Abstract
Genetic modification of the vectorial capacity of mosquito vectors of human disease requires promoters capable of driving gene expression with appropriate tissue and stage specificity. We report on the characterization in transgenic Aedes aegypti of two mosquito gut-specific promoters. A 1.4-kb DNA fragment adjacent to the 5′ end of the coding region of the Ae. aegypti carboxypeptidase (AeCP) gene and a corresponding 3.4-kb DNA fragment at the 5′ end of the Anopheles gambiae carboxypeptidase (AgCP) gene were linked to a firefly luciferase reporter gene and introduced into the Ae. aegypti germ line by using Hermes and mariner (Mos1) transposons. Six independent transgenic lines were obtained with the AeCP construct and one with the AgCP construct. Luciferase mRNA and protein were abundantly expressed in the guts of transgenic mosquitoes in four of the six AeCP lines and in the AgCP line. Expression of the reporter gene was gut-specific and reached peak levels at about 24 h post-blood ingestion. The AeCP and AgCP promoters can be used to drive the expression of genes that hinder parasite development in the mosquito gut.
Keywords: malaria, Hermes, mariner, promoter function
Mosquitoes are responsible for the transmission of major human diseases such as malaria, yellow fever, and dengue. The recent development of transgenic mosquitoes allows for an unprecedented analysis of the molecular interactions between mosquitoes and the pathogens they transmit. Two transposon-mediated germ-line transformation systems have been shown to produce stable integration of DNA into the genome of the yellow fever mosquito, Aedes aegypti (1–3). Ae. aegypti is a vector for avian malaria, dengue, and yellow fever viruses. A remaining technical hurdle for the genetic manipulation of mosquitoes has been the development of appropriate stage- and tissue-specific promoters to drive the expression of pathogen-targeting gene products. A significant advance was the demonstration of reporter gene expression in the salivary glands of mosquitoes that were transformed with the transposons Hermes and mariner (Mos1) (1, 2).
Mosquito-borne diseases are propagated when the mosquito ingests an infected blood meal. The ingestion of blood leads to a dramatic activation of many genes encoding digestive enzymes (4–6). The availability of gut-specific promoters to drive the expression of antimalarial proteins in the mosquito midgut would open a new frontier to hinder Plasmodium development in the mosquito. Here we report that Ae. aegypti and Anopheles gambiae carboxypeptidase (AeCP and AgCp, respectively) promoters can be used to drive strong and blood-inducible gene expression in the gut of transgenic Ae. aegypti.
Materials and Methods
Plasmid Construction.
For Hermes transformation, a 3.4-kb ApaI/SalI fragment containing ≈1.4 kb of the AeCP upstream region (7) fused to 2.0 kb of luciferase reporter gene of pGL3 BASIC (Promega) was inserted into pHermes [cn] (ref. 3; gift of M. Q. Benedict, Centers for Disease Control, Chamblee, GA) into the unique ApaI and XhoI sites. For Mos1 transformation, a ≈5.4-kb NotI/SalI fragment containing 3.4 kb upstream of the AgCP upstream region (8) fused to the same 2.0 kb of luciferase reporter gene was ligated into pΔM SLFC [cn] (ref. 1; gift of C. J. Coates, Texas A&M University, College Station) into the unique NotI and XhoI sites.
Embryo Microinjection and Mosquito Rearing.
Embryos of homozygous white-eyed khw mosquitoes (obtained from the University of Notre Dame stock center) were injected as described (1, 9). Embryos were injected with a mixture of the transposon construct (0.5 mg/ml) and either the Hermes helper plasmid, pHSHH1.9 (0.3 mg/ml) or the Mos1 helper plasmid, pKhsp82MOS (0.5 mg/ml), in 5 mM KCl and 0.1 mM NaH2PO4 (pH 6.8). Microinjected embryos were exposed to heat shock (42°C for 1 h) to induce the expression of Hermes or Mos1 transposases, which are under the control of the Drosophila melanogaster hsp70 and hsp82 gene promoters, respectively. Embryos were kept for 5 days at 27°C and 80% relative humidity before hatching.
Mosquitoes were reared by the methods described (10). Individual adult G1 males with colored eyes were crossed with five virgin noninjected khw females. Approximately five G1 virgin females with colored eyes were pooled and mated with 2–4 noninjected khw males. Females were allowed to feed on blood, and G1 eggs were collected. Transformants were selected by screening the eye color of adults. This was continued for every generation to establish homozygosity of the transgenic lines.
Southern Blot Analysis.
Total genomic DNA was isolated from G6 transgenic and white-eyed mosquitoes as described (11). DNA digested with EcoRI and BamHI was separated on a 1% agarose gel, blotted, and hybridized with α-32P dCTP-labeled luciferase probe (≈1.6-kb fragment from pGL3 BASIC, double digested with XbaI/NcoI). To verify DNA loading in each lane, the blot was hybridized with a 0.7-kb Ae. aegypti late trypsin probe (12).
Reverse Transcriptase (RT)–PCR.
To assay for the presence of luciferase mRNA, total RNA was extracted from guts of transgenic AeCP and khw mosquitoes 24 h after blood feeding. RT (SuperScript II, GIBCO/BRL) reactions were performed after treating the RNA with RQ1 RNase-free DNase (Promega). Primers specific to the AeCP coding region (7) (CPRTF: 5′-TTCATTCATACTCTCAACTTCTCTTGTTCCCGTAT-3′ and CPRTR: 5′-GTCGCAGTTCGTAGGTGTAGGCAATCTTTA-3′) were used together with others specific to luciferase (Lux1105F: 5′-TTTGAAGCGAAGGTTGTGGAT-3′ and Lux1462R: 5′-GTCATCGTCTTTCCGTGCTC-3′) in a PCR (95°C, 1 min; 55°C, 1:30 min; 72°C, 1 min, 30 cycles).
Northern Blot Analysis.
Mosquito guts and carcasses (whole body minus gut) were dissected from 4- to 5-day-old females. Male guts and pupae also were collected, and all tissues were frozen in an ethanol/dry ice bath and immediately stored at −80°C. To determine the temporal profile of luciferase expression in transgenic mosquitoes, guts were dissected at increasing time intervals after a blood meal. Total RNA was extracted with TRI-Reagent (Molecular Research Center, Cincinnati). RNA from ≈10 mosquitoes was separated in each lane of 1.5% agarose-formaldehyde gels and blotted by capillary action to a nylon membrane (Gene Screen, New England Nuclear). Hybridization was performed first with the luciferase probe (described above) and then with a mitochondrial rRNA probe (5, 13) as a loading control.
Western Blot Analysis.
Guts were dissected 24 h after a blood meal and homogenized in PBS. The equivalent of 10 guts per lane was analyzed by electrophoresis on a 10% polyacrylamide/SDS gel, followed by electrotransfer to a poly(vinylidene fluoride) membrane. The membrane was incubated with a rabbit antiluciferase polyclonal antibody (Accurate Chemicals), and the signal was detected with a goat anti-rabbit Ig conjugated to alkaline phosphatase (Promega).
Polysome Analysis.
Two samples of guts from transgenic mosquitoes, dissected 24 h after a blood meal, were homogenized in 10 mM Tris⋅HCl (pH 7.0), 5 mM MgOAc, 100 mM KCl, 1% Triton, and 0.5% deoxycholic acid in the presence or absence of 10 mM EDTA (15). The samples were centrifuged for 5 min at 13,600 g, and the resulting postmitochondrial fractions were loaded onto a 10–50% sucrose gradient. Samples were centrifuged at 100,000 g on a SW 28.1 rotor in a Beckman L7–55 ultracentrifuge for 4.5 h. Gradients were analyzed with an ISCO UV-5 monitor with continuous recording at 254 nm. Fourteen fractions were collected. RNA was extracted from each fraction with phenol/chloroform. The RNAs were analyzed by Northern blotting using a luciferase probe.
Results
Germ-Line Transformation.
The AeCP and AgCP genes both are expressed specifically in the mosquito gut and strongly induced by ingestion of a blood meal (7, 8). We investigated whether the upstream regions of the carboxypeptidase genes are sufficient to drive the expression of a transgene with a tissue and temporal pattern similar to that of the endogenous genes. Constructs with a luciferase reporter gene under the control of the putative AeCP or AgCP promoters were cloned into vectors containing Hermes or Mos1 transposable elements, respectively. These plasmids were injected into Ae. aegypti embryos. Six transgenic lines, designated 2T, 13T, 42T, 46T, 64T, and 76T, were obtained for the AeCP construct, four of which had dark eye color and two (42T and 64T) had either faint orange eye color or displayed mosaicism (Table 1). One transgenic line, AgCP, was obtained with the Mos1 construct. Mosaicism in Hermes-transformed Ae. aegypti has been previously observed (3). Although survival was lower for the AgCP transformation experiment, transformation efficiency was similar. Transformation efficiency, based on percentage of transgenic lines derived from individual male family founders (4.3% for the Hermes and 6.6% for the Mos1 constructs), are consistent with percentages reported in previous studies (1, 3, 14).
Table 1.
Microinjection results of AeCP and AgCP constructs
| AeCP in Hermes | AgCP in Mos1 | |
|---|---|---|
| Embryos injected | 1,594 | 1,579 |
| Adults emerged | 200 (12.54%)* | 19 (1.2%)* |
| G0 families started | 109 | 15 |
| Transformed lines established | 6 (4.3%)† | 1 (6.6%)† |
Percentage of injected embryos that hatched into adults.
Percentage of the lines derived from individual male family founders that underwent a stable transformation.
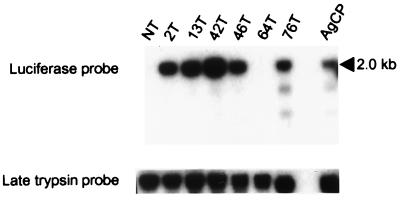
Integration of the constructs into the germ line of the transformed mosquitoes was verified by Southern blot experiments (Fig. 1). Using a luciferase probe, a 2-kb band was detected in five of the six AeCP (Hermes) transgenic lines and in the AgCP (Mos1) line. Loading of DNA was verified by probing the same blots with a trypsin probe. Luciferase sequences were not detected in AeCP line 64T, which also exhibited mosaic eye color.
Figure 1.
Verification of transgene integration by Southern blot analysis. Genomic DNA from the nontransformed and transformed mosquito lines was digested with EcoRI and BamHI and analyzed on a Southern blot with a 1.6-kb luciferase probe. The predicted 2.0-kb luciferase fragment is observed in five of the six AeCP transgenic lines and in the AgCP line but not in the nontransformed line. To verify loading of DNA in all lanes, the blot subsequently was hybridized with a late trypsin probe (loading control). The source of DNA is indicated at the top of each lane. NT: DNA from khw/khw (nontransformed) mosquitoes. Numbers followed by a T indicate the designation of each transformed mosquito line. AgCP: DNA from mosquitoes transformed with the AgCP construct.
The Homologous AeCP Promoter Drives Strong Blood-Inducible Expression of Luciferase mRNA Only in the Midgut.
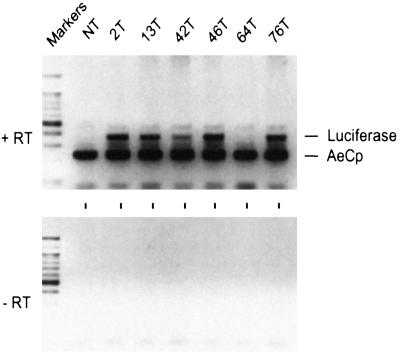
Expression of the integrated genes was measured first by RT-PCR. PCR was conducted with a mixture of two sets of primers: one specific for the sequence of the luciferase transgene, and another specific for the sequences of the endogenous carboxypeptidase gene, to serve as an internal positive control. A carboxypeptidase amplification product was observed from all gut RNA samples (Fig. 2). A luciferase amplification product was observed from gut RNA samples isolated from all but one (64T) transgenic lines. This was expected, because Southern analysis indicated that luciferase sequences were absent in line 64T (Fig. 1). No luciferase product was detected with the nontransformed strain, as expected. The signal from line 42T was consistently weaker when compared with other lines.
Figure 2.
Assessment of AeCP expression by RT-PCR. RT-PCR was conducted with a mixture of two sets of primers: one specific for luciferase that generates a 376-nt fragment (luciferase), and one specific for the endogenous carboxypeptidase that generates a 237-nt fragment (AeCP). The template was total RNA extracted from guts 24 h after a blood meal. Luciferase mRNA was detected in five of the six transgenic lines. No luciferase signal was detected with RNA from nontransformed mosquitoes (NT), as expected. The source of RNA is indicated at the top of each lane (see Fig. 1 legend). DNA size markers were run in the first lane of each panel. +RT: PCRs done after RT of the RNA; −RT: RT was omitted.
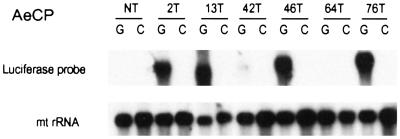
Tissue-specific expression was analyzed on Northern blots (Fig. 3). Abundant luciferase mRNA was detected in guts (but not in carcasses) from four of the six AeCP transgenic lines. Weak expression was detected in line 42T, and no expression was detected in line 64T and in nontransformed mosquitoes.
Figure 3.
Northern blot analysis of luciferase expression in mosquitoes transformed with the AeCP construct. RNA extracted from dissected blood-fed guts (G) and carcasses (C) (nongut tissues) was analyzed by Northern blotting with a radioactive luciferase probe. RNA loading in each lane was verified by hybridization of the same blot with a mitochondrial rRNA (mt rRNA) probe (5).
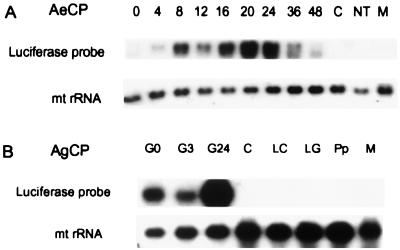
Expression from the carboxypeptidase promoters was strongly induced by blood ingestion. The time course of luciferase induction from the AeCP promoter (Fig. 4A) closely resembles that of the endogenous carboxypeptidase gene (7).
Figure 4.
Analysis of transgenic mRNA accumulation. (A) Time course of luciferase mRNA accumulation after a blood meal. Total RNA extracted from guts (or carcasses) dissected at different times after a blood meal was fractionated by electrophoresis on agarose gels and blotted to a nylon membrane. The membrane was first hybridized with a radioactive luciferase DNA probe and then with a mitochondrial rRNA probe, used as a loading control. Numbers above the lanes refer to times after a blood meal when AeCP transgenic mosquitoes were dissected. 0: Guts from sugar-fed mosquitoes; C: carcasses (nongut tissues); NT: guts from nontransformed mosquitoes dissected 24 h after a blood meal; M: male gut. (B) Assessment by Northern blot analysis of luciferase expression in mosquitoes transformed with the AgCP construct. RNA was extracted from different tissues, developmental stages, or sexes and analyzed by Northern blotting with a radioactive luciferase probe. RNA loading was verified as above. G: Guts (numbers indicate hours after a blood meal); C: carcass; LC: larval carcass; LG: larval gut; Pp; pupae; M: male gut.
The Heterologous AgCp Promoter Drives Strong Luciferase Expression with the Correct Developmental, Tissue, and Sex Specificity.
As for AeCP, expression from the heterologous AgCP promoter is strongly induced by blood ingestion (Fig. 4B). However, peak induction is reached late (24 h) after a blood meal in transgenic Ae. aegypti (Fig. 4B) rather than early (3 h) as the endogenous carboxypeptidase promoter in An. gambiae (8). Moreover, Fig. 4B provides evidence for the correct tissue (gut) specificity, correct developmental regulation (adults only), and sex specificity (females only) of the AgCP promoter.
Robust Luciferase Protein Expression in the Guts of Transgenic Mosquitoes.
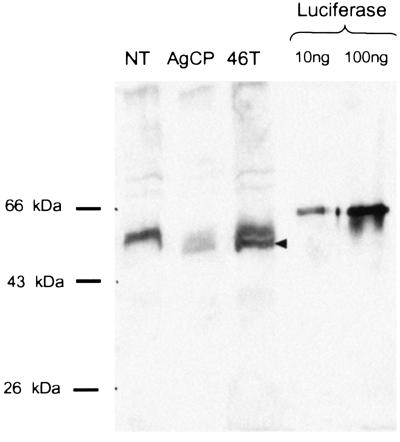
Assays of gut homogenates from the transformed mosquitoes led to the surprising finding that luciferase enzyme activity was absent from all transgenic lines, despite the presence of abundantly expressed mRNA (see above). Further analysis revealed that because of a cloning artifact a mutation occurred in an early step of assembling the constructs that resulted in a deletion of a T at residue 468 of the luciferase gene. This mutation caused a frame shift that resulted in the premature termination of the protein at amino acid 493. Western blot analysis with a luciferase antibody confirmed this finding (Fig. 5). In addition to several common cross-reacting bands in all samples, a truncated protein of about 55 kDa recognized by the antiluciferase antibody is present only in guts of the AgCP and AeCP transgenic lines. This is the size predicted from the sequence analysis described above.
Figure 5.
Western blot analysis of luciferase protein expression. Guts from the nontransformed recipient mosquito line (NT), the AgCP line, or an AeCP line (46T) were dissected from mosquitoes 24 h after a blood meal. Gut homogenates were analyzed by Western blotting with an antiluciferase antibody. The two lanes on the right contain recombinant luciferase (10 and 100 ng), used as a positive control. Migration of molecular weight markers is indicated on the left. The arrowhead indicates the position of migration of the predicted truncated protein (see text), detected only in the transgenic lines.
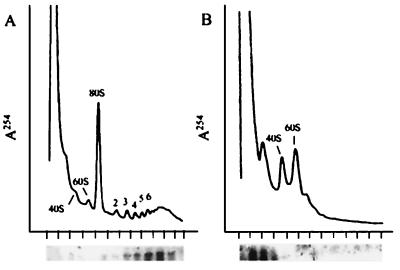
Translation of the recombinant luciferase mRNA in the guts of transgenic mosquitoes was independently verified by measuring its association with polysomes. We found that the vast majority of the luciferase mRNA is polysome-associated (Fig. 6A). This association was EDTA-sensitive, indicating that association of the luciferase mRNA was functionally significant and probably not caused by the cosedimentation of aggregated particles.
Figure 6.
Association of the luciferase mRNA with polysomes. Guts from mosquitoes transformed with the AeCP construct were dissected at 24 h after a blood meal, homogenized, and divided into two equal aliquots. One was analyzed on a sucrose gradient without further additions (A), and the other was analyzed after dissociation of polysomes by addition of EDTA to 10 mM (B). The upper part of each panel shows the A254 profile of the gradient after centrifugation, and the lower part shows the Northern blot analysis of each fraction of the gradient by using a radioactive luciferase probe. The short vertical marks below the abscissae indicate the position of each fraction. The numbers above the absorption profiles indicate the position of migration of the ribosomal subunits (40S and 60S), whole ribosomes (80S), or the number of ribosomes per polysome.
Discussion
Using the transposable elements Hermes and Mos1, we generated transgenic Ae. aegypti mosquitoes capable of driving robust sex-, tissue- and stage-specific mRNA and protein expression in the midgut epithelium. Carboxypeptidase promoters from Ae. aegypti and the distantly related An. gambiae both were functional. Furthermore, both promoters were strongly induced by blood ingestion. This finding is significant because Plasmodium ookinetes reach the midgut epithelium at a time of peak transgene expression. Considering that the midgut epithelium is also the source of the transgenic protein, this is an ideal temporal pattern for driving the expression of antimalarial gene products that target ookinetes. Thus, the promoters characterized in this work constitute a valuable tool for the genetic modification of mosquitoes.
The strength of the promoters was measured not only by the abundance of mRNA that accumulated in the midgut epithelium, but also by abundant protein expression. Based on the comparison of the signal of the transgenic luciferase on Western blots with that of a reference enzyme, it can be calculated that approximately 2 ng of luciferase protein accumulated per gut at 24 h after a blood meal. Based on a 1-μl volume in the midgut lumen, that would yield a concentration of 2 μg/ml. Concentration at the periphery, near the midgut epithelium, is probably much higher if diffusion of the secreted protein across the peritrophic matrix and into the blood bolus is slow. It is therefore feasible that a protein expressed from the carboxypeptidase promoter will accumulate at sufficiently high concentrations in the midgut lumen to act as an effective inhibitor of Plasmodium development.
Ae. aegypti and An. gambiae bear only a distant evolutionary relationship. These mosquitoes last shared a common ancestor more than 140 million years ago (16). Thus it was impossible to predict whether the AgCP promoter, which does not contain any apparent sequence similarity to the AeCP promoter, would drive gut-specific expression in the Ae. aegypti gut. There are precedents for such functional conservation of tissue-specific promoters (17–19). Although most properties of the AgCP promoter were maintained in transgenic Ae. aegypti, one significant difference was observed. Although induction of the AgCP promoter by a blood meal is rapid (3 h) in An. gambiae (8), it was much slower (24 h) in Ae. aegypti (Fig. 4B). Possibly Ae. aegypti transcription factors may not recognize the appropriate An. gambiae promoter regulatory sequences. Alternatively, some promoter sequences may be missing from the ≈3.4-kb fragment used for these experiments. In any event, transformation of anopheline mosquitoes, which are the relevant mosquitoes for human malaria transmission, recently has been achieved (20). Analysis of the carboxypeptidase promoters in anopheline mosquitoes should clarify these issues. The early induction of the AgCP promoter in anophelines would allow transgene expression at a much earlier stage of parasite development, before maturation of the peritrophic matrix (which may be a physical barrier to transgenic protein diffusion) and before the induction of the main digestive enzymes (which may promote degradation of transgenic products). Ideally, expression of an antimalarial gene product would be driven by both promoters, thus maximizing protein production during most of the parasite development in the mosquito midgut.
In summary, we report on the functional characterization of two mosquito gut-specific promoters. They are able to drive robust expression of mRNA and protein in a blood-inducible manner. These properties make the carboxypeptidase promoters ideal for use in the genetic modification of mosquitoes to refractoriness to the malaria parasite.
Acknowledgments
We gratefully acknowledge Lynn Horton for support with the polysome sedimentation analysis and Marilyn Donnely-Doman for expert technical assistance. This work was supported by grants from the National Institute of Allergy and Infectious Diseases (AI31478 and AI45123 to M.J.-L.; AI29746 and AI44238 to A.A.J.) and the MacArthur Foundation (to M.J.-L. and A.A.J.).
Abbreviations
- AeCP
Aedes aegypti carboxypeptidase
- AgCP
Anopheles gambiae carboxypeptidase
- RT
reverse transcriptase
References
- 1.Coates C J, Jasinskiene N, Miyashiro L, James A A. Proc Natl Acad Sci USA. 1998;95:3748–3751. doi: 10.1073/pnas.95.7.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coates C J, Jasinskiene N, Pott G B, James A A. Gene. 1999;226:317–325. doi: 10.1016/s0378-1119(98)00557-5. [DOI] [PubMed] [Google Scholar]
- 3.Jasinskiene N, Coates C J, Benedict M Q, Cornel A J, Rafferty C S, James A A, Collins F H. Proc Natl Acad Sci USA. 1998;95:3743–3747. doi: 10.1073/pnas.95.7.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Müller H-M, Crampton J M, Torre A D, Sinden R, Crisanti A. EMBO J. 1993;12:2891–2900. doi: 10.1002/j.1460-2075.1993.tb05951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lemos F J A, Cornel A J, Jacobs-Lorena M. Insect Biochem Mol Biol. 1996;26:651–658. doi: 10.1016/s0965-1748(96)00014-8. [DOI] [PubMed] [Google Scholar]
- 6.Jiang Q, Hall M, Noriega F, Wells M. Insect Biochem Mol Biol. 1997;27:283–289. doi: 10.1016/s0965-1748(97)00001-5. [DOI] [PubMed] [Google Scholar]
- 7.Edwards M J, Moskalyk L A, Donelly-Doman M, Vlaskova M, Noriega F G, Walker V K, Jacobs-Lorena M. Insect Mol Biol. 2000;9:33–38. doi: 10.1046/j.1365-2583.2000.00159.x. [DOI] [PubMed] [Google Scholar]
- 8.Edwards M J, Lemos F J A, Donnely-Doman M, Jacobs-Lorena M. Insect Biochem Mol Biol. 1997;27:1063–1072. doi: 10.1016/s0965-1748(97)00093-3. [DOI] [PubMed] [Google Scholar]
- 9.Morris A C. In: The Molecular Biology of Insect Vectors of Disease. Crampton J M, Beard C B, Louis C, editors. London: Chapman & Hall; 1997. pp. 423–429. [Google Scholar]
- 10.Munstermann L E. In: The Molecular Biology of Insect Vectors of Disease. Crampton J M, Beard C B, Louis C, editors. London: Chapman & Hall; 1997. pp. 13–20. [Google Scholar]
- 11.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 12.Barillas-Mury C, Graf R, Hagedorn H H, Wells M A. Insect Biochem. 1991;21:825–831. [Google Scholar]
- 13.Beard C B, Hamm D M, Collins F H. Insect Mol Biol. 1993;2:103–124. doi: 10.1111/j.1365-2583.1993.tb00131.x. [DOI] [PubMed] [Google Scholar]
- 14.Pinkerton A C, Michel K, O'Brochta D A, Atkinson P W. Insect Mol Biol. 2000;9:1–10. doi: 10.1046/j.1365-2583.2000.00133.x. [DOI] [PubMed] [Google Scholar]
- 15.Mangus D A, Jacobson A. Methods. 1999;17:28–37. doi: 10.1006/meth.1998.0704. [DOI] [PubMed] [Google Scholar]
- 16.Service M W. In: Medical Insects and Arachnids. Lane R P, Crosskey R W, editors. London: Chapman & Hall; 1993. pp. 120–240. [Google Scholar]
- 17.Skavdis G, Siden-Kiamos I, Müller H-M, Crisanti A, Louis C. EMBO J. 1996;15:344–350. [PMC free article] [PubMed] [Google Scholar]
- 18.Xiong B, Jacobs-Lorena M. Proc Natl Acad Sci USA. 1995;92:9393–9317. doi: 10.1073/pnas.92.20.9313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fenerjian M G, Kafatos F C. Dev Biol. 1994;161:37–47. doi: 10.1006/dbio.1994.1005. [DOI] [PubMed] [Google Scholar]
- 20.Catteruccia F, Nolan T, Loukeris T G, Blass C, Savakis C, Kafatos F C, Crisanti A. Nature (London) 2000;405:959–962. doi: 10.1038/35016096. [DOI] [PubMed] [Google Scholar]