Abstract
We consider the effect of cross-linking a small fraction of lipids, either saturated or unsaturated, in a mixture of saturated and unsaturated lipids and cholesterol. The change in phase behavior is examined utilizing a recent phenomenological model of the ternary system, which is extended to include a fourth component representing the cross-linked lipids. These lipids are taken to be identical to monomeric ones except for their reduced entropy of mixing. We find that even a relatively small amount of cross-linked lipids, less than 5 mol %, is sufficient to significantly expand the range of compositions within which there is coexistence between liquid-ordered and liquid-disordered phases. Equivalently, the cross-linking of lipids increases the liquid-liquid miscibility transition temperature, and therefore could bring about phase separation at a temperature at which, before cross-linking, there was only a single liquid phase.
Introduction
Compositional inhomogeneities in the lipid content of the plasma membrane, known as lipid rafts, are thought to be involved in a number of signal transduction processes (1,2). Many of these processes have in common that they are initiated by the cross-linking of membrane components (3), which suggests that these cross-linking events influence the formation and composition of the lipid rafts themselves. Indeed, insofar as rafts have been shown to exist in the complex environments of plasma membranes, through detergent resistance assays (4), fluorescent resonance energy transfer (5), and other methods (6,7), the evidence indicates that the cross-linking of signaling proteins can affect their partitioning into rafts (8) or lead to the appearance of micron-scale domains (9,10) denoted “clustered rafts” (2) in the literature.
Properties of lipid rafts in membranes have been inferred from the study of liquid-liquid phase separation in model lipid bilayers. These systems, much simpler than the plasma membranes they are designed to mimic, better lend themselves to rigorous characterization in terms of phase diagrams (11–13) and partition coefficients (14). Just as cross-linking of membrane components was shown to affect raft formation in the complex plasma membrane environment, so too have experiments demonstrated that cross-linking can influence the liquid-liquid phase behavior (15,16) of model membranes.
Of great interest (17) is the observation that raft formation is promoted not only by the cross-linking of lipids usually associated with rafts, e.g., as in the case of monosialotetrahexosylganglioside (GM1) (15), but also by the cross-linking of lipids which are not raft-associated, such as phosphatidyl-inositol 4,5 biphosphate, PIP2 (16). One would like to understand why this should be so.
Several mechanisms by which aggregation or cross-linking of saturated, raft-associated lipids could induce raft formation have been proposed, and are discussed by Kusumi et al. (18). They address in particular the dynamics of raft formation. For example, a cluster could act as a nucleation center for the raft, or the cluster could prevent other saturated lipids from diffusing out of it. In contrast, in this article we propose a simple thermodynamic mechanism by which cross-linking increases the phase space in which raft formation occurs, namely the reduction in entropy of mixing which occurs as a consequence of cross-linking. We examine its effects by utilizing a simple phenomenological model, proposed recently (19), of ternary systems of saturated and unsaturated lipids and cholesterol. The model is extended to include a fourth component, cross-linked lipids. We first show that even a relatively small fraction of cross-linked saturated lipids can have a significant effect on the phase diagram, increasing the composition and temperature range over which liquid-liquid phase separation can occur. We then demonstrate that this increase in range over which raft formation should be found also occurs if it is the non-raft-associated unsaturated lipids that are cross-linked.
Model of Ternary Lipid Bilayers and Its Extension
We begin with the phenomenological model (19) of a ternary system of cholesterol, of concentration c, and saturated and unsaturated lipids of concentrations s and u, respectively. The saturated chains are characterized by an order parameter, δ, which is intended to encapsulate their conformational order. The form of the free energy per molecule, in units of kBT, is taken to be
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
The first term, , is the contribution to the free energy density of the entropy of mixing. The next term, , describes the interactions of the saturated chains with one another. Because the configurational order parameter is not controlled externally, the value it takes, δ(T, u, s), is that which minimizes the free energy, . The free energy per molecule of the system, f(T, u, s), is then . In the system consisting of saturated lipids only, the order parameter would take the value unity.
The third term, , describes the interaction of the saturated lipids with the other two components and contains the essence of the theory:
-
1.
The strength of the repulsion between saturated and unsaturated lipids, the interaction driving the phase separation, depends upon the configuration of the saturated chains.
-
2.
The configurational order of the saturated chains increases with the cholesterol concentration.
We extend this phenomenological model by adding a fourth component to the mixture, which represents a cluster of p cross-linked lipids. We first consider the case in which these lipids are saturated, or raft-associated. Let the variable z denote the mole fraction of individual saturated lipids belonging to cross-linked clusters of p saturated lipids. We assume that the cross-linked saturated lipids interact with their surroundings in exactly the same way as monomeric ones. In the free energy density of the four-component system, therefore, only the entropy of mixing will be changed, and the terms describing the behavior of the order parameter and the molecular interactions will not distinguish between the mole fractions of monomeric (s) and cross-linked (z) saturated lipids:
| (6) |
| (7) |
| (8) |
Note that the functions and are the same as defined above. The mixing entropy has an additional term for the cross-linked saturated lipids, whose translational entropy is reduced by a factor of p. This form is well known in the Flory-Huggins theory of polymers where it represents the reduction of translational entropy of p individual monomers that have been joined to form a polymer (20). Again, the order parameter takes the value δ(T, u, s, z), which minimizes the free energy and the Helmholtz free energy per molecule is given by
| (9) |
Note that we have taken our model free energy per molecule to be independent of the area per molecule, a, because we do not believe the phase behavior of the system depends crucially upon it; the area per molecule does not vary greatly from one phase to another. Further, its absence simplifies the description of the system. Our assumption of a free energy that is independent of the area per molecule can be viewed, equivalently, as restricting the system to a particular, constant, value of a (21).
Coexistence between two phases characterized by u1, s1, z1, and u2, s2, z2, respectively, is determined by the conditions that the three independent chemical potentials of the components are equal in each phase
| (10) |
| (11) |
| (12) |
and that the surface tension, γ (T, u, s, z), be equal in each phase,
| (13) |
The product of the surface tension and the constant area per molecule is simply a Legendre transform of the free energy per molecule,
| (14) |
Equations 10–13 determine four of the six quantities specifying the two phases. Thus, the coexistence region is spanned by two independent variables for a given temperature.
The resulting phase diagram involves three independent compositions, and must therefore be plotted in a three-dimensional space, such as the interior of a regular tetrahedron. It is more convenient for us to plot a relevant two-dimensional slice through the boundary of the two-phase region, as in Fig. 1. Compositions are plotted based on the mole fractions of unsaturated lipids, u, of cholesterol, c, and of all saturated lipids, s + z, irrespective of whether they are cross-linked or not. Because of the constraint u + c + s + z = 1, the phase diagram can be plotted in the usual equilateral triangle. As to the choice of coupling strengths in our free energy, Jss can be set arbitrarily to unity, but Jus, and Jcs are constrained such that there be no phase separation in the binary u, s lipid system and in the binary c, s system. Other than this, they and the parameters k1 and k2 of the free energy, are adjusted so that they give phase diagrams that resemble very well those of experiment.
Figure 1.
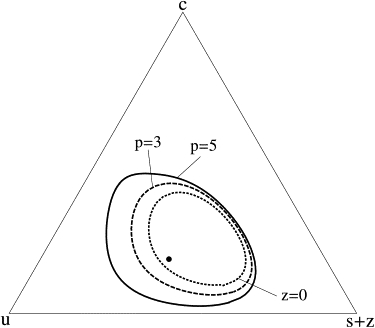
Effect of different saturated-lipid cross-linkers on the boundary of the liquid-liquid two-phase region. The temperature is above that of the main chain transition of the saturated lipid. The dotted line shows the boundary of the two-phase region for a system with no cross-linkers. The dashed and solid lines show the boundaries for concentrations z = 0.03 of cross-linkers with p = 3 and p = 5, respectively. Compositions are plotted in terms of mole fractions of unsaturated lipids (u), cholesterol (c), and total saturated lipids, including cross-linked lipids (s + z). The particular composition of s = 0.37, u = 0.45, and c = 0.18, noted in the text, is shown with a dot. In all cases, the parameters used in Eqs. 3 and 4 are Jss = 1.0, k1 = 1.0, Jus = 1.8, Jcs = 2.4, and k2 = 0.21.
The solid line in Fig. 1 shows, for p = 5, the boundary where the two-phase region intersects the plane z = 0.03. In other words, all points in the triangle shown represent compositions with 3 mol % of cross-linked saturated lipids belonging to clusters of five molecules each. The saturated-lipid-rich, liquid-ordered phase with z = 0.03 coexists with a saturated-lipid-poor, liquid-disordered phase that contains a much smaller concentration of cross-linked, saturated lipids. Similarly, the liquid-disordered phase with a concentration z = 0.03 of saturated lipids that have been cross-linked coexists, in general, with a liquid-ordered phase with a much larger concentration of cross-linked saturated lipids, one that we find can be as large as z = 0.29. For comparison with this phase boundary, that of the two-phase region without cross-linking, z = 0, is shown with a dotted line. The temperature is taken to be above that of the main chain transition of the saturated lipids.
Cross-linking saturated lipids expands the region of liquid-liquid phase coexistence. Thus, the cross-linking of some saturated lipids in a previously uniform bilayer can trigger liquid-liquid phase separation. Note that the expansion of the phase boundary is greater in the disordered liquid, which is rich in unsaturated lipids, than in the ordered liquid, which is rich in saturated ones. Presumably this is due to the fact that the concentration of saturated lipids is much less in the former than in the latter, so that the cross-linking of a certain fixed concentration of saturated lipid affects a larger fraction of these lipids in the disordered liquid phase than in the ordered one.
The effect of varying the number, p, of saturated lipids which are cross-linked into one cluster, is also shown in Fig. 1 where the phase boundary for the case p = 3 is shown by a dashed line. The concentration is z = 0.03, just as in the case p = 5, also shown there. Note, therefore, that the total number of saturated lipids cross-linked is the same in the two cases. For p = 5, however, the number of cross-linked clusters is only 60% of that in the system with smaller, p = 3 clusters. As the number of lipids in a given cluster, p, is increased, the region of phase space is made larger, in which cross-linking transforms what was a one-phase region into a region of two-phase coexistence.
Because the phase boundaries are significantly altered by cross-linking, it follows that there will be a change in the partition coefficients, Ki, of the components, which is simply the ratio of the concentration of the component in the liquid-ordered phase to that in the liquid-disordered phase. The size of this change depends, of course, upon the particular average concentration of the system within the coexistence region. For purposes of illustration, we have chosen concentrations s = 0.37, u = 0.45, and c = 0.18, a point shown in Fig. 1 by a dot. In the system with no cross-linkers, the concentration of saturated lipids in the two phases is 0.52 and 0.36, respectively, so that the partition coefficient is Ks = 1.44. After p = 5 cross-linking of 3 mol % of saturated lipids, the concentration, s + z, of all saturated lipids, cross-linked or not, is 0.63 and 0.31 so that the partition coefficient is now Ks+z = 2.03, an increase of ∼40%.
The effect of increasing the concentration of cross-linkers of a given kind is shown in Fig. 2, which plots the extent of stability of one-phase regions for the case of p = 5 cross-linkers with a concentration z = 0.03 (dashed line) as in Fig. 1, and a concentration of z = 0.05 (solid line). The effect of increasing the concentration of cross-linkers is again to increase the region of two-phase coexistence. Note that with this larger fraction of cross-linkers, the unsaturated lipids can undergo separation from the cross-linked saturated ones in the complete absence of cholesterol (the locus c = 0 in the triangle) or in the complete absence of unlinked, saturated lipids (the locus s + z = 0.05, i.e., s = 0.) This separation is completely analogous to that which occurs very commonly in binary polymer blends due to the small entropy of mixing of the polymers.
Figure 2.
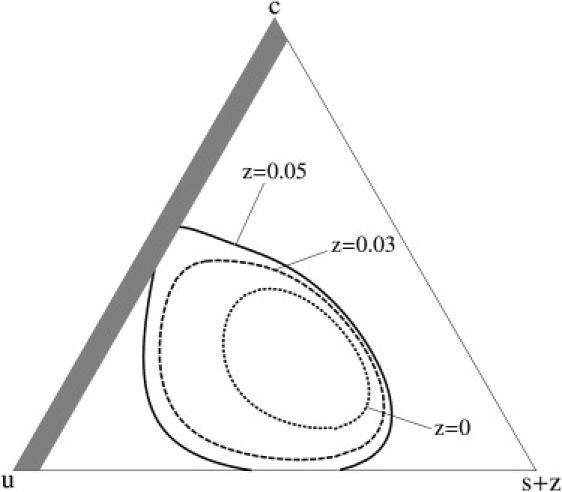
Effect of different compositions of the same saturated-lipid cross linker, p = 5, on the boundary of liquid-liquid two-phase coexistence. Again, the dotted line shows the phase boundary in the absence of any cross-linkers, while the dashed and solid lines show the boundaries for concentrations of z = 0.03 and z = 0.05. In the latter case, the concentration s + z cannot be less than 0.05, so that region is shown shaded. The parameters are the same as in Fig. 1.
Next, we consider a system below the temperature at which the pure saturated lipids undergo a transition to the gel phase. The extension of the free energy to this case follows that of Putzel and Schick (19), and will not be repeated here. In Fig. 3, the cross-linkers of p = 5 are present in a concentration of z = 0 (dashed line) and z = 0.03 (solid line), as in Fig. 1. One notes that cross-linking does not affect greatly the phase boundary of the gel phase, presumably because this phase is rich in saturated lipids, so that the cross-linking of a fixed small concentration of them causes only a small relative change in the number of unlinked, saturated, lipids.
Figure 3.
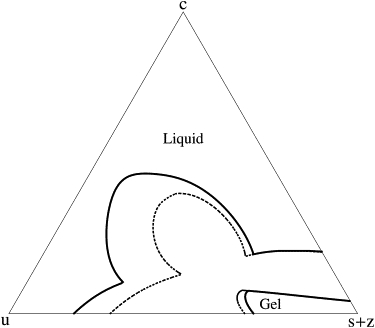
Effect of a saturated-lipid cross-linker with p = 5 on the phase boundaries of the system below the temperature of transition to the gel phase of the pure, saturated lipid system. Again, dotted lines show the phase boundaries without cross-linkers, while solid lines show the boundaries for a concentration z = 0.03 of cross-linkers. The values of the parameters are (see (19)) Jss = 1.0, k1 = 1.0, Jus = 1.8, Jcs = 2.4, k2 = 0.21, k3 = −0.25, J′us = 0.7, and J′cs = 0.0.
The case in which it is the unsaturated, non-raft-associating lipids, which are cross-linked, is easily dealt with. The free energy(see Eqs. 6–8) in this case is
| (15) |
| (16) |
| (17) |
The results for the case of simple dimerization, i.e., p = 2, are shown in Fig. 4 for a temperature above that of the main chain transition of the saturated lipid. The concentration of lipids which have been dimerized is z = 0.03 (solid line). Again, the case with no cross-linking is shown for comparison (dashed line). Note that it is now predominantly the region of large saturated lipid concentration where the increase in two-phase coexistence occurs. Again, this is presumably because the concentration of unsaturated lipids is smaller there, so that the effect of cross-linking a certain fraction of them is larger. We also note that the effect of cross-linking the unsaturated lipids is, if anything, even greater than the cross-linking of the saturated ones.
Figure 4.
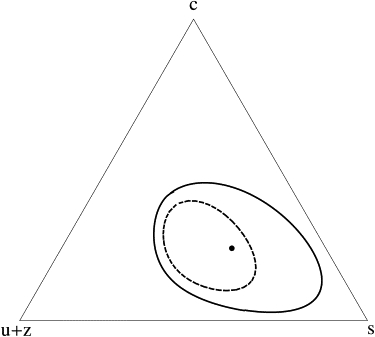
Effect of an unsaturated-lipid cross linker with p = 2 on the phase boundary of the system above the main-chain transition temperature. The dashed line shows the boundary of one-phase stability for no cross-linkers, while the solid line is for a concentration z = 0.03 of cross-linked unsaturated lipids. Compositions are plotted in terms of mole fractions of total unsaturated lipids, including cross-linked ones (u + z), cholesterol (c), and saturated lipids (s). The particular composition of s = 0.49, u = 0.27, and c = 0.24, noted in the text, is shown with a dot. Parameters of the system are the same as in Fig. 1.
An illustration of the effect on the partition coefficient of the unsaturated lipids is provided by a composition s = 0.49, u = 0.27, and c = 0.24, a point shown with a dot in Fig. 4. Before cross-linking, the concentrations of the unsaturated lipids in the liquid-ordered and -disordered phases are 0.21 and 0.47, respectively, so that their partition coefficient is Ku = 0.45. After p = 2 cross-linking of 3 mol % of unsaturated lipids, the total amount of unsaturated lipids, u + z, cross-linked or not, is 0.17 and 0.54 so that the partition coefficient is decreased to Ku+z = 0.31, a 30% reduction.
Discussion
We have proposed a simple thermodynamic mechanism, the reduction in entropy of mixing, by which cross-linking of lipids, either saturated or unsaturated, leads to an increase in the range of compositions and temperatures over which liquid-liquid coexistence occurs. The workings of the mechanism are easily understood, as is the reason that cross-linking of either raft- or non-raft-associated lipids is effective. Rafts are identified with one of the two phases that arise from the phase separation of saturated and unsaturated lipids. This separation is opposed by the entropy of mixing. Therefore any process, such as cross-linking, which decreases the entropy of mixing of either component enhances the tendency to phase-separate.
Our results are in accord with experiments on the cross-linking of raft-associated GM1 by cholera toxin (15) and on the cross-linking of non-raft-associated PIP2 (16). We found that the effect was strong even if the number of lipids cross-linked was only two, i.e., as in dimerization.
We note also that the mechanism is also effective when the lipids that are cross-linked are far apart, as might be expected to be the case when the linker is actin. That the mechanism does not depend upon proximity of the lipids cross-linked distinguishes it from other mechanisms which rely upon the effects of cross-linking on raft dynamics (18).
There is one other point to be made which relates to the efficacy of the mechanism when applied to non-raft-associated lipids, those which comprise the major part of the inner leaflet of the plasma membrane (22,23). Because of the coupling of the inner and outer leaves of the membrane, a membrane phase must be specified by the compositions of both leaves. Similarly, the difference between phases which coexist must be specified by, inter alia, composition differences in both leaves. Given the large population of unsaturated lipids in the inner leaf, it is conceivable that the difference in inner leaflet composition between “raft” and “sea” could be small even in the presence of a large difference in outer leaflet composition (21,24–26). Were this the case, such a raft would not be very useful as the small composition difference in inner leaflet would make it difficult for any chain which anchors there to distinguish one region from another. However, as we have shown, the cross-linking of unsaturated lipids tends to amplify the composition difference between phases, and therefore could turn a useless raft into a functional one.
Acknowledgments
We thank Sarah Keller and Michael Elbaum for fruitful conversations.
We thank the National Science Foundation for support under grant No. DMR-0803956.
References
- 1.Brown D.A., London E. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- 2.Simons K., Toomre D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000;1:31–41. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 3.Lemmon M.A. Membrane recognition by phospholipid-binding domains. Nature Rev. Mol. Cell Biol. 2008;9:99–111. doi: 10.1038/nrm2328. [DOI] [PubMed] [Google Scholar]
- 4.Hooper N. Detergent-insoluble glycosphingolipid/cholesterol-rich membrane domains, lipid rafts and caveolae. Mol. Membr. Biol. 1999;16:145–156. doi: 10.1080/096876899294607. [DOI] [PubMed] [Google Scholar]
- 5.Varma R., Mayor S. GPI-anchored proteins are organized in submicron domains at the cell surface. Nature. 1998;394:798–801. doi: 10.1038/29563. [DOI] [PubMed] [Google Scholar]
- 6.Schutz G., Kada G., Pastushenko V.P., Schindler H. Properties of lipid microdomains in a muscle cell membrane visualized by single molecule microscopy. EMBO J. 2000;19:892–901. doi: 10.1093/emboj/19.5.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pralle A., Keller P., Florin E.-L., Simons K., Hoerber J. Sphingolipid-cholesterol rafts diffuse as small entities in the plasma membrane of mammalian cells. J. Cell Biol. 2000;148:997–1007. doi: 10.1083/jcb.148.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holowka D., Gosse J.A., Hammond A.T., Han X., Sengupta P. Lipid segregation and IgE receptor signaling: a decade of progress. Biochim. Biophys. Acta. 2005;1746:252–259. doi: 10.1016/j.bbamcr.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Stauffer T.P., Meyer T. Compartmentalized IgE receptor-mediated signal transduction in living cells. J. Cell Biol. 1997;139:1447–1454. doi: 10.1083/jcb.139.6.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holowka D., Sheets E.D., Baird B. Interactions between FcɛRI and lipid raft components are regulated by the actin cytoskeleton. J. Cell Sci. 2000;113:1009–1019. doi: 10.1242/jcs.113.6.1009. [DOI] [PubMed] [Google Scholar]
- 11.Dietrich C., Bagatolli L., Volovyk Z.N., Thompson N., Jacobson K. Lipid rafts reconstituted in model membranes. Biophys. J. 2001;80:1417–1428. doi: 10.1016/S0006-3495(01)76114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veatch S.L., Keller S.L. Organization in lipid membranes containing cholesterol. Phys. Rev. Lett. 2002;89:268101. doi: 10.1103/PhysRevLett.89.268101. [DOI] [PubMed] [Google Scholar]
- 13.Veatch S.L., Keller S.L. Seeing spots: complex phase behavior in simple membranes. Biochim. Biophys. Acta. 2005;1746:172–185. doi: 10.1016/j.bbamcr.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 14.Wang T.Y., Leventis R., Silvius J.R. Fluorescence-based evaluation of the partitioning of lipids and lipidated peptides into liquid-ordered lipid microdomains: a model for molecular partitioning into “lipid rafts”. Biophys. J. 2000;79:919–933. doi: 10.1016/S0006-3495(00)76347-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammond A.T., Heberle F., Baumgart T., Holowka D., Baird B. Cross-linking a lipid raft component triggers liquid ordered-liquid disordered phase separation in model plasma membranes. Proc. Natl. Acad. Sci. USA. 2005;102:6320–6325. doi: 10.1073/pnas.0405654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu A.P., Fletcher D.A. Actin polymerization serves as a membrane domain switch in model lipid bilayers. Biophys. J. 2006;91:4064–4070. doi: 10.1529/biophysj.106.090852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edidin M. Switching sides: the actin/membrane lipid connection. Biophys. J. 2006;91:3963–3964. doi: 10.1529/biophysj.106.094078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kusumi A., Koyama-Honda I., Suzuki K. Molecular dynamics and interactions for creation of stimulation-induced stabilized rafts from small unstable steady-state rafts. Traffic. 2004;5:213–230. doi: 10.1111/j.1600-0854.2004.0178.x. [DOI] [PubMed] [Google Scholar]
- 19.Putzel G., Schick M. Phenomenological model and phase behavior of saturated and unsaturated lipids and cholesterol. Biophys. J. 2008;95:4756–4762. doi: 10.1529/biophysj.108.136317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Gennes P.-G. Cornell University Press; Ithaca, NY: 1979. Scaling Concepts in Polymer Physics, Ch. 4. [Google Scholar]
- 21.Putzel G., Schick M. Phase behavior of a model bilayer membrane with coupled leaves. Biophys. J. 2008;94:869–877. doi: 10.1529/biophysj.107.116251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rothman J.E., Lenard J. Membrane asymmetry. Science. 1977;195:743–753. doi: 10.1126/science.402030. [DOI] [PubMed] [Google Scholar]
- 23.Devaux P. Static and dynamic lipid asymmetry in cell membranes. Biochemistry. 1991;30:1163–1173. doi: 10.1021/bi00219a001. [DOI] [PubMed] [Google Scholar]
- 24.Collins M., Keller S. Tuning lipid mixtures to induce domains across leaflets of unsupported asymmetric bilayers. Proc. Natl. Acad. Sci. USA. 2007;105:124–128. doi: 10.1073/pnas.0702970105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collins M. Interleaflet coupling mechanisms in bilayers of lipids and cholesterol. Biophys. J. 2008;95:L32–L34. doi: 10.1529/biophysj.107.124362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagner A.J., Loew S., May S. Influence of monolayer-monolayer coupling on the phase behavior of a fluid lipid bilayer. Biophys. J. 2007;93:4268–4277. doi: 10.1529/biophysj.107.115675. [DOI] [PMC free article] [PubMed] [Google Scholar]


