Abstract
The existence of a small population of ‘cancer initiating cells (CICs)’ responsible for tumour maintenance has been firmly demonstrated in leukaemia. This concept is currently being tested in solid tumours. Leukaemia-initiating cells (LICs), particularly those which are in a quiescent state, are thought to be resistant to chemotherapy and targeted therapies resulting in disease relapse. Chronic myeloid leukaemia (CML) is a paradigmatic haematopietic stem cell (HSC) disease in which the LIC pool is not eradicated by current therapy, leading to disease relapse upon drug discontinuation. Here we define the critical role of the promyelocytic leukaemia protein (PML) tumour suppressor in HSC maintenance and present a new therapeutic approach for targeting quiescent LICs and possibly CICs by pharmacological inhibition of PML.
The existence of cancer initiating cells (CICs), a minor subpopulation of cells responsible for tumour initiation and maintenance, was proposed over 40 years ago1. In leukaemia in particular, increasing evidence suggests that out of the bulk of leukaemic cells, only a rare population of leukaemia initiating cells (LICs) propagate the disease2–7. LICs are rare and share many properties of normal haemopoietic stem cells (HSCs), such as self-renewal, pluripotency and quiescence4–6. A fundamental problem in treating leukaemia lies in the fact that LICs remain untouched by both conventional chemotherapy and even by targeted therapies7. The quiescent LIC subpopulation is thought to be particularly resistant to drugs that would normally target cells in active DNA replication7. Hence leukaemia relapse may occur because therapies eliminate proliferating cells that constitute the bulk of the tumour, but fail to eradicate quiescent LICs that can reinitiate malignancy after a period of latency. Therefore, development of novel therapeutic approaches targeting CICs and LICs may have a profound impact on cancer eradication.
CML is one of the most extensively investigated and paradigmatic stem cell disorders7. It is characterized by the presence of the Philadelphia chromosome (Ph+), which results from a chromosomal translocation between the BCR gene on chromosome 22 and the ABL gene on chromosome 98,9. This translocation generates the fusion protein BCR-ABL that displays constitutive kinase activity10. The tyrosine kinase inhibitor, imatinib, remarkably improves the prognosis of CML patients11,12. However, imatinib preferentially targets dividing cells, while non-dividing leukaemic cells are resistant to imatinib-mediated apoptosis6. Surviving leukaemia stem and progenitor cells are a potential source for relapse. This is demonstrated by the fact that if therapy is discontinued, the disease inevitably relapses in the vast majority of cases including those showing good responses without signs of disease progression13–18.
The PML gene, which is involved in the t(15;17) chromosomal translocation of acute promyelocytic leukaemia (APL), encodes a protein localizing to PML nuclear bodies (PML-NBs), a subnuclear macromolecular structure19. PML functions as a tumour suppressor that controls fundamental processes such as apoptosis, cellular proliferation and senescence20,21. Recent data demonstrated that PML is involved in neoangiogenesis and acts as a negative regulator of mTOR22. However, its role in stem cells biology has not been investigated. Here, we studied the role of PML in HSCs and LICs biology and obtained unexpected data that have implications for the eradication of LICs and CICs in human cancer.
PML expression is high in HSC and CML blasts and loss of PML predicts favorable outcome in CML
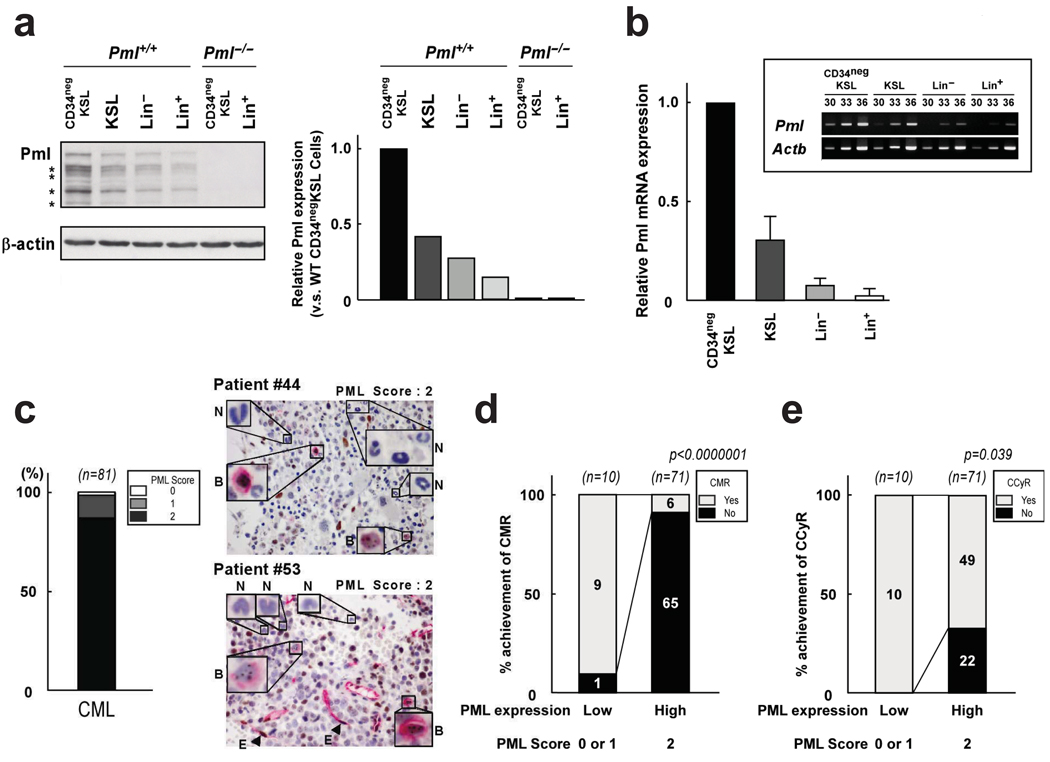
To understand whether PML expression is modulated during haematopoiesis, we analyzed Pml protein levels in various haematopoietic cell lineages in the mouse. To detect Pml levels in rare HSCs, we sorted different cell lineages directly into a sample buffer. Western blot analysis showed that Pml is highly expressed in the HSC compartment (Fig. 1a). Immunofluorescence analysis also showed increased numbers of PML-NBs in HSCs compared to committed cells (Supplementary Fig. 1a). Pml mRNA levels were also higher in the HSC population, indicating that Pml expression during haematopoiesis is regulated at the transcriptional level (Fig. 1b). High PML expression in the HSC compartment was also observed in primary human bone marrow samples (Supplementary Fig. 1b, c).
Figure 1.
PML is highly expressed in HSCs and CML. a, Fractionated mouse haematopoietic cells were flow-sorted into protein sample buffer and immunoblotted with anti-Pml antibody. Representative blots are shown in the left panel and relative Pml protein level normalized to β-actin are shown in the right panel. Asterisks indicate Pml isoforms. b, Levels of Pml and Actb transcripts were measured by q-RT-PCR in haematopoietic cells. Bar graph represents normalized expression of Pml mRNA. Experiments were performed twice and a representative result is presented. High Pml expression was also confirmed by PCR (inset). c, Bone marrow samples of CML patients in chronic phase (n = 81) were stained with anti-PML antibody (brown) and anti-CD34 (red). The Left graph shows the percentage of PML-positive samples and representative cases are on the right (arrowheads indicate endothelial cells (E) as a positive control). Insets show PML-staining in blasts (B) and differentiated neutrophils (N). d, e, Higher CMR (d) and CCyR (e) were observed in chronic phase CML patients with low PML expression. P-value was generated by a chi-square test. Absolute numbers are also indicated.
We next evaluated PML expression in samples of patients with haematopoietic malignancies. Loss of PML is frequently observed in human cancers such as prostate and lung cancer23,24. However, to our surprise most CML chronic phase (CP) samples expressed high levels of PML (Fig. 1c). Moreover, PML expression was barely detected in differentiated neutrophils whereas abundant PML expression was seen in blasts expressing CD34 (Fig. 1c and Supplementary Fig. 1d). An unexpected association was found between PML positivity and clinical outcome: CML patients with low PML expression displayed higher complete molecular response (CMR) and complete cytogenetic response (CCyR) compared with patients with high PML expression (Fig. 1d, e). Furthermore, low PML expression was strikingly predictive of better overall survival in CML (Supplementary Fig. 2). These results indicate that in CML, low PML expression predicts better clinical outcome contrary to what was observed in prostate cancer and other solid tumours23,24. These results prompted us to analyze the role of PML in haematopoiesis.
PML is required for HSC maintenance
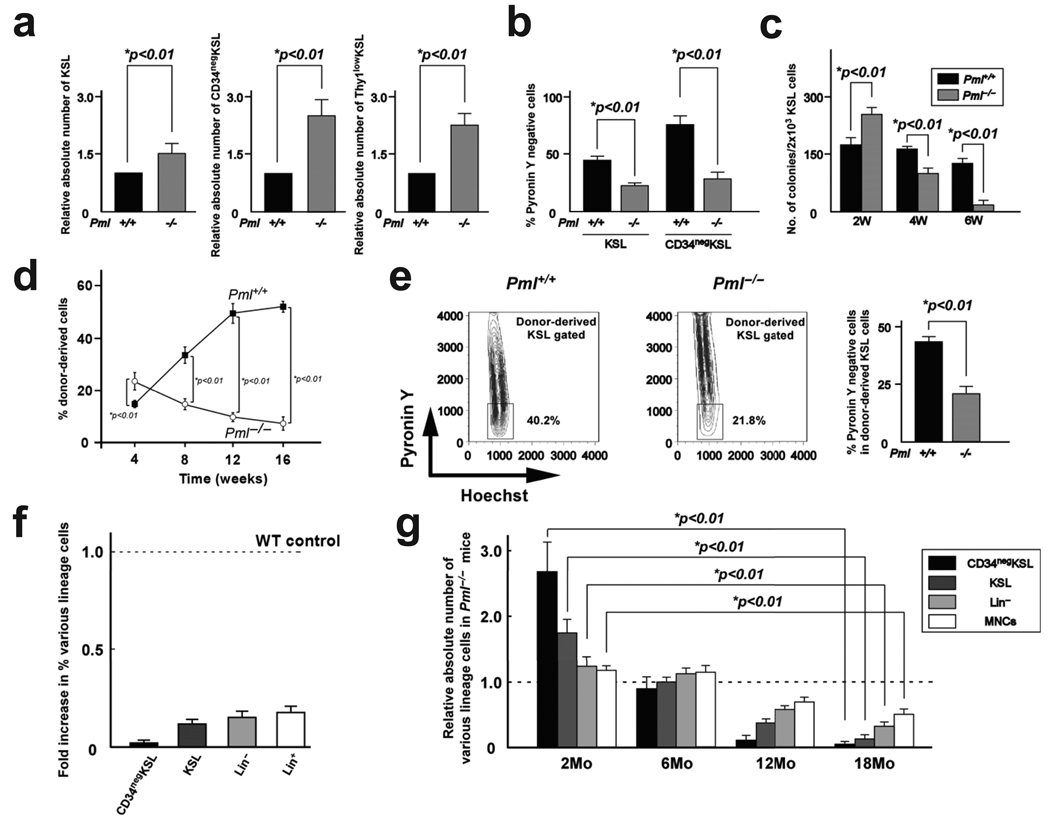
Genetic loss of Pml did not induce significant changes in the number of haematopoietic cells in peripheral blood (data not shown) and in the quantity or quality of progenitors in the bone marrow in 8-week-old mice (Supplementary Fig. 3a–d). However, an increased number of cells in the c-Kit+Sca-1+Lin− (KSL) stem cell compartment was found in 8-week-old Pml−/− mice (Fig. 2a). In particular, the number of long-term (LT) repopulating HSCs measured as CD34neg and Thy1low KSL cells was significantly higher in 8-week-old Pml-deficient mice (Fig. 2a). The proportion of cells in G0 among KSL and CD34neg KSL cells, as evaluated by Pyronin Y staining25, was markedly lower in Pml−/− mice than in wild type (WT) mice (Fig. 2b), indicating that Pml−/− HSCs are not quiescent. Consistent with these data, the number of colony-forming cells from Pml−/− KSL cells was higher than WT KSL cells after short-term culture on stromal cells (less than 2 weeks). However, the number of colonies from Pml−/− KSL cells decreased significantly after 6 weeks of culture (Fig. 2c). These results suggest that increased cycling of Pml−/− HSCs results in their exhaustion. To assess the repopulating ability of Pml−/− HSCs in vivo, we performed a competitive reconstitution assay. Flow cytometric analysis revealed that Pml−/− KSL cells contributed to haematopoietic reconstitution more than competitor cells 4 weeks after transplantation (Fig. 2d). However, the percentage of Pml−/− KSL cells significantly decreased 16 weeks after transplantation (Fig. 2d). These results indicate that Pml acts to maintain HSCs and that Pml−/− HSCs lack long-term repopulating capacity. This defect affected both myeloid as well as B and T lineages (Supplementary Fig. 4a). Cell cycle analysis of recipient BM revealed that more HSCs from Pml−/− donors were cycling than those from WT donors (Fig. 2e), indicating that Pml−/− HSCs are not quiescent in the BM of recipient mice. Analysis of chimerism revealed that all haematopoietic lineages from Pml−/− donors were affected but the greatest reductions were seen in the HSC compartment (Fig. 2f). In addition, the contribution of Pml−/− HSCs to more committed cells was more significantly impaired at later time points after transplantation (Supplementary Fig. 4b).
Figure 2.
PML is essential for HSC maintenance. a, Relative numbers ± s.d. of KSL (left), CD34negKSL (middle) and Thy1lowKSL (right) cells in Pml−/− BM and Pml+/+ BM at 8 weeks (n = 3). b, Pyronin Y negative cells in KSL cells and CD34negKSL cells of Pml+/+ or Pml−/− mice (n = 3). c, Colony forming ability of WT and Pml−/− KSL cells after long-term culture (n= 3). d, Reconstitution of WT and Pml−/− bone marrow cells after competitive transplantation assay. e, Frequency of WT and Pml−/− quiescent cells in recipient mice. Right: representative flow cytometry data. Left: mean percentages ± s.d. of Pyronin Y negative cells in donor-derived KSL population. f, Relative percentage ± s.d. of donor-derived cells in the bone marrows of recipient mice 4 months after transplantation (n = 3). g, Relative numbers ± s.d. of fractionated haematopoietic cells in Pml−/− mice at the indicated ages normalized over WT mice (n = 3).
The impact of Pml deficiency on long-term repopulation was determined carrying out a second competitive bone marrow transplantation (BMT). Pml−/− donor-derived cells could not reconstitute the bone marrow of recipient mice in second BMT (Supplementary Fig. 4c). Consistent with these data, defects in progenitor function were observed in Pml−/− donor-derived cells after BMT (Supplementary Fig. 4d).
To assess HSC function under normal homeostatic conditions, we examined the effect of Pml deficiency on haematopoiesis in older mice. Older Pml−/− mice exhibited a progressive decrease in cellularity, with a mean ratio of BM mononuclear cells (MNCs) compared to WT mice of 0.67±0.07 and 0.50±0.06 at 12 and 18 months. Additionally, in contrast to the increased number of KSL cells seen at 2 months of age, a significant reduction of HSCs was evident in Pml−/− BM at 18 months (Fig. 2g), accompanied by marked progenitor dysfunction (Supplementary Fig. 4e). Finally, repopulating cells from 18-month-old Pml−/− BM were not detected in recipient mice even 4 weeks after transplantation (Supplementary Fig. 4f). Thus, our data indicate that chronic Pml deficiency in vivo results in progressive impairment of HSC function due to defective maintenance of quiescence.
PML is indispensable for LIC maintenance
LICs have notable mechanistic similarities to normal stem cells2,4,26. Therefore, since high PML expression was seen in CML blasts (Fig. 1c), we investigated the function of PML in CML LICs,.
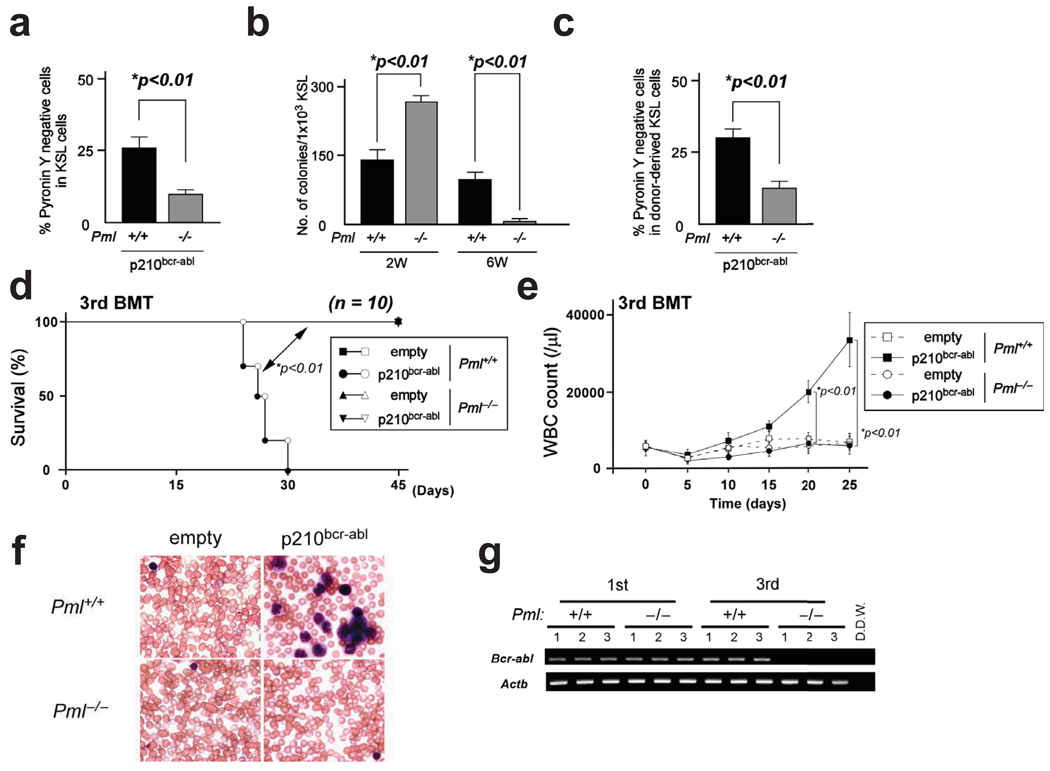
Pml+/+ and Pml−/− BM cells were transduced with p210bcr-abl, and then cultured on stromal cells to enrich LICs. Pyronin Y staining of KSL cells revealed a significant reduction in the number of quiescent cells in Pml-deficient cells compared to WT cells (Fig. 3a). Consistent with these data, Pml null LICs showed increased colony forming capacity after short-term culture but remarkable reduction in colony number after long-term culture on stromal cells when compared with WT LICs (Fig. 3b). To investigate the function of Pml in LICs in vivo, we serially transplanted BM cells transduced with p210bcr-abl to recipient mice every 2 weeks. Retroviral transduction of p210bcr-abl results in transformation of bone marrow cells, resulting in CML-like disease27. In the first BMT, Pml−/− LICs promoted earlier CML-like disease in recipient mice than WT LICs (Supplementary Fig. 5a, b). When cell cycle status was investigated, significantly fewer Pml deficient than WT LICs appeared in G0 in recipient mice (Fig. 3c).
Figure 3.
PML is essential for LIC maintenance. a, Pml+/+ or Pml−/− BM cells transduced with p210bcr-abl were co-cultured with stromal cells for two weeks. Data shown are mean percentages ± s.d. of Pyronin Y negative cells in KSL cells. b, Colony formation after long-term culture of p210bcr-abl-transduced BM cells (n = 3). c, Cell cycle status of donor-derived KSL cells transduced with p210bcr-abl 2 weeks after BMT. d, e, Survival of recipient mice receiving transduced BM cells from Pml+/+ or Pml−/− mice in 3rd round BMT (d). Log rank statistical analysis was performed to obtain p. WBC counts at indicated times after BMT are shown (e). f, Smears of PB in 3rd round BMT stained with Wright-Giemsa. g, MRD in 3rd BMT recipient mice with WT or Pml−/− bone marrow cells overexpressing p210bcr-abl was analyzed by nested PCR in 3 randomly selected recipient mice.
In the second BMT, no significant difference in survival was observed (Supplementary Fig. 5c). In the third serial BMT, however, Pml−/− LICs failed to generate CML-like disease, contrary to WT LICs (Fig. 3d–f and Supplementary Fig. 5d). In addition, minimal residual disease (MRD) was not detected in recipient mice transplanted with Pml−/− LICs (Fig. 3g). Remarkably, WT LICs retained the potential to develop CML-like disease even in the fourth serial BMT (Supplementary Fig. 5e). These results indicate that Pml-deficient LICs undergo intensive cell cycling, resulting in impairment of LIC maintenance.
As2O3 reversibly decreases PML expression in HSCs
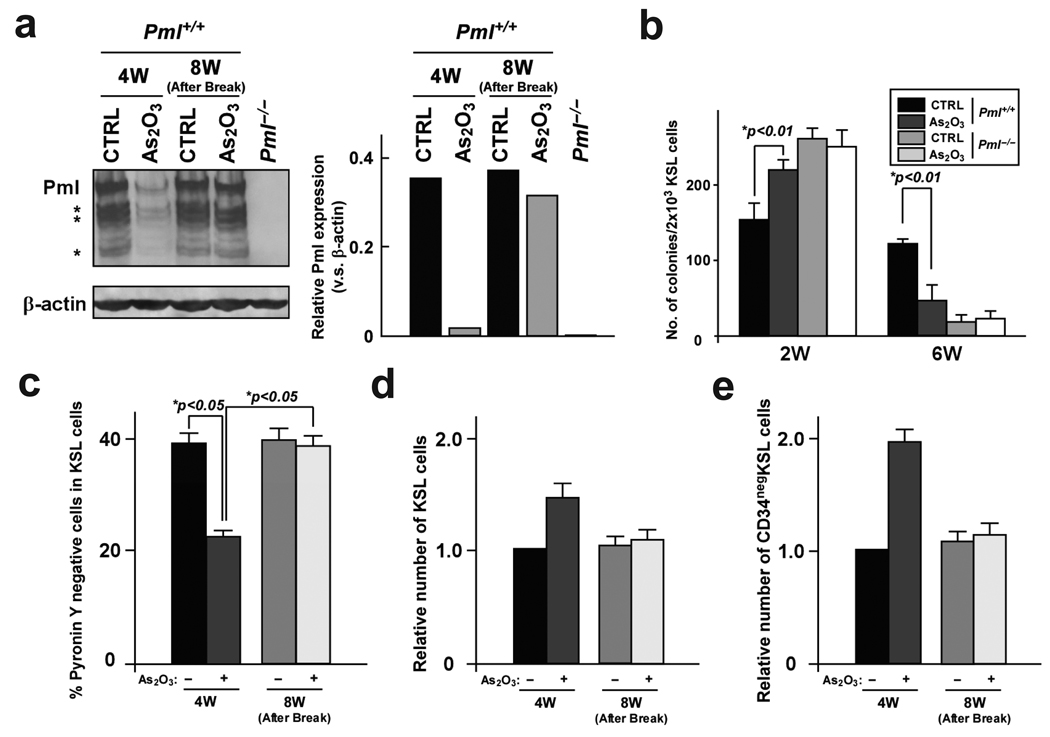
The inorganic arsenite arsenic trioxide (As2O3) has been used as a therapeutic agent for centuries28. From the 1700s through the early 1900s, arsenicals were a mainstay in the treatment of leukaemia29. The dramatic ability of arsenic to cure APL was reported in the mid-1990’s30–32. Arsenic has been shown to target PML for degradation33. Indeed, when we analyzed the effect of As2O3 treatment on HSCs, we found that it reversibly decreased Pml expression in the HSC compartment in vitro (Fig. 4a). Reduction of Pml expression by As2O3 dramatically attenuated colony-formation ability of WT KSL cells compared to control cells after 6 weeks on stromal cells, whereas an increase in colony formation was observed after short-term culture (Fig. 4b). Importantly, As2O3 treatment did not affect colony formation from Pml−/− HSCs (Fig. 4b), indicating that this effect is mostly Pml-dependent. In vivo treatment with As2O3 also reversibly reduced Pml expression (Supplementary Fig. 6), and resulted in impaired HSC quiescence and increase in the number of KSL cells (Fig. 4c, d). Furthermore, the number of LT repopulating HSCs was significantly more elevated after As2O3 treatment (Fig. 4e).
Figure 4.
Reduction of PML by As2O3 treatment abrogates maintenance of HSC quiescence. a, KSL cells from 8-week-old mice were sorted and co-cultured with stromal cells and As2O3 for 4 weeks (4W). As2O3 treatment was discontinued and co-culture continued for 4 weeks without treatment (8W; After Break). Proteins from sorted KSL cells were analyzed by Western blot (left). Normalized Pml protein levels v.s. β-actin is shown on the right. b, Pml+/+ and Pml−/− KSL cells were cultured on stromal cells with As2O3 for the indicated weeks (W) and tested for colony formation (n = 3). c–e, As2O3 reversibly inhibits quiescence of normal HSCs in vivo. Mice were treated with As2O3 from 8- to 12-weeks (4W) and left untreated from week 12 to 16 (8W; After Break). Cell cycle status of HSCs was analyzed by Pyronin Y staining (c). Mean numbers ± s.d. of KSL (d) and CD34neg KSL cells (e) are also shown.
Rapamycin rescues the phenotype of Pml-deficient HSCs and LICs
Recent data has demonstrated that PML acts as a repressor of neoangiogenesis by repressing mTOR activity in conditions of hypoxia22. Since mTOR plays an essential role in HSC maintenance as well as leukaemogenesis34,35, we examined mTOR activity in Pml−/− HSCs. Increased activity of mTOR was observed in Pml-deficient compared to WT HSCs (Supplementary Fig. 7a). In vitro treatment with the mTOR inhibitor rapamycin substantially restored colony-forming capacity in long-term cultures of Pml−/− HSCs, while it did not affect WT HSCs (Supplementary Fig. 7b). In vivo administration of rapamycin increased the quiescence of Pml−/− HSCs (Supplementary Fig. 7c), and resulted in decreased numbers of Pml−/− LT HSCs (Supplementary Fig. 7d). Moreover rapamycin treatment dramatically restored the capacity of Pml-deficient HSCs to provide long-term BM reconstitution to irradiated mice (Supplementary Fig. 7e, f). These results indicate that Pml plays an important role in maintenance of HSCs by repressing mTOR activity.
We next examined the effect of rapamycin on Pml-deficient LICs. Administration of rapamycin significantly prevented the exhaustion of Pml−/− LICs, leading to restored colony-forming capacity in long-term culture (Supplementary Fig. 7g). In addition, Pml−/− LICs treated with rapamycin gave rise to CML-like disease in the third serial BMT, although in the first BMT disease onset was delayed (Supplementary Fig. 7 h–j). Notably, rapamycin also accelerated CML-like disease by WT LICs in the third serial BMT (Supplementary Fig. 7i). In summary, these results suggest that Pml acts as a repressor of mTOR activity in LICs and mTOR super-activation impairs LIC-maintenance.
As2O3-dependent down-regulation of PML is an effective approach for LICs eradication
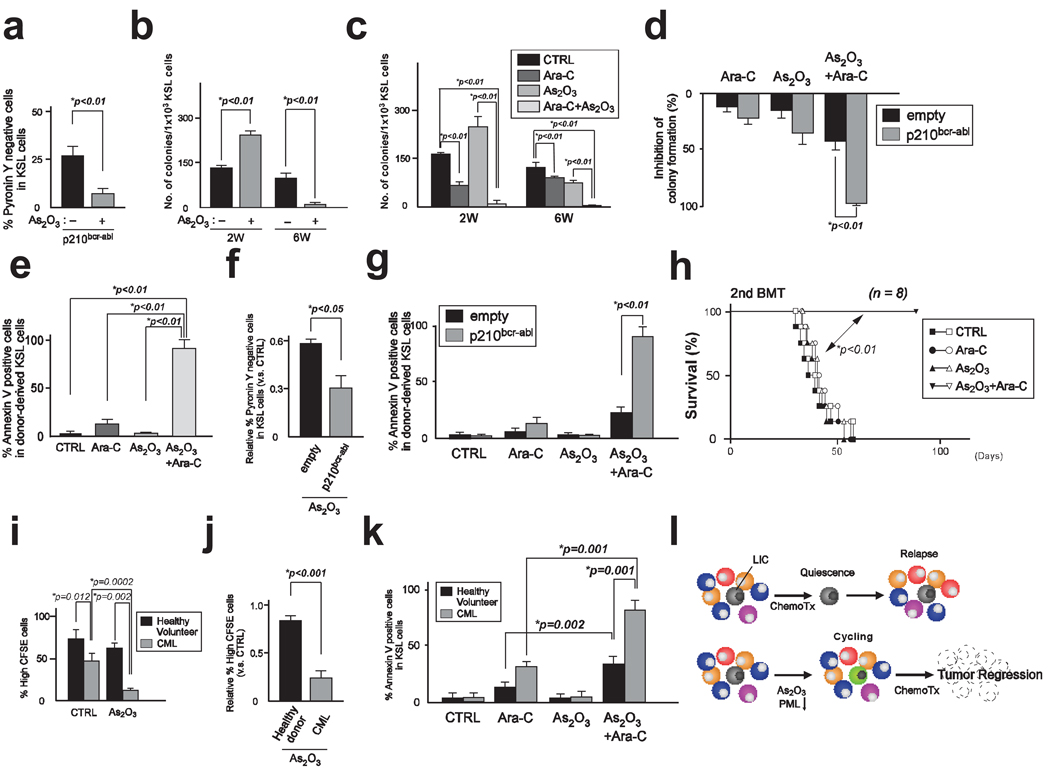
Interventions that enhance cycling of quiescent, chemotherapy-insensitive LICs are expected to facilitate their elimination. Therefore we investigated the therapeutic effect of As2O3-mediated PML reduction in LICs. As2O3 treatment significantly decreased the number of quiescent LICs without inducing apoptosis (Fig. 5a and Supplementary Fig. 8). Consistently, long-term culture-initiating cell assays revealed a remarkable inhibitory effect of As2O3 on LIC maintenance (Fig. 5b).
Figure 5.
Combination therapy with Ara-C and As2O3 eliminates LICs. a, Cell cycle analysis of BM cells transduced with p210bcr-abl co-cultured with stromal cells and As2O3 for two weeks. b, Colony formation of transduced KSL cells after long-term culture with As2O3 (n = 3). c, Transduced KSL cells co-cultured with stromal cells were treated with As2O3 for 9 days and with As2O3 and Ara-C for 5 days (2W). Treatment was discontinued and co-culture continued for 4 weeks (6W). Results are mean colony numbers ± s.d. (n = 3). d, Colony formation of As2O3 and Ara-C-treated compared to untreated BM cells infected by empty vector or p210bcr-abl in vitro (n = 3). e, Apoptosis in donor-derived transduced KSL cells in recipient mice treated with As2O3 and Ara-C (n = 3). f, Quiescence of KSL cells transduced with empty vector or p210bcr-abl after As2O3 treatment in vivo. g, Annexin V staining of HSC or LIC in recipient mice treated with As2O3 and Ara-C (n = 3). h, Survival of recipient mice transplanted with transduced BM cells at the second round of BMT. p was obtained by log rank statistical analysis. i, Percentage of non-dividing cells in Lin−CD34+CD38− cells from patients with CML and healthy volunteers cultured for 3 days (n = 3). j, Relative percentages of non-dividing cells in cells treated with As2O3 versus untreated cells. k, Apoptosis in Lin−CD34+CD38− cells co-cultured with As2O3 and Ara-C (n = 4). l, A model for As2O3-induced sensitization to therapy. Conventional chemotherapy (Chemo Tx) does not affect quiescent LICs. As2O3 abrogates LICs maintenance by reducing PML levels. Combining an anti-leukaemic treatment with As2O3 increases the sensitivity of LICs to chemotherapy and results in tumour regression.
To verify whether As2O3-induced cycling could increase the pro-apoptotic effect of chemotherapy on LICs, we combined arsenic and Ara-C treatment. Arsenic followed by Ara-C exposure significantly increased the efficacy of Ara-C-mediated induction of apoptosis, resulting in eradication of LICs even 4 weeks after treatment discontinuation (Fig. 5c and Supplementary Fig. 9a).
To analyze the effect of combination therapy on the persistence of LT-repopulating LICs, we treated LICs ex vivo, and next carried out serial transplantation assays. In the second round of BMT, mice transplanted with LICs treated with Ara-C succumbed around 20 days after BMT (Supplementary Fig. 9b). However, when donor LICs were treated with As2O3 and Ara-C, CML-like disease was not observed in recipient mice up to 40 days after BMT. These results indicate that induction of cell cycle entry by As2O3 remarkably enhances the effect of Ara-C, leading to significant increase in survival. Furthermore, we not only observed a marked survival advantage, but also a complete cure in more than half of recipient mice (Supplementary Fig. 9b). Notably, residual disease was not detected in these mice (Supplementary Table 1).
Interestingly, we observed that there are fewer quiescent cells in p210bcr-abl expressing than in control KSL cells (Supplementary Fig. 10a, b), indicating that the reservoir of quiescent cells is higher in HSCs than LICs. Moreover and importantly, exit from quiescence induced by As2O3 treatment was significantly more profound in LICs than in HSCs (Supplementary Fig. 10c). Similarly, a more profound reduction in LICs quiescence was observed in the Pml null setting (Supplementary Fig. 10d). Taken together, these findings suggest that LICs are more sensitive to induction of cell cycle entry by As2O3 than HSCs. Consequently, through induction of apoptosis, combination therapy with As2O3 and Ara-C affected LIC function, significantly more than normal HSC function in long term culture assays (Fig. 5d and Supplementary Fig. 10e).
We next investigated the effect of combination therapy on LICs maintenance in a serial transplantation model. After BMT of cells transduced with p210bcr-abl or empty vector, in vivo administration of As2O3 to recipient mice followed by Ara-C treatment induced remarkable apoptosis in LICs (Fig. 5e). Transduced KSL cells were also much more prone to cell cycle entry by As2O3 treatment than control KSL cells in the transplantation model (Fig. 5f) and significantly more apoptosis was observed in LICs treated with As2O3 and Ara-C than in HSCs (Fig. 5g).
Consequently, complete cure with no detectable residual disease was achieved in all recipient mice treated with combination therapy in the second and third round of BMT (Fig. 5h and Supplementary Figure 11a–b, Supplementary Table 2). Interestingly, in the third BMT As2O3 alone caused longer survival than Ara-C alone (Supplementary Fig. 11b), implying that inhibition of maintenance may be more effective for tumour regression than targeting cycling cells with chemotherapy.
Finally, we analyzed the impact of As2O3 treatment on stem cells isolated from human CML patients. First, similarly to murine LICs, fewer quiescent cells were observed in LICs from CML patients compared to HSCs from healthy volunteers (Fig. 5i). In addition, As2O3 treatment induced cell cycle induction more remarkably in LICs than HSCs, and was accompanied by downregulation of PML (Fig. 5i, j and Supplementary Fig. 12a, b). A more pronounced exit from quiescence in LICs compared to HSCs was also confirmed at the single cell level (Supplementary Fig. 12c). Finally, in vitro pre-treatment of human CML LICs with As2O3 followed by Ara-C induced significantly more apoptosis than Ara-C treatment alone, and a more profound apoptotic response was observed in LICs than in HSCs from healthy volunteers (Fig. 5k).
Discussion
It has been suggested that a rare population of leukaemic cells with stem characteristics (LICs) sustains the development of at least some form of leukaemia, including CML2. These cells are unresponsive to therapy and have been suggested as a cause of disease relapse6,7,36. Therefore, therapeutic strategies that target LICs are necessary to eradicate residual disease and to prevent leukaemia relapse.
We utilized a CML mouse model to analyze LIC function in the absence of the tumour suppressor Pml and revealed that PML plays an indispensable role in maintaining LICs quiescence. Pml-deficient LICs become exhausted with time and are incapable of generating CML in transplanted animals. Hence we hypothesized that there could be a therapeutic window in targeting PML for therapy.
Based on this assumption we utilized As2O3, a drug that downregulates PML expression by targeting it for degradation33and is currently used for the treatment of APL with very limited toxicity30, to mimic loss of Pml. Inhibition of Pml by As2O3 disrupted LICs maintenance and increased the efficacy of anti-leukaemic therapy by sensitizing LICs to pro-apoptotic stimuli. Consistent with the notion that targeting the quiescent LICs might be an effective strategy to cure CML, administration of growth factors or bryostatin-1 was previously shown to reduce quiescent CML cells and residual disease after imatinib treatment ex vivo6,37. Treatment with As2O3 in CML might prove therapeutically beneficial because this agent is already in the clinic where it already proved to be extremely well tolerated in extensive preclinical trials in mouse models33,38,39 and in human APL30–32.
Finally, although loss of Pml and As2O3 treatment also induce cycling of HSCs, Pml−/− HSCs are less affected than LICs and can sustain a normal life span in the mouse. On the basis of our findings, we therefore propose that As2O3 or novel PML lowering drugs should be utilized transiently at leukaemia onset, along with, or followed by, a standard of care regimens.
In previous studies, we showed that loss of Pml leads to an acceleration of APL in mouse models40,41. These data are coherent with the initial acceleration of Pml-deficient CML reported in this work (Supplementary Fig. 5a, b). Although there is no clear evidence that APL originates from a HSC, future work will be important to establish if Pml deficiency or As2O3 treatment leads to the exhaustion of the APL LIC in serial transplantation experiments. This is a plausible hypothesis coherent with the fact that PML-RARα may not inhibit PML function completely and that As2O3 is extremely effective in the treatment of APL.
In conclusion, our data demonstrate an unexpected and critical role for PML in stem cell biology and points at its therapeutic targeting as a promising avenue to eradicate LICs in leukaemia. It remains to be determined whether PML exerts a similar role in stem cells in other tissues and in CICs in other tumours, and if the transient use of As2O3 may represent therefore a more global strategy to target the CIC in other forms of cancer.
Methods summary
Mice
Generation of Pml-deficient mice (129Sv) has been described42. As2O3 (2.5 mg/kg body weight per day) was administered by intraperitoneal (i.p.) injection as described39.
Western blot
Each lineage compartment was flow-sorted directly into individual wells of a U-bottom 96-well plate containing 2x protein sample buffer. The lysate was briefly boiled and analyzed by immunoblotting. The following antibodies were used: anti-β-actin (A-5316, Sigma), anti-mouse Pml (S36 and S37 monoclonal antibodies, kindly provided by S. Lowe) and anti-human PML (Chemicon, rabbit polyclonal antibody) for human PML. Proteins were visualized using the SuperSignal western blotting kit (PIERCE). Relative protein expression signals were normalized by comparison with β-actin signals.
Bone marrow infection and transplantation experiments
Bone marrow infection and transplantation into lethally irradiated Ly45.1 congenic mice were performed as reported43. For serial transplantation, 2.5×106 BM MNCs were collected from recipient mice 2 weeks after BMT (first BMT) and transplanted into other recipient mice (second BMT). Subsequent transplantations were performed in the same manner. In some experiments, recipient mice were intraperitoneally injected with As2O3.
Primary patient sample assay
Bone marrow samples from normal volunteers and patients with CML-CP before any therapy at diagnosis were obtained according to appropriate Human Protection Committee validation at the Keio University School of Medicine (Tokyo, Japan) and at the Beth Israel Deaconess Medical Center (Boston, MA, U.S.A.) with written informed consent. Cells were maintained in serum free medium with a cytokine mixture containing 100 ng/ml of stem cell factor (SCF), 100 ng/ml of Flt-3 ligand (Peprotech) and 100 ng/ml of thrombopoietin (TPO) (Peprotech). To investigate division of HSCs and LICs, sorted Lin−CD34+CD38low/neg cells from healthy volunteers and CML patients were stained with CFSE (Molecular Probes, Eugene, OR). After three days of culture, fluorescence intensity of CFSE was analyzed by FACS.
Methods
Mice
Generation of Pml-deficient mice (129Sv) has been described42. C57BL/6 mice (B6-CD45.2) and C57BL/6 mice congenic for the CD45 locus (B6-CD45.1) were purchased from The Jackson Laboratory and crossed with 129Sv mice. F3 B6/Sv129 mice were used as recipients in transplantation assays. As2O3 (2.5 mg/kg body weight per day) was administered by intraperitoneal (i.p.) injection as described39.
Long-term cultures and colony-forming assays
For long-term cultures, KSL cells were co-cultured with stromal cells in Minimum Essential Media alpha modification (αMEM Sigma) containing 12.5% FCS (JRH Bioscience), 12.5% horse serum (Gibco BRL) and 1.0nM dexamethasone. After 2, 4 or 6 weeks of culture, cells were harvested and used for haematopoietic colony-forming assays as described44. For some experiments, 0.15µM As2O3 (Sigma) or 0.10µM Ara-C (Sigma) was added to cultures.
Western blot
Each lineage compartment was flow-sorted directly into individual wells of a U-bottom 96-well plate containing 50µl 2x protein sample buffer. The lysate was briefly boiled and analyzed by immunoblotting. The following antibodies were used: anti-β-actin (A-5316, Sigma), anti-mouse Pml (S36 and S37 monoclonal antibodies, kindly provided by S. Lowe) and anti-human PML (Chemicon, rabbit polyclonal antibody) for human PML, anti-PS6 (S235/236), anti-S6 (Cell Signaling). Proteins were visualized using the SuperSignal western blotting kit (PIERCE). Signal intensity was measured using ImageJ 1.34S software (http://rsb.info.nih.gov/ij/). Relative protein expression signals were normalized by comparison with β-actin signals.
Bone marrow infection and transplantation experiments
Transfection of the retroviral vector p210bcr-abl, bone marrow isolation from 8-week-old wild-type and Pml−/− mice, prestimulation and infection, and transplantation into lethally irradiated Ly45.1 congenic mice were performed as reported43. For serial transplantation, 2.5×106 BM MNCs were collected from recipient mice 2 weeks after BMT (first BMT). Collected cells were transplanted into other recipient mice (second BMT). Subsequent transplantations were performed in the same manner. In some experiments, recipient mice were intraperitoneally injected with As2O3. For MRD, a two-round nested PCR reaction was applied as described45.
Primary patient sample assay
Bone marrow samples from normal volunteers (3 males and 1 female, median age 31.5: range 29 to 36) and patients with CML-CP before any therapy at diagnosis (2 males and 2 females, median age at diagnosis 32.5: range 29 to 37; % blasts: 1.5 to 3.8; cytogenetics at diagnosis: Ph1 detected in all patients) were obtained according to appropriate Human Protection Committee validation at the Keio University School of Medicine (Tokyo, Japan) and at the Beth Israel Deaconess Medical Center (Boston, MA, U.S.A.) with written informed consent. Mononuclear cells were separated by Lymphoprep (Nycomed Pharma As, Oslo, Norway). Cells were maintained in serum free medium with a cytokine mixture containing 100 ng/ml of stem cell factor (SCF), 100 ng/ml of Flt-3 ligand (Peprotech) and 100 ng/ml of thrombopoietin (TPO) (Peprotech). For some experiments, 0.15µM As2O3 (Sigma) (day 1–7) or 0.10µM Ara-C (Sigma) (day 3–7) was added to cultures. To investigate division of HSCs and LICs, sorted Lin− CD34+CD38low/neg cells from healthy volunteers and CML patients were stained with CFSE (Molecular Probes, Eugene, OR). CFSE-stained cells were plated on 96 well U-bottom plates. After three days of culture, fluorescence intensity of CFSE was analyzed by FACS. To assay cell division at the single cell level, single cell sorting of Lin − CD34+CD38low/negc-Kit+ cells was performed and cells were cultured with SCF+TPO+Flt-3L. Cell division was monitored daily.
Statistical analysis
P-values were calculated using the unpaired Student's t-test.
Supplementary Material
Acknowledgements
We thank Shahiba Ogilvie for analysis of patient samples and data management, and all members of the Pandolfi laboratory for comments and discussion. K.I. was supported by a JSPS postdoctoral fellowship for research abroad. R.B. is supported by a K01 NIH grant. This work was supported by NIH grants to P.P.P.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
References
- 1.Bruce WR, van der Gaag H. A quantitative assay for the number of murine lymphoma cells capable of proliferation in vivo. Nature. 1963;199:79–80. doi: 10.1038/199079a0. [DOI] [PubMed] [Google Scholar]
- 2.Huntly BJ, Gilliland DG. Leukaemia stem cells and the evolution of cancer-stem-cell research. Nature Rev. Cancer. 2005;5:311–321. doi: 10.1038/nrc1592. [DOI] [PubMed] [Google Scholar]
- 3.Scadden DT. Cancer stem cells refined. Nature Immunol. 2004;5:701–703. doi: 10.1038/ni0704-701. [DOI] [PubMed] [Google Scholar]
- 4.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 5.Hope KJ, Jin L, Dick JE. Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nature Immunol. 2004;5:738–743. doi: 10.1038/ni1080. [DOI] [PubMed] [Google Scholar]
- 6.Holtz M, Forman SJ, Bhatia R. Growth factor stimulation reduces residual quiescent chronic myelogenous leukemia progenitors remaining after imatinib treatment. Cancer Res. 2007;67:1113–1120. doi: 10.1158/0008-5472.CAN-06-2014. [DOI] [PubMed] [Google Scholar]
- 7.Wang JC, Dick JE. Cancer stem cells: lessons from leukemia. Trends Cell Biol. 2005;15:494–501. doi: 10.1016/j.tcb.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 8.Rowley JD. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973;243:290–293. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- 9.de Klein A, et al. A cellular oncogene is translocated to the Philadelphia chromosome in chronic myelocytic leukaemia. Nature. 1982;300:765–767. doi: 10.1038/300765a0. [DOI] [PubMed] [Google Scholar]
- 10.Deininger MW, Goldman JM, Melo JV. The molecular biology of chronic myeloid leukemia. Blood. 2000;96:3343–3356. [PubMed] [Google Scholar]
- 11.Druker BJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N. Engl. J. Med. 2001;344:1031–1037. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 12.Kantarjian H, et al. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N. Engl. J. Med. 2002;346:645–652. doi: 10.1056/NEJMoa011573. [DOI] [PubMed] [Google Scholar]
- 13.Rousselot P, et al. Imatinib mesylate discontinuation in patients with chronic myelogenous leukemia in complete molecular remission for more than 2 years. Blood. 2007;109:58–60. doi: 10.1182/blood-2006-03-011239. [DOI] [PubMed] [Google Scholar]
- 14.Ghanima W, Kahrs J, Dahl TG, 3rd, Tjonnfjord GE. Sustained cytogenetic response after discontinuation of imatinib mesylate in a patient with chronic myeloid leukaemia. Eur J Haematol. 2004;72:441–443. doi: 10.1111/j.1600-0609.2004.00257.x. [DOI] [PubMed] [Google Scholar]
- 15.Cortes J, O'Brien S, Kantarjian H. Discontinuation of imatinib therapy after achieving a molecular response. Blood. 2004;104:2204–2205. doi: 10.1182/blood-2004-04-1335. [DOI] [PubMed] [Google Scholar]
- 16.Mauro MJ, Druker BJ, Maziarz RT. Divergent clinical outcome in two CML patients who discontinued imatinib therapy after achieving a molecular remission. Leuk Res. 2004;S1:S71–S73. doi: 10.1016/j.leukres.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 17.Merante S, et al. Outcome of four patients with chronic myeloid leukemia after imatinib mesylate discontinuation. Haematologica. 2005;90:979–981. [PubMed] [Google Scholar]
- 18.Higashi T, et al. Imatinib mesylate-sensitive blast crisis immediately after discontinuation of imatinib mesylate therapy in chronic myelogenous leukemia: report of two cases. Am J Hematol. 2004;76:275–278. doi: 10.1002/ajh.20096. [DOI] [PubMed] [Google Scholar]
- 19.Bernardi R, Pandolfi PP. Structure, dynamics and functions of promyelocytic leukaemia nuclear bodies. Nature Rev. Mol Cell Biol. 2007;8:1006–1016. doi: 10.1038/nrm2277. [DOI] [PubMed] [Google Scholar]
- 20.Salomoni P, Pandolfi PP. The role of PML in tumor suppression. Cell. 2002;108:165–170. doi: 10.1016/s0092-8674(02)00626-8. [DOI] [PubMed] [Google Scholar]
- 21.Wang ZG, et al. PML is essential for multiple apoptotic pathways. Nature Genet. 1998;20:266–272. doi: 10.1038/3073. [DOI] [PubMed] [Google Scholar]
- 22.Bernardi R, et al. PML inhibits HIF-1alpha translation and neoangiogenesis through repression of mTOR. Nature. 2006;442:779–785. doi: 10.1038/nature05029. [DOI] [PubMed] [Google Scholar]
- 23.Gurrieri C, et al. Loss of the tumor suppressor PML in human cancers of multiple histologic origins. J. Natl Cancer Inst. 2004;96:269–279. doi: 10.1093/jnci/djh043. [DOI] [PubMed] [Google Scholar]
- 24.Scaglioni PP, et al. A CK2-dependent mechanism for degradation of the PML tumor suppressor. Cell. 2006;126:269–283. doi: 10.1016/j.cell.2006.05.041. [DOI] [PubMed] [Google Scholar]
- 25.Arai F, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nature Rev. Cancer. 2003;3:895–902. doi: 10.1038/nrc1232. [DOI] [PubMed] [Google Scholar]
- 27.Daley GQ, Van Etten RA, Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990;247:824–830. doi: 10.1126/science.2406902. [DOI] [PubMed] [Google Scholar]
- 28.Klaassen CD. Heavy metals and heavy-metal antagonists. In: Hardman JG, Gilman AG, Limbird LE, editors. The Pharmacological Basis of Therapeutics. New York: McGraw-Hill; 1996. pp. 1649–1672. [Google Scholar]
- 29.Aronson SM. Arsenic and old myths. R I Med. 1994;77:233–234. [PubMed] [Google Scholar]
- 30.Mathews W, et al. Single-agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia: durable remissions with minimal toxicity. Blood. 2006;107:2627–2632. doi: 10.1182/blood-2005-08-3532. [DOI] [PubMed] [Google Scholar]
- 31.Soignet SL, et al. Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N. Engl. J. Med. 1998;339:1341–1348. doi: 10.1056/NEJM199811053391901. [DOI] [PubMed] [Google Scholar]
- 32.Shen ZX, et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II. Clinical efficacy and pharmacokinetics in relapsed patients. Blood. 1997;89:3354–3360. [PubMed] [Google Scholar]
- 33.Lallemand-Breitenbach V, et al. Role of promyelocytic leukemia (PML) sumolation in nuclear body formation, 11S proteasome recruitment, and As2O3-induced PML or PML/retinoic acid receptor alpha degradation. J. Exp. Med. 2001;193:1361–1371. doi: 10.1084/jem.193.12.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yilmaz OH, et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J, et al. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature. 2006;441:518–522. doi: 10.1038/nature04747. [DOI] [PubMed] [Google Scholar]
- 36.Bhatia R, et al. Persistence of malignant hematopoietic progenitors in chronic myelogenous leukemia patients in complete cytogenetic remission following imatinib mesylate treatment. Blood. 2003;101:4701–4707. doi: 10.1182/blood-2002-09-2780. [DOI] [PubMed] [Google Scholar]
- 37.Jørgensen HG, et al. Enhanced CML stem cell elimination in vitro by bryostatin priming with imatinib mesylate. Exp Hematol. 2005;33:1140–1146. doi: 10.1016/j.exphem.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 38.Lallemand-Breitenbach V, et al. Retinoic acid and arsenic synergize to eradicate leukemic cells in a mouse model of acute promyelocytic leukemia. J. Exp. Med. 1999;189:1043–1052. doi: 10.1084/jem.189.7.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rego EM, He LZ, Warrell RP, Jr, Wang ZG, Pandolfi PP. Retinoic acid (RA) and As2O3 treatment in transgenic models of acute promyelocytic leukemia (APL) unravel the distinct nature of the leukemogenic process induced by the PML-RARalpha and PLZF-RARalpha oncoproteins. Proc. Natl Acad. Sci. USA. 2000;97:10173–10178. doi: 10.1073/pnas.180290497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rego EM, et al. Role of promyelocytic leukemia (PML) protein in tumor suppression. J. Exp. Med. 2001;193:521–529. doi: 10.1084/jem.193.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gurrieri C, et al. Mutations of the PML tumor suppressor gene in acute promyelocytic leukemia. Blood. 2004;103:2358–2362. doi: 10.1182/blood-2003-07-2200. [DOI] [PubMed] [Google Scholar]
- 42.Wang ZG, et al. Role of PML in cell growth and the retinoic acid pathway. Science. 1998;279:1547–1551. doi: 10.1126/science.279.5356.1547. [DOI] [PubMed] [Google Scholar]
- 43.Di Cristofano A, et al. p62(dok), a negative regulator of Ras and mitogen-activated protein kinase (MAPK) activity, opposes leukemogenesis by p210(bcr-abl) J. Exp. Med. 2001;194:275–284. doi: 10.1084/jem.194.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ito K, et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nature Med. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- 45.Cross NC, et al. Minimal residual disease after allogeneic bone marrow transplantation for chronic myeloid leukaemia in first chronic phase: correlations with acute graft-versus-host disease and relapse. Br. J. Haematol. 1993;84:67–74. doi: 10.1111/j.1365-2141.1993.tb03026.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.