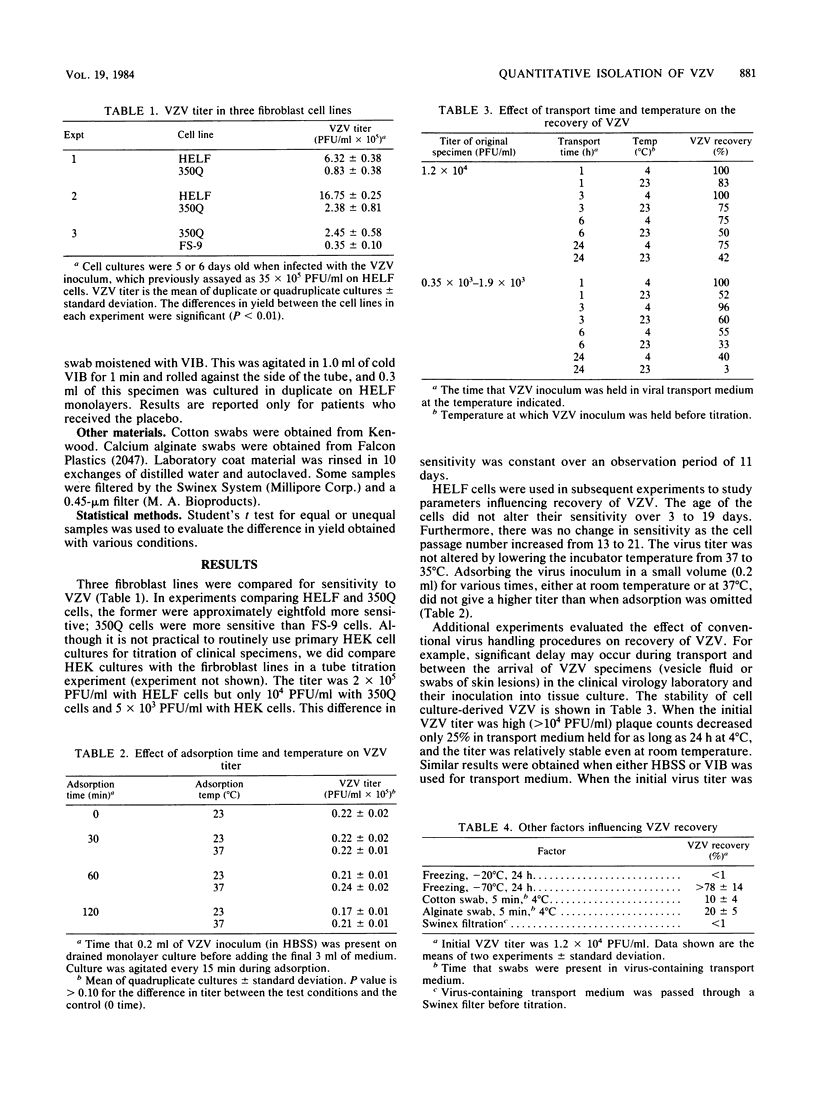
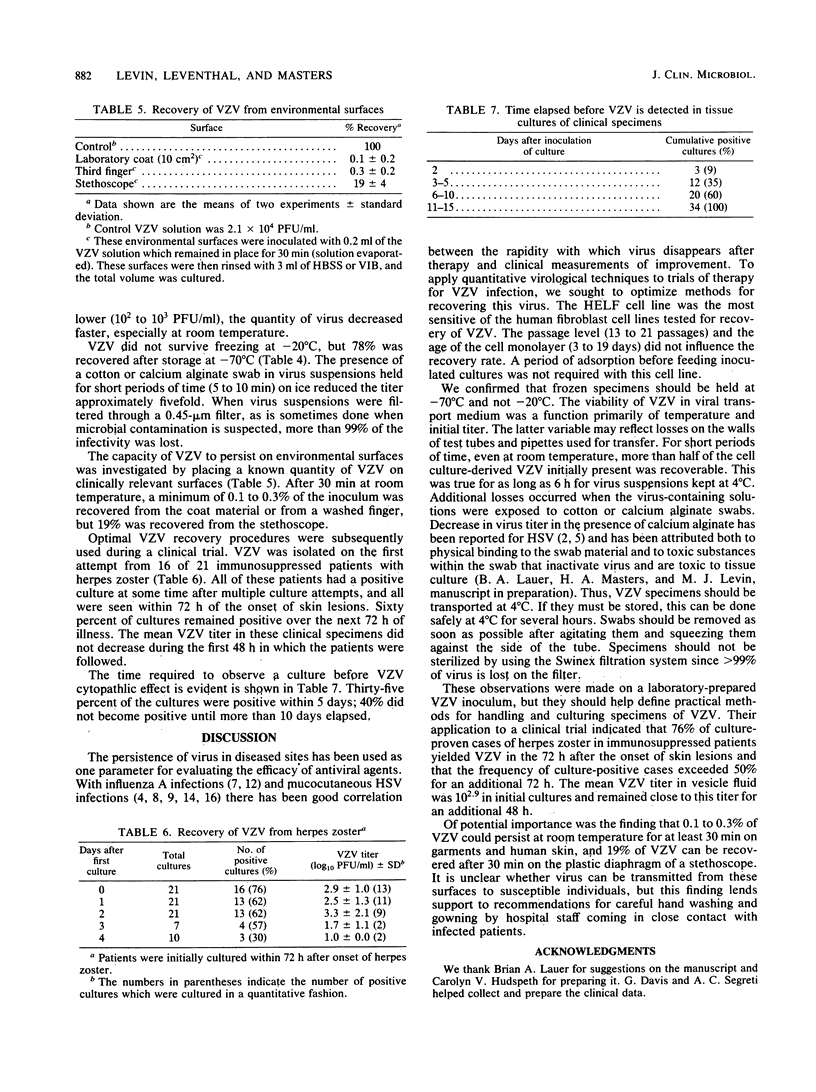
Abstract
Optimal conditions are described for the recovery of cell culture-derived varicella-zoster virus (VZV). Of the cells tested, human embryonic lung fibroblasts were the most sensitive. Storage and handling procedures were examined to determine the stability of VZV in viral transport medium. When the initial VZV titer was high (2 X 10(4) PFU/ml) 40% of the VZV survived room temperature for 24 h and 75% of the VZV remained viable for this long at 4 degrees C. Recovery was 5- to 10-fold less at lower initial VZV titers (less than 2 X 10(3) PFU/ml). Other factors which influenced VZV recovery included freezing at -20 degrees C, the use of cotton or calcium alginate swabs, and filtration to remove bacterial contaminants. The tissue culture methods described were used in a reconstruction experiment to demonstrate that VZV could be recovered from a laboratory coat or human skin (0.1 to 0.3% of input VZV) or from a stethoscope (19% of input VZV) as late as 30 min after inoculation. During a clinical trial using optimal VZV recovery procedures, 76% of the patients with herpes zoster yielded VZV when first cultured, and 60% remained culture positive for an additional 48 h.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bean B., Braun C., Balfour H. H., Jr Acyclovir therapy for acute herpes zoster. Lancet. 1982 Jul 17;2(8290):118–121. doi: 10.1016/s0140-6736(82)91090-x. [DOI] [PubMed] [Google Scholar]
- Bettoli E. J., Brewer P. M., Oxtoby M. J., Zaidi A. A., Guinan M. E. The role of temperature and swab materials in the recovery of herpes simplex virus from lesions. J Infect Dis. 1982 Mar;145(3):399–399. doi: 10.1093/infdis/145.3.399. [DOI] [PubMed] [Google Scholar]
- Bryson Y. J., Dillon M., Lovett M., Acuna G., Taylor S., Cherry J. D., Johnson B. L., Wiesmeier E., Growdon W., Creagh-Kirk T. Treatment of first episodes of genital herpes simplex virus infection with oral acyclovir. A randomized double-blind controlled trial in normal subjects. N Engl J Med. 1983 Apr 21;308(16):916–921. doi: 10.1056/NEJM198304213081602. [DOI] [PubMed] [Google Scholar]
- Corey L., Nahmias A. J., Guinan M. E., Benedetti J. K., Critchlow C. W., Holmes K. K. A trial of topical acyclovir in genital herpes simplex virus infections. N Engl J Med. 1982 Jun 3;306(22):1313–1319. doi: 10.1056/NEJM198206033062201. [DOI] [PubMed] [Google Scholar]
- Crane L. R., Gutterman P. A., Chapel T., Lerner A. M. Incubation of swab materials with herpes simplex virus. J Infect Dis. 1980 Apr;141(4):531–531. doi: 10.1093/infdis/141.4.531. [DOI] [PubMed] [Google Scholar]
- Knight V., Fedson D., Baldini J., Douglas R. G., Couch R. B. Amantadine therapy of epidemic influenza a(2) (Hong Kong). Infect Immun. 1970 Feb;1(2):200–204. doi: 10.1128/iai.1.2.200-204.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers J. D., Wade J. C., Mitchell C. D., Saral R., Lietman P. S., Durack D. T., Levin M. J., Segreti A. C., Balfour H. H., Jr Multicenter collaborative trial of intravenous acyclovir for treatment of mucocutaneous herpes simplex virus infection in the immunocompromised host. Am J Med. 1982 Jul 20;73(1A):229–235. doi: 10.1016/0002-9343(82)90097-3. [DOI] [PubMed] [Google Scholar]
- Mitchell C. D., Bean B., Gentry S. R., Groth K. E., Boen J. R., Balfour H. H., Jr Acyclovir therapy for mucocutaneous herpes simplex infections in immunocompromised patients. Lancet. 1981 Jun 27;1(8235):1389–1392. doi: 10.1016/s0140-6736(81)92569-1. [DOI] [PubMed] [Google Scholar]
- Nilsen A. E., Aasen T., Halsos A. M., Kinge B. R., Tjøtta E. A., Wikström K., Fiddian A. P. Efficacy of oral acyclovir in the treatment of initial and recurrent genital herpes. Lancet. 1982 Sep 11;2(8298):571–573. doi: 10.1016/s0140-6736(82)90658-4. [DOI] [PubMed] [Google Scholar]
- Prober C. G., Kirk L. E., Keeney R. E. Acyclovir therapy of chickenpox in immunosuppressed children--a collaborative study. J Pediatr. 1982 Oct;101(4):622–625. doi: 10.1016/s0022-3476(82)80725-7. [DOI] [PubMed] [Google Scholar]
- Togo Y., Hornick R. B., Felitti V. J., Kaufman M. L., Dawkins A. T., Jr, Kilpe V. E., Claghorn J. L. Evaluation of therapeutic efficacy of amantadine in patients with naturally occurring A2 influenza. JAMA. 1970 Feb 16;211(7):1149–1156. [PubMed] [Google Scholar]
- Turner R., Shehab Z., Osborne K., Hendley J. O. Shedding and survival of herpes simplex virus from 'fever blisters'. Pediatrics. 1982 Oct;70(4):547–549. [PubMed] [Google Scholar]
- Wade J. C., Newton B., McLaren C., Flournoy N., Keeney R. E., Meyers J. D. Intravenous acyclovir to treat mucocutaneous herpes simplex virus infection after marrow transplantation: a double-blind trial. Ann Intern Med. 1982 Mar;96(3):265–269. doi: 10.7326/0003-4819-96-3-265. [DOI] [PubMed] [Google Scholar]
- Whitley R. J., Soong S. J., Dolin R., Betts R., Linnemann C., Jr, Alford C. A., Jr Early vidarabine therapy to control the complications of herpes zoster in immunosuppressed patients. N Engl J Med. 1982 Oct 14;307(16):971–975. doi: 10.1056/NEJM198210143071602. [DOI] [PubMed] [Google Scholar]
- Whitley R., Barton N., Collins E., Whelchel J., Diethelm A. G. Mucocutaneous herpes simplex virus infections in immunocompromised patients. A model for evaluation of topical antiviral agents. Am J Med. 1982 Jul 20;73(1A):236–240. doi: 10.1016/0002-9343(82)90098-5. [DOI] [PubMed] [Google Scholar]
- Yeager A. S., Morris J. E., Prober C. G. Storage and transport of cultures for Herpes simplex virus, type 2. Am J Clin Pathol. 1979 Dec;72(6):977–979. doi: 10.1093/ajcp/72.6.977. [DOI] [PubMed] [Google Scholar]


