Abstract
We have observed the seropositive rate of Taenia solium cysticercosis in residents at Nabo Village, Tiandong County, Guangxi Zhuang Autonomous Region, China by enzyme linked immunosorbent assay. The village had been found to be a relatively high endemic area of porcine cysticercosis among roaming pigs. Of 202 persons examined four males aged 15, 25, 35 and 41 year-old exhibited absorbance (abs) at 0.18, 0.20, 0.35 and 0.55, respectively. In addition, two females whose ages were 35 and 39 years revealed specific antibody levels of abs 0.26 and 0.41 in their sera. Overall positive rate among the people was 2.97%. All of these persons agreed that they had ingested the pork infected with T. solium metacestode (TsM), while history of proglottid discharge was not noticed from all of them. Three males and one female complained of intermittent headache. Our findings reinforced not only that the prevalence of cysticercosis might be related with roaming pigs infected with TsM but also that behavioral and environmental practices in local community constituted risk factors for transmission of the infection.
Keywords: Taenia solium metacestode, roaming pigs, swine cysticercosis, epidemiology, seroprevalence
INTRODUCTION
Taenia solium neurocysticercosis (NC), a disease caused by the T. solium metacestode (TsM), comprised one of the most common parasitic diseases in the central nervous system (CNS). The disease has recently gained significant recognition in several communities due to its reemergence by increased immigration and frequent travel to endemic areas (Maguire, 2004). Clinical manifestation of the disease is highly pleiomorphic and non-specific according to the number and location of worms infected in the CNS as well as the stage of the infection (White, 2000). However, NC is mainly manifested with seizure, symptom complex due to increased intracranial pressure and focal neurologic deficits, and is a leading cause of adult-onset seizure where the disease is endemic (Garcia and Del Brutto, 2000; Bucardo et al., 2005).
NC is diagnosed largely by characteristic neuroimaging findings such as computerized tomography (CT) and magnetic resonance (MR). Since the number, size and location of the infected cysts and stage of infections are variable in many patients, however, imaging diagnosis is often confusing and inconclusive in some extents. In such cases, detection of specific antibodies circulated in the sera or cerebrospinal fluids (CSF) by enzyme linked immunosorbent assay (ELISA) or by immunoblot is helpful to confirm or to exclude the diagnosis (Garcia et al., 2002). In addition, serological test is useful for epidemiological survey in a large scale in endemic areas due to its easy applicability and high reproducibility.
In the present study, we have observed the specific antibody levels against TsM cyst fluid (CF) in residents at Nabo Village, Tiandong Province, Guangxi Zhuang Autonomous Region, China to exploit the seroepidemiological prevalence of cysticercosis. The village is approximately 200-km far from Nanning, Capital City of Guangxi Zhuang Autonomous Region. In the village, we had observed many roaming pigs and previously found two pigs heavily infected with TsM out of 30 pigs in local slaughterhouses. Since the prevalence of cysticercosis might be highly related with domestic roaming pigs infected with TsM in local areas, survey on the seroprevalence in such area would be helpful to understand the actual relationship between the swine infection rate and human cysticercosis.
MATERIALS AND METHODS
Serum collection and history taking from the residents
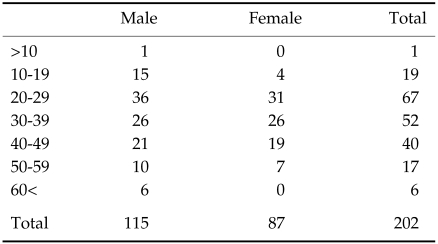
A total of 202 sera from residents in Nabo Village, Tiandong Province, Guangxi Zhuang Autonomous Region, China, were collected in June, 1999. The subjects were comprised of 115 male (56.9%) and 87 female (43.1%) (Table 1). All of serum samples collected were delivered to the Department of Molecular Parasitology, Sungkyunkwan University School of Medicine, Suwon, Korea at 4℃ and stored at -70℃ until used. The residents were concomitantly asked if they had a history of ingestion of pigs infected with TsM, and/or whether they suffered from headache and/or seizure, whether they experienced a presence of subcutaneous nodule(s) and finally whether they had a history of proglottid discharge. The study protocol was approved by the Institutional Review Board of Guangxi Center for Diseases Control, Chinese Academy of Sciences, China.
Table 1.
Age and sex distribution of persons subjected in this study
Collection of cyst fluid (CF) of Taenia solium metacestode (TsM)
TsM CF was collected from the naturally infected pigs at the village. Intact cysts were harvested from the measled pork and washed in physiologic saline more than 10 times. The cysts were individually punctured using aseptic needles. The collected CF was centrifuged at 20,000 g for 1 hr and the supernatant was used as CF antigen. All procedures were carried out at 4℃. The CF was stored at -70℃ until used.
Enzyme-linked immunosorbent assay (ELISA)
Antibody tests were carried out by micro-ELISA against three antigens of TsM CF, crude extracts of adult Paragonimus westermani and sparganum. Antigens were coated at 2.5 µg/ml of protein concentrations in carbonate-bicarbonate buffer (pH 9.6). The sera were diluted at 1:100 and reacted for 2 hr at 37℃. Peroxidase conjugated anti-human IgG was diluted at 1:1,000 and further reacted for 2 hr at 37℃. The reaction was developed with o-phenylene diamine chromogen (Sigma, St. Louis, MO, USA). Absorbance (abs) was read at 490 nm using micro-titer plate (M3550, Bio-Rad, Hercules, CA, USA). Cut-off abs was set at 0.18 for cysticercosis, 0.25 for paragonimiasis and 0.22 for sparganosis, respectively.
Immunoblotting
The CF proteins were separated by 4-20% SDS-PAGE in reducing conditions and were transferred to nitrocellulose membranes (Schleicher & Schuell, Germany). Membranes were cut into strips and incubated overnight with individual sera in a dilution of 1:200 in Tris-HCl (100 mM, pH 8.0) supplemented with 1% casein (Calbiochem, La Jolla, CA, USA). The strips were further incubated with peroxidase-conjugated anti-human IgG (heavy- and light-chain specific, Cappel, West Chester, PA, USA) for 3-4 hr at a dilution of 1:1,000. The reactions were then developed by 4-chloro-1-naphthol as a chromogen (4C1N, Sigma).
RESULTS
Age and sex distribution of the study subject
Table 1 summarized the distribution of 202 persons subjected in this study by age and sex. People aged between third and fifth decades comprised the major constituents whose proportion reached 33.2, 25.7 and 19.8%, respectively. The statistical difference between male and female was not observed.
History taking and physical examination of the residents at Nabo Village
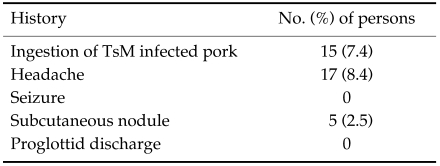
We investigated the possibility of cysticercosis by inquiring the residents whether there has been possible preponderance. As shown in Table 2, 15 people (7.4%) stated that they had a history of ingestion of pork infected with TsM. Seventeen people experienced the episode of intermittent headache while no person revealed a history of any kind of seizure. Subcutaneous nodules were recognized in five persons (2.5%), but these nodules were hardly differentiable from nodules caused by other diseases such as lipoma, sebaceous cyst, neurofibroma, fibromatosis and etc. No one had a history of proglottid discharge.
Table 2.
Results of history taking among 202 subjected persons
Serum levels of specific antibodies against antigens of TsM CF, and crude extracts of adult Paragonimus westermani and sparganum
We checked specific antibody levels in sera from 202 residents. Serum antibody levels against TsM CF were 0.09 ± 0.07; those against crude extracts of P. westermani and the sparganum were 0.04 ± 0.02 and 0.04 ± 0.03, respectively.
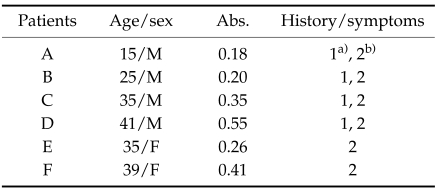
We further analyzed the specific IgG antibody levels against the TsM CF in the individual sera. Most of the study subjects showed the abs below 0.1 (161/202 cases, 79.7%). Thirty-six people showed the abs between 0.1 and 0.17 (17.8%). Only four male (3.5%) and two female (2.3%) exhibited higher levels of serum antibody titers over 0.18 of positive criteria. Most people showed specific antibody levels ranging from 0.06 to 0.08. No significant statistical difference was recognized by age and sex. Of 202 persons tested, four male persons whose age were 15, 25, 35 and 41 years old revealed abs at 0.18, 0.20, 0.35 and 0.55, respectively, and two females aged 35 and 39 showed the specific antibody titer of 0.26 and 0.41 in their sera (Table 3). Overall positive rate in these people was 2.97%. Table 3 summarized the results of history taking from persons who showed higher antibody levels in their sera than positive criteria. All of the male persons had an experience of eating pork infected with TsM. They were also complained of intermittent headache. Other two females agreed that they had a history of ingestion of pork infected with TsM. However, history of proglottid discharge was not noticed from all of these persons.
Table 3.
Results of history taking whose sera showed positive reaction against TsM CF antigen
a)Headache.
b)History of ingestion of pork infected with TsM.
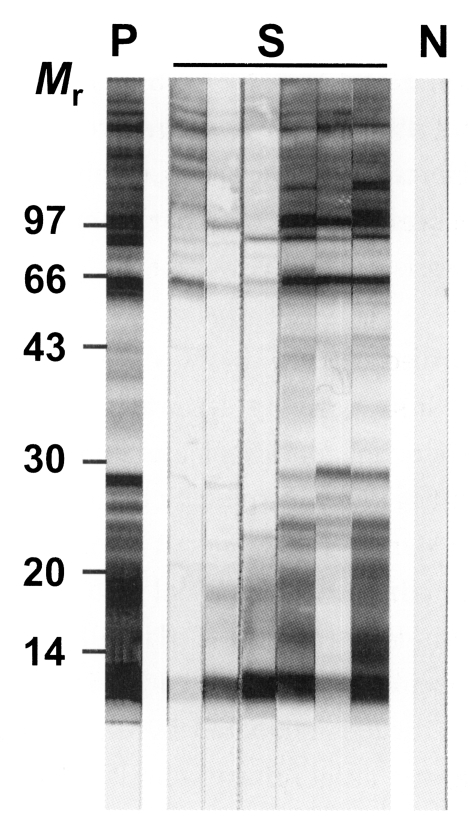
Fig. 1 demonstrated an immunoblot finding obtained from each person who showed high antibody titers by micro-ELISA. All of the sera examined showed positive antibody reactions to the multiple bands.
Fig. 1.
Immunoblot analysis performed with six serum samples, each from persons who showed high antibody titers by micro-ELISA. Multiple bands are reactive. P; positive control, S; samples tested, N; negative control, Mr; relative molecular mass in kDa.
DISCUSSION
Prevalence of cysticercosis and taeniasis may be related with gender, age, residential area as well as pork consumption and contact with the people who infected with the adult worm. In addition, higher levels of human infection are closely associated to porcine cysticercosis and inadequate sanitary infrastructure (Garcia-Noval et al., 1996). In the first instance, we had expected that quite a high infection rate might be observed in Nabo Village because swine cysticercosis was relatively high and the village was deficient of sanitary environment. However, the rate of seroprevalence was lower as 2.97% than previous studies, which were reported as 9.5% in Guangxi (Mai et al., 1999). It has been suggested that seropositive rates generally increased with age and were highest in person over seventh decades according to previous reports in China (Cao et al., 1996, 1997; Zhou, 1998). Therefore, the relatively low seropositive rate might be attributed to the fact that we are not able to test the people over the seventh decades due either to their reluctance or difficulties in the blood sampling.
The TsM infection invokes NC, which is one of the major causes of neurological diseases worldwide (White, 2000). Although the symptoms and signs of NC are highly protean, parenchymal NC is commonly presented with seizures and headaches. One remarkable sign of cysticercosis is subcutaneous nodule, which represented cysticerci in underlying muscles; on the while, these nodules represented highly non-specific clinical manifestations, which were readily caused by several space occupying lesions. We encountered five persons with subcutaneous nodules in this study, whereas specific antibody levels in their sera were below abs of 0.12. Therefore, we thought that these nodules might be resultant from other pathologic conditions. However, there were no neuroimaging medical facilities in the village and neuroimaging diagnosis of these serologically suspected cases or characterization of disease stage was not available. In this medical environment, ELISA may be a useful clue for detection of T. solium cysticercosis. Clinical and serological follow-up study for these persons may be important to observe their physical status.
The seroprevalence of cysticercosis suggested that there was a high risk of contact with pigs infected with TsM in Nabo Village. Four male persons have seropositive abs by ELISA, and were complained of intermittent headache. It is not clear whether these suspected cases had exposed to the T. solium eggs or pork infected with TsM. However, history taking revealed that all of these persons had been exposed to pork meat infected with the parasite. In addition, the present result showed that there is high possibility of cysticercosis in the village because cysticercosis is a common but often asymptomatic infection that affects both humans and pigs (Diaz et al., 1992).
In conclusion, this is the first seroepidemiological study of cysticercosis in the Nabo, Guangxi Zhuang Autonomous Region, China. Our findings suggest that cysticercosis is not highly but relatively endemic in this area, and community behavioural and environmental practices are attributable to continued transmission of this parasitic infection. To prevent further transmission of cysticercosis especially where the disease is endemic, the cultural traditions including human behaviour and sanitary environment should be carefully reorganized.
Footnotes
This work was supported by a 5-year NGO Program (1996-2000), Ministry of Foreign Affairs and Trade and also by a grant from the Korea Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (02-PJ1-PG3-20204-0006).
References
- 1.Bucardo F, Meza-Lucas A, Espinoza F, Garcia-Jeronimo RC, Garcia-Rodea R, Correa D. The seroprevalence of Taenia solium cysticercosis among epileptic patients in Leon, Nicaragua, as evaluated by ELISA and western blotting. Ann Trop Med Parasitol. 2005;99:41–45. doi: 10.1179/136485905X19856. [DOI] [PubMed] [Google Scholar]
- 2.Cao WC, van der Ploeg CP, Gao CL, Xu JF, Cao XC, Cui ZH, Ren ZX, Habbema JD. Seroprevalence and risk factors of human cysticercosis in a community of Shandong, China. Southeast Asian J Trop Med Public Health. 1996;27:279–285. [PubMed] [Google Scholar]
- 3.Cao WC, van der Ploeg CP, Xu J, Gao C, Ge L, Habbema JD. Risk factors for human cysticercosis morbidity: a population-based case-control study. Epidemiol Infect. 1997;119:231–235. doi: 10.1017/s0950268897007619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diaz F, Garcia HH, Gilman RH, Gonzales AE, Castro M, Tsang VC, Pilcher JB, Vasquez LE, Lescano M, Carcamo C. Epidemiology of taeniasis and cysticercosis in a Peruvian village. Am J Epidemiol. 1992;135:875–882. doi: 10.1093/oxfordjournals.aje.a116383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia HH, Del Brutto OH. Taenia solium cysticercosis. Infect Dis Clin North Am. 2000;14:97–119. doi: 10.1016/s0891-5520(05)70220-8. [DOI] [PubMed] [Google Scholar]
- 6.Garcia HH, Evans CA, Nash TE, Takayanagui OM, White AC, Jr, Botero D, Rajshekhar V, Tsang VC, Schantz PM, Allan JC, Flisser A, Correa D, Sarti E, Friedland JS, Martinez SM, Gonzalez AE, Gilman RH, Del Brutto OH. Current consensus guidelines for treatment of neurocysticercosis. Clin Microbiol Rev. 2002;15:747–756. doi: 10.1128/CMR.15.4.747-756.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia-Noval J, Allan JC, Fletes C, Moreno E, DeMata F, Torres-Alvarez R, Soto de Alfaro H, Yurrita P, Higueros-Morales H, Mencos F, Craig PS. Epidemiology of Taenia solium taeniasis and cysticercosis in two rural Guatemalan communities. Am J Trop Med Hyg. 1996;55:282–289. doi: 10.4269/ajtmh.1996.55.282. [DOI] [PubMed] [Google Scholar]
- 8.Maguire JH. Tapeworms and seizures-treatment and prevention. N Engl J Med. 2004;350:215–217. doi: 10.1056/NEJMp038204. [DOI] [PubMed] [Google Scholar]
- 9.Mai FZ, Xie ZY, Lu XG. The seroepidemiological survey of Taenia solium taeniasis/cysticercosis in the minority focusing region of Guangxi. Guangxi J Prev Med. 1999;5:121. [Google Scholar]
- 10.White AC., Jr Neurocysticercosis: Updates on epidemiology, pathogenesis, diagnosis, and management. Annu Rev Med. 2000;51:187–206. doi: 10.1146/annurev.med.51.1.187. [DOI] [PubMed] [Google Scholar]
- 11.Zhou KX. The epidemiology and prevention of Taenia solium taeniasis/cysticercosis in China. Chin J Prev Care Parasitic Dis. 1998;11:344–345. [Google Scholar]