Abstract
A 29 kDa cysteine protease of Taenia solium metacestodes was purified by Mono Q anion-exchanger and Superose 6 HR gel filtration chromatography. The enzyme was effectively inhibited by cysteine protease inhibitors, such as iodoacetic acid (IAA) and trans-epoxy-succinyl-L-leucyl-amido (4-guanidino) butane (E-64) while inhibitors acting on serine- or metallo-proteases did not affect the enzyme activity. The purified enzyme degraded human immunoglobulin G (IgG), collagen and bovine serum albumin (BSA), but human IgG was more susceptible for proteolysis by the enzyme. To define the precise biological roles of the enzyme, more detailed biochemical and functional studies would be required.
Keywords: Taenia solium, metacestode, cysteine protease, human IgG
Human neurocysticercosis is a neurological disease caused by invasion of the T. solium metacestode (TsM), a larval pork tapeworm, to the central nervous system. Human becomes infected with the parasite by ingestion of food contaminated with eggs of the parasite. The main clinical manifestations of the disease are headache, focal and generalized seizure, hydrocephalus and nonspecific neurological deficits according to the number and location of the parasite in the brain. The inflammatory responses of the host also played essential roles in several clinical manifestations (Carpio, 2002; Del Brutto, 2005).
Cysteine proteases of parasites play major roles in tissue penetration, nutrient uptake and immune evasion from their host (Tort et al., 1999; Sajid and McKerrow, 2002). The cysteine proteases of Paragonimus westermani metacercariae exert their crucial roles in metacercarial excystment (Chung et al., 1995), host IgG degradation (Chung et al., 1997a) as well as stimulation of human eosinophils to induce degranulation (Shin et al., 2005). A 43 kDa cysteine protease purified from T. crassiceps has been shown to degrade host IgG (White et al., 1997). In addition, cysteine protease secreted by T. solium metacestodes depleted CD4 lymphocytes in vitro and induced apoptosis in human CD4+ T-cells (Molinari et al., 2000; Tato et al., 2004). To define the precise biological roles of the enzymes, more detailed biochemical studies would be essential, but these efforts have been hampered by the lack of the purified enzymes. In the present study, we purified a 29 kDa cysteine protease from T. solium metacestodes and partially characterized its biochemical properties.
TsM were collected from the naturally infected pigs. Intact metacestodes were extensively washed with sterile saline and homogenized in a Teflon-pestle homogenizer with 20 mM Tris-HCl buffer (pH 7.2) followed by centrifuged at 15,000 rpm for 30 min. All procedure was done at 4℃. The resulting supernatant was used as crude extracts and stored at -70℃ until use.
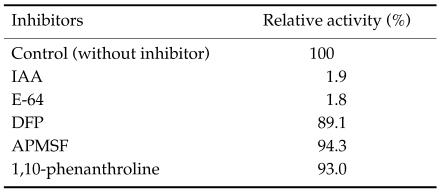
Cysteine protease activity was assayed using synthetic dipeptide substrate carbobenzoyl-phenylalanyl-arginyl-7-amino-4-methylcoumarin (Cbz-Phe-Arg-AMC) with 2 mM dithioreitol (DTT) (Chung et al., 1997b). The inhibitor test was done with respective protease inhibitors such as IAA (20 µM), E-64 (10 µM), di-isopropylfluorophosphate (DFP, 2 mM), 4-(amidinophenyl)methanesulphonyl fluoride (APMSF, 10 µM), 1,10-phenanthroline (2 mM). All chemicals were purchased from the Sigma (St. Louis, MO, USA).
For the purification of cysteine protease, the crude extract was loaded onto Mono Q HR 5/5 anion exchanger column previously equilibrated with 20 mM Tris-HCl buffer (pH 7.2). The column was washed with the same buffer and absorbed proteins were eluted with increasing of NaCl molarity up to 1 M performed by ÄKTA FPLC system (Amersham Pharmacia Biotech, Piscataway, NJ, USA). Fractions showing protease activities were pooled and loaded onto Superose 6 HR gel filtration column equilibrated with 20 mM sodium acetate buffer (pH 6.4). The column was eluted with the same buffer by flow rate of 0.2 ml/min. The fractions showing proteolytic activities were then analyzed by 7.5-15% SDS-PAGE.
For the in-gel activity staining of cysteine protease, gelatin (final 0.2%, v/v) was incorporated into the gel and SDS-PAGE was performed under non-reducing condition with or without cysteine protease inhibitor. After electrophoresis, the gels were transferred into 2.5% Triton X-100 and incubated in 0.1M sodium acetate buffer (pH 6) at 37℃ overnight. After incubation, the gels were stained with Coomassie brilliant blue.
To investigate the activity of the enzyme against macromolecular substrates, the purified enzyme was incubated with bovine serum albumin, human IgG and type I collagen. The reaction mixtures were incubated at 37℃ with increasing incubation times for 1, 3, 5 hr and overnight and then, the reaction products were analyzed by SDS-PAGE.
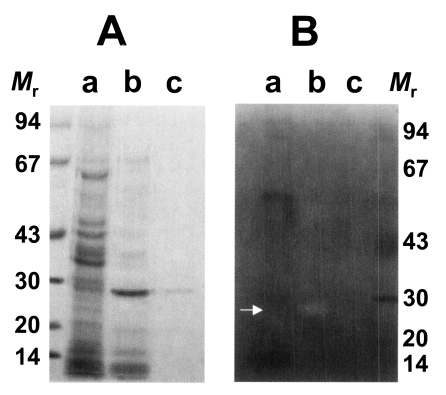
As shown in Fig. 1A, the purified enzyme was migrated at 29 kDa by reducing SDS-PAGE analysis. It had a moderate activity against synthetic peptide substrate, Cbz-Phe-Arg-AMC. In inhibitors study, the activity of the purified enzyme was inhibited by cysteine protease inhibitors such as E-64 and IAA, while serine- or metalloprotease inhibitors including DFP, APMSF and 1,10-phenanthroline did not affect the activity of the enzyme (Table 1). These results indicated that the purified enzyme belongs to the cysteine protease family and had a less enzymatic activity against Cbz-Phe-Arg-AMC than cysteine protease of other tissue invading parasites such as P. westermani and sparganum. In addition, the activity of the purified enzyme was decreased when the enzyme was stored at 4℃ (data not shown).
Fig. 1.
SDS-PAGE analysis of purified cysteine protease of Taenia solium metacestode (A) and gelatinolysis by the purified cysteine protease (B). The gel was analyzed on 7.5-15% gradient gel. Mr, standard marker proteins. Panel A, lanes a, crude extracts; b, partially purified cysteine protease through Mono Q column; and c, purified cysteine protease. Panel B, lanes a, crude extracts; b, purified cysteine protease without inhibitor; and c, purified cysteine protease with respective inhibitor, IAA. Proteolytic activity was shown on white area on gelatin gel (white arrow).
Table 1.
Relative activities of purified cysteine protease by various inhibitors
Substrate incorporating gel study showed that the 29 kDa cysteine protease could degrade gelatin in the acidic pH in the presence of DTT and the enzyme could not degrade gelatin in the presence of cysteine protease inhibitor, IAA (Fig. 1B). This result also demonstrates that purified 29 kDa protease was probed to be cysteine protease. Cysteine and metalloprotease activities from excretory-secretory products of the metacestodes have been detected in gelatin containing SDS-PAGE (Molinari et al., 2000). In our study, however, the purified 29 kDa cysteine protease was only assessed activity against gelatin containing SDS-PAGE. The detection and properties of other serine- or metallo-protease will be required on-going study.
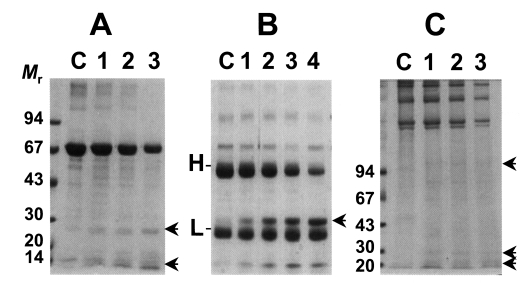
As shown in Fig. 2, the cysteine protease could degrade macromolecular substrates such as BSA and human IgG. BSA was slowly degraded by the enzyme with increasing incubation periods (Fig. 2A). On the other hands, the purified enzyme could degrade human IgG in 1 hr and heavy chain of human IgG could be cleaved mostly in overnight incubation (Fig. 2B). In reaction with collagen, the purified cysteine protease could also cleave collagen, however, the purified cysteine protease showed an ability of a little collagenolysis as in case of BSA (Fig. 2C). In these experiments, it is revealed that the cysteine protease exerts a little proteolytic activity against BSA, collagen while it selectively exerts relatively high ability of degradation against IgG. Taken together with cleavage ability of synthetic peptide substrate, it is presumed that the role of 29 kDa cysteine protease of T. solium metacestodes might be more involved in the evasion mechanism against host immune effector systems than penetration and nutrient uptake in its host and more precise experiments are required for the elucidation of physiological roles of cysteine protease in T. solium metacestodes.
Fig. 2.
Degradation of macromolecules by the purified cysteine protease. Mr, standard marker protein. Panel A, degradation of BSA. Lane C, control BSA; lanes 1-3, 1, 3, 5 hr incubation with purified cysteine protease, respectively. Arrows indicate degradation products. Panel B, cleavage of human IgG. Lane C, control IgG; lanes 1-4, 1, 3, 5 hr and overnight incubation with the enzyme, respectively. H & L denote heavy and light chain of IgG. Arrow indicates degradation product of IgG. Panel C, degradation of Type I collagen. Lane C, control collagen; lanes 1-3, 1, 3, 5 hr incubation with the enzyme, respectively. Arrows indicate degradation product of collagen.
ACKNOWLEDGMENTS
Authors are grateful to professor Yoon Kong, Sungkyunkwan University School of Medicine, for providing some materials and valuable comments.
Footnotes
This study was supported by the Korea Research Foundation Grant (KRF-2002-002-E00027).
References
- 1.Carpio A. Neurocysticercosis: an update. Lancet Infect Dis. 2002;2:751–762. doi: 10.1016/s1473-3099(02)00454-1. [DOI] [PubMed] [Google Scholar]
- 2.Del Brutto OH. Neurocysticercosis. Semin Neurol. 2005;25:243–251. doi: 10.1055/s-2005-917661. [DOI] [PubMed] [Google Scholar]
- 3.Chung YB, Kong Y, Joo IJ, Cho SY, Kang SY. Excystment of Paragonimus westermani metacercariae by endogenous cysteine protease. J Parasitol. 1995;81:137–142. [PubMed] [Google Scholar]
- 4.Chung YB, Yang HJ, Kang SY, Kong Y, Cho SY. Activities of different cysteine proteases of Paragonimus westermani in cleaving human IgG. Korean J Parasitol. 1997a;35:139–142. doi: 10.3347/kjp.1997.35.2.139. [DOI] [PubMed] [Google Scholar]
- 5.Chung YB, Kong Y, Yang HJ, Kang SY, Cho SY. Cysteine protease activities during maturation stages of Paragonimus westermani. J Parasitol. 1997b;83:902–907. [PubMed] [Google Scholar]
- 6.Molinari JL, Mejia H, White AC, Jr, Garrido E, Borgonio VM, Baig S, Tato P. Taenia solium: A cysteine protease secreted by metacestodes depletes human CD4 lymphocytes in vitro. Exp Parasitol. 2000;94:133–142. doi: 10.1006/expr.2000.4490. [DOI] [PubMed] [Google Scholar]
- 7.Sajid M, McKerrow JH. Cysteine proteases of parasitic organisms. Mol Biochem Parasitol. 2002;120:1–21. doi: 10.1016/s0166-6851(01)00438-8. [DOI] [PubMed] [Google Scholar]
- 8.Shin MH, Chung YB, Kita H. Degranulation of human eosinophils induced by Paragonimus westermani-secreted protease. Korean J Parasitol. 2005;43:33–37. doi: 10.3347/kjp.2005.43.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tato P, Fernandez AM, Solano S, Borgonio V, Garrido E, Supulveda J, Molinari JL. A cysteine protease from Taenia solium metacestodes induce apoptosis in human CD4+ T-cells. Parasitol Res. 2004;92:197–204. doi: 10.1007/s00436-003-1008-1. [DOI] [PubMed] [Google Scholar]
- 10.Tort J, Brindley PJ, Knox D, Wolfe KH, Dalton JP. Proteinases and associated genes of parasitic helminths. Adv Parasitol. 1999;43:161–266. doi: 10.1016/s0065-308x(08)60243-2. [DOI] [PubMed] [Google Scholar]
- 11.White AC, Jr, Baig S, Chappell CL. Characterization of a cysteine proteinase from Taenia crassiceps cysts. Mol Biochem Parasitol. 1997;85:243–253. doi: 10.1016/s0166-6851(96)02839-3. [DOI] [PubMed] [Google Scholar]