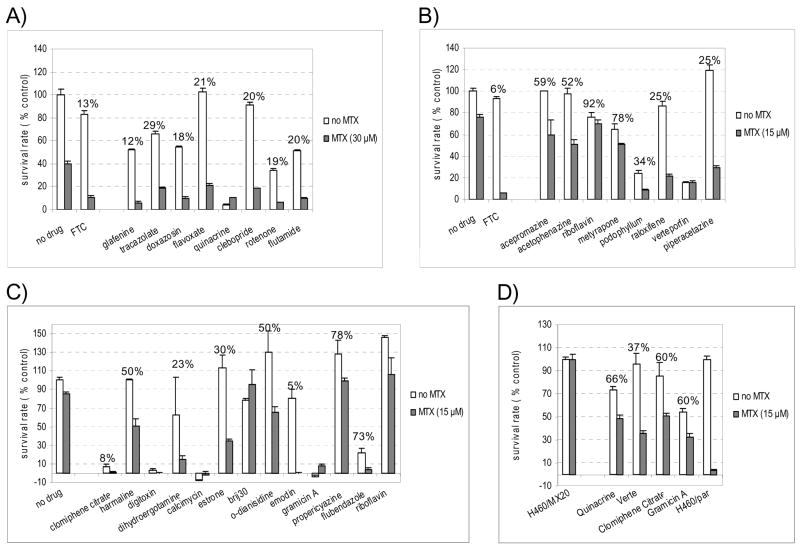
Twenty-eight novel candidate ABCG2 inhibitors identified in the BLI screen were tested by MTX resensitization assay, a hallmark of ABCG2 inhibitor function (4). Both ABCG2 overexpressing H460/MX20 cells and the parent line were treated with MTX (15 or 30μM) for three days. As expected, H460/MX20 cells survived exposure to MTX better than the parent cells due to the induced expression of ABCG2 (~ 40% vs. ~ 9% survival in 30 μM MTX, ~ 80% vs < 20% in 15 μM MTX). The potent, selective ABCG2 inhibitor FTC restored the sensitivity of H460/MX20 cells to MTX and significantly reduced their survival rate. Twenty-eight novel candidate inhibitors were initially tested at 20μM for three days. Twenty demonstrated a similar capacity to sensitize H460/MX20 cells to MTX, confirming that they are indeed ABCG2 inhibitors (Figure 1A, 1B and 1C). Brij 30 was found to resensitize H460/MX20 cells to MTX significantly after two days of incubation (data not shown). The most active inhibitors were glafenine and doxazosin mesylate, which, at concentrations of 20 μM, reduced the survival of H460/MX20 cells to 12% and 18%, respectively. These results were consistent with their considerable activity in the BLI screen (20- and 16-fold signal enhancement, respectively). That suggests that the magnitude of BLI signal enhancement can reflect the potency of ABCG2 inhibitors.
Figure 1.
Mitoxantrone (MTX) resensitization assay. NCI-H460/MX20 (ABCG2-overexpressing, human non-small cell lung carcinoma cells) cells were treated for three days with or without MTX (30 μM in panel A, and 15 μM in panels B, C and D), in the presence of a potential inhibitor (20 μM in panel A, B and C, 1 μM in panel D), and surviving cells were assessed with the XTT assay. Survival rates were expressed as percentages normalized by the data obtained in the negative control where no MTX or any compound was added. 10 μM FTC was used as a positive control. Numbers on top of bar pairs are survival rates caused by each compound in the presence of MTX relative to the compound alone. Data are presented as mean ± SEM, n = 3.
Six compounds, including quinacrine, verteporfin, digitoxin, clomiphene citrate, calcimycin and gramicin A, were too cytotoxic to be tested at 20 μM (Figure 1A, 1B and 1C). Each was tested again at 1 μM with 15μM MTX for two days. At this lower concentration, quinacrine, verteporfin, clomiphene citrate, and gramicin A showed resensitization of H460/MX20 cells to MTX, confirming them as ABCG2 inhibitors (Figure 1D). The other two, digitoxin and calcimycin, were tested at even lower concentrations (0.3, 0.1 and 0.03 μM). They were no longer cytotoxic at 0.1 and 0.03 μM, but did not reduce the survival rate of H460/MX20 cells significantly after two days when co-incubated with 15 μM MTX (data not shown). However, at these lower concentrations (0.03 and 0.1 μM), they enhance BLI signal minimally (data not shown).
In summary, 26 of the 28 candidate compounds identified in the BLI assay were confirmed by the MTX resensitization assay to be new ABCG2 inhibitors. Compounds designated as false positive were actually difficult to test in the MTX resensitization assay by virtue of their direct cytotoxicity, and had to be tested at very low (non-cytotoxic) doses below which their ability to inhibit ABCG2 was also compromised.
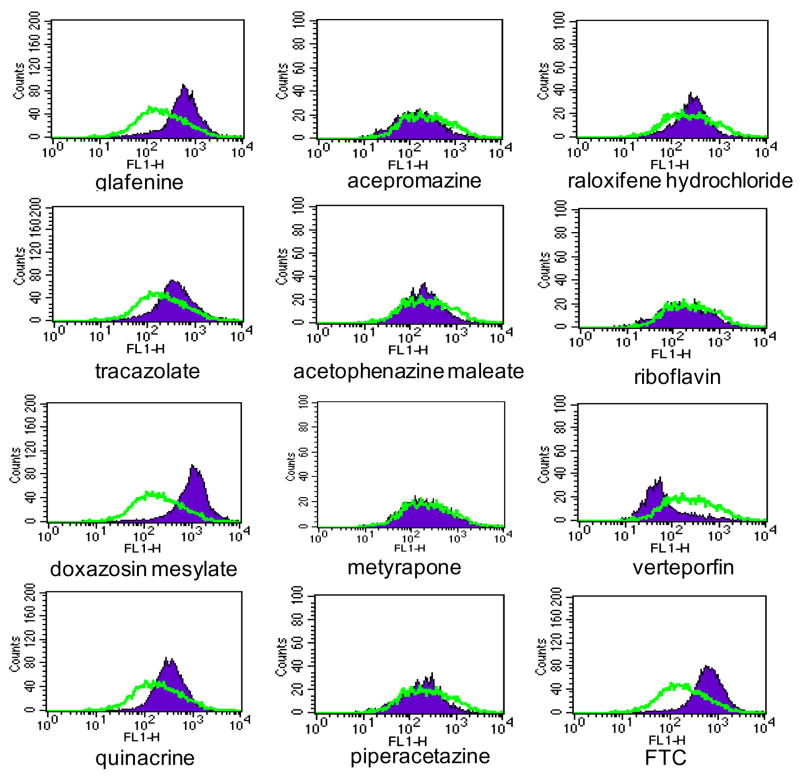
Sixteen of the candidate ABCG2 inhibitors were also tested with the BODIPY-prazosin assay. HEK293/ABCG2 cells were incubated with BODIPY-prazosin and each test compound, and then subjected to flow cytometry. Nine of the 16 compounds, glafenine, tracazolate, doxazosin mesylate, quinacrine, clebopride, flutamide, flavoxate hydrochloride, rotenone, and podophyllum resin were positive by this assay. Notably, seven compounds identified by the BLI assay and confirmed by MTX resensitization assay, acepromazine, acetophenazine maleate, metyrapone, piperacetazine, raloxifene hydrochloride, riboflavin and verteporfin were negative according to this assay (Figure 2). However, all seven were confirmed by the MTX resensitization assay. The results of the MTX resensitization and the BODIPY-prazosin assays are compared in Table 2.
Figure 2.
Effect of selected positive hits on ABCG2 function shown by flow cytometry analysis of the BODIPY-prazosin dye uptake assay. HEK293/ABCG2 cells were incubated in BODIPY-prazosin in the absence (open curve) or presence of a compound (20 μM, filled curve) as described in the Materials and Methods. FTC (10 μM) was used as a positive control.
Table 2.
Results of the mitoxantrone (MTX) resensitization assay* and the BODIPY-prazosin dye uptake (BP) assays.
| Compound | MTX | BP |
|---|---|---|
| glafenine | Y | Y |
| tracazolate | Y | Y |
| doxazosin mesylate | Y | Y |
| verteporfin | Y | N |
| flavoxate hydrochloride | Y | Y |
| quinacrine | Y | Y |
| clebopride maleate | Y | Y |
| metyrapone | Y | N |
| rotenone | Y | Y |
| acepromazine | Y | N |
| flutamide | Y | Y |
| podophyllum resin | Y | Y |
| piperacetazine | Y | N |
| acetophenazine maleate | Y | N |
| raloxifene hydrochloride | Y | N |
| riboflavin | Y | N |
Y = yes, N = no
Sensitivity of the BLI assay
The BLI assay was further evaluated by searching the HDL for previously known ABCG2 inhibitors. Due to the relatively recent characterization of ABCG2, relatively few ABCG2 inhibitors are known (20). Twenty five previously known ABCG2 inhibitors/substrates were found to be included in the HDL. In addition to the ten compounds listed in Table 1A producing significant BLI signal, fifteen additional, known ABCG2 inhibitors are present in the HDL (Supplementary Table 2). Twenty two of those compounds enhanced the BLI signal significantly (from 2.3- to 19-fold), and only three, naringenin, acacetin and genistein, enhanced the BLI signal less than two-fold (1.9-, 1.8- and 1.2-fold, respectively).
Further characterization of selected new ABCG2 inhibitors
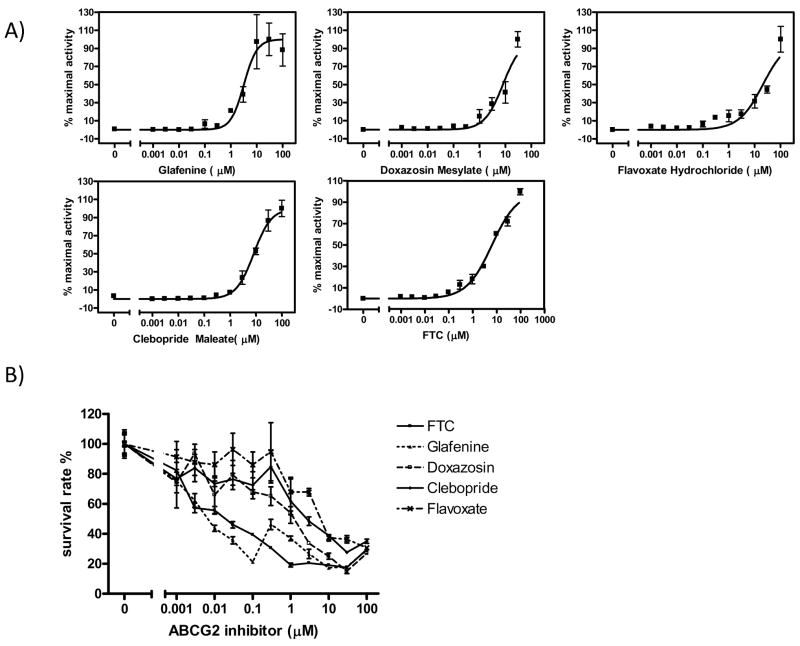
As described previously (12), an ABCG2 inhibitor can enhance fLuc-based BLI signal in a dose-dependent manner. The BLI signal-enhancing effect of selected ABCG2 inhibitors was evaluated within the range of 0.001 μM – 100 μM, with HEK293/ABCG2/fLuc cells and 50 μg/mL D-luciferin. The data obtained at 40 min after imaging commencement were chosen arbitrarily to be plotted (Figure 3A). The IC50 value of glafenine as an ABCG2 inhibitor was calculated to be 3.2 μM. For three other ABCG2 inhibitors, doxazosin mesylate, flavoxate hydrochloride, and clebopride maleate, the BLI signal did not reach a plateau, even at concentrations as high as 100 μM. Assuming that the BLI signal produced by each compound at 100 μM approaches a maximum value, the IC50 values of doxazosin mesylate, flavoxate hydrochloride, and clebopride maleate can be calculated to be 8.0 μM, 20 μM, and 8.2 μM, respectively. The same assay was used to calculate the IC50 value of FTC, and it was determined to be 6.6μM using the 30 min data. Although that value deviates from the IC50 values reported for FTC in literature (0.3 – 1.3 μM), the discrepancy may be caused by the fact that the assays involve different substrates. In terms of its ability to inhibit ATPase, Robey et al. measured the IC50 value of FTC to be 1 μM (13), while Ozvegy et al. obtained values of 1.3 μM (21) and 0.4 μM (22). The IC50 value of FTC was also reported to be 0.8 μM using the pheophorbide A fluorescent dye uptake assay (23). According to those previous reports, FTC reached the plateau of its ABCG2-inhibiting effect at a concentration of 10 μM, but the BLI assay indicates that higher concentrations would be needed to provide a maximal inhibitory effect (Figure 3A).
Figure 3.
ABCG2 inhibitors cause a dose-dependent response in assays measuring the activity of ABCG2 inhibitors. A) Enhancement of bioluminescence signal in HEK293/ABCG2 cells expressing fLuc. Cells were imaged in medium containing 50 μg/mL D-luciferin and increasing concentrations of glafenine, doxazosin mesylate, flavoxate hydrochloride, clebopride maleate, or FTC, and bioluminescence signal was quantified. The data were plotted and the IC50 value of each ABCG2 inhibitor was calculated with GraphPad Prism version 4.0 for Windows (GraphPad Software, San Diego, CA) using variable-slope logistic nonlinear regression analysis. B) Resensitization of ABCG2-overexpressing HEK293 cells to MTX treatment. Cells were plated at a density of 1 × 104 cells per well in 96-well plates, and allowed to attach before incubated in medium containing an ABCG2 inhibitor and/or MTX for 3 days. Cell viabilities were assessed with the XTT assay and expressed as percentages of the control that was treated with MTX alone. Mean ± SEM, n = 3.
The dose-dependent effect of ABCG2 inhibitors was also evaluated with the MTX resensitization assay. ABCG2 overexpressing H460/MX20 cells were incubated for three days with increasing concentrations of each ABCG2 inhibitor in addition to 15 μM MTX, and the survival rates were plotted against the concentration of each compound (Figure 3B). Consistent with the IC50 values of each ABCG2 inhibitor obtained from BLI assay, glafenine proved a more potent ABCG2 inhibitor than FTC, doxazosin mesylate, clebopride maleate, and flavoxate hydrochloride.
To check whether newly identified ABCG2 inhibitors were specific to ABCG2 as opposed to inhibiting other MDR pumps generally, they were also tested for their ability to inhibit ABCB1/Pgp and ABCC1/MRP1. The resensitization assay was performed with MDCKII cells overexpressing Pgp or MRP1 (24) using Col, a Pgp and MRP1 substrate (25, 26). MDCKII cells overexpressing Pgp or MRP1 were incubated for two days in medium containing 1 μM (for Pgp) or 0.3 μM (for MRP1) Col and increasing concentrations of each ABCG2 inhibitor. As shown in Supplementary Figure 1A, compared to VP, glafenine is a more potent Pgp inhibitor, doxazosin mesylate has similar potency, and clebopride maleate and flavoxate hydrochloride demonstrate weak Pgp-inhibiting ability at relatively high concentration (30 μM). Glafenine and doxazosin mesylate have similar potencies to VP for MRP1 inhibition, while clebopride maleate and flavoxate hydrochloride proved weak, even at relatively high concentration (30 μM) (Supplementary Figure 1B). However, all of these ABCG2 inhibitors are specific for ABCG2 at low concentrations (≤1 μM). For example, glafenine can effectively resensitize H460/MX20 cells to MTX at a concentration as low as 0.001 μM (Figure 1B), but does not provide resensitization of MDCKII/Pgp or MDCKII/MRP1 cells to Col until 1 μM or 10 μM, respectively.
In vivo inhibition of ABCG2 activity by selected new ABCG2 inhibitors
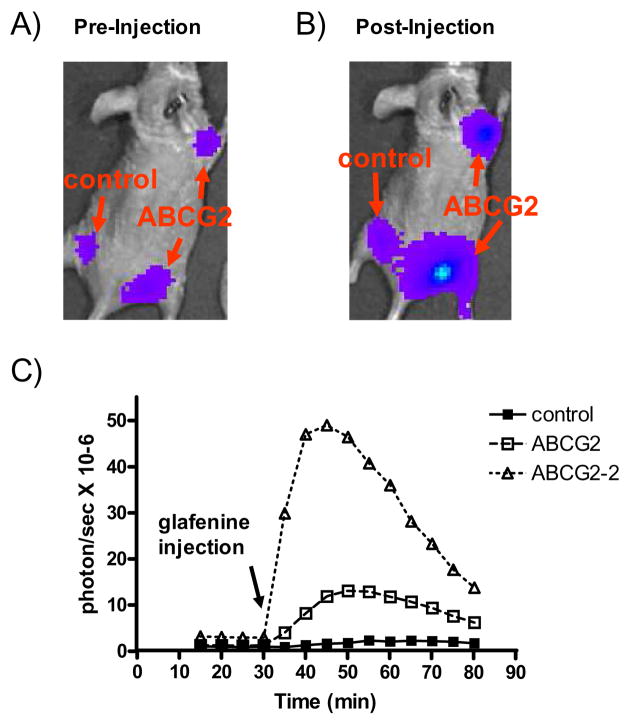
Two of the newly identified ABCG2 inhibitors, glafenine and doxazosin mesylate, were tested further for their ability to inhibit ABCG2 function in vivo. We have previously shown that administration of FTC in vivo can significantly enhance D-luciferin-dependent BLI signal output of xenografts derived from ABCG2-overexpressing HEK293 cells (6). Here we used the same strategy to test the effect of these new ABCG2 inhibitors in vivo. HEK293/empty/fLuc and HEK293/ABCG2/fLuc cells were implanted subcutaneously into opposite flanks of female nude mice. Five mice were implanted to generate 10 ABCG2-overexpressing xenografts and five controls. Animals were imaged after D-luciferin administration, which was followed by a bolus injection of a single dose of ABCG2 inhibitor and continued imaging. After glafenine injection (25 mg/kg i.v.), nine out of 10 ABCG2-overexpressing xenografts showed enhanced BLI signal over the control in the same mouse. Those 10 xenografts showed an average of ~ 2.9-fold signal enhancement over the control with the highest approaching 6.7-fold (Figure 4). Glafenine caused increases in BLI signal of up to ~ 11.6- and ~ 17.4-fold in two separate HEK293/ABCG2/fLuc xenografts (right front and rear flanks) in the same mouse compared to the signals generated by those xenografts immediately before injection. By contrast, the BLI signal of the HEK293/empty/fLuc xenograft in the left flank increased by only ~ 2.6-fold (Figure 4). Doxazosin mesylate injection caused a similar but weaker BLI signal enhancement of ABCG2-overexpressing xenografts in vivo (data not shown).
Figure 4.
Glafenine inhibits ABCG2 activity in a living mouse as shown by BLI. HEK293/empty/fLuc (control) and HEK293/ABCG2/fLuc cells were implanted to the flanks (left and right, respectively) of immunocompromised (nude) mice. A) A representative mouse showing BLI acquired 30 min after administration of D-luciferin i.p., immediately before administration of glafenine (25mg/kg). B) The same mouse as in (A) imaged 15 min after i.v. glafenine administration. C) Time course of BLI signal from both control and ABCG2 overexpressing xenografts before and after glafenine injection. The BLI signal from ABCG2 transfected xenografts increased up to ~ 11.6- and ~17.4-fold (right front and rear, respectively), while the BLI signal from the control xenograft increased only ~ 2.6-fold, compared to their signals immediately before glafenine injection. The arrow indicates the time of glafenine injection.
Discussion
We have developed and evaluated a cell-based, high-throughput assay to uncover new inhibitors of ABCG2. This assay builds upon our finding that D-luciferin, the substrate of fLuc, is a specific substrate of ABCG2, and uses BLI to screen for ABCG2 inhibitors (6). The screening of 3,273 compounds identified 219 candidate ABCG2 inhibitors with at least a two-fold signal enhancement over controls, ~ 60% of which have been previously reported as ABCG2 inhibitors, including gefitinib, prazosin, and harmine. The ability to identify known ABC transporter inhibitors, both potent and weak, proved that the assay is sensitive and reliable. The results also demonstrate the ability of the assay to identify previously unknown ABCG2 inhibitors. Approximately 40% of the 219 potent and ~ 84% of the ~ 150 less potent compounds have never been reported previously as being inhibitors or substrates of an ABC transporter. The less potent compounds, in particular, may be difficult to identify with other methods.
The power of the BLI assay was further demonstrated by the confirmation of the majority (26/28) of the new ABCG2 inhibitors uncovered, indicating a low false-positive rate. Twenty eight candidate ABCG2 inhibitors with over five-fold signal enhancement were subjected to the MTX resensitization assay, and 16 of them were also tested with the BODIPY-prazosin dye uptake assay. All but two were confirmed by the MTX resensitization assay. The low false positive rate may be explained by a previous report, where a screen of more than 70,000 compounds by an assay using pure fLuc found no activator of the luciferase-coupled reaction that could enhance the luminescent signal (27). The signal enhancement seen in our BLI assay is attributed only to the increased intracellular concentration of D-luciferin upon administration of putative ABCG2 inhibitors from our library. However, caution is still required because ABCG2 may not be the only membrane transporter that affects the permeability of D-luciferin. In addition, pore-forming agents or detergents that disrupt cell membranes may cause false positives because of the leakage of D-luciferin into cells. Among the two compounds identified in this screen that could not be confirmed by the MTX resensitization assay, calcimycin is a cation ionophore, and falls into this category.
The BLI-based assay is very sensitive with no false negatives obtained. While the results of the MTX resensitization assay were as expected, those of the BODIPY-prazosin dye uptake assay were intriguing. Only nine out of 16 compounds tested were confirmed by this fluorescence-based assay and seven (~ 44%) failed this assay altogether. All of the seven compounds were confirmed by the MTX resensitization assay (Table 2). Notably, MTX resistance is the hallmark of the ABCG2-inhibiting phenotype (28), (29), therefore, this discrepancy suggests a high false negative rate for the BODIPY-prazosin assay. To understand the discrepancy better, we analyzed the structures of the seven compounds that could not be confirmed by the BODIPY-prazosin assay. Metyrapone, acepromazine, piperacetazine and acetophenazine have an aromatic ketone functional group, which can act as an electron acceptor and deactivate the singlet state of BODIPY via an intermolecular electron-transfer process (30), (31). Porphyrin in verteporin and benzopteridine in riboflavin can quench the fluorescence of BODIPY by way of photoinduced electron transfer (32) (33). It has been reported previously that the fluorescence of several dyes used to probe mitochondrial transmembrane potential can be quenched by some anticancer drugs, including adaphostin, MTX and amsacrine (34). Accordingly, fluorescence-based assays must be cautiously applied. The implication of this finding is significant. Since fluorescence-based assays have seen the most use in discovering new ABCG2 inhibitors (13), (23), (35), (36), it is possible that many ABCG2 inhibitors that quench fluorescence have been missed. The BLI-based screening assay described here has the advantage of not being prone to such an artifact. That advantage was demonstrated by our search of the HDL for previously known ABCG2 inhibitors, which revealed that the BLI-based assay missed none of them.
The BLI-based assay is efficient due to the elimination of incubation and wash steps. Several hundred drugs can be screened in one day using the current system, with many thousands of drugs possible if the technique were automated. False negatives caused by cytotoxicity in extended incubation are not a concern.
Candidate ABCG2 inhibitors obtained from our screen of the HDL are categorized based on their therapeutic effects, and can be clustered into several classes, including drugs affecting the cardiovascular and central nervous systems, the gastrointestinal system, among others (Supplementary Table 3). Previously, it has been reported that ABCG2 is expressed in brain, colon, small and large intestine, venous endothelium, and in capillaries, suggesting that the expression pattern indicates that ABCG2 plays a protective role in these tissues. However, further evidence is needed to support that hypothesis (4). Our discovery that many drugs treating diseases associated with these tissues are ABCG2 inhibitors is consistent with the possible functions of ABCG2 indicated by its expression pattern. The involvement of ABCG2 in some of the processes represented by the classes of agents uncovered in our assay has been recognized previously. Many anti-HIV drugs inhibit ABCG2 (37) and a bidirectional interaction between EGFR and ABCG2 has been reported (38). The roles that ABCG2 may play in most of the other processes have not been studied extensively. Information obtained from this screen may shed light on new mechanisms of action of drugs designed for other purposes. Since most of the compounds in the HDL library have been approved by the FDA, certain candidates could be immediately used in conjunction with standard cancer chemotherapy to help combat the effects of ABC transporter expression, which often confound such treatment.
Supplementary Material
Acknowledgments
We would like to thank Dr. Robert Robey from the National Cancer Institute for helpful discussions. We also thank The Maryland Stem Cell Research Foundation, NIH NS43987 and U24 92871 for financial support.
References
- 1.Doyle LA, Yang W, Abruzzo LV, et al. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(26):15665–70. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nature reviews. 2006;5(3):219–34. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 3.Benderra Z, Faussat AM, Sayada L, et al. Breast cancer resistance protein and P-glycoprotein in 149 adult acute myeloid leukemias. Clin Cancer Res. 2004;10(23):7896–902. doi: 10.1158/1078-0432.CCR-04-0795. [DOI] [PubMed] [Google Scholar]
- 4.Robey RW, Polgar O, Deeken J, To KW, Bates SE. ABCG2: determining its relevance in clinical drug resistance. Cancer metastasis reviews. 2007;26(1):39–57. doi: 10.1007/s10555-007-9042-6. [DOI] [PubMed] [Google Scholar]
- 5.Ailles LE, Weissman IL. Cancer stem cells in solid tumors. Current opinion in biotechnology. 2007;18(5):460–6. doi: 10.1016/j.copbio.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Bressler JP, Neal J, et al. ABCG2/BCRP expression modulates D-Luciferin based bioluminescence imaging. Cancer research. 2007;67(19):9389–97. doi: 10.1158/0008-5472.CAN-07-0944. [DOI] [PubMed] [Google Scholar]
- 7.Rettig GR, McAnuff M, Liu D, Kim JS, Rice KG. Quantitative bioluminescence imaging of transgene expression in vivo. Analytical biochemistry. 2006;355(1):90–4. doi: 10.1016/j.ab.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 8.Rabindran SK, Ross DD, Doyle LA, Yang W, Greenberger LM. Fumitremorgin C reverses multidrug resistance in cells transfected with the breast cancer resistance protein. Cancer research. 2000;60(1):47–50. [PubMed] [Google Scholar]
- 9.Brovko LY, Griffiths MW. Illuminating cellular physiology: recent developments. Science progress. 2007;90(Pt 2–3):129–60. doi: 10.3184/003685007X215986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chong CR, Chen X, Shi L, Liu JO, Sullivan DJ., Jr A clinical drug library screen identifies astemizole as an antimalarial agent. Nature chemical biology. 2006;2(8):415–6. doi: 10.1038/nchembio806. [DOI] [PubMed] [Google Scholar]
- 11.Chong CR, Qian DZ, Pan F, et al. Identification of type 1 inosine monophosphate dehydrogenase as an antiangiogenic drug target. Journal of medicinal chemistry. 2006;49(9):2677–80. doi: 10.1021/jm051225t. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Laterra J, Pomper MG. Hedgehog pathway inhibitor HhAntag691 is a potent inhibitor of ABCG2/BCRP and ABCB1/Pgp. Neoplasia (New York, NY. 2009;11(1):96–101. doi: 10.1593/neo.81264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robey RW, Honjo Y, van de Laar A, et al. A functional assay for detection of the mitoxantrone resistance protein, MXR (ABCG2) Biochimica et biophysica acta. 2001;1512(2):171–82. doi: 10.1016/s0005-2736(01)00308-x. [DOI] [PubMed] [Google Scholar]
- 14.Zhang JH, Chung TD, Oldenburg KR. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J Biomol Screen. 1999;4(2):67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 15.Robey RW, Honjo Y, Morisaki K, et al. Mutations at amino-acid 482 in the ABCG2 gene affect substrate and antagonist specificity. British journal of cancer. 2003;89(10):1971–8. doi: 10.1038/sj.bjc.6601370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakamura Y, Oka M, Soda H, et al. Gefitinib (“Iressa”, ZD1839), an epidermal growth factor receptor tyrosine kinase inhibitor, reverses breast cancer resistance protein/ABCG2-mediated drug resistance. Cancer research. 2005;65(4):1541–6. doi: 10.1158/0008-5472.CAN-03-2417. [DOI] [PubMed] [Google Scholar]
- 17.Zhou S, Schuetz JD, Bunting KD, et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nature medicine. 2001;7(9):1028–34. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Gupta A, Wang H, et al. BCRP transports dipyridamole and is inhibited by calcium channel blockers. Pharmaceutical research. 2005;22(12):2023–34. doi: 10.1007/s11095-005-8384-4. [DOI] [PubMed] [Google Scholar]
- 19.Limtrakul P, Chearwae W, Shukla S, Phisalphong C, Ambudkar SV. Modulation of function of three ABC drug transporters, P-glycoprotein (ABCB1), mitoxantrone resistance protein (ABCG2) and multidrug resistance protein 1 (ABCC1) by tetrahydrocurcumin, a major metabolite of curcumin. Molecular and cellular biochemistry. 2007;296(1–2):85–95. doi: 10.1007/s11010-006-9302-8. [DOI] [PubMed] [Google Scholar]
- 20.Ahmed-Belkacem A, Pozza A, Macalou S, Perez-Victoria JM, Boumendjel A, Di Pietro A. Inhibitors of cancer cell multidrug resistance mediated by breast cancer resistance protein (BCRP/ABCG2) Anti-cancer drugs. 2006;17(3):239–43. doi: 10.1097/00001813-200603000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Ozvegy C, Litman T, Szakacs G, et al. Functional characterization of the human multidrug transporter, ABCG2, expressed in insect cells. Biochemical and biophysical research communications. 2001;285(1):111–7. doi: 10.1006/bbrc.2001.5130. [DOI] [PubMed] [Google Scholar]
- 22.Ozvegy C, Varadi A, Sarkadi B. Characterization of drug transport, ATP hydrolysis, and nucleotide trapping by the human ABCG2 multidrug transporter. Modulation of substrate specificity by a point mutation. The Journal of biological chemistry. 2002;277(50):47980–90. doi: 10.1074/jbc.M207857200. [DOI] [PubMed] [Google Scholar]
- 23.Henrich CJ, Bokesch HR, Dean M, et al. A high-throughput cell-based assay for inhibitors of ABCG2 activity. J Biomol Screen. 2006;11(2):176–83. doi: 10.1177/1087057105284576. [DOI] [PubMed] [Google Scholar]
- 24.Evers R, Kool M, Smith AJ, van Deemter L, de Haas M, Borst P. Inhibitory effect of the reversal agents V-104, GF120918 and Pluronic L61 on MDR1 Pgp-, MRP1- and MRP2-mediated transport. British journal of cancer. 2000;83(3):366–74. doi: 10.1054/bjoc.2000.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annual review of pharmacology and toxicology. 1999;39:361–98. doi: 10.1146/annurev.pharmtox.39.1.361. [DOI] [PubMed] [Google Scholar]
- 26.Shen DW, Cardarelli C, Hwang J, et al. Multiple drug-resistant human KB carcinoma cells independently selected for high-level resistance to colchicine, adriamycin, or vinblastine show changes in expression of specific proteins. The Journal of biological chemistry. 1986;261(17):7762–70. [PubMed] [Google Scholar]
- 27.Auld DS, Southall NT, Jadhav A, et al. Characterization of chemical libraries for luciferase inhibitory activity. Journal of medicinal chemistry. 2008;51(8):2372–86. doi: 10.1021/jm701302v. [DOI] [PubMed] [Google Scholar]
- 28.Doyle LA, Ross DD. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2) Oncogene. 2003;22(47):7340–58. doi: 10.1038/sj.onc.1206938. [DOI] [PubMed] [Google Scholar]
- 29.Bates SE, Robey R, Miyake K, Rao K, Ross DD, Litman T. The role of half-transporters in multidrug resistance. Journal of bioenergetics and biomembranes. 2001;33(6):503–11. doi: 10.1023/a:1012879205914. [DOI] [PubMed] [Google Scholar]
- 30.Perez-Prieto J, Galian RE, Morant-Minana MC. Aromatic ketones as photocatalysts: combined action as triplet photosensitiser and ground state electron acceptor. Chemphyschem. 2006;7(10):2077–80. doi: 10.1002/cphc.200600328. [DOI] [PubMed] [Google Scholar]
- 31.Matsumoto T, Urano Y, Shoda T, Kojima H, Nagano T. A thiol-reactive fluorescence probe based on donor-excited photoinduced electron transfer: key role of ortho substitution. Organic letters. 2007;9(17):3375–7. doi: 10.1021/ol071352e. [DOI] [PubMed] [Google Scholar]
- 32.Ulrich G, Ziessel R, Harriman A. The chemistry of fluorescent bodipy dyes: versatility unsurpassed. Angewandte Chemie (International ed. 2008;47(7):1184–201. doi: 10.1002/anie.200702070. [DOI] [PubMed] [Google Scholar]
- 33.Mario Koenig BP, Zieg Harald, Ritter Tobias, Bouas-Laurent Henri, Bonneau Roland, Desvergne Jean-Pierre. Photoinduced Electron Transfer in a Phenothiazine- Riboflavin Dyad Assembled by Zinc-Imide Coordination in Water. Journal of the American Chemical Society. 1999;121(8):7. [Google Scholar]
- 34.Le SB, Holmuhamedov EL, Narayanan VL, Sausville EA, Kaufmann SH. Adaphostin and other anticancer drugs quench the fluorescence of mitochondrial potential probes. Cell death and differentiation. 2006;13(1):151–9. doi: 10.1038/sj.cdd.4401732. [DOI] [PubMed] [Google Scholar]
- 35.Rajagopal A, Simon SM. Subcellular localization and activity of multidrug resistance proteins. Molecular biology of the cell. 2003;14(8):3389–99. doi: 10.1091/mbc.E02-11-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mogi M, Yang J, Lambert JF, et al. Akt signaling regulates side population cell phenotype via Bcrp1 translocation. The Journal of biological chemistry. 2003;278(40):39068–75. doi: 10.1074/jbc.M306362200. [DOI] [PubMed] [Google Scholar]
- 37.Weiss J, Rose J, Storch CH, et al. Modulation of human BCRP (ABCG2) activity by anti-HIV drugs. The Journal of antimicrobial chemotherapy. 2007;59(2):238–45. doi: 10.1093/jac/dkl474. [DOI] [PubMed] [Google Scholar]
- 38.Liu H, Cheng D, Weichel AK, et al. Cooperative effect of gefitinib and fumitremorgin c on cell growth and chemosensitivity in estrogen receptor alpha negative fulvestrant-resistant MCF-7 cells. International journal of oncology. 2006;29(5):1237–46. [PubMed] [Google Scholar]
- 39.Yanase K, Tsukahara S, Asada S, Ishikawa E, Imai Y, Sugimoto Y. Gefitinib reverses breast cancer resistance protein-mediated drug resistance. Molecular cancer therapeutics. 2004;3(9):1119–25. [PubMed] [Google Scholar]
- 40.Zamek-Gliszczynski MJ, Nezasa K, Tian X, et al. The important role of Bcrp (Abcg2) in the biliary excretion of sulfate and glucuronide metabolites of acetaminophen, 4-methylumbelliferone, and harmol in mice. Molecular pharmacology. 2006;70(6):2127–33. doi: 10.1124/mol.106.026955. [DOI] [PubMed] [Google Scholar]
- 41.Litman T, Brangi M, Hudson E, et al. The multidrug-resistant phenotype associated with overexpression of the new ABC half-transporter, MXR (ABCG2) Journal of cell science. 2000;113(Pt 11):2011–21. doi: 10.1242/jcs.113.11.2011. [DOI] [PubMed] [Google Scholar]
- 42.Chearwae W, Shukla S, Limtrakul P, Ambudkar SV. Modulation of the function of the multidrug resistance-linked ATP-binding cassette transporter ABCG2 by the cancer chemopreventive agent curcumin. Molecular cancer therapeutics. 2006;5(8):1995–2006. doi: 10.1158/1535-7163.MCT-06-0087. [DOI] [PubMed] [Google Scholar]
- 43.Zhou XF, Yang X, Wang Q, Coburn RA, Morris ME. Effects of dihydropyridines and pyridines on multidrug resistance mediated by breast cancer resistance protein: in vitro and in vivo studies. Drug metabolism and disposition: the biological fate of chemicals. 2005;33(8):1220–8. doi: 10.1124/dmd.104.003558. [DOI] [PubMed] [Google Scholar]
- 44.van Herwaarden AE, Wagenaar E, Merino G, et al. Multidrug transporter ABCG2/breast cancer resistance protein secretes riboflavin (vitamin B2) into milk. Molecular and cellular biology. 2007;27(4):1247–53. doi: 10.1128/MCB.01621-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cooray HC, Janvilisri T, van Veen HW, Hladky SB, Barrand MA. Interaction of the breast cancer resistance protein with plant polyphenols. Biochemical and biophysical research communications. 2004;317(1):269–75. doi: 10.1016/j.bbrc.2004.03.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.