Abstract
We used live cell imaging to compare the fate of human nontransformed (RPE-1) and cancer (HeLa, U2OS) cells as they entered mitosis in nocodazole or taxol. In the same field, and in either drug, a cell in all lines could die in mitosis, exit mitosis and die within 10 h, or exit mitosis and survive ≥10 h. Relative to RPE-1 cells, significantly fewer HeLa or U2OS cells survived mitosis or remained viable after mitosis: in nocodazole concentrations that inhibit spindle microtubule assembly, or in 500 nM taxol, 30% and 27% of RPE-1 cells, respectively, died in or within 10 h of exiting mitosis while 90% and 49% of U2OS and 78% and 81% of HeLa died. This was even true for clinically relevant taxol concentrations (5 nM) which killed 93% and 46%, respectively, of HeLa and U2OS cells in mitosis or within 10 h of escaping mitosis, compared to 1% of RPE-1 cells. Together these data imply that studies using HeLa or U2OS cells, harvested after a prolonged block in mitosis with nocodazole or taxol, are significantly contaminated with dead or dying cells. We also found that the relationship between the duration of mitosis and survival is drug and cell type specific and that lethality is related to the cell type and drug used to prevent satisfaction of the kinetochore attachment checkpoint. Finally, work with a pancaspase inhibitor suggests that the primary apoptotic pathway triggered by nocodazole during mitosis in RPE-1 cells is not active in U2OS cells. Cell Motil. Cytoskeleton 2008.
Keywords: mitosis, apoptosis, kinetochore attachment checkpoint, mitotic slippage, nocodazole, taxol
INTRODUCTION
In 1906 Dixon noted in his “Manual of Pharmacology” (p 96) that one effect of colchicine was to excite karyokinesis (mitosis) in most body tissues. That this increased number of mitotic figures results from an accumulation of arrested mitoses and not from a stimulation of mitosis was not evident, however, until live cell culturing methods became widespread in the 1930s [see Ludford, 1936]. In 1991 the mitotic arrest induced by colcemid and other microtubule (MT) poisons was shown to be mediated by a bonafide intrinsic cell cycle checkpoint control [Hoyt et al., 1991; Li and Murray, 1991] that is activated at the beginning of mitosis. Subsequently it was found that this checkpoint monitors kinetochore attachment [Rieder et al., 1994, 1995] and it delays chromatid separation (anaphase onset) and exit from mitosis (telophase) until all kinetochores are saturated with and stably attached to spindle MTs [reviewed in Musacchio and Salmon, 2007]. Since MT poisons prevent the stable accumulation of MTs at kinetochores, they arrest or delay progress through mitosis via this kinetochore attachment checkpoint (KAC).
The signal transduction cascade behind the KAC ultimately prevents E3 ubiquitin ligase assemblies, known as anaphase-promoting complexes (APCs), from targeting for destruction those proteins required for maintaining chromatid cohesion (shugoshin, securin) and the mitotic condition (cyclin B). As for other checkpoints, an active KAC is not normally capable of blocking exit from mitosis indefinitely. Instead, when the KAC cannot be satisfied, as in the complete absence of spindle MTs, many normal nontransformed cells exit mitosis after a prolonged delay and enter the next G1 as micronucleated tetraploid cells [reviewed in Rieder and Maiato, 2004]. The process by which cells escape mitosis when they cannot satisfy the KAC is termed “mitotic slippage” and it occurs because even when active the KAC is not 100% efficient at preventing a slow but continuous APC-mediated destruction of cyclin B [Brito and Rieder, 2006].
Through the years many have explored how cells, especially those from tumors, respond as they enter mitosis in MT poisons. Although some of these studies contained live cell analyses [e.g., Pulkkinen et al., 1996; Chen et al., 2003; Shi et al., 2008] the bulk were based on indirect data garnered from fixed cell populations prepared, e.g., for light microscopic or fluorescence activated cell sorting (FACS) analyses, or for protein assays like immunoprecipitation/blotting [see Rieder and Maiato, 2004]. However, in the absence of live cell data indirect methods can produce vague or erroneous conclusions. This is especially true when estimations of cell survival are based on the reattachment of viable cells without considering those that die and float away between time-points. This largely neglected consideration can lead to a gross underestimation of cell death. Likewise, although FACS is heavily used for exploring how drugs impact entry into and exit from mitosis, most FACS studies use fluorescent probes, like DAPI, propidium iodide or phosphohistone H3 antibody, that do not differentiate G2 from mitotic cells. (It is well established that histone H3 phosphorylation begins in very early G2 [Hendzel et al., 1997; Crosio et al., 2002; Shinohara et al., 2006] and not during mitosis.) As a result many previous studies on how cells respond during mitosis to MT poisons not only produced confusing data on “G2/M” cells, but they also lacked reliable data on whether those cells that died did so during mitosis or the subsequent G1. Lastly, the issue of whether conclusions made from one cell type, usually a tumor cell like HeLa, are applicable to other cancer or even normal cells is seldom addressed.
Drugs that perturb MT function, like taxanes and vinca alkaloids, are used to treat certain cancers [reviewed in Jordan and Wilson, 2004]. Although their mechanism(s) of action remain vague, they are thought to work by delaying or preventing satisfaction of the KAC, which in turn leads to a prolonged mitosis and subsequent cell death [e.g., Rowinsky and Donehower, 1991; Panvichian et al., 1998; Mollinedo and Gajate, 2003; Shi et al., 2008]. Recently we reported, based on live cell analyses, that when nontransformed human RPE-1 cells are exposed to low concentrations of MT poisons (i.e., 50 nM nocodazole, 5 nM vinblastine or 5 nM taxol) mitosis is, as expected, prolonged. Surprisingly, however, at these drug concentrations 90–100% of the cells constructed over a 2–4 h period abnormal but functional spindles that satisfied the KAC, and 80–90% of these subsequently divided into two or more aneuploid daughters [Brito et al., 2008]. Others have reported that some cancer cell types (e.g., A549 and HeLa) also complete a similarly abnormal division in 5–10 nM taxol [Jordan et al., 1993; Chen and Horwitz, 2002; Ikui et al., 2005].
These recent studies suggest that at low (clinically relevant) concentrations MT poisons do not kill non-transformed or cancer cells in mitosis. However the relative survival percentages of normal or cancer cells during or after division in low drug concentrations remain to be determined. At the same time the link between the duration of a drug-induced prolonged mitosis (i.e., a D-mitosis) and cell death, whether death occurs in mitosis or after slippage, the mechanisms behind this death, and how they vary between normal and cancer cells and with different drugs and drug concentrations are only just beginning to be directly studied in detail [Rieder and Maiato, 2004; Jordan and Kamath, 2007; Shi et al., 2008]. To address some of these questions we have conducted a comparative live-cell study on the behavior and fate of human cells from a nontransformed (RPE-1) and two popular cancer (U2OS, HeLa) cell lines, as they enter mitosis in the presence of nocodazole or taxol, two widely used spindle poisons.
MATERIALS AND METHODS
Cell Culture and Drug Treatment
HeLa (cervical carcinoma), U2OS (osteosarcoma) and hTERT-RPE-1 (RPE-1; retinal pigment epithelial) cell lines were cultured as monolayers in DMEM supplemented with 10% fetal bovine serum (FBS). All cells were maintained in a humidified incubator at 37°C in a 5% CO2 environment. Prior to experiments cells were subcultured onto 25 mm2 glass coverslips and grown as above for at least 16 h.
For filming, coverslip cultures were mounted in Rose chambers and recordings were made at 37°C as previously described [Khodjakov and Rieder, 2006]. Before imaging, nocodazole or taxol (Calbiochem, La Jolla, CA) was added to the medium at the concentrations noted in the text. The general caspase inhibitor (20 μM), Q-VD-OPh (R&D Systems, Minneapolis, MN; Cat # OPH001), was added to the medium 1 h before adding nocodazole.
Live-Cell Microscopy
Time-lapse recordings of RPE-1, HeLa and U2OS cells were captured every 2 (untreated controls) to 10 (experimental) minutes at 37°C with a low magnification (10X 0.3NA) PlanFluor phase-contrast objective lens mounted on a Nikon Diaphot microscope equipped with a Spot Insight QE video camera and fast UniBlitz shutters (Vincent Associates, Rochester, NY). The hardware was driven by Image-Pro Plus 5.1.020 (Media Cybernetics, Silver Spring, MD). Image sequences were compiled with ImageJ 1.35c (NIH), and contrast-adjustment was conducted using Photoshop CS2 (Adobe Systems).
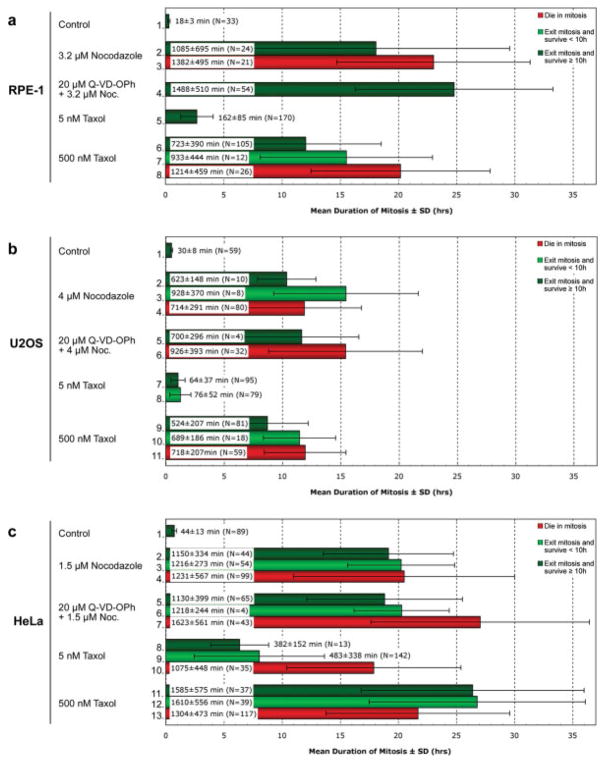
In our graphs N refers to the total numbers of cells pooled from ≥2 separate long-term recordings obtained on different days, with the exception of those experiments using the pancaspase inhibitor, Q-VD-OPh, which were from single experiments. In some cases the number of cells noted in the lanes of our timing graph (Fig. 2) is different from that of our survival graph (Fig. 4) because clustering of adjacent cells sometimes prohibited determining an accurate duration of mitosis for a particular cell but not its ultimate fate.
Fig. 2.
In the presence of nocodazole or taxol, U2OS cells spend significantly less time in mitosis than RPE-1 or HeLa cells. Phase-contrast recordings of RPE-1 (a), U2OS (b) and HeLa (c) cultures continuously incubated with nocodazole or taxol in the presence or absence of a pancaspase inhibitor (Q-VD-OPh) were scored for cells that died in mitosis (red), died within 10 h of escaping mitosis (light green), or survived ≥10 h after escaping mitosis (dark green). Percentages were calculated from data pooled from ≥ 2 separate long-term recordings obtained on different days. Q-VD-OPh data are from single experiments. The white box in each bar defines the mean duration of mitosis in minutes ± the standard deviation, while N defines the total number of cells obtained in each category (see also Materials and Methods). See text for details.
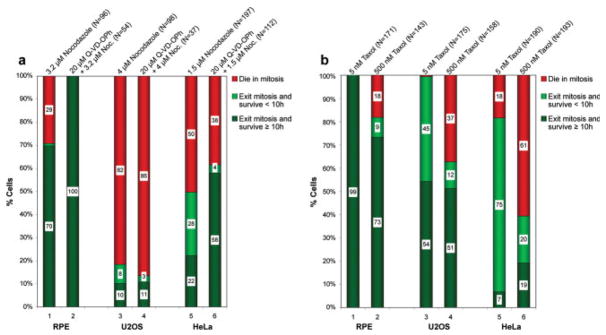
Fig. 4.
Nontransformed RPE-1 cells show higher survival rates when dividing in the presence of nocodazole or taxol than U2OS or HeLa cells. Phase-contrast recordings of RPE-1, U2OS and HeLa cultures incubated with nocodazole (a) or taxol (b) were scored (cf. Fig. 2) for cells that died in mitosis (red), died within 10 h of escaping mitosis (light green), or survived ≥10 h after escaping mitosis (dark green). Numbers in the white box within each subset of an experimental treatment note the percentage of cells in that subset. (a) RPE-1, U2OS and HeLa cells treated with nocodazole alone (lanes 1, 3, and 5) or nocodazole and Q-VD-OPh (a caspase inhibitor; lanes 2, 4, and 6). Concentrations and total cell number are indicated above each column. Note that relative to RPE-1 cells, nocodazole concentrations that inhibit spindle MT assembly kill substantially more U2OS and HeLa cells during or shortly after mitosis. (b) RPE-1, U2OS and HeLa cultures treated with either 5 nM (lanes 1, 3, and 5) or 500 nM (lanes 2, 4, and 6) taxol. As for nocodazole, compared to RPE-1 cells U2OS and HeLa have a more difficult time surviving mitosis in the presence of taxol. Note also that taxol kills substantially fewer U2OS than HeLa cells during or after mitosis, and that only 7% of HeLa cells dividing in 5 nM taxol are alive 10 h later.
Immunofluorescence
Microtubule staining was performed as previously described [Brito and Rieder, 2006]. As primary and secondary antibodies we used mouse anti-α-tubulin (DM1α, Sigma) and AlexaFluor 594 (Molecular Probes), respectively. DNA was counterstained with Hoechst 33342 (Molecular Probes).
After staining, cells were imaged as a Z-series (200 nm apart) on an Olympus IX70 inverted microscope equipped with a CM350 camera (Photometrics). The images were deblurred using the SoftWorx 2.5 deconvolution algorithm (Applied Precision, Issaquah, WA), and presented as maximal intensity projections.
RESULTS AND DISCUSSION
Human RPE-1 cells express telomerase reverse transcriptase which extends their replicative lifespan beyond senescence without inducing aneuploidy or neoplastic transformation [Jiang et al., 1999]. Recently we reported that when RPE-1 enter mitosis in the presence of 3.2 μM nocodazole, the minimal concentration that inhibits all spindle MT assembly, most (~70%) ultimately slip through mitosis and enter the next G1 as micronucleated 4N cells [Brito and Rieder, 2006; Brito et al., 2008].
In the complete absence of MTs the duration of a D-mitosis reflects the “strength” of the KAC [Weaver and Cleveland, 2005]. Given this we sought to determine the fate of U2OS and HeLa, two widely used cancer cell lines, when they enter mitosis under conditions in which the KAC cannot be satisfied. At the same time we asked if the strength of the KAC in HeLa and U2OS differs from that of a nontransformed (RPE-1) cell. To obtain this data we treated RPE-1, U2OS and HeLa cultures with, respectively, 3.2 μM, 4 μM, and 1.5 μM nocodazole, concentrations near the minimum that completely inhibit spindle MT assembly in each cell type (e.g., Fig. 1a). We then used low magnification phase-contrast time-lapse light microscopy to follow fields of cells at 37°C, for 48–72 h. We also used two taxol concentrations that altered spindle morphology to different extents: one (5 nM; Fig. 1b) that allows the KAC to become satisfied after several hours [Brito et al., 2008], and another (500 nM; Fig. 1c) that produces a more durable delay in mitosis. In our studies we defined the duration of mitosis as the period between nuclear envelope breakdown and initiation of the violent plasma membrane blebbing characteristic of telophase/cytokinesis, a feature that also precedes reflattening as cells treated with MT poisons slip through mitosis. At 37°C mitosis in untreated RPE-1, U2OS and HeLa cells averaged, respectively, 18 ± 3 min, 30 ± 8 min and 44 ± 13 min (Figs. 2a–c, lanes 1). These timings nicely illustrate that, relative to nontransformed cells the average duration of mitosis is prolonged in most cancer cell lines [Yang et al., 2008].
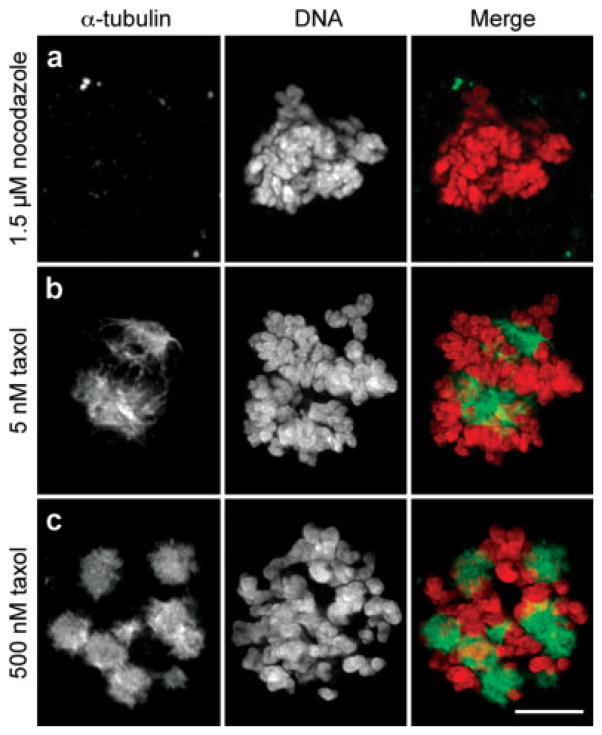
Fig. 1.
Nocodazole inhibits spindle microtubule formation whereas taxol promotes microtubule stability. HeLa cells were incubated overnight with 1.5 μM nocodazole (a), 5 nM (b) taxol, or 500 nM (c) taxol prior to fixation and immunostaining for α-tubulin. First column, α-tubulin; second column, DNA; third column, merged images. HeLa that enter mitosis in the presence of 1.5 μM nocodazole fail to form spindle microtubules. The same is true for RPE-1 and U2OS cells treated, respectively, with 3.2 and 4.0 μM nocodazole (not shown). When exposed continuously to 5 nM taxol HeLa cells form abnormal but functional spindles that ultimately satisfy the kinetochore attachment checkpoint (b) while in 500 nM taxol multiple stable radial microtubule arrays (asters) are formed that the chromosomes attach to (c). The same is true for RPE-1 and U2OS (not shown). Bar = 5 μm.
Cells That Enter Mitosis in Nocodazole or Taxol Can Either Die in Mitosis or Slip into the Next G1 Where They Die Within 10 h or Survive ≥10 h
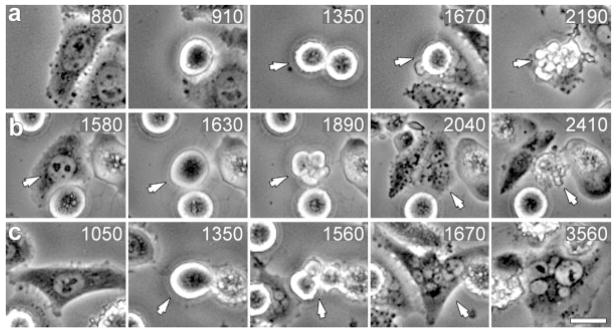
Death in mitosis was easily recognizable in time-lapse records since under most conditions it was manifested by a sudden violent blebbing of the rounded cell followed by a cytoplasmic contraction with a resultant change in refractivity (Fig. 3a). Those cells that survived mitosis in the presence of spindle poisons reflattened onto the substrate and then either died within (Fig. 3b), or survived for more than (Fig. 3c), 10 h (a duration chosen because it approximates that of G1 in a normal cycling cell). [Some of the cells that survived ≥10 h after slipping through mitosis were still alive when filming was terminated while others died within the time course of the experiment.] All three of these fates were seen in the same field of view with both drugs in all three cell lines.
Fig. 3.
Human cells in the same population that enter mitosis in the presence of microtubule poisons exhibit one of three fates. Selected frames from the same phase-contrast video recording of HeLa cells entering mitosis in 5 nM taxol. (a) This cell enters mitosis at 910 min, and then dies in mitosis at the 2190 min time point (arrow). (b) This cell enters mitosis at 1630 min, escapes mitosis (blebbing in 1890 min) to form a 4N cell (2040 min) that subsequently dies (2410 min) <10 h after exiting mitosis. (c) This cell enters mitosis at 1350 min (arrow), exits at 1560 min (arrow), and survives ≥10 h (until filming was terminated). Time, relative to addition of taxol, is shown in minutes. Bar = 10 μm.
The KAC in Cancer Cells can be Similar to or Weaker Than That of Nontransformed Cells
If a weaker KAC promotes cell transformation, or if tumorgenesis selects for a weaker KAC [see Weaver and Cleveland, 2005], then in the permanent presence of unattached kinetochores, as occurs during our nocodazole treatments, cancer cells should spend less time in a D-mitosis than nontransformed cells. However, of the cells that slipped through mitosis in nocodazole that were still alive after 10 h RPE-1 and HeLa averaged ~18–19 h in mitosis while U2OS averaged only ~10 h (cf. Fig. 2a–c, lanes 2). [The durations of mitosis in all cells under our experimental conditions are summarized in Fig. 2.] Similarly, those 4N G1 RPE-1 cells that were still alive ≥10 h after slipping through mitosis in 500 nM taxol averaged ~12 h in mitosis (Fig. 2a, lane 6), while HeLa were blocked for significantly longer (~26 h; Fig. 2c, lane 11) and U2OS for shorter (~9 h; Fig. 2b, lane 9). These results and the work of others [e.g., Tighe et al., 2001] reveal that a weaker KAC is not always selected for during tumorgenesis: in some cancer cell lines (like HeLa) it can be similar to that of nontransformed (RPE-1) cells, while in other cancer lines (like U2OS) it can be weaker. One question raised by our data is why, in RPE-1 cells that survived for at least 10 h after mitosis, completely preventing MT assembly with nocodazole blocks them for ~18 h (Fig. 2a, lane 2) while stabilizing spindle MTs with 500 nM taxol blocks them in mitosis for only ~12 h (Fig. 2a, lane 6)? Although the answer is unknown, it is possible that in 500 nM taxol many RPE-1, but not HeLa or U2OS cells, exit mitosis prematurely relative to those cells lacking MTs because they ultimately satisfy the KAC [see Brito et al., 2008]. This is supported by our finding that RPE-1 cells that died in mitosis in the presence of 500 nM taxol, i.e., that failed to slip, did so after ~20 h (Fig. 2a, lane 8).
The length of the mitotic delay induced by MT poisons was cell type specific even for 5 nM taxol (Figs. 2a–c): relative to untreated controls (Figs. 2a–c, lanes 1), in 5 nM taxol mitosis was prolonged 9X in RPE-1 (Fig. 2a, lane 5) and HeLa (Fig. 2c, lane 8) cells but only 2X in U2OS (Fig. 2b, lane 7). Why, compared to RPE-1 and HeLa, do U2OS cells spend so little time in mitosis in the presence of 5 nM taxol? One possibility is that the membrane drug pumps in U2OS cells are more efficient than those in RPE-1 and HeLa in eliminating the drug from the cell. Another is that, as noted above, compared to RPE-1 and HeLa the checkpoint machinery in U2OS is significantly weakened. In turn this may allow for an accelerated destruction of cyclin B even when some kinetochores are not stably attached to MTs [see Weaver and Cleveland, 2005]. In this regard one or more of the KAC proteins involved in generating the “wait anaphase” signal are known to be mutated or down-regulated in many types of cancers [e.g., Cahill et al., 1998; Wang et al., 2008; reviewed in Weaver and Cleveland, 2005]. Although the KAC in these cells is commonly referred to as being “inactivated”, in most cases (as in U2OS) it is simply impaired in that it can still delay exit from mitosis for many hours but not for the highly protracted period characteristic of normal cells.
Compared to Nontransformed (RPE-1) Cells U2OS and HeLa are Much Less Likely to Survive Mitosis When Treated with MT Poisons
Although some cells in all three lines slipped through mitosis in MT poisons, compared to RPE-1 cells death in mitosis was significantly enhanced in both cancer lines: whereas in nocodazole (the absence of MTs) 71% of RPE-1 slipped through mitosis (Fig. 4a, lane 1), only 18% of U2OS (lane 3) and 50% of HeLa (lane 5) did so. This was also true for 500 nM taxol where 82% of RPE-1 cells survived to exit mitosis (Fig. 4b, lane 2), while only 63% of U2OS (lane 4) and 39% of HeLa (lane 6) survived mitosis. These findings are not consistent with conclusions based on less direct data that, when the KAC cannot be satisfied the state of MT assembly influences whether a cell will slip through or die in mitosis [Andreassen and Margolis, 1994; Trielli et al., 1996; Chen and Horwitz, 2002]. Instead, we find that similar numbers of RPE-1 cells slipped through mitosis whether MTs were absent (nocodazole) or present (500 nM taxol), and this was also true for HeLa, but not U2OS (cf., Fig. 4a, lane 3; Fig. 4b, lane 4). Thus, relative to nontransformed cells, when the KAC cannot be satisfied U2OS and HeLa cells have a more difficult time surviving mitosis, and survivability differs dramatically between the lines.
Similar conclusions were recently reported by Mitchison and colleagues [Shi et al., 2008] who used live cell imaging to study the fate of several cell types as they enter mitosis in the presence of MT poisons. Although these workers report no timing data they concluded, as we do, that for nocodazole or taxol the fraction of cells dying in mitosis versus dying after exiting mitosis was different for each cell line and drug. They also reported that for nocodazole (500 nM) and taxol (150 nM) the ratio of HeLa cells that died in mitosis versus after slippage was 30:1, while the majority of U2OS cells died after exiting mitosis. We found, by contrast, that in 1.5 μM nocodazole or 500 nM taxol 50% and 61%, respectively, of HeLa cells died in mitosis (Fig. 4a, lane 5; Fig. 4b, lane 6), while in 4 μM nocodazole and 500 nM taxol 82% and 37%, respectively, of U2OS cells died in mitosis (Fig. 4a, lane 3; Fig. 4b, lane 4). The reasons for the discrepancies between the Shi et al. (2008) study and ours are unknown but they may be due to different drug concentrations used in the two studies or to different cell strains.
As reported previously [Brito et al., 2008], after a delay of ~3 h, 99% of RPE-1 cells that entered mitosis in the presence of 5 nM taxol ultimately satisfied the KAC (Fig. 2a, lane 5; Fig. 4b, lane 1) and 90% of these divided into two or more daughter cells (Fig. 5). When we repeated this study on U2OS and HeLa we found that 99% of U2OS (Fig. 4b, lane 3) and 82% of HeLa (Fig. 4b, lane 5) cells survived mitosis in 5 nM taxol and >70% of these survivors divided into two or more daughters (Fig. 5) after remaining in division, respectively, for an average of 1 and 6 h (Fig. 2b, lane 7; Fig. 2c, lane 8). Depending on the line 25–40% of the cells formed multipolar spindles (Fig. 5) and, in fixed cell preparations many anaphase cells were seen to contain >2 groups of chromosomes and/or lagging chromosomes which led to aneuploid daughter cells. Surprisingly, unlike for RPE-1 in which 99% of the daughter cells were still alive 10 h after exiting mitosis in the continuous presence of 5 nM taxol (Fig. 4b, lane 1), only 54% of U2OS (lane 3) and 7% of HeLa (lane 5) were alive 10 h after mitosis. Thus, compared to nontransformed human cells, cancer cells respond very differently to a delay in mitosis induced by 5 nM taxol, as do different cancer cell lines—which may partly explain the clinical efficacy of taxol.
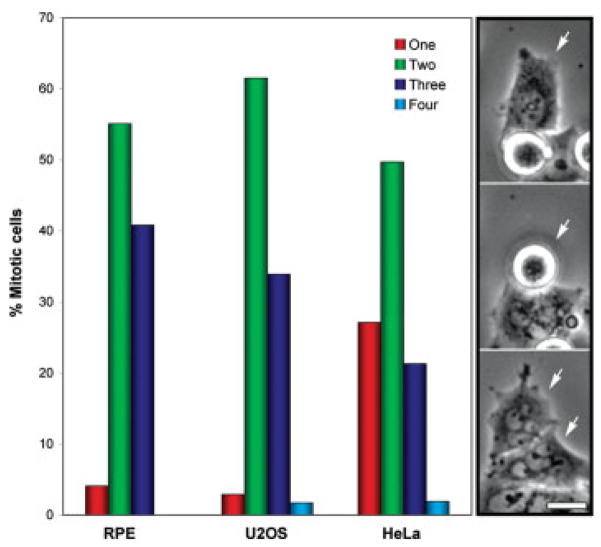
Fig. 5.
Low magnification time-lapse recordings of RPE-1, U2OS and HeLa cells dividing in the presence of 5 nM taxol were analyzed for cells that exited mitosis without dividing (red bars), or that exited mitosis and divided into two (green bars), three (blue bars) or four daughters (light blue bars). Images on the right depict selected frames of a HeLa cell that divided in the presence of 5 nM taxol into two daughters (white arrows in bottom frame) both of which were micro-nucleated. Bar = 10 μm.
At this point we can conclude, as others have [see Rieder and Maiato, 2004; Brito et al., 2008] that within a range, the lower the concentrations of a MT poison the shorter the duration of mitosis. In this regard the IC50 is the molar concentration of a drug that produces 50% of the maximum possible inhibitory response for that drug. In the case of cells treated with drugs, the IC50 is generally defined as the concentration that inhibits proliferation by 50%. The IC50 for taxol is reported to be 8 nM for HeLa [Jordan et al., 1993; Woods et al., 1995] and 9 nM for U2OS [Kelling et al., 2003]. This being the case we can conclude that these two cell lines exhibit very different fates in response to entering mitosis in 5 nM taxol: while 54% of U2OS cells were still alive 10 h after mitosis (Fig. 4b, lane 3) only 7% of HeLa remained viable (Fig. 4b, lane 5) and similar results were also seen with 500 nM taxol (Fig. 4b, lanes 4 and 6). Thus, our data reveal that HeLa are much more sensitive to low concentrations of taxol than U2OS.
Another commonly used index for MT inhibitors is the Kmet [e.g., Jordan et al., 1992; Kelling et al., 2003] or EC50 [Shi et al., 2008] which defines the drug concentration that produces over a 20–24 h treatment period an accumulation of mitotic cells to 50% the maximal level (Emax). Here it is noteworthy that the Kmet/EC50 for a particular drug can vary considerably depending on how the data was collected. For example, for HeLa treated with taxol the Kmet/EC50 as determined by direct counts of mitotic cells is 8 nM with an Emax of ~90% [Jordan et al., 1992], while the Kmet/EC50 for nocodazole is 54 nM with an Emax of ~95% [Jordan et al., 1992]. On the other hand, flow cytometry of propidium iodide stained HeLa cells, which does not differentiate G2 from M cells or cells that have slipped, gives an Kmet/EC50 for taxol of 11 nM with an Emax of 70% but an inexplicable Kmet/EC50 for nocodazole of 167 nM with an Emax of 75% [Shi et al., 2008].
In cancer (but not in nontransformed) cell lines ~100% of the cells within a population cycle into mitosis in a 20–24 h period, even in the complete absence of MTs [Uetake and Sluder, 2007]. Thus, at the end of 20–24 h the Kmax for a particular cell line should be >90% if the cells remained blocked in mitosis for on average 20–24 h. This is certainly true for HeLa which exhibit a Kmax >90% [Schimke et al., 1991; Jordan et al., 1993]. This being the case the Kmet/EC50 really simply defines the drug concentration that delays individual cells in mitosis for half the possible maximum duration. From our timing data on HeLa (Fig. 2c, lane 2) this would be ~10 h instead of ~20 h, while for U2OS (Fig. 2b, lane 2) it would be ~5 h instead of ~10 h. The reason the Kmax for HeLa (~90%) and U2OS [~65%; Kelling et al., 2003; Shi et al., 2008] are so different is that even under conditions in which they cannot satisfy the KAC, U2OS average just 10 h in mitosis while HeLa average 20 h. As a result although ~100% of U2OS cells will have entered mitosis after a 24 h treatment with a concentration of nocodazole that prohibits spindle MT assembly, only ~50% will still be in mitosis at the 24 h time point. By contrast, ~90% of HeLa, which are arrested in mitosis for >20 h, will still be in mitosis at the end of 24 h.
Lethality is Related to the Drug Used to Prevent Satisfaction of the KAC in U2OS but Not in HeLa or RPE-1 cells
For RPE-1 the ratio of cells that lived ≥10 h after slipping through mitosis in the presence of nocodazole (71%) versus 500 nM taxol (82%) was 71/82 (c.f., Fig. 4a, lane 1; Fig. 4b, lane 2; Fig. 2). By contrast, for U2OS and HeLa this ratio was, respectively, 18/63 (Fig. 4a, lane 3; Fig. 4b, lane 4) and 50/39 (Fig. 4a, lane 5; Fig. 4b, lane 6). If we subtract from this analysis those cells that died within 10 h of slippage, the nocodazole/taxol survival ratio for RPE-1 approaches 1/1 (70/73) as it does in HeLa (22/19), whereas in U2OS (10/51) it remains very different. Thus, while nontransformed RPE-1 cells show a similar and relatively low sensitivity to concentrations of nocodazole and taxol that prohibit satisfaction of the KAC, U2OS are much more sensitive to nocodazole than taxol while HeLa are highly sensitive to both drugs. Although it is well established that the fate of a D-mitosis depends on the drug concentration (see above), time of exposure and cell type [Rieder and Maiato, 2004], the assumption that it is largely independent of the type of MT poison used [Mollinedo and Gajate, 2003] is clearly not valid for some cancer cells [see also Shi et al., 2008].
The Relationship Between the Duration of a D-Mitosis and Survival is Drug and Cell Type Specific
Drug washout studies suggest that the longer a cell spends in D-mitosis the less likely it is to survive mitosis or the next G1 [see Rieder and Maiato, 2004]. If true, this implies that a death switch is thrown at some point during division after which the cell is destined to die even if it escapes mitosis. Indeed, we find that the duration of mitosis for those cells that die in mitosis in 500 nM taxol is significantly longer, compared to cells that survive long-term, for RPE-1 (cf. Fig. 2a, lanes 6 and 8; P < 0.001) and U2OS (Fig. 2b, lanes 9 and 11; P < 0.002) cells. Although this relationship also exists in HeLa dividing in 5 nM taxol (Fig. 2c, lanes 8 and 11) it is not true for 500 nM taxol (lanes 11 and 13). Furthermore, there was no statistical difference between the duration of a nocodazole-induced D-mitosis and long (≥10 h) or short (<10 h) term survival (Fig. 2) for HeLa (P = 0.28) or U2OS (P = 0.37). Thus, only some cell types exhibit a correlation between the duration of a D-mitosis and survival, and then only for some MT poisons. Nevertheless, the fact that some cancer cell lines show this relationship for taxol explains why many cells that have a weakened KAC are resistant to taxol [Panvichian et al., 1998; Masuda et al., 2003; Sudo et al., 2004]: since the delay in mitosis is not sufficient for the death switch to be thrown they enter the next G1 as viable entities. Finally, where there is a relationship between the duration of D-mitosis and survival, in different cells the death switch can be thrown at very different times: the 18% of RPE-1 cells that die during mitosis in 500 nM taxol (Fig. 4b, lane 2) average ~20 h in mitosis (Fig. 2a, lane 8) whereas, under the same conditions 37% of U2OS cells die in mitosis (Fig. 4b, lane 4) after just ~12 h (Fig. 2b, lane 11). Thus, even though the KAC is weaker in U2OS than in RPE-1, the putative death switch shows an accelerated timing relative to normal cells.
The Short-Term Fate of a Cell Undergoing a D-Mitosis Does Not Depend on p53
Some have concluded from indirect data that lethality to taxol or nocodazole during a “G2/M arrest” requires functional p53 [Cross et al., 1995; Kienitz et al., 2005], while others claim that p53 plays no role in the death of human cells exposed to MT poisons [Minn et al., 1996; Lanni and Jacks, 1998; Casenghi et al., 1999]. Our data reveal directly that the presence (RPE-1, U2OS) or the functional absence (HeLa) of p53 has no influence on whether a cell survives mitosis or the following G1 (Fig. 4). Concerning this issue, FACS studies frequently fail to differentiate between cells in mitosis and those that slip through into G1 (both are 4N), and in the presence of functional p53 the latter remain arrested in G1 during which they ultimately die by apoptosis, senescence or other mechanisms [Woods et al., 1995; Uetake and Sluder, 2004].
One of the Major Death Pathways Triggered in HeLa by Nocodazole During Mitosis or After Mitotic Slippage is Not Active in U2OS Cells
Drugs that prevent satisfaction of the KAC often induce a caspase-dependent apoptotic response in tumor cells [Rieder and Maiato, 2004; Allan and Clarke, 2007]. As a first step towards investigating if the death mechanism(s) during or after mitotic slippage vary with the cell type we pretreated RPE-1, HeLa and U2OS cultures with 20 μM Q-VD-OPh, before exposing them to nocodazole. Although zVAD-fmk and Boc-D-fmk are more commonly used broad-spectrum pancaspase inhibitors, Q-VD-OPh even at one tenth the concentration, is significantly more effective in preventing apoptosis and, in addition to its enhanced effectiveness, it is not toxic at typical (10–20 μM) working concentrations. It has been shown to irreversibly inhibit recombinant caspases 1, 3, 8, 9, 10, and 12 with IC50 values ranging from 25 to 400 nM and to be equally effective in preventing apoptosis mediated by three major apoptotic pathways based, respectively, on caspase 9/3, caspase 8/10 and caspase 12 [Caserta et al., 2003].
We found that inhibiting caspase activity with Q-VD-OPh prevented nocodazole-treated RPE-1 cells from dying in or after mitosis, i.e., it increased the long term survival rate from 70% to 100% (Fig. 4a, lane 2). Thus, those nocodazole-treated RPE-1 cells that would normally die before (29%), or after (1%), mitotic slippage in nocodazole do so via apoptosis mediated by one of the above caspases. In contrast to conclusions based on less direct observations, and consistent with the recent report by Shi et al [2008], this result shows that cells need not slip through mitosis to die via apoptosis induced by MT poisons [Wang et al., 2000; Chen et al., 2003].
Compared to nontransformed RPE-1 cells we found that inhibiting caspases before treating U2OS cultures with nocodazole had no effect on short or long term cell viability (Fig. 4a, lanes 3 and 4; P > 0.5): in nocodazole alone 82% died in mitosis whereas in nocodazole and Q-VD-OPh 86% died in mitosis. By contrast, pretreating HeLa cultures with Q-VD-OPh before adding nocodazole significantly increased the percentage of cells that were still alive 10 h after mitosis from 22% to 58% (Fig. 4a, lanes 5 and 6; P < 0.001). It did this by decreasing both the percentage that died in mitosis (50–38%) as well as the number that died within 10 h of exiting mitosis (28–4%). Thus at least one of the primary death pathways triggered in HeLa during or shortly after mitotic slippage in 1.5 μM nocodazole is inhibited by Q-VD-OPh, and this pathway is active in nocodazole-treated RPE-1 but not U2OS cells.
It is evident from Fig. 2a (lanes 2 and 4) that although Q-VD-OPh treatment allows 100% of RPE-1 cells to survive slippage in the absence of MTs (in 3.2 μM nocodazole), it prolongs mitosis ~1.6 fold (P = 0.005). This implies either that caspase activity is required for nocodazole-treated RPE-1 cells to ubiquitinate cyclin B in a timely manner, or that our nocodazole/20 μM Q-VD-OPh cocktail somehow retards cyclin B proteolysis in RPE-1 but not U2OS or HeLa cells. Regardless, our data reveal that inhibiting caspase 3 does not abrogate the KAC in any of our cell lines, as recently reported from less direct observations on human hepatoma cells [Hsu et al., 2006]. Our findings also contrast sharply with a recent FACS-based report that pancaspase inhibitors significantly prolong mitosis in HeLa treated with nocodazole [Kim et al., 2005]. More likely the increase noted by these authors in G2/M cells in the presence of both nocodazole and a pancaspase inhibitor, which was ascribed to a prolongation of mitosis, was due instead to an enhanced survival of 4N G1 cells created by slippage.
In summary, our live cell observations reveal a number of fundamental differences in the behavior and fate of nontransformed RPE-1 cell line and two popular cancer cell lines (HeLa and U2OS) as they enter mitosis in the presence of MT poisons. They emphasize the importance of augmenting indirect observations with live cell studies and they caution against extrapolating conclusions between drug studies on different cell lines. One of our key conclusions is that compared to nontransformed RPE-1 cells, HeLa and U2OS cancer cells are much less likely to survive mitosis in nocodazole or (even 5 nM) taxol, and those that do are more likely to die shortly after entering the next G1. These findings lead to an important cautionary note: studies ranging from the biochemistry of mitosis to the KAC and mitotic slippage often utilize HeLa cell populations harvested in mitosis after a prolonged (24–48 h) treatment with MT poisons (often nocodazole). However, our live cell studies reveal that 50% and 61% of HeLa cells die within 24 h of entering mitosis in the presence, respectively, of 1.5 μM nocodazole or 500 nM taxol (Figs. 4a and 4b), and this becomes 78% and 81% within 40 h. This means that studies using HeLa cells harvested after long-term nocodazole treatment are significantly contaminated with dead or dying cells. The situation for U2OS, another popular cell for research on the KAC and mitotic progression, is no better: in nocodazole 82% of these cells die in mitosis within an average of 12 h (cf. Fig. 4a, lane 3; Fig. 4b, lane 4)—a fact that has not been considered in any publication on U2OS to date.
Our data provide a potential explanation for why taxol is effective against some tumors (Fig. 4b): in clinically relevant (5 nM) concentrations 93% of HeLa (lane 5) and 46% of U2OS (lane 3) cells either die in mitosis, or their progeny die within 10 h of exiting mitosis. By contrast under the same conditions 99% of nontransformed RPE-1 cells satisfy the KAC and produce progeny that survive for at least 10 h (Fig. 4b, lane 1). An important question for the future is what becomes of those progeny produced from a division of normal cells in 5-nM taxol.
Acknowledgments
Contract grant sponsor: NIH/GMS; Contract grant number: 40198; Contract grant sponsor: Fundacao para a Ciencia e a Tecnologia; Contract grant number: SFRH/BD/13663/2003.
Abbreviations used
- APCs
anaphase-promoting complexes
- D-mitosis
delay in mitosis
- FACS
fluorescence activated cell sorting
- KAC
kinetochore attachment checkpoint
- MT
microtubule
References
- Allan LA, Clarke PR. Phosphorylation of caspase-9 by CDK1/cyclin B1 protects mitotic cells against apoptosis. Mol Cell. 2007;26:301–310. doi: 10.1016/j.molcel.2007.03.019. [DOI] [PubMed] [Google Scholar]
- Andreassen PR, Margolis RL. Microtubule dependency of p34cdc2 inactivation and mitotic exit in mammalian cells. J Cell Biol. 1994;127:789–802. doi: 10.1083/jcb.127.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito DA, Rieder CL. Mitotic checkpoint slippage in vertebrates occurs via cyclin B destruction in the presence of an active checkpoint. Curr Biol. 2006;16:1194–1200. doi: 10.1016/j.cub.2006.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito DA, Yang Z, Rieder CL. Microtubules do not promote mitotic slippage when the spindle assembly checkpoint cannot be satisfied. J Cell Biol. 2008;182:623–629. doi: 10.1083/jcb.200805072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill DP, Lengauer C, Yu J, Riggins GJ, Wilson JK, Markowitz SD, Kinzler KW, Vogelstein B. Mutations of mitotic checkpoint genes in human cancers. Nature. 1998;392:300–303. doi: 10.1038/32688. [DOI] [PubMed] [Google Scholar]
- Casenghi M, Mangiacasale R, Tuynder M, Fauquet PC, Elhajoui A, Lavia P, Mousset S, Kirsch-Volders M, Cundari E. p53-dependent apoptosis and p53-dependent block of DNA re-replication following mitotic spindle inhibition in human cells. Exp Cell Res. 1999;250:339–350. doi: 10.1006/excr.1999.4554. [DOI] [PubMed] [Google Scholar]
- Caserta TM, Smith AN, Gultice AD, Reedy MA, Brown TL. Q-VD-OPh, a broad spectrum capsase inhibitor with potent anti-apoptotic properties. Apoptosis. 2003;8:345–352. doi: 10.1023/a:1024116916932. [DOI] [PubMed] [Google Scholar]
- Chen J-G, Horwitz SB. Differential mitotic responses to microtubule-stabilizing and -destabilizing drugs. Cancer Res. 2002;62:1935–1938. [PubMed] [Google Scholar]
- Chen J-G, Yang C-PH, Cammer M, Horwitz SB. Gene expression and mitotic exit induced by microtubule-stabilizing drugs. Cancer Res. 2003;63:7891–7899. [PubMed] [Google Scholar]
- Crosio C, Firma GM, Loury R, Kimura M, Okano Y, Zhou H, Sen S, Allis CD, Sassone-Corsi P. Mitotic phosphorylation of histone H3, spatio-temporal regulation by mammalian Aurora kinases. Mol Cell Biol. 2002;22:874–885. doi: 10.1128/MCB.22.3.874-885.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross SM, Sanchez CA, Morgan CA, Shimke MK, Ramel S, Idzerda RL, Raskind WH, Reid BJ. A p53-dependent mouse spindle checkpoint. Science. 1995;267:1353–1356. doi: 10.1126/science.7871434. [DOI] [PubMed] [Google Scholar]
- Dixon WE. A Manual of Pharmacology. London: Edward Arnold, Pub; 1906. p. 451. [Google Scholar]
- Hendzel MJ, Wei Y, Mancini MA, Hooser AV, Ranalli T, Brinkley BR, Bazett-Jones DP, Allis CD. Mitosis-specific phosporylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma. 1997;106:348–360. doi: 10.1007/s004120050256. [DOI] [PubMed] [Google Scholar]
- Hoyt AM, Totis L, Roberts BT. S. cerevisiae genes required for cell cycle arrest in response to loss of microtubule function. Cell. 1991;66:507–517. doi: 10.1016/0092-8674(81)90014-3. [DOI] [PubMed] [Google Scholar]
- Hsu S-L, Yu C-TR, Yin S-C, Tang M-J, Tien A-C, Wu Y-M, Huang C-YF. Caspase 3, periodically expressed and activated at G2/M transition, is required for nocodazole-induced mitotic checkpoint. Apoptosis. 2006;11:765–771. doi: 10.1007/s10495-006-5880-x. [DOI] [PubMed] [Google Scholar]
- Ikui AE, Yang C-PH, Matsumoto T, Horwitz SB. Low concentrations of taxol cause mitotic delay followed by premature dissociation of p55CDC from Mad2 and BubR1 and abrogation of the spindle checkpoint, leading to aneuploidy. Cell Cycle. 2005;4:1385–1388. doi: 10.4161/cc.4.10.2061. [DOI] [PubMed] [Google Scholar]
- Jiang XR, Jimenez G, Chang E, Frolkis M, Kusler B, Sage M, Beeche M, Bodnar AG, Wahl GM, Tlsty TD, Chiu CP. Telomerase expression in human somatic cells does not induced changes associated with a transformed phenotype. Nature Genetics. 1999;21:111–114. doi: 10.1038/5056. [DOI] [PubMed] [Google Scholar]
- Jordan MA, Kamath K. How do microtubule-targeted drugs work? An overview. Curr Cancer Drug Targets. 2007;7:730–742. doi: 10.2174/156800907783220417. [DOI] [PubMed] [Google Scholar]
- Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nature Rev Cancer. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- Jordan MA, Thrower D, Wilson L. Effects of vinblastine, podo-phyllotoxin and nocodazole on mitotic spindles: implications for the role of microtubule dynamics in mitosis. J Cell Sci. 1992;102:401–416. doi: 10.1242/jcs.102.3.401. [DOI] [PubMed] [Google Scholar]
- Jordan MA, Toso RJ, Thrower DA, Wilson L. Mechanism of mitotic block and inhibition of cell proliferation by taxol at low concentrations. Proc Nat Acad Sci USA. 1993;90:9552–9556. doi: 10.1073/pnas.90.20.9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelling J, Sullivan KF, Wilson L, Jordan MA. Suppression of centromere dynamics by Taxol in living osteosarcoma cells. Cancer Res. 2003;63:2794–2801. [PubMed] [Google Scholar]
- Khodjakov A, Rieder CL. Imaging the division process in living tissue culture cells. Methods. 2006;38:2–16. doi: 10.1016/j.ymeth.2005.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienitz A, Vogel C, Morales I, Muller R, Bastians H. Partial downregulation of mad1 causes spindle checkpoint inactivation and aneuploidy, but does not confer resistance towards taxol. Oncogene. 2005;24:4301–4310. doi: 10.1038/sj.onc.1208589. [DOI] [PubMed] [Google Scholar]
- Kim M, Murphy K, Liu F, Parker SE, Dowling ML, Baff W, Kao GD. Caspase-mediated specific cleavage of BubR1 is a determinant of mitotic progression. Mol Cell Biol. 2005;25:9232–9248. doi: 10.1128/MCB.25.21.9232-9248.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanni JS, Jacks T. Characterization of the p53-dependent post-mitotic checkpoint following spindle disruption. Mol Cell Biol. 1998;18:1055–1064. doi: 10.1128/mcb.18.2.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Murray AW. Feedback control of mitosis in budding yeast. Cell. 1991;66:519–531. doi: 10.1016/0092-8674(81)90015-5. [DOI] [PubMed] [Google Scholar]
- Ludford RJ. The action of toxic substances upon the division of normal and malignant cells in vitro and in vivo. Arch F Exp Zelif. 1936;18:411–441. [Google Scholar]
- Masuda A, Maeno K, Nakagawa T, Saito H, Takahashi T. Association between mitotic spindle checkpoint impairment and susceptibility to the induction of apoptosis by anti-microtubule agents in human lung cancers. Am J Pathol. 2003;163:1109–1116. doi: 10.1016/S0002-9440(10)63470-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minn AJ, Boise LH, Thompson CB. Expression of Bcl-xL and loss of P53 can cooperate to overcome a cell cycle checkpoint induced by mitotic spindle damage. Genes Dev. 1996;10:2621–2631. doi: 10.1101/gad.10.20.2621. [DOI] [PubMed] [Google Scholar]
- Mollinedo F, Gajate C. Microtubules, microtubule-interfering agents and apoptosis. Apoptosis. 2003;8:413–450. doi: 10.1023/a:1025513106330. [DOI] [PubMed] [Google Scholar]
- Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nature Rev Mol Cell Biol. 2007;8:379–393. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- Panvichian R, Orth K, Day ML, Day KC, Pilat MJ, Pienta KJ. Paclitaxel-associated multimininucleation is permitted by the inhibition of caspase activation: A potential early step in drug resistance. Cancer Res. 1998;58:4667–4672. [PubMed] [Google Scholar]
- Pulkkinen JO, Elomaa L, Joensuu H, Martikainen P, Servomaa K, Grenman R. Paclitaxel-induced apoptotic changes followed by time-lapse video microscopy in cell lines established from head and neck cancer. J Cancer Res Clin Oncol. 1996;122:214–218. doi: 10.1007/BF01209648. [DOI] [PubMed] [Google Scholar]
- Rieder CL, Maiato H. Stuck in division or passing through: What happens when cells cannot satisfy the spindle assembly checkpoint. Dev Cell. 2004;7:637–651. doi: 10.1016/j.devcel.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Rieder CL, Schultz A, Cole R, Sluder G. Anaphase onset in vertebrate somatic cells is controlled by a checkpoint that monitors sister kinetochore attachment to the spindle. J Cell Biol. 1994;127:1301–1310. doi: 10.1083/jcb.127.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder CL, Cole RW, Khodjakov A, Sluder G. The checkpoint delaying anaphase in response to chromosome monoorientation is mediated by an inhibitory signal produced by unattached kinetochores. J Cell Biol. 1995;130:941–948. doi: 10.1083/jcb.130.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimke RT, Kung AL, Rush DF, Sherwood SW. Differences in mitotic control among mammalian cells. Cold Sprin Harb Symp Quant Biol. 1991;65:417–425. doi: 10.1101/sqb.1991.056.01.049. [DOI] [PubMed] [Google Scholar]
- Shi J, Orth JD, Mitchison T. Cell type variation in response to antimitotic drugs that target microtubuels and kinesin-5. Cancer Res. 2008;68:3269–3276. doi: 10.1158/0008-5472.CAN-07-6699. [DOI] [PubMed] [Google Scholar]
- Rowinsky EK, Donehower RC. The clinical pharmacology and use of antimicrotubule agents in cancer chemotherapeutics. Pharm Ther. 1991;52:35–84. doi: 10.1016/0163-7258(91)90086-2. [DOI] [PubMed] [Google Scholar]
- Shinohara M, Mikhailov AV, Aguirre-Ghiso JA, Rieder CL. Extracellular signal-regulated kinase 1/2 activity is not required in mammalian cells during late G2 for timely entry into or exit from mitosis. Mol Biol Cell. 2006;17:5227–5240. doi: 10.1091/mbc.E06-04-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo T, Nitta M, Saya H, Ueno NT. Dependence of paclitaxel sensitivity on a functional spindle assembly checkpoint. Cancer Res. 2004;64:2502–2508. doi: 10.1158/0008-5472.can-03-2013. [DOI] [PubMed] [Google Scholar]
- Tighe A, Johnson VL, Albertella M, Taylor S. Aneuploid colon cancer cells have a robust spindle checkpoint. EMBO Rep. 2001;21:609–614. doi: 10.1093/embo-reports/kve127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trielli MO, Andreassen PR, Lacroix FB, Margolis RL. Differential Taxol-dependent arrest of transformed and nontransformed cells in the G1 phase of the cell cycle, and specific-related mortality of transformed cells. J Cell Biol. 1996;135:689–700. doi: 10.1083/jcb.135.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uetake Y, Sluder G. Cell cycle progression after cleavage failure: mammalian somatic cells do not possess a “tetraploidy checkpoint”. J Cell Biol. 2004;165:609–615. doi: 10.1083/jcb.200403014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uetake Y, Sluder G. Cell cycle progression without an intact microtubule cytoskeleton. Curr Biol. 2007;17:2081–2086. doi: 10.1016/j.cub.2007.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TH, Wang H-S, Soong Y-K. Paclitaxel-induced cell death. Cancer. 2000;88:2619–2628. doi: 10.1002/1097-0142(20000601)88:11<2619::aid-cncr26>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Wang X, Cheung HW, Chun AC, Wong YC. Mitotic checkpoint defects in human cancers and their implications to chemotherapy. Front Biosci. 2008;13:2103–2114. doi: 10.2741/2827. [DOI] [PubMed] [Google Scholar]
- Weaver BAA, Cleveland DW. Decoding the links between mitosis, cancer, and chemotherapy: The mitotic checkpoint, adaptation, and cell death. Cancer Cell. 2005;8:7–12. doi: 10.1016/j.ccr.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Woods CM, Zhu J, McQueney PA, Bollag D, Lazarides E. Taxol-induced mitotic block triggers rapid onset of a p53-independent apoptotic pathway. Mol Med. 1995;1:506–526. [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Loncarek J, Khodjakov A, Rieder CL. Extra centrosomes and/or chromosomes prolong mitosis in human cells. Nature Cell Biol. 2008;10:748–751. doi: 10.1038/ncb1738. [DOI] [PMC free article] [PubMed] [Google Scholar]