Abstract
In recent years, ontologies have become a mainstream topic in biomedical research. When biological entities are described using a common schema, such as an ontology, they can be compared by means of their annotations. This type of comparison is called semantic similarity, since it assesses the degree of relatedness between two entities by the similarity in meaning of their annotations. The application of semantic similarity to biomedical ontologies is recent; nevertheless, several studies have been published in the last few years describing and evaluating diverse approaches. Semantic similarity has become a valuable tool for validating the results drawn from biomedical studies such as gene clustering, gene expression data analysis, prediction and validation of molecular interactions, and disease gene prioritization.
We review semantic similarity measures applied to biomedical ontologies and propose their classification according to the strategies they employ: node-based versus edge-based and pairwise versus groupwise. We also present comparative assessment studies and discuss the implications of their results. We survey the existing implementations of semantic similarity measures, and we describe examples of applications to biomedical research. This will clarify how biomedical researchers can benefit from semantic similarity measures and help them choose the approach most suitable for their studies.
Biomedical ontologies are evolving toward increased coverage, formality, and integration, and their use for annotation is increasingly becoming a focus of both effort by biomedical experts and application of automated annotation procedures to create corpora of higher quality and completeness than are currently available. Given that semantic similarity measures are directly dependent on these evolutions, we can expect to see them gaining more relevance and even becoming as essential as sequence similarity is today in biomedical research.
Introduction
Comparison and classification have been central pillars of biology since Linnaeus proposed his taxonomy and Darwin observed the mockingbirds on the Galapagos Islands. Like most scientific knowledge, biological laws and models are derived from comparing entities (such as genes, cells, organisms, populations, species) and finding their similarities and differences. However, biology is unlike other sciences in that its knowledge can seldom be reduced to mathematical form. Thus, biologists either record their knowledge in natural language—for example, in scientific publications—or they must seek other forms of representation to organize it, such as classification schemes. When new entities arise, biologists approach them by comparing them to known entities and making inferences according to their degree of similarity.
Comparing entities is not always trivial. For instance, while the sequences or structures of two gene products can be compared directly (through alignment algorithms), the same is not true of their functional aspects. The difference is that sequences and structures have an objective representation and measurable properties, whereas functional aspects have neither. This does not mean that it is impossible to compare functional aspects, but that to be compared they must be expressed in a common and objective form.
The advent of automated sequencing has had deep repercussions on knowledge representation in biology. As experimental methods shift in scope from the gene level to the genomic level, computational analysis is proving essential in handling the increasing amount of data. Thus it has become crucial to adopt common and objective knowledge representations to help knowledge sharing and computer reasoning. This need led to the development of ontologies for goals such as annotating gene products (Gene Ontology), annotating sequences (Sequence Ontology), and annotating experimental assays (Microarray and Gene Expression Data Ontology).
The adoption of ontologies for annotation provides a means to compare entities on aspects that would otherwise not be comparable. For instance, if two gene products are annotated within the same schema, we can compare them by comparing the terms with which they are annotated. While this comparison is often done implicitly (for instance, by finding the common terms in a set of interacting gene products), it is possible to do an explicit comparison with semantic similarity measures. Within the context of this article, we define a semantic similarity measure as a function that, given two ontology terms or two sets of terms annotating two entities, returns a numerical value reflecting the closeness in meaning between them.
The Gene Ontology (GO) [1] is the main focus of investigation of semantic similarity in molecular biology, not only because it is the ontology most widely adopted by the life sciences community, but also because comparing gene products at the functional level is crucial for a variety of applications. Semantic similarity applied to the GO annotations of gene products provides a measure of their functional similarity. From this point forward, we will use the term “functional similarity” when referring to the similarity between two gene products given by the semantic similarity between the sets of GO terms with which they are annotated. As such, the semantic similarity measures and the studies reviewed in this article are presented in the context of GO, notwithstanding the fact that they are applicable to other biological ontologies.
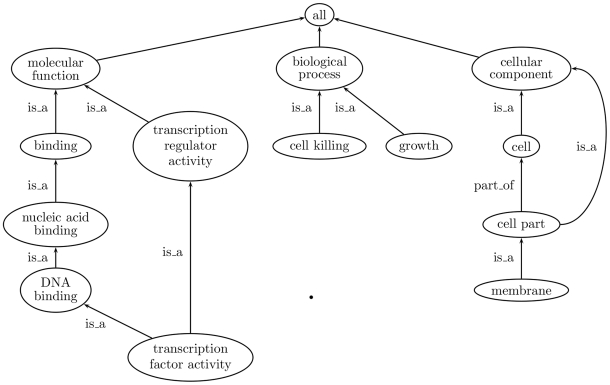
GO provides a schema for representing gene product function in the cellular context. Figure 1 shows how GO is structured as three independent directed acyclic graphs (DAGs) that correspond to orthogonal categories of gene product function: molecular function, biological process, and cellular component. The nodes in the graph represent terms that describe components of gene product function. GO links the terms to each other by relationships, most commonly of the types ‘is a’ and ‘part of’, the former expressing a simple class–subclass relationship and the latter expressing a part–whole relationship. Gene products that are described by GO terms are said to be annotated with them, either directly or through inheritance, since annotation to a given term implies annotation to all of its ancestors (true path rule). The Gene Ontology Consortium is responsible for developing and maintaining GO terms, their relationships, and their annotations to genes and gene products of the collaborating databases. Moreover, GO Consortium is also responsible for developing tools that support the creation, maintenance, and use of all this information.
Figure 1. Section of the GO graph showing the three aspects (molecular function, biological process, and cellular component) and some of their descendant terms.
The fact that GO is a DAG rather than a tree is illustrated by the term “transcription factor activity” which has two parents. An example of a part of relationship is also shown between the terms cell part and cell.
Classification of Semantic Similarity Measures
Several approaches are available to quantify semantic similarity between terms or annotated entities in an ontology represented as a DAG such as GO. This article distinguishes these approaches in the following way:
Scope: Which entities they intend to compare, that is, GO terms versus gene products;
Data source: Which components of the ontology they use, i.e., edges versus nodes;
Metric: How they quantify and combine the information stored on those components.
Comparing Terms
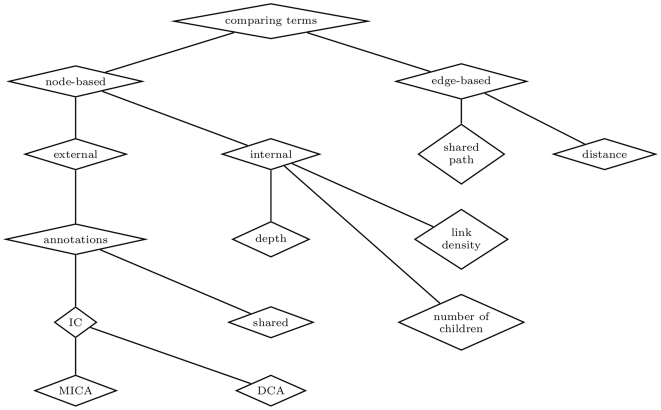
There are essentially two types of approaches for comparing terms in a graph-structured ontology such as GO: edge-based, which use the edges and their types as the data source; and node-based, in which the main data sources are the nodes and their properties. We summarize the different techniques employed in these approaches in Figure 2 and describe them in the following sections. Note that there are other approaches for comparing terms that do not use semantic similarity—for example, systems that select a group of terms that best summarize or classify a given subject based on the discrete mathematics of finite partially ordered sets [2].
Figure 2. Main approaches for comparing terms: node-based and edge-based and the techniques used by each approach.
DCA, disjoint common ancestors; IC, information content; MICA, most informative common ancestor.
Edge-based
Edge-based approaches are based mainly on counting the number of edges in the graph path between two terms [3]. The most common technique, distance, selects either the shortest path or the average of all paths, when more than one path exists. This technique yields a measure of the distance between two terms, which can be easily converted into a similarity measure. Alternatively, the common path technique calculates the similarity directly by the length of the path from the lowest common ancestor of the two terms to the root node [4].
While these approaches are intuitive, they are based on two assumptions that are seldom true in biological ontologies: (1) nodes and edges are uniformly distributed [5], and (2) edges at the same level in the ontology correspond to the same semantic distance between terms. Several strategies have been proposed to attenuate these issues, such as weighting edges differently according to their hierarchical depth, or using node density and type of link [6]. However, terms at the same depth do not necessarily have the same specificity, and edges at the same level do not necessarily represent the same semantic distance, so the issues caused by the aforementioned assumptions are not solved by those strategies.
Node-based
Node-based approaches rely on comparing the properties of the terms involved, which can be related to the terms themselves, their ancestors, or their descendants. One concept commonly used in these approaches is information content (IC), which gives a measure how specific and informative a term is. The IC of a term c can be quantified as the negative log likelihood,
where p(c) is the probability of occurrence of c in a specific corpus (such as the UniProt Knowledgebase), being normally estimated by its frequency of annotation. Alternatively, the IC can also be calculated from the number of children a term has in the GO structure [7], although this approach is less commonly used.
The concept of IC can be applied to the common ancestors two terms have, to quantify the information they share and thus measure their semantic similarity. There are two main approaches for doing this: the most informative common ancestor (MICA technique), in which only the common ancestor with the highest IC is considered [8]; and the disjoint common ancestors (DCA technique), in which all disjoint common ancestors (the common ancestors that do not subsume any other common ancestor) are considered [9].
Approaches based on IC are less sensitive to the issues of variable semantic distance and variable node density than edge-based measures [8], because the IC gives a measure of a term's specificity that is independent of its depth in the ontology (the IC of a term is dependent on its children but not on its parents). However, the IC is biased by current trends in biomedical research, because terms related to areas of scientific interest are expected to be more frequently annotated than other terms. Nevertheless, the use of the IC still makes sense from a probabilistic point of view: it is more probable (and less meaningful) that two gene products share a commonly used term than an uncommonly used term, regardless of whether that term is common because it is generic or because it is related to a hot research topic.
Other node-based approaches include looking at the number of shared annotations, that is, the number of gene products annotated with both terms [10]; computing the number of shared ancestors across the GO structure; and using other types of information such as node depth and node link density (i.e., node degree) [11].
Comparing Gene Products
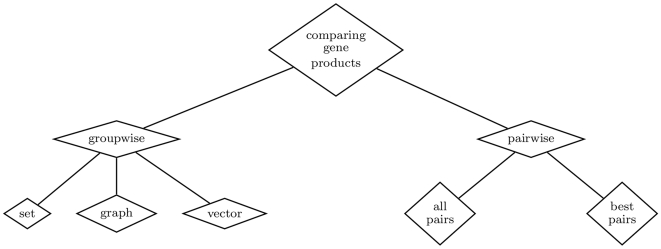
Gene products can be annotated with several GO terms within each of the three GO categories. Gene product function is often described by several molecular function terms, and gene products often participate in multiple biological processes and are located in various cellular components. Thus, to assess the functional similarity between gene products (within a particular GO category) it is necessary to compare sets of terms rather than single terms. Several strategies have been proposed for this, which we have divided into two categories: pairwise and groupwise approaches, as shown in Figure 3.
Figure 3. Main approaches for comparing gene products: pairwise and groupwise and the techniques used by each approach.
Pairwise
Pairwise approaches measure functional similarity between two gene products by combining the semantic similarities between their terms. Each gene product is represented by its set of direct annotations, and semantic similarity is calculated between terms in one set and terms in the other (using one of the approaches described previously for comparing terms). Some approaches consider every pairwise combination of terms from the two sets (all pairs technique), while others consider only the best-matching pair for each term (best pairs technique). A global functional similarity score between the gene products is obtained by combining these pairwise semantic similarities, with the most common combination approaches being the average, the maximum, and the sum.
Groupwise
Groupwise approaches do not rely on combining similarities between individual terms to calculate gene product similarity, but calculate it directly by one of three approaches: set, graph, or vector.
In set approaches only direct annotations are considered and functional similarity is calculated using set similarity techniques.
In graph approaches gene products are represented as the subgraphs of GO corresponding to all their annotations (direct and inherited). Functional similarity can be calculated either using graph matching techniques or, because these are computationally intensive, by considering the subgraphs as sets of terms and applying set similarity techniques.
In vector approaches a gene product is represented in vector space, with each term corresponding to a dimension, and functional similarity is calculated using vector similarity measures. Vectors can be binary, with each dimension denoting presence or absence of the term in the set of annotations of a given gene product, or scalar, with each dimension representing a given property of the term (for example, its IC).
Survey of Semantic Similarity Measures
Since the first application of semantic similarity in biology, by Lord et al. [12], several semantic similarity measures have been developed for use with GO, as shown in Table 1. The following sections present a survey of semantic similarity measures proposed within the context of GO.
Table 1. Summary of term measures, their approaches, and their techniques.
| Measure | Approach | Techniques |
| Resnik [8] | Node-based | MICA |
| Lin [13] | Node-based | MICA |
| Jiang and Conrath [14] | Node-based | MICA |
| GraSM [9] | Node-based | DCA |
| Schlicker et al. [16] | Node-based | MICA |
| Wu et al. [22] | Edge-based | Shared path |
| Wu et al. [23] | Edge-based | Shared path; distance |
| Bodenreider et al. [17] | Node-based | Shared annotations |
| Othman et al. [11] | Hybrid | IC/depth/number of children; distance |
| Wang et al. [25] | Hybrid | Shared ancestors |
| Riensche et al. [18] | Node-based | IC/MICA; shared annotations |
| Yu et al. [20] | Edge-based | Shared path |
| Cheng et al. [21] | Edge-based | Shared path |
| Pozo et al. [24] | Edge-based | Shared path |
Measures for Terms
Node-based
The most common semantic similarity measures used with GO have been Resnik's, Lin's, and Jiang and Conrath's, which are node-based measures relying on IC [8],[13],[14]. They were originally developed for the WordNet, and then applied to GO [12],[15]. Resnik measures similarity between two terms as simply the IC of their most informative common ancestor (MICA):
| (1) |
While this measure is effective in determining the information shared by two terms, it does not consider how distant the terms are from their common ancestor. To take that distance into account, Lin's and Jiang and Conrath's measures relate the IC of the MICA to the IC of the terms being compared:
| (2) |
| (3) |
However, being relative measures, 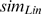 and
and 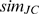 are displaced from the graph. This means that these measures are proportional to the IC differences between the terms and their common ancestor, independently of the absolute IC of the ancestor.
are displaced from the graph. This means that these measures are proportional to the IC differences between the terms and their common ancestor, independently of the absolute IC of the ancestor.
To overcome this limitation, Schlicker et al. [16] have proposed the relevance similarity measure, which is based on Lin's measure, but uses the probability of annotation of the MICA as a weighting factor to provide graph placement.
| (4) |
A constraint all of these measures share is that they look only at a single common ancestor (the MICA) despite the fact that GO terms can have several DCA. To avoid this, Couto et al. [9] proposed the GraSM approach, which can be applied to any of the measures previously described, and where the IC of the MICA is replaced by the average IC of all DCA.
Bodenreider et al. [17] developed a node-based measure that also uses annotation data but does not rely on information theory. It represents each GO term as a vector of all gene products annotated with it, and measures similarity between two terms by computing the scalar product of their vectors.
Riensche et al. used coannotation data to map terms between different GO categories and calculate a weighting factor, which can then be applied to a standard node-based semantic similarity measure [18].
Edge-based
Within the edge-based approaches, Pekar and Staab proposed a measure based on the length of the longest path between two terms' lowest common ancestor and the root (maximum common ancestor depth), and on the length of the longest path between each of the terms and that common ancestor [19]. It is given by the expression
| (5) |
where δ(c 1,c 2) is the length in number of edges of the longest distance between term c 1 and term c 2. This measure was first applied to GO by Yu et al. [20].
Cheng et al. also proposed a maximum common ancestor depth measure, but weighted each edge to reflect depth [21]. Wu et al. proposed a nonweighted maximum common ancestor depth measure [22]. An adjustment of this measure was proposed by Wu et al., introducing the distance to the nearest leaf node and the distance to the lowest common ancestor to take term specificity into account [23].
A distinct approach was proposed by Pozo et al. [24], where a “Functional Tree” for molecular function terms is first derived from their co-occurrence in the same set of Interpro entries, and semantic similarity between two terms is calculated from the height of their lowest common ancestor in this “Functional Tree” rather than in the GO graph. With this approach, the authors intend to reveal natural biological links between the terms.
Hybrid
Wang et al. [25] developed a hybrid measure in which each edge is given a weight according to the type of relationship. For a given term c 1 and its ancestor ca, the authors define the semantic contribution of ca to c 1, as the product of all edge weights in the “best” path from ca to c 1, where the “best” path is the one that maximizes the product. Semantic similarity between two terms is then calculated by summing the semantic contributions of all common ancestors to each of the terms and dividing by the total semantic contribution of each term's ancestors to that term. Othman et al. proposed a hybrid distance measure in which each edge is weighted by node depth, node link density, and difference in IC between the nodes linked by that edge [11].
Measures for Gene Products
Several measures for calculating the functional similarity between gene products have also been developed, as shown in Tables 2 and 3.
Table 2. Summary of pairwise approaches.
| Measure | Approach | Techniques | Term Comparison |
| Lord et al. [12] | All pairs | Average | Resnik/Lin/Jiang |
| Sevillla et al. [26] | All pairs | Maximum | Resnik/Lin/Jiang |
| Riensche et al. [18] (XOA) | All pairs | Maximum | XOA |
| Azuaje et al. [27] | Best pairs | Average | Resnik/Lin/Jiang |
| Couto et al. [9] | Best pairs | Average | GraSM+(Resnik/Lin/Jiang) |
| Schlicker et al. [16] (funSim) | Best pairs | Average | simRel |
| Wang et al. [25] | Best pairs | Average | Wang |
| Tao et al. [61] (ITSS) | Best pairs | Average Min. threshold | Lin |
| Pozo et al. [24] | Best pairs | Average | Pozo |
| Lei et al. [29] | All pairs Best pairsa | Average Max, Sum | Depth of LCA |
Lei et. al also consider exact matches only.
Table 3. Summary of groupwise approaches.
| Measure | Approach | Techniques | Weighting |
| Lee et al. [30] (TO) | Graph-based | Term overlap | None |
| Mistry et al. [31] (NTO) | Graph-based | Term overlap, Normalized | None |
| Gentleman [33] (simLP) | Graph-based | Shared-path | None |
| Gentleman [33] (simUI) | Graph-based | Jaccard | None |
| Martin et al. [32] (GOToolBox) | Graph-based | Czekanowski-Dice, Jaccard | None |
| Pesquita et al. [44] (simUI) | Graph-based | Jaccard | IC |
| Ye et al. [35] | Graph-based | LCA, Normalized | None |
| Cho et al. [36] | Graph-based | LCA | IC |
| Lin et al. [37] | Graph-based | Intersection | Annotation set probability |
| Yu et al. [38] | Graph-based | LCA | Annotation set probability |
| Sheehan et al. [39] (SSA) | Graph-based | Resnik, Lin | Annotation set probability |
| Huang et al. [40] | Vector-based | Kappa-statistic | None |
| Chabalier et al. [41] | Vector-based | Cosine | IC |
Pairwise
The most common methods of measuring gene product functional similarity have been pairwise approaches based on node-based term measures, namely, Resnik's, Lin's, and Jiang and Conrath's. Lord et al. were the first to apply these measures, using the average of all pairwise similarities as the combination strategy [12]; Sevilla et al. applied them using the maximum of the pairwise similarities instead [26]; while Couto et al. and Azuaje et al. opted for a composite average in which only the best-matching term pairs are considered (best-match average) [9],[27]. Schlicker et al. all proposed a variation of the best-match average, by combining semantic similarities for both molecular function and biological process aspects of GO [16]; while Tao et al. used a threshold of minimum similarity for selecting best-matching term pairs, and considered only reciprocal pairs to reduce the noise [28]. Riensche et al. also employed the maximum combination strategy, but introduced a variation to allow comparison of terms from different aspects of GO (see node-based term measures) [18].
Pairwise approaches have also been applied to edge-based measures: Wang et al. and Pozo et al. used a best-match average combination strategy with their measures [24],[25], and Lei et al. tested a number of different combination approaches, including the average, maximum, and sum for all pairs, best pairs, and only exact matches [29].
Of the several combination strategies employed in pairwise measures, the best-match average variants are the best overall. The maximum approach can answer the question of whether two gene products share a functional aspect, but is unsuitable to assess their global similarity, as it is indifferent to the number of functional aspects they share and to the number of functional aspects in which they differ. For instance, a gene product A with terms t1 and t2 will be considered 100% similar to a gene product B with terms t1 and t3 under the maximum approach, which obviously does not reflect the differences between the gene products. As for the average approach, because it makes an all-against-all comparison of the terms of two gene products, it will produce counterintuitive results for gene products that have several distinct functional aspects. For instance, two gene products, A and B, that share the same two unrelated terms, t1 and t2, will be 50% similar under the average approach, because similarity will be calculated between both the matching and the opposite terms of the two gene products. The best-match average approach provides a good balance between the maximum and average approaches by considering all terms but only the most significant matching.
Groupwise
Purely set-based approaches are not common, because few measures consider only direct annotations, but many graph-based approaches use set similarity techniques to simplify the problem of graph matching. The first graph-based measure to be applied to GO was that of Lee et al. [30], in which the similarity between gene products is defined by the number of terms they share (term overlap [TO]). More recently, Mistry et al. [31] proposed a normalized version of Lee's measure (NTO), in which the number of overlapping terms is divided by the annotation set size for the gene with the lower number of annotations. GOToolBox also implements some set similarity techniques applied to GO graphs, namely Czekanowski-Dice and Jaccard [32]. Gentleman's simLP and simUI measures were also among the first graph-based measures to be applied to GO [33]: simLP extends the maximum common ancestor depth concept to gene products, so two gene products are as similar as the depth of the term that is the lowest common ancestor to all the terms' direct annotations; whereas simUI considers gene products as the set of terms in their annotation subgraphs, and uses the Jaccard index to calculate the similarity between them:
| (6) |
Based also on the Jaccard index, Pesquita et al. have proposed the simGIC measure, in which each GO term is weighted by its IC [34].
Ye et al. proposed a normalized version of simLP that takes into account the minimum and maximum depths within each GO category [35].
Cho et al. developed a simpler groupwise approach, in which the semantic similarity between two gene products is given by the information content of the most informative term they share [36]. This produces the same result as Resnik's measure with the maximum combination strategy, but is simpler to apply to gene products, as it does not require computing pairwise term similarities.
Other graph-based measures consider the probability of a gene product being annotated with a particular set of terms (annotation set probability). Lin et al. calculate the similarity between two gene product subgraphs as the frequency of occurrence of the graph resulting from the intersection of both subgraphs, that is, the frequency of gene products whose annotation subgraph contains the intersect graph [37]. Yu et al. proposed the “total ancestry similarity” measure, a probabilistic approach in which similarity is given by the probability that any two gene products have exactly the same set of lowest common ancestors as the two gene products being compared [38]. The SSA algorithm by Sheehan et al. is based on the probability of any given gene product being annotated with the nearest common ancestors of two gene products. This probability is then transformed into an IC measure that the authors use to compute Resnik's and Lin's measures to obtain a final gene product similarity value [39]. This algorithm also considers the types of relations between the terms in the subgraphs and corrects the number of annotations for the parent term in a part_of relation, if its number of annotations is smaller than its child's, to comply with the logic that if the part exists, necessarily the whole does too.
As for vector-based approaches, Huang et al. developed a gene product similarity measure based on annotation profiles that includes GO terms as well as many other derived from varied sources for the tool DAVID [40]. Each gene product is represented as a binary vector with each term having the value 1 if it is annotated to the gene product or 0 otherwise. Similarity is calculated through kappa-statistics, which are a co-occurrence probability measure corrected for chance.
Chabalier et al. also consider gene products as a vector of all GO terms, but weight them according to their IC. Semantic similarity is then calculated through the cosine similarity algorithm, which is commonly used to measure document similarity in information retrieval [41]:
| (7) |
Evaluation of Semantic Similarity Measures
Given the variety of approaches and measures for semantic similarity, a fundamental question arises: How well does each measure capture the similarity in function between two gene products?
Addressing this question is not trivial, because there is no direct way to ascertain the true functional similarity between two gene products. If there were, there would be no need to apply semantic similarity in the first place. However, there are independent properties, such as sequence similarity or coexpression data, that can be used as measures of similarity at different levels, and by correlating semantic similarity with such properties it is feasible to assess how well a given measure captures the similarity between gene products.
The choice of how to evaluate measures is still a subject of debate, because no gold standard and few global comparative studies exist. Furthermore, most authors test only a few measures of interest for their particular applications. Table 4 summarizes the most prominent studies and the best measure identified by them.
Table 4. Summary of assessment studies performed on semantic similarity measures in GO, detailing the properties used in the evaluation and the best performing measures.
| Study | Standard | Best Measure |
| Lord et al. [15] | Sequence similarity | Resnik (average) |
| Wang et al. [42] | Gene expression | None |
| Sevillla et al. [26] | Gene expression | Resnik (max) |
| Couto et al. [9] | Family similarity | Jiang and Conrath |
| Schlicker et al. [16] | Sequence and family similarity | Schlicker et al. |
| Lei et al. [29] | Subnuclear location | TO |
| Guo et al. [43] | Human regulatory pathways | Resnik (average) |
| Wang et al. [25] | Clustering | Wang et al. |
| Pesquita et al. [44] | Sequence similarity | simGIC |
| Xu et al. [45] | PPI/gene expression | Resnik(Max) |
| Mistry et al. [31] | Sequence similarity | TO/Resnik(Max) |
The first assessment study was done by Lord et al., who tested Resnik's, Lin's, and Jiang and Conrath's measures against sequence similarity using the average combination approach [15]. They used linear correlation as the evaluation criterion, concluding that it was highest for the molecular function aspect, as would be expected given the strong relationship between function and sequence. Of the three measures tested, Resnik's measure had consistently the highest correlation.
Later, Wang et al. tested the same measures against gene coexpression data, also using linear correlation. Although a high correlation was found, no conclusion was drawn about the relative performance of the measures [42].
Sevilla et al. also used gene coexpression data, but tested these measures with the maximum combination approach rather than the average. In agreement with Lord et al., they found Resnik's measure to perform best, and found the biological process aspect to have the highest correlation with gene coexpression. This result was far from unexpected, since genes with similar expression patterns are likely to be involved in the same biological processes [26].
Couto et al. tested the GraSM variation of these measures using a best-match average combination strategy, correlating sequence similarity with the number of Pfam families shared. They found the highest correlation with Jiang and Conrath's measure and the biological process aspect [9].
Schlicker et al. compared their measure with Resnik's measure by viewing the distribution of sequence similarity over semantic similarity, considering discrete levels. They found their measure to perform best, particularly in distinguishing orthologous gene products from gene products with other levels of sequence similarity, for both molecular function and biological process aspects [16].
Lei et al. tested several semantic similarity measures in an application for predicting subnuclear location, using the multi-class classification results (Matthews correlation coefficient) as an evaluation criterion [29]. Regarding term measures, these authors compared Resnik's measure with the simLP measure (adapted for term similarity) and an exact term-matching approach, finding them not to be significantly different in performance. They also tested several combination strategies for applying simLP to gene product similarity, concluding that the sum of exact-matching term pair similarities produced the best results. This outcome is not surprising, given the precise nature of their application, as the subnuclear location of a gene product will often be related to the presence of specific GO terms.
Guo et al. evaluated simUI, simLP, Resnik's, Lin's, and Jiang and Conrath's measures on their ability to characterize human regulatory pathways. They concluded that the pairwise approaches tested performed better than groupwise approaches, with Resnik's measure being the best performing overall [43].
Wang et al. tested their measure against Resnik's by clustering gene pairs according to their semantic similarity and comparing the resulting clusters with a human perspective. They showed that their measure produced results closer to human expectations [25].
Recently, Pesquita et al. have done an evaluation of semantic similarity measures by modeling their behavior as function of sequence similarity. These authors compared Resnik's, Lin's, and Jiang and Conrath's measures, using several combination strategies, and also the groupwise measures simUI and simGIC, showing that the relationship between semantic similarity and sequence similarity is not linear for any of these measures. They found that the average and maximum combination strategies produce worse results than the best-match average and, consistent with Lord's and Sevilla's results, found that Resnik's measure performs better than the other two term-based measures. Overall the best measure for gene product similarity was shown to be simGIC, slightly surpassing Resnik's measure using the “pairwise-best pairs-average” approach in resolution [44].
Xu et. al have also conducted an evaluation study using protein–protein interactions and gene expression datasets of Saccharomyces cerevisiae as the standard [45]. They tested the maximum and average techniques with Resnik's term similarity, and also Tao's, Schlicker's, and Wang's measures with receiver operator characteristic (ROC) analysis. The maximum approach was found to be the best performer in all GO categories. A positive influence of the number of annotations per gene product was also found.
Mistry et al. evaluated eleven measures: Resnik, Lin, and Jiang and Conrath with both the average and maximum approaches; three vector-based measures (cosine, weighted cosine, and kappa); and TO and NTO. They investigated the correlation between measures and the correlation with sequence similarity. They found a good correlation between TO and Resnik's maximum and average. These three measures also correlated well to sequence similarity, with TO presenting the highest correlation.
What we can draw from these studies is that there is no clear best measure for comparing terms or gene products. Different measures have performed differently under different circumstances, and a given measure can be well suited for a specific task but perform poorly in another. For instance, simUI was found by Guo et al. to be the weakest measure when evaluated for its ability to characterize human regulatory pathways, while Pesquita et al. found it to be fairly good when evaluated against sequence similarity. However, one result has been obtained consistently: pairwise measures using Resnik's term similarity outperform Lin's and Jiang & Conrath's methods in all studies except family similarity.
There is also no clear best strategy for evaluating GO-based semantic similarity measures; there are arguments for and against most of the strategies employed. For instance, sequence similarity is well known to be related to functional similarity, but it is just as well known that there are gene products with similar sequences but distinct functions and vice-versa. Another example are Pfam families, which are related to global functional aspects of gene products, but will likely not be suitable to compare with detailed GO annotations.
Semantic Similarity Implementations
The rise in number of semantic similarity measures was accompanied by the development of tools to calculate them. Currently available tools fall into three categories: Web tools, standalone tools, or R packages (see Table 5).
Table 5. Tools for GO-based semantic similarity measures.
| Tool | Format Available | Measures Implemented | Input Sizea | Annotation Types | Extras |
| FuSSiMeG | Web | Several | 2 | All | None |
| GOToolBox | Web | Several | Unlimited | Single | Representation, Clustering, Semantic retrieval |
| ProteInOn | Web | Several | 10 | All/manual | Protein interaction |
| G-SESAME | Web | Wang et al. [25] | 2 | All manual, Single manual | Clustering, Filter by species |
| FunSimMat | Web | Several | Unlimited | All | Filter by protein family, Filter by species |
| DynGO | Standalone | AVG(Resnik) | Unlimited | All ECs | Visualization, Browsing, Semantic retrieval |
| UTMGO | Standalone | Othman et al. [11] | NA | IEA/non-IEA | Semantic retrieval of terms |
| SemSim | R | Several | NA | all/non-IEA | Support for clustering, filter by species |
| GOvis | R | simLP+simUI | NA | All | Visualization |
| csbl.go | R | Several | NA | NA | Clustering |
Acceptable number of terms or gene products.
Web tools, such as FuSSiMeG (http://xldb.fc.ul.pt/rebil/ssm) [46], GOToolBox (http://burgundy.cmmt.ubc.ca/GOToolBox) [32], ProteInOn (http://xldb.fc.ul.pt/biotools/proteinon) [47], FunSimMat (http://funsimmat.bioinf.mpi-inf.mpg.de) [48], and G-SESAME (http://bioinformatics.clemson.edu/G-SESAME) [25], are readily available and simple to use, and their maintenance and updating are at the expense of the provider. However, they are limited to a pre-set number of options.
Standalone applications, such as DynGO (http://gauss.dbb.georgetown.edu/liblab/dyngo.html) [49] and UTMGO (available upon request) [11], have the advantages that they are not limited in the size of input data (unlike many Web tools) and can support heavier computations (DynGO supports semantic retrieval of both similar terms and gene products, while UTMGO supports retrieval of similar terms). However, they require a local installation, which can be less appealing for some users (DynGO in particular works as a server–client application), and must be updated by the end user.
The R packages SemSim (http://www.bioconductor.org/packages/2.2/bioc/html/SemSim.html) and GOvis (http://bioconductor.org/packages/2.3/bioc/html/GOstats.html) are part of the Bioconductor project, which comprises many R packages for bioinformatics and biostatistics applications. A more recent R based package for semantic similarity, csbl.go (http://csbi.ltdk.helsinki.fi/csbl.go/) [50], also implements semantic similarity-based clustering, but it relies on users to load annotation data. The main advantage of these implementations is the possibility of integration between the semantic similarity and other packages, such as visualization tools or statistical analysis.
Although no single tool implements all existing semantic similarity measures, FuSSiMeG, ProteInOn, FunSimMat, SemSim, and csbl.go provide a wide range of them, and enable the user to choose one or even to compare several (in the case of FunSimMat). Most of the tools mentioned above also provide other types of services and information, such as protein interactions (ProteInOn), GO graph visualization (GOvis, DynGO), GO browsing (DynGO), and clustering (GOToolBox, G-SESAME, csbl.go).
Semantic Similarity Applications
The application of semantic similarity allows gene products to be compared at different levels. This section presents several scenarios in which GO-based semantic similarity measures have been successfully applied.
For instance, GO-based semantic similarity can be used to compare gene products by their biochemical function (molecular function), the cellular and supracellular processes in which they are involved (biological process), and their cellular or extracellular location (cellular component). Comparing the molecular function aspect, we can measure the functional similarity between gene products and gain insight into function-related characteristics such as domains and active sites. The biological process aspect can be related to protein interaction, both physical and indirect (involved in the same process network), and thus can be used to predict them and to analyze coexpression data. The cellular component aspect can be linked to colocalization and in that context be used to validate physical interaction and localization-dependent functions and processes. Overall, GO-based semantic similarity measures have been applied mainly for validating and predicting functions and interactions, and for analyzing transcriptomics and proteomics data.
Automated prediction of gene product function has been a cornerstone of genome annotation, because experimental methods are too costly and time consuming to be able to cope with the size and continuous growth of genetic data [51]. Semantic similarity can be used to assess the performance of automated function prediction methods (as was used in the Automated Function Prediction 2005 Special Interest Group meeting of ISMB 2005 in Detroit, USA) and to validate their results [52],. It has also been used as a component of several function prediction systems, based on protein–protein interactions [54], on structural similarity of protein surface [55], and on clustering using semantic similarity [56]; and to validate automatic annotations [57]. Tao et al. have developed a function prediction system in which annotations are transferred between proteins with the only criterion being their semantic similarity [28].
Semantic similarity can also play an important role in both predicting and validating gene product interactions and interaction networks. Regarding prediction, some authors developed methods based solely on semantic similarity [23],[41], whereas others combined semantic similarity with gene expression data [43],[58],[59]. As for validation of interactions, semantic similarity has been used to select a negative dataset of noninteracting proteins to assess prediction methods [60], to improve the performance of predictions by excluding false positives [61], and to assess the quality of predicted interaction networks by comparing them to experimentally verified interactions [48]. Also in the context of interactions, semantic similarity has been used to extract functional modules from interaction networks [62], to align biological pathways [63] to generate functionally meaningful network subsets [64], and to characterize protein interaction networks to support breast cancer outcome prediction [65].
In the analysis of transcriptomics and proteomics data, the main role of semantic similarity has been to improve the clustering of coexpressed gene products by taking into account their functional similarity [56], [66]–[69]. However, it can also be used to link and compare results from different assays [30], to improve data quality [70], and to validate gene selection for biomedical purposes [71].
Other biological applications of semantic similarity include determining interfold similarity based on sequence/structure fragments [72], evaluating the biological significance of coexpressed chromosome domains [73], predicting domain distances [2], and predicting cellular localization [29]. There are also other applications such as integration of semantic search [75],[76].
Unfortunately, most application studies use only one measure and results are not comparable across studies, making it difficult to assess which measure is best for which purpose. However, based on the few comparative studies that exist, we can identify the most successful measures so far in the three main applications of GO-based semantic similarity: function prediction/validation, protein–protein interaction prediction/validation, and cellular location prediction (see Table 6).
Table 6. Best measures for the main applications of GO-based semantic similarity measures.
| Application | Best Measure | Reference |
| Functiona p/v | BMA(Resnik)/simGIC | [44] |
| Protein-protein interaction p/v | Max(Resnik) | [43],[45] |
| Cellular location prediction | SUM(EM) | [29] |
Identified by sequence similarity.
p/v, prediction/validation.
Discussion
Semantic similarity measures are dependent on the quality and completeness of both the ontology and the annotated corpus they use. Biomedical ontologies have several irregularities, such as variable edge length (edges at the same level convey different semantic distances), variable depth (terms at the same level have different levels of detail), and variable node density (some areas of the ontology have a greater density of terms than others). These are due to the irregular nature of biomedical knowledge and our limited understanding of it, and should be taken into account by semantic similarity measures, particularly edge-based measures, which are sensible to these issues. There are also problems with the use of annotated corpora in node-based measures, because these are biased by biomedical research interests (since terms related to subjects of research focus are expected to be more frequently annotated than other terms) and limited by current experimental techniques (if the currently most popular technique can discover gene product functional aspects only to a certain level of detail, then more detailed terms will seldom be used for annotation). Despite these issues, the use of IC on semantic similarity measures still makes sense probabilistically, if not always semantically, because commonly used terms are more likely to be shared by gene products than uncommonly used terms.
Another issue particular to GO is that not all annotations are equal, because evidence codes show the type of evidence that supports the annotation. While most evidence codes symbolize experimental methods and the corresponding annotations are manually curated, the most prevalent evidence code (IEA) indicates the annotation was inferred electronically (by sequence similarity, from other databases, or from keyword mapping files) and not manually curated. Whether IEA annotations should be used or disregarded for semantic similarity is still a subject of open debate, because using them entails a loss of precision but not using them entails a loss of coverage (over 97% of all annotations in the GOA-UniProt database are IEA, and less than 2% of the GO terms have non-IEA annotations [77]). As the proportion of IEA annotations continues to increase, and they steadily improve in quality (up to 91%–100% precision having been reported [78]), there will be fewer reasons to ignore them, and they will eventually be widely adopted. Since IEA annotations are usually made by inference through similarity to sequences of model organisms, improvements in the experimental annotation of model organisms will result in higher-quality IEA annotations. Meanwhile, perhaps the best way to decide whether to include or discard IEA annotations for a particular study is to first analyze the corpus in question and verify if the gene products of interest are well characterized with manually curated annotations or if IEA annotations are essential for their characterization. Note that using different sets of annotations (such as all, or just the manually curated ones, etc.) will have an impact in the IC values calculated for the terms, preventing their comparison. It is also important to stress that only results obtained with the same versions of GO's ontology and annotation data are comparable, since changes to both the ontologies and the annotations made with them affects semantic similarity .
An important issue in the evaluation of semantic similarity measures based on the IC is that of data circularity between the data used to evaluate the measures and the GO annotations. For instance, if a given measure is evaluated by correlation with sequence similarity, then using annotations based on sequence similarity (those with evidence codes ISS and IEA) to calculate the IC leads to data circularity, as there is direct dependency between both data sources. The same is true for the use of annotations inferred by physical interaction (IPI) when a measure is evaluated by correlation with protein-protein interactions, and other similar cases. To minimize the effect of data circularity, evaluation studies should (and usually do) remove annotations based on evidence of the same nature as the data used to evaluate the measures.
Data circularity is not the only problem in evaluating GO-based semantic similarity measures. The lack of a gold standard for evaluating semantic similarity makes it hard to assess the quality of the different measures and to find out which are best for which goals. One of the reasons for the continued lack of a gold standard is that measures are often developed for specific goals and evaluated only in that context. Furthermore, there are pros and cons to all data sources used to evaluate semantic similarity. The best solution is likely that a gold standard be designed by experts to cover most applications of semantic similarity, not based on proxies for true functional similarity, such as sequence similarity or gene coexpression.
How to Choose a Semantic Similarity Measure
Researchers who wish to employ semantic similarity in their work need to spend some time defining their requirements to choose an adequate measure. Since different measures interpret ontology and annotation information in different ways, researchers need to understand the differences and decide which interpretation is best suited to their needs. Below, we outline some of the steps that should be taken before choosing a semantic similarity measure.
Identify your scope: Comparing one aspect versus comparing multiple aspects of gene products;
Identify your level of detail: Comparing gene products in specific functions or overall similarity; and
Analyze the annotations of your dataset: Determining the number of annotations per gene product, including and excluding IEA annotations and annotation depth.
When wishing to compare single aspects of gene products, researchers should opt for maximum approaches (“pairwise–all pairs–maximum”). These will give a measure of how similar two gene products are at their most similar aspect. For comparing multiple aspects, the best measures are “pairwise–best pairs–average” or groupwise approaches, since they allow for the comparison of several terms. However, depending on the level of detail desired, “pairwise–best pairs–average” or “groupwise–set” should be used for a higher degree of specificity (since only direct annotations are used) and “groupwise–vector” or graph for a more generalized similarity (since all annotations are used). To further minimize the relevance of specificity, unweighted graph or vector measures can be employed, so that high-level GO terms are not considered less relevant.
However, having to analyze the dataset before deciding which measure to use can be cumbersome to researchers who just need a “quick and dirty” semantic similarity calculation. In this case, researchers should resort to one of the several semantic similarity tools available and use their good judgment in analyzing the results. Most semantic similarity measures proposed so far have shown fair if not good results, and for less detailed analyses any one of them can give a good overview of the similarities between gene products.
Conclusions
Over the last decade, ontologies have become an increasingly important component of biomedical research studies, because they provide the formalism, objectivity, and common terminology necessary for researchers to describe their results in a way that can be easily shared and reused by both humans and computers. One benefit of the use of ontologies is that concepts, and entities annotated with those concepts, can be objectively compared through the use of semantic similarity measures. Although the use of semantic similarity in the biomedical field is recent, there is already a wide variety of measures available that can be classified according to the approach they use.
There are two main semantic similarity approaches for comparing concepts: edge-based, which rely on the structure of the ontology; and node-based, which rely on the terms themselves, using information content to quantify their semantic meaning. Node-based measures are typically more reliable in the biomedical field because most edge-based measures assume that all relationships in an ontology are either equidistant or have a distance as function of the depth, neither of which is true for existing biomedical ontologies.
Because biomedical entities are often annotated with several concepts, semantic similarity measures for comparing entities need to rely on sets of concepts rather than single concepts. There are two main approaches for this comparison: pairwise, in which entities are represented as lists of concepts that are then compared individually; and groupwise, in which the annotation graphs of each entity are compared as a whole.
Several studies have been conducted to assess the performance of different similarity measures, by correlating semantic similarity with biological properties such as sequence similarity, or other classification schemas such as Pfam. Most measures were shown to perform well, but as few comprehensive studies have been conducted, it is difficult to draw any conclusion about which measure is best for any given goal.
Until now, most research efforts in this area developed novel measures or adapted preexisting ones to biomedical ontologies, with most novel measures sporting an increased complexity compared to previous ones. This increased complexity is mainly a result of more recent measures combining several strategies to arrive at a final score. Although the need for improved measures is unquestionable, this trend fails to answer the most pressing community needs: (1) easy application to both small and large datasets, which would be best achieved by developing tools that are at once easy to use and powerful; and (2) elucidation of which measure is better fitted to the researcher's needs, which would imply comparative studies of all existing measures and approaches.
Although important efforts in these two areas have already been made, semantic similarity is still far from reaching the status of other gene product similarities, such as sequence-based ones, in which fast and reliable algorithms coupled with the development of ever-growing databases has made them the cornerstone of present day molecular biology. One important step forward would be the development of a gold standard for gene product function that would allow the effective comparison of semantic similarity measures. Nevertheless, semantic similarity is not restricted to gene products and it can be expected that, as more biomedical ontologies are developed and used, these measures will soon be applied to different scenarios. It is then crucial that bioinformaticians focus on strategies to make semantic similarity a practical, useful, and meaningful approach to biological questions.
Footnotes
The authors have declared that no competing interests exist.
This work was supported by the Portuguese Fundacao para a Ciencia e Tecnologia through the Multiannual Funding Programme, and the PhD grants SFRH/BD/42481/2007 and SFRH/BD/29797/2006. The funder approved the PhD plans of the two students. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.GO-Consortium. The Gene Ontology (GO) database and informatics resource. Nucleic Acids Research. 2004;32:D258–D261. doi: 10.1093/nar/gkh036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joslyn C, Mniszewski S, Fulmer A, Heaton G. The gene ontology categorizer. Bioinformatics. 2004;20:i169–177. doi: 10.1093/bioinformatics/bth921. [DOI] [PubMed] [Google Scholar]
- 3.Rada R, Mili H, Bicknell E, Blettner M. Development and application of a metric on semantic nets. 1989. pp. 17–30. In: IEEE Transaction on Systems, Man, and Cybernetics. 19.
- 4.Wu Z, Palmer MS. Verb semantics and lexical selection. Proceedings of the 32nd. Annual Meeting of the Association for Computational Linguistics (ACL 1994) 1994. pp. 133–138. URL http://dblp.uni-trier.de/db/conf/acl/acl94.html#WuP94.
- 5.Budanitsky A. Lexical semantic relatedness and its application in natural language processing. 1999. URL http://citeseer.ist.psu.edu/budanitsky99lexical.html.
- 6.Richardson R, Smeaton AF, Murphy J. Using WordNet as a knowledge base for measuring semantic similarity between words. 1994. Technical Report CA-1294, Dublin, Ireland. URL citeseer.ist.psu.edu/richardson94using.html.
- 7.Seco N, Veale T, Hayes J. An intrinsic information content metric for semantic similarity in wordnet. ECAI. 2004. pp. 1089–1090.
- 8.Resnik P. Using information content to evaluate semantic similarity in a taxonomy. Proc. of the 14th International Joint Conference on Artificial Intelligence. 1995. pp. 448–453.
- 9.Couto FM, Silva MJ, Coutinho PM. Semantic similarity over the gene ontology: Family correlation and selecting disjunctive ancestors. Proc. of the ACM Conference in Information and Knowledge Management as a short paper. 2005.
- 10.Budanitsky A. Semantic distance in wordnet: An experimental, application-oriented evaluation of five measures. 2001. URL citeseer.ist.psu.edu/budanitsky01semantic.html.
- 11.Othman R, Deris S, Illias R. A genetic similarity algorithm for searching the gene ontology terms and annotating anonymous protein sequences. J Biomed Inform. 2007;23:529–38. doi: 10.1016/j.jbi.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Lord P, Stevens R, Brass A, Goble C. Investigating semantic similarity measures across the Gene Ontology: the relationship between sequence and annotation. Bioinformatics. 2003;19:1275–1283. doi: 10.1093/bioinformatics/btg153. [DOI] [PubMed] [Google Scholar]
- 13.Lin D. Proc. of the 15th International Conference on Machine Learning. San Francisco, CA: Morgan Kaufmann; 1998. An information-theoretic definition of similarity. pp. 296–304. [Google Scholar]
- 14.Jiang J, Conrath D. Semantic similarity based on corpus statistics and lexical taxonomy. In: Proc. of the 10th International Conference on Research on Computational Linguistics, Taiwan. 1997.
- 15.Lord P, Stevens R, Brass A, Goble C. Semantic similarity measures as tools for exploring the gene ontology. Proc. of the 8th Pacific Symposium on Biocomputing. 2003. pp. 601–612. [DOI] [PubMed]
- 16.Schlicker A, Domingues FS, Rahnenführer J, Lengauer T. A new measure for functional similarity of gene products based on gene ontology. BMC Bioinformatics. 2006;7 doi: 10.1186/1471-2105-7-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bodenreider O, Aubry M, Burgun A. Non-lexical approaches to identifying associative relations in the gene ontology. Pac Symp Biocomput. 2005:91–102. URL http://view.ncbi.nlm.nih.gov/pubmed/15759617. [PMC free article] [PubMed] [Google Scholar]
- 18.Riensche RM, Baddeley BL, Sanfilippo AP, Posse C, Gopalan B. Xoa: Web-enabled cross-ontological analytics. 2007. pp. 99–105. In: Services, 2007 IEEE Congress on.
- 19.Pekar V, Staab S. Proceedings of the 19th international conference on Computational linguistics. Morristown, NJ, USA: Association for Computational Linguistics; 2002. Taxonomy learning: factoring the structure of a taxonomy into a semantic classification decision. pp. 1–7. doi: http://dx.doi.org/10.3115/1072228.1072318. [Google Scholar]
- 20.Yu H, Gao L, Tu K, Guo Z. Broadly predicting specific gene functions with expression similarity and taxonomy similarity. Gene. 2005;352:75–81. doi: 10.1016/j.gene.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 21.Cheng J, Cline M, Martin J, Finkelstein D, Awad T, et al. A knowledge-based clustering algorithm driven by gene ontology. Journal of Biopharmaceutical Statistics. 2004;14:687–700. doi: 10.1081/bip-200025659. [DOI] [PubMed] [Google Scholar]
- 22.Wu H, Su Z, Mao F, Olman V, Xu Y. Prediction of functional modules based on comparative genome analysis and gene ontology application. Nucleic Acids Res. 2005;33:2822–2837. doi: 10.1093/nar/gki573. URL http://view.ncbi.nlm.nih.gov/pubmed/15901854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu X, Zhu L, Guo J, Zhang DY, Lin K. Prediction of yeast proteinprotein interaction network: insights from the gene ontology and annotations. Nucleic Acids Research. 2006;34:2137–2150. doi: 10.1093/nar/gkl219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pozo AD, Pazos F, Valencia A. Defining functional distances over gene ontology. BMC Bioinformatics. 2008;9 doi: 10.1186/1471-2105-9-50. doi: http://dx.doi.org/10.1186/1471-2105-9-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang JZZ, Du Z, Payattakool R, Yu PSS, Chen CFF. A new method to measure the semantic similarity of go terms. Bioinformatics. 2007 doi: 10.1093/bioinformatics/btm087. doi: http://dx.doi.org/10.1093/bioinformatics/btm087. [DOI] [PubMed] [Google Scholar]
- 26.Sevilla JL, Segura V, Podhorski A, Guruceaga E, Mato JM, et al. Correlation between gene expression and go semantic similarity. IEEE/ACM Transactions on Computational Biology and Bioinformatics. 2005. [DOI] [PubMed]
- 27.Azuaje F, Wang H, Bodenreider O. Ontology-driven similarity approaches to supporting gene functional assessment. Proceedings of the ISMB 2005 SIG meeting on Bio-ontologies. 2005.
- 28.Tao Y, Sam L, Li J, Friedman C, Lussier Y. Information theory applied to the sparse gene ontology annotation network to predict novel gene function. Bioinformatics. 2007;23:529–38. doi: 10.1093/bioinformatics/btm195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lei Z, Dai Y. Assessing protein similarity with gene ontology and its use in subnuclear localization prediction. BMC Bioinformatics. 2006;7 doi: 10.1186/1471-2105-7-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee HK, Hsu AK, Sajdak J, Qin J, Pavlidis P. Coexpression analysis of human genes across many microarray data sets. Genome Res. 2004;14:1085–1094. doi: 10.1101/gr.1910904. doi: http://dx.doi.org/10.1101/gr.1910904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mistry M, Pavlidis P. Gene ontology term overlap as a measure of gene functional similarity. BMC Bioinformatics. 2008;9 doi: 10.1186/1471-2105-9-327. URL http://www.biomedcentral.com/1471-2105/9/327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin D, Brun C, Remy E, Mouren P, Thieffry D, et al. Gotoolbox: functional analysis of gene datasets based on gene ontology. Genome Biol. 2004;5 doi: 10.1186/gb-2004-5-12-r101. doi: http://dx.doi.org/10.1186/gb-2004-5-12-r101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gentleman R. Visualizing and Distances Using GO. 2005. URL http://www.bioconductor.org/docs/vignettes.html.
- 34.Pesquita C, Faria D, Bastos H, Falcão AO, Couto F. Evaluating gobased semantic similarity measures. ISMB/ECCB 2007 SIG Meeting Program Materials. International Society for Computational Biology. 2007.
- 35.Ye P, Peyser BD, Pan X, Boeke JD, Spencer FA, et al. Gene function prediction from congruent synthetic lethal interactions in yeast. Molecular Systems Biology. 2005;1:msb4100034–E1–msb4100034–E12. doi: 10.1038/msb4100034. doi: http://dx.doi.org/10.1038/msb4100034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cho YR, Hwang W, Ramanathan M, Zhang A. Semantic integration to identify overlapping functional modules in protein interaction networks. BMC Bioinformatics. 2007;8:265+. doi: 10.1186/1471-2105-8-265. doi: http://dx.doi.org/10.1186/1471-2105-8-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin N, Wu B, Jansen R, Gerstein M, Zhao H. Information assessment on predicting protein-protein interactions. BMC Bioinformatics. 2004;5 doi: 10.1186/1471-2105-5-154. doi: http://dx.doi.org/10.1186/1471-2105-5-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu H, Jansen R, Gerstein M. Developing a similarity measure in biological function space. Bioinformatics. 2007 URL http://view.ncbi.nlm.nih.gov/pubmed/17540677. [Google Scholar]
- 39.Sheehan B, Quigley A, Gaudin B, Dobson S. A relation based measure of semantic similarity for gene ontology annotations. BMC Bioinformatics. 2008;9 doi: 10.1186/1471-2105-9-468. doi: http://dx.doi.org/10.1186/1471-2105-9-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang DW, Sherman BT, Tan Q, Collins JR, Alvord GW, et al. David gene functional classification tool: A novel biological modulecentric algorithm to functionally analyze large gene list. Genome Biology. 2007;8:R183+. doi: 10.1186/gb-2007-8-9-r183. doi: http://dx.doi.org/10.1186/gb-2007-8-9-r183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chabalier J, Mosser J, Burgun A. A transversal approach to predict gene product networks from ontology-based similarity. BMC Bioinformatics. 2007;2:235. doi: 10.1186/1471-2105-8-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang H, Azuaje F, Bodenreider O, Dopazo J. Gene expression correlation and gene ontology-based similarity: an assessment of quantitative relationships. Computational Intelligence in Bioinformatics and Computational Biology, 2004. CIBCB '04. Proceedings of the 2004 IEEE Symposium on. 2004. pp. 25–31. URL http://ieeexplore.ieee.org/xpls/abs all.jsp?arnumber=1393927. [DOI] [PMC free article] [PubMed]
- 43.Guo X, Liu R, Shriver CD, Hu H, Liebman MN. Assessing semantic similarity measures for the characterization of human regulatory pathways. Bioinformatics. 2006;22:967–973. doi: 10.1093/bioinformatics/btl042. doi: 10.1093/bioinformatics/btl042. URL http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieven&db=pubmedn&dopt=Abstractn&list uids=16492685. [DOI] [PubMed] [Google Scholar]
- 44.Pesquita C, Faria D, Bastos H, Falcão AO, Couto F. Metrics for go based protein semantic similarity: a systematic evaluation. BMC Bioinformatics. 2008;9 doi: 10.1186/1471-2105-9-S5-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu T, Du L, Zhou Y. Evaluation of go-based functional similarity measures using s. cerevisiae protein interaction and expression profile data. BMC Bioinformatics. 2008;9 doi: 10.1186/1471-2105-9-472. doi: http://dx.doi.org/10.1186/1471-2105-9-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Couto F, Silva MJ. Implementation of a functional semantic similarity measure between gene-products. 2003. Technical Report DI/FCUL TR 3-29, FCUL. URL http://www.di.fc.ul.pt/tech-reports/03-29.pdf.
- 47.Faria D, Pesquita C, Couto F, Falcão A. Proteinon: A web tool for protein semantic similarity. 2007. DI/FCUL TR 07–6, Department of Informatics, University of Lisbon. URL http://www.di.fc.ul.pt/tech-reports/07-6.pdf.
- 48.Schlicker A, Albrecht M. Funsimmat: a comprehensive functional similarity database. Nucl Acids Res. 2007:gkm806+. doi: 10.1093/nar/gkm806. doi: http://dx.doi.org/10.1093/nar/gkm806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu H, Hu ZZ, Wu CH. Dyngo: a tool for visualizing and mining of gene ontology and its associations. BMC bioinformatics. 2005;6 doi: 10.1186/1471-2105-6-201. doi: http://dx.doi.org/10.1186/1471-2105-6-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ovaska K, Laakso M, Hautaniemi S. Fast gene ontology based clustering for microarray experiments. BioData mining. 2008;1 doi: 10.1186/1756-0381-1-11. doi: http://dx.doi.org/10.1186/1756-0381-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Friedberg I. Automated protein function prediction-the genomic challenge. Briefings in Bioinformatics. 2006;7:225–242. doi: 10.1093/bib/bbl004. [DOI] [PubMed] [Google Scholar]
- 52.Duan ZH, Hughes B, Reichel L, Shi T. IMSCCS '06: Proceedings of the First International Multi-Symposiums on Computer and Computational Sciences - Volume 1 (IMSCCS'06) Washington, DC, USA: IEEE Computer Society; 2006. The relationship between protein sequences and their gene ontology functions. pp. 76–83. doi: http://dx.doi.org/10.1109/IMSCCS.2006.133. [Google Scholar]
- 53.Ye P, Peyser BD, Pan X, Boeke JD, Spencer FA, et al. Gene function prediction from congruent synthetic lethal interactions in yeast. Molecular Systems Biology. 2005;1:msb4100034–E1–msb4100034–E12. doi: 10.1038/msb4100034. doi: http://dx.doi.org/10.1038/msb4100034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu M, Gao L, Guo Z, Li Y, Wang D, et al. Globally predicting protein functions based on co-expressed protein-protein interaction networks and ontology taxonomy similarities. Gene. 2007;391:113–119. doi: 10.1016/j.gene.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 55.Liu ZP, Wu LY, Wang Y, Chen L, Zhang XS. Predicting gene ontology functions from protein's regional surface structures. BMC Bioinformatics. 2007;8 doi: 10.1186/1471-2105-8-475. doi: http://dx.doi.org/10.1186/1471-2105-8-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen JL, Liu Y, Sam LT, Li J, Lussier YA. Evaluation of highthroughput functional categorization of human disease genes. BMC Bioinformatics. 2007;8(Suppl 3) doi: 10.1186/1471-2105-8-S3-S7. doi: http://dx.doi.org/10.1186/1471-2105-8-S3-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Couto F, Silva M, Lee V, Dimmer E, Camon E, et al. Goannotator: linking protein go annotations to evidence text. Journal of Biomedical Discovery and Collaboration. 2006;1:19. doi: 10.1186/1747-5333-1-19. doi: 10.1186/1747-5333-1-19. URL http://www.biomedcentral.com/1747-5333/1/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee PH, Lee D. Modularized learning of genetic interaction networks from biological annotations and mrna expression data. Bioinformatics. 2005;21:2739–2747. doi: 10.1093/bioinformatics/bti406. URL http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieven&db=pubmedn&dopt=Abstractn&list uids=15797909. [DOI] [PubMed] [Google Scholar]
- 59.Brown KR, Jurisica I. Online predicted human interaction database. Bioinformatics. 2005;21:2076–2082. doi: 10.1093/bioinformatics/bti273. URL http://view.ncbi.nlm.nih.gov/pubmed/15657099. [DOI] [PubMed] [Google Scholar]
- 60.Hur AB, Noble W. Choosing negative examples for the prediction of protein-protein interactions. BMC Bioinformatics. 2006;7 doi: 10.1186/1471-2105-7-S1-S2. doi: http://dx.doi.org/10.1186/1471-2105-7-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mahdavi MA, Lin YH. False positive reduction in protein-protein interaction predictions using gene ontology annotations. BMC Bioinformatics. 2007;8 doi: 10.1186/1471-2105-8-262. doi: http://dx.doi.org/10.1186/1471-2105-8-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zheng H, Wang H, Glass DH. Integration of genomic data for inferring protein complexes from global protein-protein interaction networks. IEEE Trans Syst Man Cybern B Cybern. 2008;38:5–16. doi: 10.1109/TSMCB.2007.908912. doi: http://dx.doi.org/10.1109/TSMCB.2007.908912. [DOI] [PubMed] [Google Scholar]
- 63.Gamalielsson J, Olsson B. B.: Gosap: Gene ontology based semantic alignment of biological pathways. 2005. Technical report, Skovde University. [DOI] [PubMed]
- 64.Lubovac Z, Gamalielsson J, Olsson B. Combining functional and topological properties to identify core modules in protein interaction networks. Proteins: Structure, Function, and Bioinformatics. 2006;64:948–959. doi: 10.1002/prot.21071. doi: http://dx.doi.org/10.1002/prot.21071. [DOI] [PubMed] [Google Scholar]
- 65.Taylor IWW, Linding R, Warde-Farley D, Liu Y, Pesquita C, et al. Dynamic modularity in protein interaction networks predicts breast cancer outcome. Nature biotechnology. 2009 doi: 10.1038/nbt.1522. doi: http://dx.doi.org/10.1038/nbt. 1522. [DOI] [PubMed] [Google Scholar]
- 66.Speer N, Spieth C, Zell A. A memetic clustering algorithm for the functional partition of genes based on the gene ontology. 2004. pp. 252–259. In: Computational Intelligence in Bioinformatics and Computational Biology, 2004. CIBCB apos;04. Proceedings of the 2004 IEEE Symposium on.
- 67.Wang H Azuaje O Fand Bodenreider. An ontology-driven clustering method for supporting gene expression analysis. 2005. pp. 389–394. In: Computer-Based Medical Systems, 2005. Proceedings. 18th IEEE Symposium on.
- 68.Kustra R, Zagdanski A. CBMS. IEEE Computer Society; 2006. Incorporating gene ontology in clustering gene expression data. pp. 555–563. [Google Scholar]
- 69.Wolting C, Mcglade JC, Tritchler D. Cluster analysis of protein array results via similarity of gene ontology annotation. BMC Bioinformatics. 2006;7 doi: 10.1186/1471-2105-7-338. doi: http://dx.doi.org/10.1186/1471-2105-7-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tuikkala J, Elo L, Nevalainen O, Aittokallio T. Improving missing value estimation in microarray data with gene ontology. Bioinformatics. 2006;22:566–572. doi: 10.1093/bioinformatics/btk019. URL http://n.uni-trier.de/db/journals/bioinformatics/bioinformatics22.html#TuikkalaENA06. [DOI] [PubMed] [Google Scholar]
- 71.Duan KB, Rajapakse JC, Wang H, Azuaje F. Multiple svm-rfe for gene selection in cancer classification with expression data. IEEE Trans Nanobioscience. 2005;4:228–234. doi: 10.1109/tnb.2005.853657. URL http://view.ncbi.nlm.nih.gov/pubmed/16220686. [DOI] [PubMed] [Google Scholar]
- 72.Friedberg I, Godzik A. Connecting the protein structure universe by using sparse recurring fragments. Structure. 2005;13:1213–1224. doi: 10.1016/j.str.2005.05.009. doi: http://dx.doi.org/10.1016/j.str.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 73.Li H, Liu Y, Shin S, Sun Y, Loring JF, et al. Transcriptome coexpression map of human embryonic stem cells. BMC Genomics. 2006;7:103. doi: 10.1186/1471-2164-7-103. URL http://www.citebase.org/abstract?id=oai:biomedcentral.com:1471-2164-7-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bjorklund AK, Ekman D, Light S, Frey-Skott J, Elofsson A. Domain rearrangements in protein evolution. Journal of Molecular Biology. 2005;353:911–923. doi: 10.1016/j.jmb.2005.08.067. doi: http://dx.doi.org/10.1016/j.jmb.2005.08.067. [DOI] [PubMed] [Google Scholar]
- 75.Cao S, Qin L, He W, Zhong Y, Zhu Y, et al. Semantic search among heterogeneous biological databases based on gene ontology. Acta Biochim Biophys Sin (Shanghai) 2004;36:365–70. doi: 10.1093/abbs/36.5.365. [DOI] [PubMed] [Google Scholar]
- 76.Vanteru B, Shaik J, Yeasin M. Semantically linking and browsing pubmed abstracts with gene ontology. BMC Genomics. 2008;9 doi: 10.1186/1471-2164-9-S1-S10. doi: http://dx.doi.org/10.1186/1471-2164-9-S1-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Camon E, Magrane M, Barrell D, Lee V, Dimmer E, et al. The gene ontology annotation (goa) database: sharing knowledge in uniprot with gene ontology. Nucleic Acids Research. 2004;32:D262. doi: 10.1093/nar/gkh021. URL http://www. citebase.org/abstract?id=oai:pubmedcentral.gov:308756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Camon EB, Barrell DG, Dimmer EC, Lee V, Magrane M, et al. An evaluation of go annotation retrieval for biocreative and goa. BMC Bioinformatics. 2005;6(Suppl 1) doi: 10.1186/1471-2105-6-S1-S17. doi: 10.1186/1471-2105-6-S1-S17. [DOI] [PMC free article] [PubMed] [Google Scholar]