Abstract
Extensive previous studies on taxonomy, behavior/bionomics and control of Anopheles sinensis are reviewed and summarized. Recent molecular identification revealed that the population of An. sinensis complex includes An. sinensis, An. pullus, An. lesteri and at least two new species, and An. yatsushiroensis is synonmy of An. pullus. An. sinensis is the main vector specie of vivax malaria in Korea. Larvae of An. sinensis breed in wide range of habitats which are naturally-made clean water, stagnant or flowing; main habitats include rice fields, ditches, streams, irrigation cannals, marshes, ponds, ground pools, etc. Their host preferences are highly zoophilic. Human blood rate is very low (0.7-1.7%); nevertheless An. sinensis readily feeds on man when domestic animals are not found near by. They feed on hosts throughout the night from dusk to dawn with a peak period of 02:00-04:00 hours; they are slightly more exophagic (biting outdoors); much larger numbers come into the room when light is on. Main resting places are outdoors such as grasses, vegetable fields and rice fields. A mark-release-recapture study resulted that 37.1% was recaptured within 1 km, 29.4% at 1-3 km, 21.1% at 3-6 km, 10.3% at 6-9 km and 2.1% at 9-12 km distance. An. sinensis hibernate outdoors (mostly under part of dense grasses) during October-March. At the end of the hibernation period (March-April) they feed on cows at daytime. Until today any single measure to effectively control An. sinensis population has not been found. Indoor residual spray with a long-lasting insecticide can not reduce vector population densities, but shorten their life spans in some degree, so contributes to malaria control.
Keywords: Anopheles sinensis, malaria, Korea
INTRODUCTION
In Korea, vivax malaria which had been prevalent throughout the country for many years was eradicated in 1979. Fifteen years later, malaria re-emerged in South Korea in 1993 and an outbreak has been occurring, with thousands of cases every year (Chai, 1999; Ree, 2000). Moreover, in North Korea hundred thousands of malaria cases have been found recently, being reported 204,428 cases and 300,000 cases in 2000 and 2001, respectively. Infection rate of the inhabitants was 32.9% in Kaepung-gun, Hwhanghaenam-do in 2001 (unpublished report).
The degree of epidemicity of malaria is decided by many factors, of which vector efficiency is one of the most important ones. The main vector species of vivax malaria in Korea is Anopheles sinensis (Ree et al., 1967; Hong, 1977; Lee et al., 2000; Strickman et al., 2001). The knowledge of behavior and bionomics of the vector species is of vital importance to understand the epidemiological features, to find effective control measures, and to interrupt vector-human contact. Studies of An. sinensis in Korea initiated by several Japanese workers in 1910s-1930s, providing very primitive informations. Thereafter, comprehensive studies were implemented by the entomological team of National Malaria Eradication Service (NMES) in 1960s and by various workers in 1990s. The author reviewed all the available study results of An. sinensis, which would contribute for the control/eradication of recent outbreak vivax malaria not only in South Korea but also in North Korea.
Anopheles sinensis Wiedmann 1828 is a member of the Anopheles hyrcanus group belonging to the Myzorhynchus series of the subgenus Anopheles, which are distinguished from other series by the presence of pale bands (usually four) on the palpi and by the presence of a tuft of dark scales on the clypeus on each side in the female adult. An. sinensis is distributed in Assam, Burma, Thailand, Malaysia, Indonesia, Kampuchea, Vietnam, Nam, China, Taiwan, Japan and Korea. Among 28 related species of the Hyrcanus group, five species was found in Korea. An. sinensis was reported in Korea as the new species, named An. yesoensis by Hasegawa (1913), and it was revealed that it was synonym of An. sinensis by Yamada (1924). In 1936 An. sineroides Yamada, 1924. was found near Seoul, and An. pullus, a new species was collected in Taejeon, Chungchongnam-do (Yamada, 1937). An. lesetri Baisa et Hu, 1936 was reported for the first time in Korea by Whang (1964). An. yatsushiroensis Miyazaki, 1951 was found by Hong and Ree (1968). Besides five related species of the Hyrcanus group, An. koreicus Yamada and Watanabe, 1918 and An. lindesai japonicus Yamada, 1958 were listed in Korean mosquito fauna by Yamada (1937) and Hong and Ree (1968), respectively. Recently An. yatsushiroensis was synonimized of An. pullus by morphological observation (Shin and Hong, 2001) and by molecular evidences (Hwang et al., 2004). Recent molecular studies revealed that An. lesteri from Korea, An. anthropophagus from China and An. lesteri from the Philippines were all same species (Wilkerson et al., 2003), and An. lesteri from Japan and An. anthropophagus from China were same species (Hwang et al., 2005). Ree et al. (2005) reported an unknown Anopheles species which was morphologically identical to An. sinensis, and Li et al. (2005) also found two unknown species. Rueda (2005) designated these two species as new species, An. belenrae sp. nov. and An. kleini sp. nov. They were morphologically identical with An. pullus and An. sinensis, respectively. Morphological identification of sinensis complex is extremely difficult, so that some numbers of An. lesteri, An. pullus (including the form yatsushiroensis) and two species (at least) are mixed in the population of An. sinensis in Korea.
TAXONOMY OF ANOPHELES HYRCANUS COMPLEX
Malaria Infection of An. sinensis
Within the range of distribution of An. sinensis its importance as a malaria vector would appear to be confined to China, Taiwan, Japan including the Ryuku Islands, and Korea. An. sinensis is not probably considered to be a malaria vector in Indonesia and Malaysia, and of little significance to human health in Thailand. In Japan, an infection rate of 0.9% was reported in Kyoto prefecture in 1903. In China various workers reported 0.006-2.58% of infection rates of An. sinensis to P. vivax. The infection rate of An. sinensis in Taiwan was 0.02% in 1947-1949.
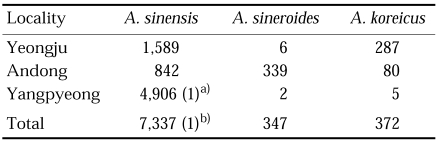
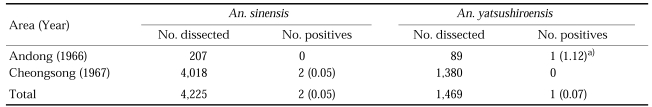
In Korea, salivary gland dissections of Anopheles mosquitoes for natural infection rate of vivax malaria were implemented in three different locations in 1960-1962 (Table 1). Total 7,517 females of An. sinensis were dissected and one positive was found, showing the infection rate of 0.01% in total of three locations (Ree et al., 1967). In 1966-1967, 4,225 An. sinensis and 1,469, An. yatsushiroensis were dissected for sporozoites and oocysts, and two and one positive cases were found from the former (0.05%) and the latter species (0.07%), respectively, as shown in Table 2 (Hong, 1977).
Table 1.
Salivary gland dissections of Anopheles mosquitoes for natural infection of vivax malaria in Korea in 1960-1962 (Ree et al., 1967)
a)positive (0.04% of infection rate).
b)positive (0.01% of infection rate).
Table 2.
Salivary gland dissections of Anopheles mosquitoes for natural infection of vivax malaria in 1966-1967 (Hong, 1977)
a)Infection rate in parenthesis.
At 14 locations of malaria endemic areas, 4,866 An. sinensis, 673 An. pullus, 58 An. sineroides and 12 An. lesteri were collected in August - September 1996. They were pooled (50 ± 5) and nested-PCR were implemented for application of P. vivax specific gene, and two positives from An. sinensis pools were found showing at minimum 0.04% of infection rate (Lee et al., 2000). Anopheles mosquitoes which were collected during landing or biting humans in Paju city, Kyonggi-do in July 1996 were studied for vivax infections by ELISA assay of circumsporozoite antigen and 0.28% (1 positive/361 females) and 0.06% (1/1,559) of the infection rate were found at Taesong-dong and Camp Bonifas, respectively (Strickman et al., 2001). None of 2,005 An. sinensis from other collecting sites were infected. None of 82 An. lesteri or 29 An. yatsushiroensis was positive for vivax malaria.
Breeding Habitat
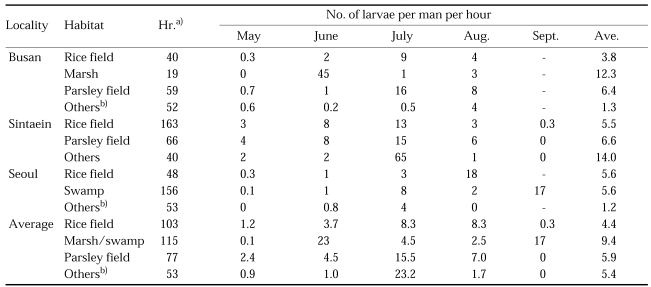
Broadly speaking, the breeding habitat of An. sinensis is everywhere much the same. The larvae are found in rice fields, open grassy ponds, ground pools, swamps, marshes, shores of lakes, stream margins, ditches, and seepages. All these are normally fresh, shallow water habitats, either stagnant or flowing, usually with emergent vegetation and exposed to sunlight. Results of larval collections of An. sinensis in May-September by dipping are given in Table 3 (NMES, 1968) and Table 4 (Self et al., 1971). Though numbers of the larvae collected in rice fields are smaller than those collected in parceley fields and marshes, the main breeding place of An. sinensis is undoubtedly the rice field, taking into account the total area size of each breeding place throughout the whole country. Comparative studies between adult population densities and size of the rice field were carried out in 13 counties of Chollabuk-do in 1985-1990 (Ree and Lee, 1993). Population densities of An. sinensis had no correlation with sizes of the rice fields (r = 0.12), whereas those of Cx. tritaeniorhynchus had a positive correlation (r = 0.62). These findings indicate that An. sinensis had many other breeding habitats together with rice fields so that density of An. sinensis population was not much influenced by the size of rice fields. Large or small temporary ground pools, particularly in rainy season, provide ideal breeding places for An. sinensis. Streams are also one of the main breeding sources. The author collected 347 larvae of An. sinensis in 30 minutes at water weed of a rapidly flowing stream in August 1992 (unpublished data).
Table 3.
Collections of An. sinensis larvae by dipping in May-September, 167 (NMES, 1968)
a)Number of weekly collections in May-September are given in parenthesis.
Table 4.
Number of 3rd-4th instar larvae of An. sinensis collected by dipping in 1971 (Self et al., 1971)
a)Total hours of the collections.
b)ground pools, ditches, streams, etc.
Recently, Claborn et al. (2002) reported that six primary habitats of An. sinensis were rice fields, irrigation ditches, drainage ditches, stream pools, irrigation pools and marshes, most of which harbored similar densities of larvae. They also reported that environmental factors, such as pH, total dissolved solids, percent of surface covered with floating vegetation, and nitrate and phosphate concentrations were not correlated with larval densities.
Seasonal Prevalence
The seasonal prevalence of An. sinensis population has been studied by many workers in Korea, mainly applying two monitoring methods, either cow biting collections throughout the night during the period of 1960s - early 1970s (Whang, 1964; Paik et al., 1965; NMES, 1966, 1968; Hong, 1967; Kim et al., 1978), or light trap collections in 1970s-1990s (Kim et al., 1978; Hong, 1983; Sohn, 1984; Lee and Ree, 1991; Yoon et al., 1994; Kim et al., 1995, 1997, 1999, 2000, 2001, 2003a, 2003b, 2004; Shim et al., 1997; Lee et al., 1999; Strickman et al., 2000; Ree et al., 2001, Lee and Kim, 2001).
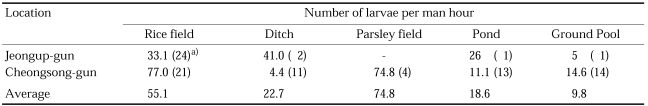
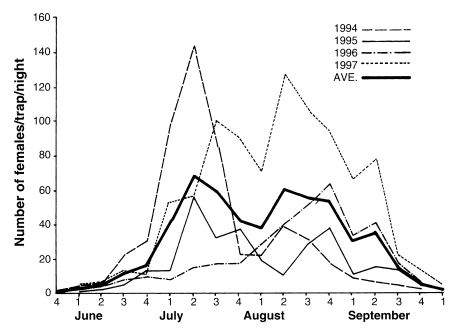
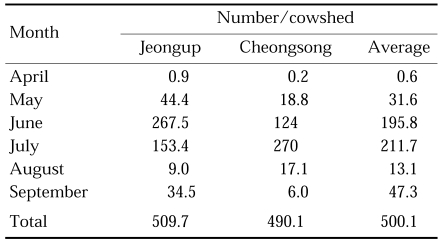
Cow biting collections with two hour intervals throughout the night were carried out at three different areas in 1964, and the peak appeared in August at Yangpyeong and Yeoju, Gyeonggi-do and in July at Okgu, Jeollabuk-do, as shown in Table 5 (Paik et al., 1965). Seasonal prevalence of this species was also studied in 1967 by means of cow biting collections with weekly intervals in Jeongup-gun, Jeollabuk-do and Cheongsong-gun, Gyeongsangbuk-do, showing the peak period at late July in both areas as shown in Table 6 (NMES, 1968). In Daegu, Gyeongsangbuk-do, the peak of An. sinensis population were appeared at the 5th week of July in 1981 and at the 3rd week of July in 1982; population density was markedly different, being 5,556 mosquitoes collected in 1981 whereas 29,755 mosquitoes in 1982 (Sohn, 1984). Most extensive studies on seasonal prevalence were done by Lee and Ree (1991), who monitored seasonal trend of mosquitoes with weekly operation of light traps at 18 cities/guns of Jeollabuk-do in 1985-1990 (Fig. 1), and also by Kim et al. (2000) who weekly operated light traps at four different locations in the same area (Munsan, Gyeonggi-do) in 1994-1997 (Fig. 2). It is noteworthy that the peak of the population density has appeared one to two weeks earlier since late 1980s, compared to the peak period of 1960s-1970s.
Table 5.
Cow biting collectionsa) of An. sinensis at Yangpyeong and Yeoju, Gyeonggi-do and Okgu, Jeollabuk-do in 1964 (Paik et al., 1965)
a)Average of 4 night collections each month.
Table 6.
Seasonal prevalence of An. sinensis by means of cow biting collections at Jeongup, Jeollabuk-do and Cheongsong, Gyeongsangbuk-do in 1967 (cow/man/8 hours) (NMES, 1968)
Fig. 1.
Seasonal prevalence of Anopheles sinensis adult populations in Jeollabuk-do in 1985-1990 (Average of 18 Si/Gun collections) (Lee and Ree, 1991).
Fig. 2.
Seasonal prevalence of Anopheles sinensis collected by New Jersey light traps at Munsan area, Gyeonggi-do in 1994-1997 (Kim et al., 2000).
Summarizing all the study results of various workers mentioned above, seasonal fluctuations and population sizes of An. sinensis are markedly different from location to location and from year to year. In general, females of the new generation start appearing in late April - early May. Population sizes steadily increase from early - mid June, reach the peak during the perod of late June - mid July, follow by a significant decline in August, appear a small secondary peak in early September, and disappear in mid-late October.
Only a few studies on weather factors influencing population dynamics of An. sinensis were carried out in Korea. Ree and Lee (1993) reported that the summer air temperature was correlated in some degree with the adult population size, but other factors such as precipitation, sunshine hours, relative humidity and winter temperature were not correlated. Lee and Kim (2001) reported that temperature and precipitation were the major factors influencing An. sinensis populations.
Resting Habit
After blood feeding, female mosquitoes rest in shaded and humid places, waiting for development of follicles during the period of the gonotrophic cycle. Gonotrophic period of An. sinensis was 2.7 days in average (2-3 days) in July-August in north Gyeonggi-do (Ree et al., 2001).
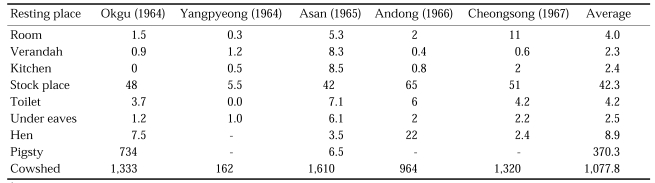
Indoor resting place collections of An. sinensis were weekly done in 10 houses and 10 cowsheds in 5 different localities in June-September 1964-1967, and the results are summarized in Table 7 (Paik et al., 1965; Won and Hong, 1968). Very small numbers were found in houses, being collected 4.0 females/room, 2.3 females/verandah, 2.4 females/kitchen and 42.3 females/stock place, whereas large numbers were collected in animal sheds, being collected 370.3 females/pigsty and 1,077.9 females/cowshed.
Table 7.
Indoor resting place collectionsa) of An. sinensis at 5 locations in June-September in 1964-1967 (Paik et al., 1965; Won and Hong, 1968)
a)Ten randomly selected houses and cowsheds were checked every week.
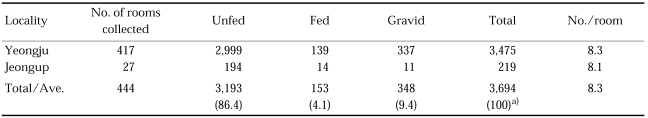
On the other hand, 8.3 females of An. sinensis resting on walls of a bedroom (living room) were collected at night (21:30-22:30 hours), as shown in Table 8 (NMES, 1966). Majority (86.4%) of total mosquitoes were unfed, and only 4.1% was blood fed, which means that most of the females entered rooms rest on walls for a while before feeding, whereas 93.4% of An. sinensis females resting on the wall of a cowshed during the night was fed ones (Hong and Kim, 1990), which means that they rest after fed for a while, before flying outside. It is not clear why a majority of females resting in living rooms were unfed whereas a majority of females resting in cowsheds were fed; it would be resulted from their animal host preference. When they rest on the wall of cowsheds, they prefer low side of the wall (65.3%) rather than upper part (34.7%).
Table 8.
Resting place collections in bedrooms at night (21:30-22:30) at Yeongju, Gyeongsangbuk-do in June-July 1963, and Jeongup, Jeollabuk-do in July 1967 (NMES, 1968)
a)Per cent in parenthesis.
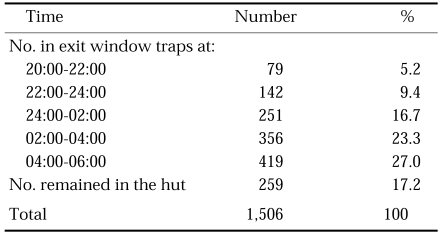
As shown in Table 9, resting place collections in cowsheds in the morning revealed that most of An. sinensis females after feeding flew out of sheds before sunlight, particularly in August, taking into account that numbers of resting mosquitoes were much smaller compared to numbers of biting mosquitoes on cows (Refer Tables 5 and 6). Experimental hut collections with exit window traps showed that only 17.2% of the total An. sinensis rested on walls of the hut, and the others (82.8%) were trapped into the exit window traps in June-July (Table 10), which means that An. sinensis is highly exophilic.
Table 9.
Resting place collections at An. sinensis in cowsheds at daytime (09:00-10:00) by month in Jeongup, Jeollabuk-do and Cheongsong, Gyeongsangbuk-do in 1967 (NMES, 1968)
Table 10.
An. sinensis collections in an experimental hut attached with exit window traps at Yeongju, Gyeongsangbuk-do in June-July, 1963 (unpublished)
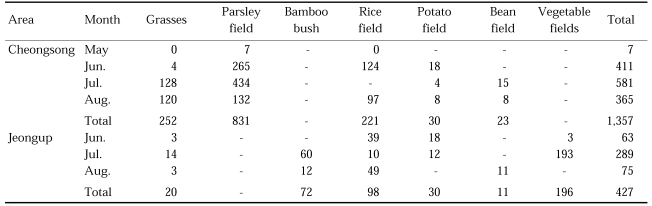
Outdoor resting mosquitoes were collected by sweeping an insect net at a wide range of habitats, such as grasses, rice fields, parsley fields, potato fields, bean fields and other vegetable fields, as shown in Table 11 (NMES, 1968). Though the highest number of the mosquitoes were collected in parsley fields, it is not a main resting place, as the size of parsley fields was very small, compared to the areas of grasses and rice fields that are the main resting places.
Table 11.
Outdoor resting place collectionsa) of An. sinensis by sweeping at Cheongsong, Gyeongsangbuk-do and Jeongup, Jeollabuk-do in 1967 (NMES, 1968)
a)Number/2 man/4 hours.
Host Preferences
It was known that An. sinensis was almost entirely zoophilic in Thailand; in comparative biting tests involving man and cow, almost none of them were attracted to man (Harrison and Scanlon, 1975). In Japan, Sasa (1951) showed that An. sinensis was strongly attracted by big animals and usually very few fed on man.
In Korea precipitin tests of the blood fed females of An. sinensis collected at outdoor resting places in Yangpyeong, Gyeonggi-do in 1962 showed that this mosquito fed exclusively bovines (54.8%) and pigs (42.5%); human blood rate was 1.7% (Table 12; Ree et al., 1967). In north Kyonggi-do in 1999 host blood analysis of An. sinensis collected at outdoor resting places by ELISA also showed similar results, giving 0.7% for human, 89.8% for bovine, 3.3% for pig (Ree et al., 2001). A significant difference between two localities was pig blood meals (42.5% vs 3.3%), which resulted from the availability of pigs for blood feeding. Lee et al. (2001) also studied host preference of An. sinensis in paju, Kyonggi-do in 2000, showing 0.8% of human blood rate (Table 12). Though human blood rates of these host blood tests were very low, they readily fed on man in great numbers where domestic animals (cows and pigs) were not near by for feeding. Number of human biting mosquitoes were 30/man/night at Taesong-dong and 130/man/ night at Camp Bonifas located in DMZ where domestic animals were not found (Strickman et al., 2001). An. sinensis fed also dogs, chicken and cats in small numbers.
Table 12.
Host blood analysis of An. sinensis collected at outdoor resting places in Yeoju, Gyeonggi-do in July 1962 (Ree et al., 1967), Goyang, Gyeonggi-do in 1999 (Ree et al., 2001), and Paju, Gyeonggi-do in 2000 (Lee et al., 2001)
a)Per cent in parenthesis.
Feeding Time and Place
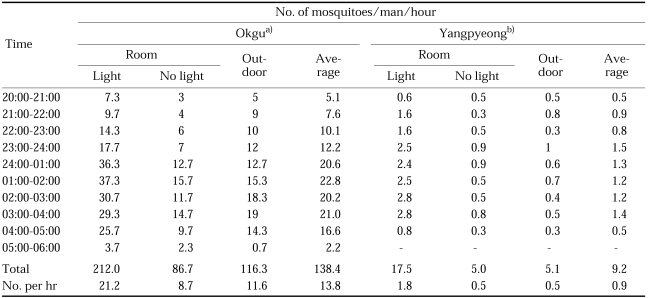
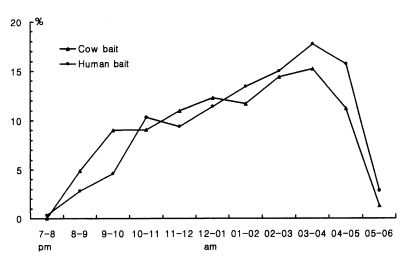
Cow biting collections of An. sinensis were hourly carried out at three different locations in 1965-1967 (Table 13; Hong, 1977). Human biting collections throughout the night were also carried out at Okgu, Jeollabuk-do and Yangpyeong, Gyeonggi-do in July 1965 (Table 15; NMES, 1966) and at several different locations of northern Gyeonggi-do in July-August 1995-1996 (Tables 14; Shim et al., 1997; Strickman et al., 2000). All of the study results showed that An. sinensis fed throughout night from dusk to dawn, apparently feeding more actively from 24:00 to 04:00. Exceptionally, the peak of feeding time appeared at 05:00 in the study result of Strickman et al. (2000). Figure 3 shows that feeding time on animal bait and human bait is not different. Feeding activity at outdoors and indoors was not much different when the room was dark as shown in Table 15. However, when the light was on, much higher numbers of An. sinensis came into the room and fed on man (212.0/man/night with light vs 86.7 without light in Okgu; 17.5 with light vs 5.0 without light at Yangpyeong).
Table 13.
Cow biting collectionsa) of An. sinensis at Asan, Chungcheongnam-do in 1965, Andong, Gyeongsangbuk-do in 1966 and Cheongsong, Gyeongsangbuk-do in 1967 (Hong, 1977)
a)Average of 3-4 collections each month at each locality.
Table 15.
Human biting collections at Okgu, Jeollabuk-do in 1964 and Yangpyeong, Gyeonggi-do in July 1965 (NMES, 1966)
a)Average of 3 nights. b)Average of 8 nights indoors and 11 nights outdoors.
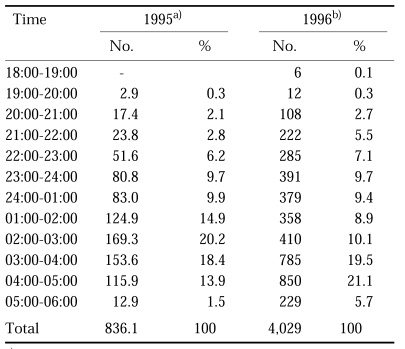
Table 14.
Human biting collections of An. sinensis at northern part of Gyeonggi-do in July-August in 1995 and 1996
a)Average of the collections at 4 locations; 2 men collections in a tent with light (Shim et al., 1997).
b)Total of 19 night collections at 5 different sites near Pammunjeom; 2 men collections outdoors (Strickman et al., 2000).
Fig. 3.
Comparison of feeding time of An. sinensis between cow biting and human biting (Hong, 1977; Shim et al., 1997; Strickman et al., 2000).
Feeding activity of An. sinensis were significantly different by month, showing that the peak time appeared in early night (2000-2300 hr) in May, June and September, whereas it appeared after midnight in July and August (Table 13). These results strongly support that feeding activity of An. sinensis largely relies upon meteorological factors, particularly air temperature, with the majority of individuals biting late at night during warm weather (>20℃) and early at night during cool weather.
It was observed that hibernated An. sinensis fed on cow during daytime (12:00-17:00) when temperature was above 12℃ in March-April (NMES, 1968). The biting activities of An. sinensis were sensitibly influenced by wind speed and direction (Whang, 1964), and negatively correlated with size of the moon (Strickman et al., 2000).
Physiological Age
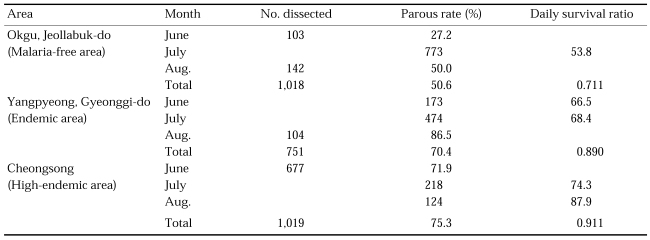
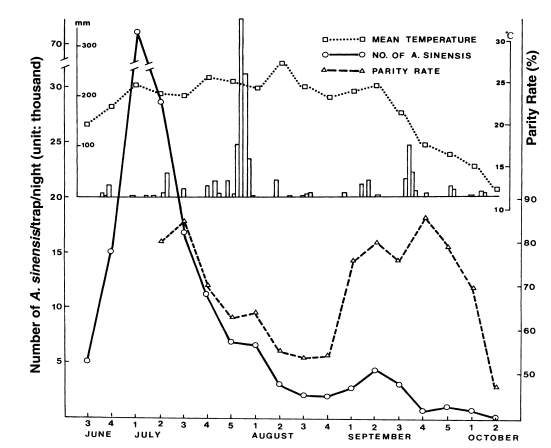
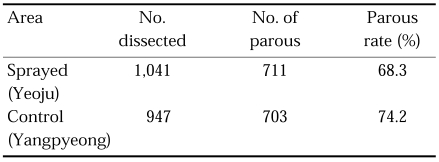
For physiological age determination, ovaries were dissected for parous rate at three topographically different areas in 1964 and 1967 (Table 16) (Paik et al., 1965; Hong, 1977). Parous rates were significantly different at three study areas. It was 50.6% (0.711 of proportion of daily survival) in Okgu, Jeollabuk-do, located in plain, malaria-free area, whereas it was 70.4% (0.890 of proportion of daily survival) in Yangpyeong, Gyeonggi-do, located in hilly, moderately endemic area of malaria, and 75.3% (0.911 of proportion of daily survival) in Cheongsong, Gyeongsangbuk-do, located in mountainous area where malaria was highly endemic. These results showed positive correlation between longevity of the vector species and degree of malaria endemicity, i.e. the longer life span of the vector mosquitoes, the more malaria cases. Recently in northern part of Gyeonggi-do, where outbreaks of malaria cases have occurred, parous rate of An. sinensis was 70.5% (0.890 of daily survival ratio) at Gusan-dong, Goyang-si, Gyeonggi-do in 1999 (Ree et al., 2001), which was very similar to 70.4% at Gaegun-myeon, Yongpyeong-gun, Gyeonggi-do in 1964. Contrary to the above result, Lee et al. (2001) reported that parous rate was 48.8% (0.787 of daily survival ratio) in Paju-si in 2000 (Table 17). Ree et al. (2001) observed that adult population density of An. sinensis and their parous rate (longevity) were positively correlated, showing that sharp decrease of the population density during late July-August resulted from the short life span of the vector mosquitoes (Fig. 4).
Table 16.
Parous rate of An. sinensis at Okgu, Jeollabuk-do and Yangpyeong, Gyeonggi-do in 1964 (Paik et al., 1965) and Cheongsong, Gyeongsangbuk-do in 1967 (Hong, 1977)
Table 17.
Parous rate of An. sinensis at two localities in Gyeonggi-do in 1999-2000
a)Collected by light traps in 1999 (Ree et al., 2001).
b)Collected by human bait in 2000 (Lee et al., 2001).
Fig. 4.
Weekly occurrence of population density and parous rate of Anopheles sinensis at Gusan-dong, Goyang-si, Gyeonggi-do, in 1999. Mean temperature by week and daily rainfall are also shown (Ree et al., 2001).
Flight Range and Dispersal
Geographical expansion of malaria transmission is largely determined by flight/dispersal activities of the vector mosquitoes, when vivid human movement is not observed. Therefore, flight/dispersal range of An. sinensis, the vector species of vivax malaria, is a vital important factor for understanding recent malaria epidemics in border areas between North and South Korea.
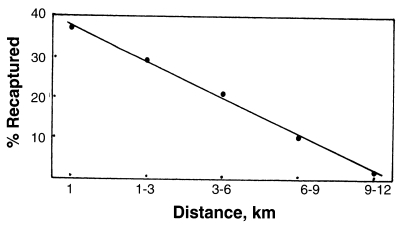
A mark-release recapture experiment of An. sinensis was carried out in Paju city, Gyeonggi-do, Korea during the period of 7-28 September 1998 (Cho et al., 2002). Total 12,772 unfed females were marked with fluorescent dye and released. Recaptures of marked females were made from following days of the release, by operating 13 light traps, each of which was set up in a cowshed at various distance from the release point. Light trap collections continued every night for 21 days. Among total 194 marked females recaptured (1.52% recapture rate), 37.1% were recaptured within 1 km from the release point, 29.4% at 1-3 km distance, 21.1% at 3-6 km, 10.3% at 6-9 km, and 2.1% (4 females) at 9-12 km. Correlation between flight distance and number of the recaptured An. sinensis was observed, shown as Fig. 5.
Fig. 5.
Correlation between flight distance (km) and per cent of An. sinensis recaptured (Cho et al., 2002; the figure drawn by Ree).
Hibernation Habit
Studies on finding hibernation places of An. sinensis have been rather extensively carried out by various workers (Whang, 1961, 1964; NMES, 1968; Hong, 1977; Ree et al., 1976; Shim et al., 1987c, 1989). Whang (1964) reported that among total 4,402 hibernating females 22 An. sinensis were found in culverts and 6 An. sinensis under bridges in November-December in 1959-1960. Two straw piles (one 2.5 × 3 × 3 m, and the other 2.7 × 3.5 × 1.4 m) were covered with specially-made extra-large mosquito nets in Jeongup, Jeollabuk-do from 11 March to 30 April 1966. A collector searched mosquitoes resting in the mosquito net every day (twice a day) and 6 females of An. sinensis and one Culex pipiens were collected (NMES, 1968). An exit window trap was covered over an abandoned well (stone-piled with 10 m deep), and 108 rat holes at banks of rice fields were covered with specially designed cone-shape trap from February to May. The results of both trials were negative (NMES, 1968). A vinyl tent was covered over grasses in December in Buan-gun, Jeollabuk-do, and collected 6 females of An. sinensis (Shim et al., 1987c). The same collection method was applied at Gosan, Cheju-do and 3 females of An. sinensis were collected (Shim et al., 1989). Stone piles were covered with a plastic tent and stones were removed one by one in December 1972 in Busan, and 15 females of An. sinensis were found together with 1 An. pullus, 31 Cx. pipiens pallens and 4 Cx. orientalis (Ree et al., 1976). A mobile vinyl tent was covered over heavy grasses of rice paddy banks at Samha-ri, Yangju-gun, Gyeonggi-do in January-March 1999. When temperature inside the tent raised high (>15℃) by sun light, hibernating mosquitoes flew out from grasses in a few minutes. At total 46 sites, 145 females of An. sinensis complex were collected. Most of them were An. pullus (Hwang et al., 2004).
The first collection of An. sinensis larvae of the first new generation of the season was on April 14 (one larva from ditch) in Cheongsong, Gyeongsangbuk-do and on April 15 (two 3rd instar larvae from rice fields) in Jeongup, Jeollabuk-do in 1967 (NMES, 1968). These larval findings in mid April indicate that hibernated females fed on hosts in March and laid eggs in late March - early April. Because temperature at night was too low to feed, cow biting collections at daytime were carried out in Jeongup, Jeollabuk-do from early March to late April 1967. An. sinensis started collecting from mid March. The results are shown in Table 18 (NMES, 1968). It is interesting to note that their feeding activity was observed during daytime even when temperature was 12-13℃.
Table 18.
Dry ice/mosquito net and cow biting collections of hibernating An. sinensis at daytime in Jeongup, Jeollabuk-do in March - April 1967 (NMES, 1968)
a)Collections in Cheongsong, Gyeongsangbuk-do.
Indoor resting place collections in cowsheds at daytime and cow biting collections at night showed that hibernating females appeared for feeding at night in small numbers in April - early May; 0.3 and 4.0 females per cow per night were collected in April and May respectively in Cheongsong, Gyeongsanbuk-do in 1967, and 3.3 females per cow per night were collected on cow bait in May in Asan, Chungcheongnam-do in 1965 (Hong, 1977).
In conclusion, females of An. sinensis hibernate outdoors at various concealed places such as grasses, straw piles, stone piles and others. Successfully survived females during several months of overwintering period appear for feeding on cows in daytime in mid March-April. They start feeding on cows when temperature reaches above 12℃ at night.
CONTROL
Two different control measures are applied for malaria vector control, one larval control and the other adult control. Principal objective of the former is to reduce absolute population density and that of the latter is to cut off vector-human contact by selective killing of old-age mosquitoes and/or reducing longevity of the vector mosquitoes. Indoor residual spray with a long-lasting insecticide is the most classical methodology for the latter purpose.
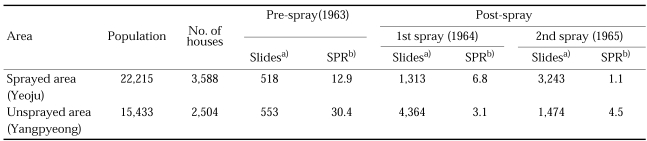
In 1964 and 1965, Daesin-myeon with a population of 22,215 in 3,586 houses were selected for the treated area in Yeoju-gun, Gyeonggi-do and the neighbouring Gaegun-myeon, Yangpyeong-gun was selected for untreated (control) area. All surfaces of the houses and animal sheds in Daesin-myeon were sprayed with 2 g/m2 of DDT in April-May each year of 1964 and 1965. Passive case detection for malaria cases was carried out in both areas and suspected fever cases were dosed with chloroquine (NMES, 1966). Relative population densities of An. sinensis were compared by cow biting collections throughout night with a week interval. The result is summarized in Table 19, showing no significant difference of densities and seasonal trends of the vector mosquitoes between in the DDT-sprayed and control areas. In other words, total coverage of the houses and animal sheds with DDT could not suppress density of An. sinensis population. Parous rate of An. sinensis was 68.3% in the treated area and 74.2% in the control area, which was slightly lower in the treated area (Table 20). Malaria cases were decreased in both the sprayed and unsprayed areas in the first year of the treatment, and then significantly decreased in the sprayed area in the second year of the treatment (Table 21). The slide positive rate (SPR) of malaria before treatment (1963) were 12.9% and 30.4% in the treated and untreated areas, respectively, and SPR in 1964 (after 1st treatment) was 6.8% and 3.1%, respectively, which would probably be resulted from the passive case detection and drug administration in both areas. SPR in 1965 (after the 2nd treatment) was further decreased (1.1%) in the sprayed area, whereas increased (4.5%) in the unsprayed area. It can be explained that PCD (case finding and treatment) could significantly reduce the cases in a short-term period only where incidence rate was high, and when the incidence rate decreased to very low level PCD alone could not reduce cases further, but DDT spray, as an additional supplemental control measure, reduced the cases further.
Table 19.
Comparison of population densities of An. sinensis between DDT-sprayed (Daesin-myeon) and control (Gaegun-myeon) areas in Gyeonggi-do, by means of cow biting collection at 2 hour intervals in June-September in 1964-1965. Total coverage of DDT indoor residual spray was completed in April-May each year (NMES, 1966)
a)Number/cow/hour, in average of 1964-1965.
Table 20.
Comparison of parous rate of An. sinensis between DDT-sprayed and control aeras in June-September 1965 (NMES, 1966)
Table 21.
Comparison of malaria cases between DDT sprayed and unsprayed areas in 1963-1965
a)Number of blood slides of the suspected fever cases by PCD.
b)Slide positive rate.
Thermal foggings which have been widely and extensively applied in villages in Korea, and permethrin-treated mosquito nets which are successfully applied in tropical countries have not been evaluated for the effectiveness of An. sinensis control. Therefore such studies are required with high priority.
For larval control of An. sinensis, susceptibility of An. sinensis larvae to various insecticides including Bacillus thuringiensis israelensis (B.t.i.) and Bacillus sphaericus was tested by many researchers (Self et al., 1974; Shim and Kim, 1980, 1981; Ree et al., 1981b; Shim et al., 1987 a, b, 1995; Lee et al., 1996; Lee and Yu, 1999; Shin et al., 2003). These studies revealed that An. sinensis has developed high level of resistance since 1980s to most of the organophosphorous and carbamate insecticides which had been extensively applied on rice fields for the control of agricultural pests. Ree et al. (1981 a, b; 1982) reported that the seasonal prevalence of An. sinensis larvae in the rice field was rather stable, not being affected by insecticide pressure at all, whereas natural predators such as larvae of Odonata and Coleoptera drastically decreased by agricultural insecticide applications. Field trials in rice fields also showed that 14 insecticides tested were ineffective to control of An. sinensis larval population except cartap and B.t.i. (Ree et al., 1981a; Yu et al., 1982a; Shim et al., 1987a, b, d). Entomological studies were performed on the community structures of aquatic animals including mosquito larvae in organically-farmed rice fields (insecticides not applied) and conventionally-farmed rice fields (insecticides applied) in Boseong, Jeollanam-do in 1995-1996 (Lee et al., 1997; Lee, 1998). The abundance of An. sinensis larvae was lower in the organically-farmed rice fields in which number of the Chinese muddy loaches (Misgurnus mizolepis), predator of mosquito larvae was high, as compared to the conventionally-farmed rice fields.
For the development of biological control measures against larval population of An. sinensis, laboratory and field tests were carried out to find effective biological control agents by Yu et al. (1981, 1982a,b, 1993), Ree and Lee (1983), Yu (1986), Shim et al. (1987a,d), Yu and Kim (1993), and Kim et al. (1994). Potential biological control agents as effective predators of An. sinensis larvae were Aphyocypris chinensis, Aplochilus latipes, Moroco oxycephalus and Misgurnus anguillicaudatus among native fishes, and nymphs of Orthetrum triagulare melania and Sympetrum sp. in Odonata, and larva of Cybister japonicus, lacophilus sp. and Hydrophilus affinis in Coleoptera.
Analyzing all available data on biological control against An. sinensis larvae, it would not practically applicable in the rice field, not only in technical but in economical ponts of view, except in a very limited area or isolated habitats.
References
- 1.Chai JY. Re-emerging Plasmodium vivax malaria in the Republic of Korea. Korean J Parasitol. 1999;37:129–143. doi: 10.3347/kjp.1999.37.3.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho SH, Lee HW, Shin EH, Lee HI, Lee WG, Kim CH, Kim JT, Lee JS, Lee WJ, Jung GG, Kim TS. A mark-release-recapture experiment with Anopheles sinensis in the northern part of Gyonggi-do, Korea. Korean J Parasitol. 2002;40:139–148. doi: 10.3347/kjp.2002.40.3.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Claborn DM, Hshieh PB, Roberts DR, Klein TA, Zeichner BC, Andre RG. Environmental factors associated with larval habitats of malaria vectors in northern Kyonggi Province, Republic of Korea. J Am Mosq Control Assoc. 2002;18(3):178–185. [PubMed] [Google Scholar]
- 4.Harrison BA, Scanlon JE. The subgenus Anopheles in Thailand. Contrib An. Entomol Instit. 1975;12:1–307. [Google Scholar]
- 5.Hasegawa Y. Malaria in Korea. J Chosun Med Soc. 1913;4:53–69. (in Japanese) [Google Scholar]
- 6.Hong HK. Bionomics of Anopheles sinensis Wiedmann in western plain area in Korea. Korean J Zool. 1967;10:76–80. (in Korean) [Google Scholar]
- 7.Hong HK. Ecological studies on Korean mosquitoes mainly found in the human habitation. PhD Thesis. Tongkook University; 1977. p. 57. (in Korean) [Google Scholar]
- 8.Hong HK. Ecological studies on the vector of Japanese encephalitis Culex tritaeniorhynchus in Korea. Korean J Epidemiol. 1983;5:29–40. (in Korean) [Google Scholar]
- 9.Hong HK, Kim CM. Studies on resting behaviour of mosquitoes in rural villages in Incheon, Korea. J Nat Sci, Incheon Univ. 1990;1:59–71. (in Korean) [Google Scholar]
- 10.Hong HK, Ree HI. Two unreported Anopheles mosquitoes in Korea. Korean J Zool. 1968;11:16–18. [Google Scholar]
- 11.Hwang UW, Yong TS, Ree HI. Molecular evidence for synonymy of Anopheles yatsushiroensis and An. pullus. J Am Mosq Control Asso. 2004;20:99–104. [PubMed] [Google Scholar]
- 12.Hwang UW, Tang LH, Kobayashi M, Yong TS, Ree HI. Molecular evidence supports that Anopheles anthropophagus from China and An. lesteri from Japan are the same species. J Am Mosq Control Assoc. 2005;21 doi: 10.2987/8756-971X(2006)22[324:MESTAA]2.0.CO;2. (in press) [DOI] [PubMed] [Google Scholar]
- 13.Kim KH, Shin HK, Ree HI. Survey for the production of the occurrance of Japanese encephalitis epidemics in Korea (1978) Korean J Virol. 1978;8:37–43. (in Korean) [Google Scholar]
- 14.Kim HC, Kim MS, Yu HS. Biological control of vector mosquitoes by the use of fish predators, Moroco oxycephalus and Misgurnus anguillicaudatus in the laboratory and semi-field rice paddy. Korean J Entomol. 1994;24:269–284. [Google Scholar]
- 15.Kim HC, Lee KW, Robert LL, Sardelis MR, Chase FE. Seasonal prevalence of mosquitoes collected from light trap in Korea (1991-1992) Korean J Entomol. 1995;25:225–234. [Google Scholar]
- 16.Kim HC, Lee KW, Jones JW, Korch GW. Seasonal prevalence of mosquitoes collected from light trap in Korea (1993-1994) Korean J Entomol. 1997;27:21–28. [Google Scholar]
- 17.Kim HC, Lee KW, Klein TA, Strickman DA. Seasonal prevalence of mosquitoes collected from light trap in Korea (1995-1996) Korean J Entomol. 1999;29:181–187. [Google Scholar]
- 18.Kim HC, Strickman CD, Lee KW. Seasonal prevalence and feeding activity of Anopheles sinensis (Diptera: Culicidae) in the northwestern part of Kyonggiprovince, Republic of Korea. Korean J Entomol. 2000;30:193–199. [Google Scholar]
- 19.Kim HC, Lee KW, Miller WB, Strickman D. Seasonal prevalence of mosquitoes collected from light traps in Korea (1997-1998) Korean J Entomol. 2001;31:7–13. [Google Scholar]
- 20.Kim HC, Lee KW, Richards RS, Schleich SS, Herman WE, Klein TA. Seasonal prevalence of mosquitoes collected from light traps in Korea (1999-2000) Korean J Entomol. 2003a;33:9–16. [Google Scholar]
- 21.Kim HC, Friendly OS, Pike JG, Schuster AL, O'Guinn ML, Klein TA. Seasonal prevalence of mosquitoes collected from light traps in Korea, 2001. Korean J Entomol. 2003b;33:189–199. [Google Scholar]
- 22.Kim HC, Chong ST, Pike JG. Seasonal prevalence of mosquitoes collected from light traps in the Republic of Korea, 2002. Entomol Res. 2004;34:177–186. [Google Scholar]
- 23.Lee DK. Effect of two rice culture methods on the seasonal occurrence of mosquito larvae and other aquatic animals in rice fields of southwestern Korea. J Vector Ecol. 1998;23:161–170. [PubMed] [Google Scholar]
- 24.Lee DK, Kim SJ. Seasonal prevalence of mosquitoes and weather factors influmencing population size of Anopheles sinensis (Diptera, Culicidae) in Busan, Korea. Korean J Entomol. 2001;31:183–188. [Google Scholar]
- 25.Lee DK, Yu HS. Susceptibility of medically important larvae and larvivorous fishes to Abate and Abate-S in Korea. Korean J Appl Entomol. 1999;38:165–169. (in Korean) [Google Scholar]
- 26.Lee DK, Jeon JH, Kang HS, Yu HS. Analysis of aquatic ecosystems in organic and conventional farming rice fields and mosquito larval populations. Korean J Entomol. 1997;27:203–214. (in Korean) [Google Scholar]
- 27.Lee DK, Cho HC, Kwon HD, Lee CN, Ha ST, Bin JH, Jung GY. Seasonal prevalence of mosquitoes collected from light trap in Pusan, Korea (1993-1995) Korean J Hlth Sci. 1999;9:79–82. [Google Scholar]
- 28.Lee HI, Lee JS, Shin EH, Lee WJ. Malaria transmission potential by Anopheles sinensis in the Republic of Korea. Korean J Parasitol. 2001;39:185–192. doi: 10.3347/kjp.2001.39.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JS, Lee WJ, Cho SH, Ree HI. Outbreak of vivax malaria in areas adjacent tot he demilitarized zone, South Korea, 1998. Am J Trop Med Hyg. 2002;66 doi: 10.4269/ajtmh.2002.66.13. (in press) [DOI] [PubMed] [Google Scholar]
- 30.Lee KW, Kim HC, Lee SH, Korch GW, Klein TA. Susceptibility and resistance to diagnostic doses of insecticides on vector and nuisance mosquitoes in Korea. Korean J Entomol. 1996;26:249–256. (in Korean) [Google Scholar]
- 31.Lee SK, Ree HI. Studies on mosquitoe population dynamics in Chollabug-do, Korea (1985-1990) - 2. Factors influencing populaton sizes of Culex tritaeniorhynchus and Anopheles sinensis - Korean J Entomol. 1991;23:185–194. [Google Scholar]
- 32.Lee WJ, Lee HW, Shin EH, Yang YC, Kim NR, Hong ST, Lee JS. Vector determination of tertian malaria Plasmodium vivax by polymerase chain reaction. Korean J Entomol. 2000;30:77–83. (in Korean) [Google Scholar]
- 33.Li C, Lee JS, Groebner JL, Kim HC, Klein TA, O'Guinn ML, Wilkerson RC. A newly recognized species in the Anopheles Hyrcanus complex and molecular identification of related species from the Republic of South Korea (Diptera: Culicidae) Zootaxa. 2005;939:1–8. [Google Scholar]
- 34.NMES. Progress Report, 1961-1965. National Malaria Eradication Service, Ministry of Health and social Affairs; 1966. Malaria pre-eradication programme in Korea; p. 74. (unpublished document) [Google Scholar]
- 35.NMES. Studies on the behaviour and possible control measures of the confirmed vector of Japanese encephalitis in Korea. National Malaria Eradication Service, the Ministry of Health and Social Affairs; 1968. p. 81. (unpublished document) [Google Scholar]
- 36.Paik YH, Song JH, Ree HI, Hong HK. Epidemiological studies on malaria situation in Korea. I. On the binomics of Anopheles sinensis and its relation to malaria in Korea. New Med J. 1965;8:53–59. (in Korean) [Google Scholar]
- 37.Ree HI. Unstable vivax malaria in Korea. Korean J Parasitol. 2000;38:119–138. doi: 10.3347/kjp.2000.38.3.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ree HI, Lee SK. Studies on mosquito population dynamics in Chollabug-do, Korea (1985-1990) II. Factors influencing population sizes of Culex tritaeniorhynchus and Anopheles sinensis. Korean J Entomol. 1993;23:185–194. [Google Scholar]
- 39.Ree HI, Lee WJ. Laboratory studies on predation efficacy of sow Odonata nymphs and Coleoptera larvae against mosquito larvae. Korean J Entomol. 1983;13:31–38. [Google Scholar]
- 40.Ree HI, Hong HK, Paik YH. Study on natural infection of Plasmodium vivax in Anopheles sinensis in Korea. Korean J Parasitol. 1967;5:3–4. doi: 10.3347/kjp.1967.5.1.3. (in Korean) [DOI] [PubMed] [Google Scholar]
- 41.Ree HI, Wada Y, Jolivet PHA, Hong HK, Self LS, Lee KW. Studies on over-wintering of Culex tritaeniorhynchus Giles in the Republic of Korea. Cah ORSTOM sér Ent Méd Parasitol. 1976;14:105–109. [Google Scholar]
- 42.Ree HI, Hong HK, Shim JC, Lee JS, Cho HW, Kim CL. A study on seasonal prevalence of the populations of the mosquito larvae and other aquatic invertebrates in rice fields in Korea. Korean J Zool. 1981a;24:151–161. [Google Scholar]
- 43.Ree HI, Shim JC, Hong HK, Lee JS, Cho HW, Kim CL. Studies on control effects of pesticides applications against the vector mosquito larvae in rice fields in Korea. Korean J Entomol. 1981b;11:39–45. [Google Scholar]
- 44.Ree HI, Shim JC, Kim CL, Lee WJ. Studies on population dynamics of vector mosquito larvae and other aquatic animals including predators breeding in rice fields in 1982. Rep NIH Korea. 1982;19:137–149. [Google Scholar]
- 45.Ree HI, Hwang UW, Lee IY, Kim TU. Daily survival and human blood index of Anopheles sinensis, the vector species of malaria in Korea. J Am Mosq Control Assoc. 2001;17:67–72. [PubMed] [Google Scholar]
- 46.Ree HI, Yong TS, Hwang UW. Identification of four species of the Anopheles hyrcanus complex (Diptera: Culicidae) found in Korea using species-specific primers for Polymerase Chain Reaction Assay. Med Entomol Zool. 2005;56 (in press) [Google Scholar]
- 47.Rueda LM. Two new species of Anopheles (Anopheles) Hyrcanus group (Diptera: Culicidae) from the Republic of South Korea. Zootaxa. 2005;941:1–26. [Google Scholar]
- 48.Sasa M. Two years' observation on the seasonal activities and zoophilism of mosquitoes in Tokyo by animal trap method. Jap J Exp Med. 1951;20:509–517. [Google Scholar]
- 49.Self LS, Shin HK, Kim KH, Lee KW, Chow CY, Hong HK. WHO/VBC/71. 1971. Ecological studies on Culex tritaeniorhynchus in the Republic of Korea in 1971; p. 332. (unpublished WHO document) [Google Scholar]
- 50.Self LS, Shim JC, Jolivet P. Susceptibility of Culex tritaeniorhynchus and six other mosquitoes to insecticides in Korea. Cah ORSTOM sér Ent méd Parasitol. 1974;12:81–92. [Google Scholar]
- 51.Shim JC, Kim CL. Studies on the susceptibility of public health insecticides against mosquitoes in Korea. Rep NIH Korea. 1980;17:357–362. (in Korean) [Google Scholar]
- 52.Shim JC, Kim CL. On the susceptibility of insecticides against vector mosquitoes. Rep NIH Korea. 1981;18:249–255. (in Korean) [Google Scholar]
- 53.Shim JC, Yoon YH, Kim CL, Lee WJ, Lee BI. Pesticides effect against the vector mosquitoes and aquatic animals in ice and parsley fields in the context of integrated control. Rep NIH Korea. 1987a;24:477–492. (in Korean) [Google Scholar]
- 54.Shim JC, Ban SJ, Eym YB, Yu HS. Development of Research on bacterial pathogen (B.t.i. and B. sphaericus) and evaluation of effectiveness against disease vector mosquitoes in Korea. Rep NIH Korea. 1987b;24:501–517. (in Korean) [Google Scholar]
- 55.Shim JC, Yoon YH, Kim CL, Lee WJ. Studies on the hibernation of vector mosquitoes in Korea. Rep NIH Korea. 1987c;24:493–500. (in Korean) [Google Scholar]
- 56.Shim JC, Yoon YH, Kim CL, Lee WJ, Lee BI, Kim SC. Integrated control of vector mosquitoes in rice fields. Korean J Entomol. 1987d;17:83–91. (in Korean) [Google Scholar]
- 57.Shim JC, Yoon YH, Kim CL, Lee WJ, Shin EH. Studies on the hibernation of vector mosquitoes in Korea. Report NIH, Korea. 1989;26:213–222. (in Korean) [Google Scholar]
- 58.Shim JC, Hong HK, Koo SH, Lee DK. Susceptibilities of Anopheles sinensis larvae (Culicidae, Diptera) to various insecticides. Korean J Entomol. 1995;25:69–76. (in Korean) [Google Scholar]
- 59.Shim JC, Shin EH, Yang DS, Lee WK. Seasonal prevalence and feeding time of mosquitoes (Diptera: Culicidae) at outbreak regions of domestic malaria (P. vivax) in Korea. Korean J Entomol. 1997;27:265–277. (in Korean) [Google Scholar]
- 60.Shin EH, Hong HK. A new synonym of Anopheles (Anopheles) pullus Yamada, 1937 - A. (A.) yatsushiroensis Miyazaki, 1951. Korean J Entomol. 2001;31:1–5. [Google Scholar]
- 61.Shin EH, Park YI, Lee HI, Lee WJ. Insecticide susceptibilities of Anopheles sinensis larvae from Paju-shi, Korea. Korean J Entomol. 2003;33:33–37. [Google Scholar]
- 62.Sohn SR. Seasonal prevalence of mosquitoes collected with light trap - At a pigshed in the vicinity of Daegu city, Korea. Korean J Zool. 1984;27:117–125. (in Korean) [Google Scholar]
- 63.Strickman D, Miller ME, Kim HC, Lee KW. Mosquito surveillance in the demilitarized zone, Republic of Korea, during an outbreak of Plasmodium vivax malaria in 1996 and 1997. J Am Mosq Cont Assoc. 2000;16:100–113. [PubMed] [Google Scholar]
- 64.Strickman D, Miller ME, Lee KW, Kim HC, Wirtz RA, Perich M, Novakoski WL, Feighner BH, Roh CS. Successful entomological intervention against Anopheles sinensis limiting transmission of Plasmodium vivax to American soldiers in the Republic of Korea. Korean J Entomol. 2001;31:189–195. [Google Scholar]
- 65.Whang CH. Hibernation of mosquitoes in Korea. Mosq News. 1961;21:17–20. [Google Scholar]
- 66.Whang CH. Studies on bionomics of Anopheles mosquitos in Korea. Korean Med J. 1964;9:49–74. (in Korean) [Google Scholar]
- 67.Wilkerson RC, Li C, Rueda LM, Kim HC, Klein TA, Song GH, Strickman D. Molecular confirmation of Anopheles (Anopheles) lesteri from the Republic of South Korea and its genetic identity with An. (Ano.) anthropophagus from China (Diptera: Culicidae) Zootaxa. 2003;378:1–14. [Google Scholar]
- 68.Won PH, Hong HK. Studies on bionomics of Korean mosquitoes Part II Ecological studies on Anopheles mosquitoes in Korea. Dongkuk University thesis. 1968;5:122–158. (in Korean) [Google Scholar]
- 69.Yamada M. A new species of Anopheles in Chosen (Korea) Keijo J Med. 1937;8:237–260. [Google Scholar]
- 70.Yamada S. A revision of the adult anopheline mosquitoes of Japan: Systematic descriptions, their habits and their relations to human diseases, together with an account of three new species. Sci Rep Gov Inst Infect Dis Tokyo. 1924;3:215–241. [Google Scholar]
- 71.Yoon JH, Park HC, Jung BG. Seasonal prevalence of mosquitoes collected with light trap - At a cow shed inthe vicinity of Taegu city, Korea. Korean J Entomol. 1994;24:7–17. (in Korean) [Google Scholar]
- 72.Yu HS. Biological control of malaria vector, Anopheles sinensis Wiedemann by the release of larvivorous fish, Aplocheilus latipes in simulated rice paddies in Korea. Korean J Entomol. 1986;16:93–99. [Google Scholar]
- 73.Yu HS, Kim HC. Integrated control of encephalitis vector (Culex tritaenior- hynchus) with native fishes (Aplocheilus latipes and Aphyocypris chinensis) and Bacillus thuringiensis (H-14) in marshes in Jindo Island of Korea. Korean J Entomol. 1993;23:221–230. [Google Scholar]
- 74.Yu HS, Yun YH, Lee DK, Lee WJ. Biological control of mosquito larvae breeding in rice paddies in the presence of fish predator, Aphyocypris chinensis in Korea. Korean J Entomol. 1981;11:29–37. [Google Scholar]
- 75.Yu HS, Lee DK, Lee WJ, Shim JC. Mosquito control evaluation of Bacillus thuringiensis var. islaelensis in the laboratory, simulated rice fields, and confined field trials in Marsh and sewage effluent in South Korea. Korean J Entomol. 1982a;12:69–82. [Google Scholar]
- 76.Yu HS, Lee DK, Lee WJ. Mosquito control by the release of fish predator, Aphyocypris chinensis in natural mosquito breeding habitats of rice paddies and stream seepage in South Korea. Korean J Entomol. 1982b;12:61–67. [Google Scholar]
- 77.Yu HS, Kim HC, Yang KH. Mosquito vector control by the use of native fish (Aphyocypris chinensis) and Bacillus thuringiensis (H-14) in natural rice fields and parsley fields in Korea. Korean J Entomol. 1993;23:231–243. [Google Scholar]