Abstract
Soluble egg antigen (SEA) from the helminth Schistosoma mansoni promotes T helper type 2 (Th2) responses by modulating antigen-presenting cell function. The Jagged/Notch pathway has recently been implicated in driving Th2 development. We show here that SEA rapidly up-regulated mRNA and protein expression of the Notch ligand Jagged-1 in both murine bone marrow-derived macrophages (BMMs) and human monocyte-derived macrophages (HMDMs). Another potential Th2-promoting factor, interleukin (IL)-33, was not transcriptionally induced by SEA in BMMs. Up-regulation of Jagged-1 mRNA by SEA was also apparent in conventional dendritic cells (DCs), although the effect was less striking than in BMMs. Conversely, SEA-pulsed DCs, but not BMMs, promoted IL-4 production upon T-cell activation, suggesting that Jagged-1 induction alone is insufficient for instructing Th2 development. A comparison of the responses initiated in BMMs by SEA and the bacterial endotoxin lipopolysaccharide (LPS) revealed common activation of extracellular signal-regulated kinase-1/2 (ERK-1/2) and p38 phosphorylation, as well as induction of Jagged-1 mRNA. However, only LPS triggered IκB degradation, phosphorylation of c-Jun N-terminal kinase (Jnk) and signal transducer and activator of transcription 1 (Stat1) Tyr701, and IL-33 and IL-12p40 mRNA up-regulation. Inducible gene expression was modified by the presence of the macrophage growth factor colony-stimulating factor (CSF)-1, which inhibited Jagged-1 induction by SEA and LPS, but enhanced LPS-induced IL-12p40 expression. Unlike LPS, SEA robustly activated signalling in HEK293 cells expressing either Toll-like receptor 2 (TLR2) or TLR4/MD2. Pharmacological inhibition of the ERK-1/2 pathway impaired SEA- and LPS-inducible Jagged-1 expression in BMMs. Taken together, our data suggest that Jagged-1 is an ERK-dependent target of TLR signalling that has a macrophage-specific function in the response to SEA.
Keywords: antigen presentation, helminths, signal transduction, Th2 cells
Introduction
The polarization of acquired immune responses towards T helper type 1 (Th1), Th2 and Th17 phenotypes in infectious disease and autoimmunity determines disease progression and severity. Several cytokines and cell-surface ligands can influence this polarization by acting on Th cells during antigen-induced activation.1 In Th2 differentiation, the most important of these is interleukin (IL)-4, which signals via signal transducer and activator of transcription 6 (Stat6) to trigger transcriptional reprogramming and Th2 development.2 The IL-1R family member ST2, which is expressed selectively on Th2 cells,3 is similarly required for primary Th2 development.4 Accordingly, the ligand for this receptor, IL-33, was found to promote Th2 responses in vivo.5,6 The Notch signalling system has also been implicated in Th2 development. For example, Th2 differentiation was impaired in mice that lack Notch signalling, and ectopic expression of the Notch ligand Jagged-1 on antigen-presenting cells (APCs) promoted Th2 development through a Stat6-independent pathway, leading to the expression of IL-4 and the transcription factor Gata-3.7 Indeed, Notch targeted the Gata-3 promoter directly8 and synergized with this transcription factor to drive IL-4 expression.9
In the context of infectious disease, Th1 and Th2 polarization by APCs is strongly influenced by the type of pathogen encountered. For example, detection of certain pathogen-associated molecular patterns (PAMPs) such as CpG DNA by APCs promotes a potent Th1 response.10 In contrast, parasitic worm infections are characteristically associated with a polarized Th2 response.11 Such effects may be partly mediated by antagonism of Th1-promoting pathways; many helminth components can down-regulate production of IL-12 by APCs in response to Th1-promoting stimuli.12,13 However, even in the absence of a Th1-polarizing stimulus, several helminth products are able to modify APC function to promote Th2 development.14–16 Perhaps most strikingly, dendritic cells (DCs) co-pulsed with both helminth products and bacterial products subsequently directed a Th2-specific response against the helminth product, but a Th1-specific response against the bacterial product in vivo.17 Such data imply that helminths directly up-regulate the production of Th2-promoting factors.
The activation of specific Toll-like receptors (TLRs) by certain PAMPs can elicit a Th2-promoting phenotype in APCs. TLR2 ligands promoted Th2 development,18,19 and low doses of the TLR4 ligand lipopolysaccharide (LPS), which is classically regarded as a Th1-promoting PAMP, also promoted Th2 responses.20,21 The Th2-promoting effects of low-dose LPS may relate to the selective activation of downstream signalling pathways. LPS, which signals through multiple adaptor proteins (MyD88, Mal, TRIF and TRAM),22 primed Th2 development in MyD88-deficient mice,22,23 implying that selective activation of MyD88-independent pathways favours the Th2 phenotype.
The helminth Schistosoma mansoni, a major pathogen of humans and livestock, initiates a potent Th2 response in the host. This Th2 response, which is both host protective and a mediator of disease pathology, is driven by antigens derived from eggs lodged in the liver and lungs [soluble egg antigens (SEAs)].24 As an adjuvant, SEA promoted Th2 cytokine and antibody production in vivo,15 an effect that appears to be mediated, at least in part, by regulation of APC function.25 Components of SEA are recognized via TLR3 and TLR4,16,26 which can both signal in the absence of MyD88.23,27,28 Expression profiling using microarrays has been used to identify SEA-responsive genes in DCs,12 but the exact genes involved in driving Th2 development remain unknown. Of the signal transduction pathways, SEA selectively activated the extracellular signal-regulated kinase-1/2 (ERK-1/2) pathway27 and transiently activated nuclear factor (NF)-κB in DCs,16 but signalling downstream of SEA has not yet been mechanistically linked to Th2 development. Although DCs are clearly central cells in T-cell polarization, in S. mansoni infections, macrophages have also been reported to contribute to the Th2 phenotype,29 and protect against immunopathology by down-regulating Th1 responses.30 In this study, we set out to determine whether SEA regulated the expression of potential Th2-promoting factors (Jagged-1 and IL-33) in macrophages and DCs. In so doing, we found that SEA robustly induced Jagged-1 expression in an ERK-dependent fashion in macrophages, in spite of which, SEA-pulsed macrophages did not promote IL-4 production from splenocytes upon T-cell activation. SEA was a relatively weak inducer of Jagged-1 in DCs, implying that Jagged-1 has a specific function in the macrophage response to SEA.
Materials and methods
Preparation of SEA from mouse livers
Ten livers from mice infected with S. mansoni eggs were frozen, and then finely sliced at room temperature and incubated overnight at 37° with 0·2 mg/ml collagenase (Sigma-Aldrich, Castle Hill, NSW, Australia), 100 U/ml penicillin and 100 μg/ml streptomycin in a total volume of 50 ml of phosphate-buffered saline (PBS). On the following day, the livers were disaggregated with a mortar and pestle, passed through a 280-μm filter and washed three times in ice-cold PBS. The resulting cell suspension was layered onto a Percoll gradient with 0·25 m sucrose in PBS and centrifuged at 2060 g for 10 min. The pellet was re-suspended in PBS with 1 mm ethyleneglycoltetraacetic acid (EGTA)/ethylenediaminetetraacetic acid (EDTA) and the Percoll layering repeated. At this point, the pellet was resuspended in deionized H2O and sonicated on ice to lyse the eggs. The lysate was then re-centrifuged at 10 000 g for 30 min at 4° and the supernatant containing the soluble antigens was collected and stored at −80°. Protein concentration was determined by a bicinchoninic acid (BCA) assay.
Cell culture and reagents
Bone marrow-derived macrophages (BMMs) and DCs were generated by culture of bone marrow cells from C57BL/6 or TLR4−/− (C57BL/6 background) mice for 6 days in ‘complete’ RPMI-1640 (medium supplemented with 10% fetal calf serum, 20 U/ml penicillin, 20 μg/ml streptomycin and 2 mm l-glutamine) containing either 10 000 U/ml colony-stimulating factor (CSF)-1 (a gift from Chiron, Emeryville, CA) for BMMs or 0·5 ng/ml granulocyte–macrophage colony-stimulating factor (GM-CSF) and IL-4 for DCs. All animal studies were performed in accordance with the University of Queensland animal ethics guidelines. Human monocyte-derived macrophages (HMDMs) were generated by culture of CD14+ monocytes purified using magnetic antibody cell sorting (MACS) Technology (Miltenyi Biotec, Sydney, NSW, Australia) in complete Iscove’s modified Dulbecco’s medium (IMDM) with 10 000 U/ml CSF-1 for 6 days. On day 6, BMMs or HMDMs were plated out and incubated overnight in 3 ml of medium without CSF-1 at 2–3 million cells per well in a six-well plate, unless otherwise specified. HEK293 cell lines stably transfected with TLR2, TLR3, TLR4/MD2 or TLR9, kindly provided by Prof. Doug Golenbock, University of Massachusetts Medical School, Worcester, MA, were cultured in complete Dulbecco’s modified Eagle’s minimal essential medium (DMEM) with 500 μg/ml G418, while parent HEK293 cells were cultured in complete DMEM alone. These cells were plated overnight in 1 ml of medium (120 000 cells per well in a 24-well plate) and treated on the following day.
Cells were treated with 10 ng/ml LPS from S. minnesota that was purified by gel filtration (Sigma-Aldrich), zymosan A from Saccharomyces cerevisiae (Sigma-Aldrich), PolyIC [double-stranded RNA (dsRNA) synthesized by Invivogen, San Diego, CA], CpG DNA (phosphorothioate-modified oligonucleotide 2006 5′-TCGTCGTTTTGTCGTTTTGTCGTT-3′ for human HEK293-TLR9 cells or phosphorothioate-modified oligonucleotide 1668S 5′-TCCATGACGTTCCTGATGCT-3′ for mouse cells; GeneWorks Pty Ltd, Hindmarsh, SA, Australia), polymyxin B sulphate (Sigma-Aldrich) or PD-98059 (Biomol International L.P., Plymouth Meeting, PA) at the concentrations indicated in the figure legends.
RNA and cDNA preparation and real-time polymerase chain reaction (PCR)
Total RNA was extracted using a QIAprep Spin Miniprep kit (Qiagen, Melbourne, Vic., Australia) and treated with DNaseI (Invitrogen, Melbourne, Vic., Australia). cDNA was generated using SuperScript III reverse transcriptase (Invitrogen) using equivalent amounts of RNA for each sample. Gene expression was determined by quantitative real-time PCR (SybrGreen or Taqman) using reagents from Applied Biosystems (Foster City, CA). Gene-specific primers were designed using primer 3 software (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi) and synthesized by GeneWorks Pty Ltd. Data were generated with the comparative threshold cycle (ΔCT) method by normalizing to hypoxanthine phosphoribosyltransferase (HPRT). Primer sequences (all shown 5′ to 3′) used were: mouse Jagged-1 forward, AGAAGTCAGAGTTCAGAGGCGTCC; mouse Jagged-1 reverse, AGTAGAAGGCTGTCACCAAGCAAC; human Jagged-1 forward, CGGGAACATACTGCCATGAAAATA; human Jagged-1 reverse, ATGCACTTGTAGGAGTTGACACCA; human HPRT forward, TCAGGCAGTATAATCCAAAGATGGT; human HPRT reverse, AGTCTGGCTTATATCCAACACTTCG; mouse IL-12p40 forward, GGAAGCACGGCAGCAGAATA; mouse IL-12p40 reverse, AACTTGAGGGAGAAGTAGGAATGG; mouse IL-33 forward, AGACCAGGTGCTACTACGCTA; mouse IL-33 reverse, ACACCGTCGCCTGATTGAC; mouse HPRT forward, CAGTCCCAGCGTCGTGATTAG and mouse HPRT reverse, AAACACTTTTTCCAAATCCTCGG. Some experiments were performed for mouse Jagged-1 used Taqman primers from Applied Biosystems (Melbourne, Vic., Australia): Jagged-1 (Mm00496902_m1) and HPRT (Mm00446968_m1).
Analysis of IL-4 production from anti-CD3-activated splenocytes
To analyse the ability of SEA-treated BMMs and DCs to promote IL-4 production from splenocytes upon T-cell activation, BMMs or DCs were treated overnight with medium, LPS, SEA or CpG DNA, co-cultured with splenocytes for 7 days (at a ratio of five splenocytes to one BMM or DC), harvested and transferred to 48-well plates coated with or without 0·5 μg of anti-mouse CD3 (Becton Dickinson, North Ryde, Australia) in fresh media containing the original stimulus (medium, LPS, SEA or CpG DNA) for a further 3 days. Supernatants were harvested and IL-4 assays conducted by sandwich enzyme-linked immunosorbent assay (ELISA) (OptEIA kit; Becton Dickinson).
HEK293-TLR activation assays
Culture supernatants were collected and stored at −20°. IL-8 levels, used as a read-out of the immunoactivation of HEK293 cells expressing different TLRs, were quantified using a sandwich ELISA kit (BD Biosciences, Sydney, NSW, Australia) in accordance with the manufacturer’s instructions. The basal levels of IL-8 produced from HEK293 cells expressing different TLRs before stimulation varied markedly; for example, ∼10 pg/ml for HEK293-TLR2 cells and ∼1000 pg/ml for HEK-TLR4/MD2 cells. For this reason, data for each cell line are presented as fold induction relative to unstimulated controls. ERK-1/2 phosphorylation in HEK293-TLR2 cells was assessed by immunoblotting as below for BMMs.
Immunoblotting
BMMs were lysed in boiling 66 mm Tris–HCl with 2% sodium dodecyl sulphate (SDS), 1 mm sodium vanadate, 1 mm sodium pyrophosphate, 1 mm sodium molybdate and 1 mm sodium fluoride. The resulting lysates were homogenized at room temperature using 21-gauge needles and centrifuged, and supernatants were stored at −70°. When required, proteins were separated via sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) (8–12%) and gels transferred to methanol-activated Immobilon-P polyvinylidene fluoride (PVDF) membranes (Millipore, Sydney, NSW, Australia). The blots were then blocked and probed with antibodies for Jagged-1 (Santa Cruz Biotechnology Inc, Santa Cruz, CA), phospho (Thr202/Tyr204) and total ERK1/2, phospho (Tyr701) Stat1, phospho (Thr183/Tyr185) c-Jun N-terminal kinase (Jnk), phospho (Thr180/Tyr182) p38 and IκB-α (all from Cell Signaling Technology Inc., Danvers, MA). Secondary antibodies for detection were horseradish peroxidase (HRP)-conjugated anti-mouse immunoglobulin G (IgG) (Cell Signaling Technology Inc.), or anti-goat IgG (Pierce Biotechnology via Quantum Scientific, Brisbane, Qld, Australia).
Assays for contaminating LPS in SEA preparations
The multiple SEA preparations that were used through the course of this study were LPS-free as determined by two approaches. Firstly, LPS levels were below the level of detection in an automated colorimetric limulus amoebocyte lysate assay (Charles River Laboratories, Wilmington, MA). Secondly, we used RAW264-hELAM-d2EGFP (RAW-ELAM) cells, in which pro-inflammatory stimuli such as LPS activate the endothelial leucocyte adhesion molecule promoter to drive inducible expression of the green fluorescent protein (GFP) reporter gene.31 Cells were cultured in complete RPMI overnight (120 000 cells in 270 μl per well of a 24-well plate). On the following day, LPS, SEA or a sodium chloride vehicle control was incubated in a total volume of 30 μl for 1 hr at 37° with or without 50 μg/ml polymyxin B sulphate, an antibiotic that binds to the lipid A portion of LPS, and then added to RAW-ELAM cells for 5–6 hr. Cells were then washed with PBS and harvested in PBS/1 mm EDTA/0·1% sodium azide, and levels of GFP expression (mean cellular fluorescence; arbitrary units) were quantified by flow cytometry. LPS-inducible expression of GFP was inhibited by polymyxin B sulphate, whilst SEA had a minimal effect on GFP expression and polymyxin B sulphate had no effect on this response.
Statistical analysis
A Student’s two-tailed t-test assuming equal variance was performed to determine statistical significance for data from pooled independent experiments, and all graphs were generated using Microsoft Excel.
Results
CSF-1-starved BMMs provide a cellular system to characterize Th2-promoting gene expression
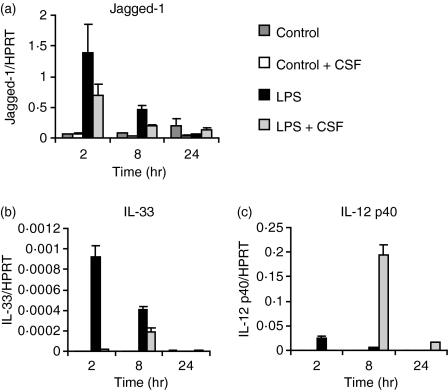
Although both Jagged-1 and IL-33 can elicit Th2 responses, very few studies have addressed whether transcription of these genes is inducible by Th2-promoting parasites in macrophages or DCs. Both genes have been reported to be LPS-inducible,5,7 and we confirmed these findings in BMMs (Fig. 1a,b). We previously reported that pretreatment of BMMs with the growth factor CSF-1 potently amplified LPS induction of IL-12p40, a subunit of the Th1-promoting cytokine IL-12;32 so we investigated the priming effect of this cytokine on candidate Th2-promoting genes. LPS-induced expression of both Jagged-1 and IL-33 mRNAs was inhibited when BMMs were cultured overnight in the presence of CSF-1 (Fig. 1a,b). In contrast, CSF-1 potently primed BMMs for LPS-inducible IL-12p40 mRNA expression (Fig. 1c), as previously observed.32 Thus, CSF-1 negatively regulates the induction of Th2-promoting genes in a selective manner.
Figure 1.
Colony-stimulating factor (CSF)-1 suppresses lipopolysaccharide (LPS)-inducible Jagged-1 and interleukin (IL)-33 mRNA expression, but increases IL-12p40 expression in murine bone marrow macrophages (BMMs). BMMs were plated overnight in the presence or absence of CSF-1 and then treated for 2, 8 and 24 hr with 10 ng/ml LPS or medium as a control. Gene expression (mean of triplicates + standard error of the mean) was quantified by real-time polymerase chain reaction (PCR) for Jagged-1 (a), IL-33 (b) and IL-12p40 (c). The results shown are representative of six similar experiments. HPRT, hypoxanthine phosphoribosyltransferase.
SEA induces Jagged-1, but not IL-33, expression in murine and human macrophages
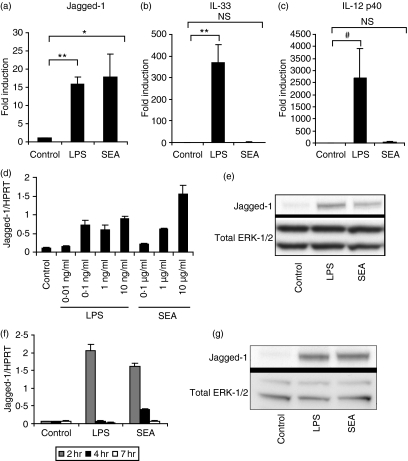
Both the IL-33/ST2 and Jagged/Notch pathways have recently been identified as regulators of Th2 development.5,7,8 We therefore determined whether SEA, which potently drives Th2 development,33 affected the expression of either IL-33 or Jagged-1. SEA rapidly up-regulated the mRNA levels of Jagged-1, but not IL-33 or IL-12p40, in CSF-1-starved BMMs (Fig. 2a,b and c). This effect was not attributable to contaminating LPS, as none of the batches of SEA used contained detectable levels of LPS as assessed by a limulus amoebocyte lysate assay or induced GFP expression in RAW-ELAM cells31 in a polymyxin B-sensitive fashion (data not shown). Further, LPS up-regulated mRNA expression of all three mediators, in contrast to the selective effects of SEA. Jagged-1 up-regulation by SEA was observed over a dose response at the mRNA level (Fig. 2d) and was also evident at the protein level using immunoblotting (Fig. 2e). Inducible mRNA expression was transient, peaking at 2 hr before declining to baseline by 8 hr (data not shown). SEA and LPS similarly induced Jagged-1 mRNA (Fig. 2f) and protein (Fig. 2g) in HMDMs, thus confirming that this response was not restricted to mouse macrophages.
Figure 2.
Soluble egg antigen (SEA) selectively up-regulates Jagged-1 mRNA and protein expression in mouse and human macrophages. Bone marrow macrophages (BMMs) [plated overnight without colony-stimulating factor (CSF)-1] were treated for 2 hr with 10 ng/ml lipopolysaccharide (LPS), 10 μg/ml SEA or medium as a control (a, b, c), or treated for 2 hr with various concentrations of LPS or SEA (d). Human monocyte-derived macrophages (HMDMs) were treated for 2, 4 and 7 hr (f). Gene expression was calculated by real-time polymerase chain reaction (PCR) for Jagged-1 (a, d, f), interleukin (IL)-33 (b) and IL-12p40 (c). For quantification of protein expression, BMMs (e) or HMDMs (g) were plated overnight in the absence of CSF-1 and treated for 4 hr with 10 ng/ml LPS, 10 μg/ml SEA or medium as a control. Total Jagged-1 and extracellular signal-regulated kinase-1/2 (ERK-1/2) protein were visualized by immunoblotting. Results for the mouse experiments are from six independent experiments, displayed as mean + SEM (a, b, c), or are representative of three or more similar experiments (d, e). Results for the human experiments (f, g) are representative of two similar experiments using different donors. *, P < 0·05; **, P < 0·01; #, P = 0·058; NS, not significant for either LPS or SEA versus control treatments.
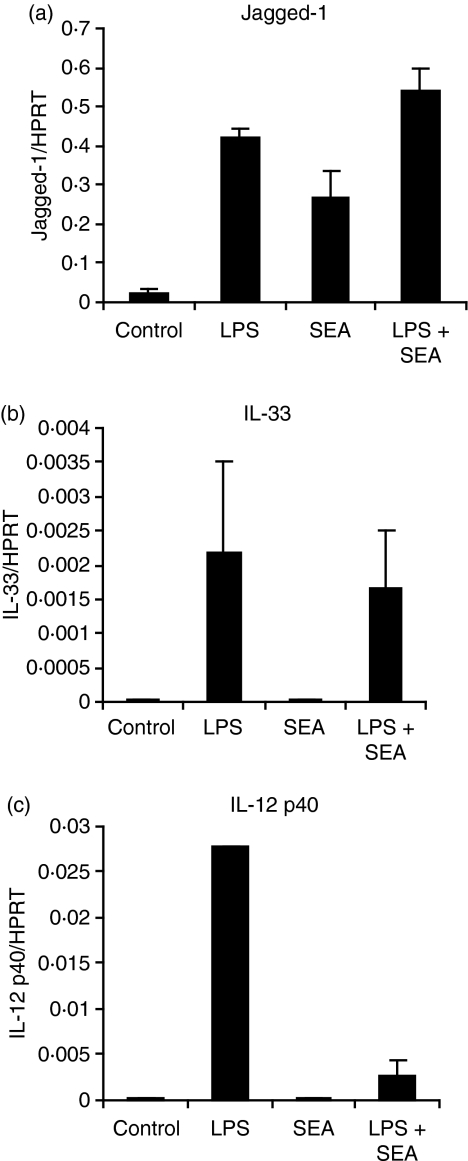
Induction of Jagged-1 by LPS and SEA is not additive in BMMs
We next determined whether the effect of LPS and SEA on Jagged-1 expression was additive or synergistic. The combination of LPS and SEA did not significantly increase Jagged-1 expression in murine BMMs over levels induced by either stimulus alone (Fig. 3a), and SEA did not amplify LPS-inducible IL-33 mRNA expression (Fig. 3b). This suggests that LPS and SEA may utilize overlapping signalling pathways to induce Jagged-1 expression. However, SEA did suppress LPS-induced IL-12p40 mRNA levels (Fig. 3c), as has previously been reported in DCs.12 This confirms that the SEA preparations used in our studies had similar activity to those used by others.
Figure 3.
Combinatorial effects of soluble egg antigen (SEA) and lipopolysaccharide (LPS) on regulated gene expression in bone marrow macrophages (BMMs). BMMs were plated overnight in the absence of colony-stimulating factor (CSF)-1, and then treated for 2 hr with 10 ng/ml LPS, 10 μg/ml SEA, both, or medium as a control. Gene expression (mean of triplicates + standard error of the mean) was calculated by real-time polymerase chain reaction (PCR) for Jagged-1 (a), interleukin (IL)-33 (b) and IL-12p40 (c). The results shown are indicative of seven similar experiments.
Induction of Jagged-1 in APCs does not correlate with permissiveness for Th2 responses
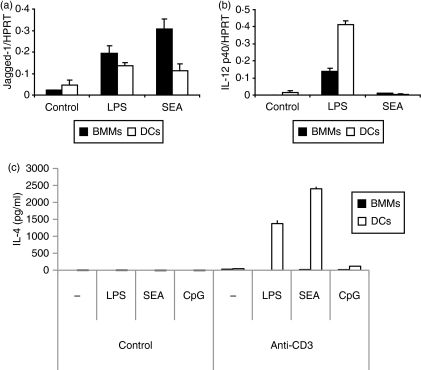
We next compared the effects of SEA on Jagged-1 expression in BMMs versus conventional, CD11c+ DCs. SEA did induce Jagged-1 mRNA expression in DCs, but the response was less pronounced than in BMMs (Fig. 4a). In contrast, LPS-inducible IL-12p40 mRNA expression was higher in DCs than in BMMs (Fig. 4b). As SEA induced Jagged-1 expression in both BMMs and DCs, we next assessed whether these cell populations could promote a Th2 response upon pulsing with SEA. Figure 4(c) demonstrates that SEA-pulsed DCs, but not SEA-pulsed BMMs, elicited robust IL-4 production from anti-CD3-activated splenocytes. Unlike SEA and LPS, the potent Th1-promoting stimulus, CpG DNA did not prime IL-4 production in these assays. Thus, Jagged-1 induction on BMMs is insufficient to direct Th2 development, and must have alternative roles in immune regulation in response to SEA. To gain some insight into the potential roles of SEA-inducible Jagged-1 expression in macrophages, we next assessed the mechanism of induction in this cell type.
Figure 4.
Jagged-1 induction in antigen-presenting cells (APCs) does not correlate with permissiveness for T helper type 2 (Th2) responses (a and b). Bone marrow macrophages (BMMs) [plated overnight without colony-stimulating factor (CSF)-1] or dendritic cells (DCs) were treated for 2 hr with 10 ng/ml lipopolysaccharide (LPS), 10 μg/ml soluble egg antigen (SEA) or medium as a control. Gene expression was calculated by real-time polymerase chain reaction (PCR) for Jagged-1 (a) and interleukin (IL)-12p40 (b). (c) BMMs or DCs were stimulated overnight with medium, 10 ng/ml LPS, 10 μg/ml SEA or 0·1 μm CpG DNA, and then co-cultured with purified mouse splenocytes for 7 days, followed by anti-CD3 stimulation in combination with a second treatment of medium, LPS, SEA or CpG DNA for 3 days. IL-4 levels in cell culture supernatants were determined by enzyme-linked immunosorbent assay (ELISA). Data are representative of three or more similar experiments.
SEA signals via both TLR2 and TLR4
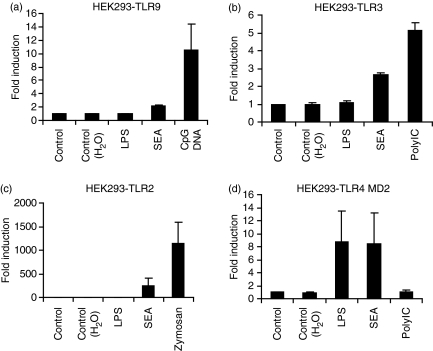
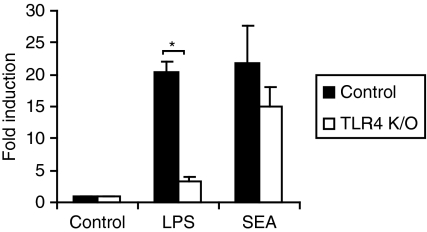
Parasite products modulate immune responses via TLR4-dependent and -independent pathways,26,34–36 so we employed HEK293 cells ectopically expressing different TLRs to investigate the TLR4 dependence of the SEA signal. All HEK293-TLR cell lines examined (HEK293-TLR2, TLR-3, TLR-4/MD2 and TLR-9) responded as expected to their corresponding PAMP (Fig. 5). SEA modestly increased IL-8 production (twofold or less) from either parent HEK293 cells or those expressing TLR9 (Table 1 and Fig. 5a). As PolyIC also triggered some IL-8 production from parent HEK293 cells (Table 1), and one report showed that SEA signalled via TLR3,26 the implication is that HEK293 cells may express some basal TLR3. We observed variation in the response of HEK293-TLR3 cells to SEA, with some batches triggering robust IL-8 production (data not shown) and some having modest effects (Fig. 5b). In contrast, all batches of SEA strongly up-regulated IL-8 production from HEK293 cells expressing either TLR-2 (Fig. 5c) or TLR-4/MD-2 (Fig. 5d). Consistent with the activation of multiple TLRs by SEA, TLR4 deletion in BMMs did not significantly affect SEA-induced Jagged-1 mRNA expression (Fig. 6). We conclude that SEA can signal via multiple TLRs, and that individual TLRs are likely to be functionally redundant for SEA-inducible Jagged-1 expression.
Figure 5.
Soluble egg antigen (SEA) signals in HEK293 cells expressing Toll-like receptor 2 (TLR2) or TLR4/MD2, but not TLR9. HEK293 cells expressing TLR9 (a), TLR3 (b), TLR2 (c) or TLR4/MD2 (d) were treated for 24 hr with 10 μg/ml SEA, TLR-specific positive controls (10 ng/ml LPS, 0·1 μg/ml CpG DNA (2006), 30 μg/ml PolyIC and 20 μg/ml zymosan) and vehicle controls. Interleukin (IL)-8 production in culture supernatants was determined by enzyme-linked immunosorbent assay (ELISA). Results from three independent experiments (except for HEK293 cells expressing TLR3, which represents four independent experiments) are displayed as fold induction (relative to unstimulated cells) + standard error of the mean.
Table 1.
Interleukin (IL)-8 production in response to soluble egg antigen (SEA) and other Toll-like receptor (TLR) ligands in HEK293 cells1
| Treatment | (IL-8) fold induction |
|---|---|
| Medium control | 1·0 ± 0·0 |
| H2O control | 0·9 ± 0·1 |
| LPS (10 ng/ml) | 0·7 ± 0·1 |
| SEA | 1·5 ± 0·2 |
| Zymosan | 0·6 ± 0·1 |
| PolyIC | 5·0 ± 0·1 |
| CpG DNA 2006 | 0·6 ± 0·1 |
HEK293 cells were plated overnight in complete Dulbecco’s modified Eagle’s minimal essential medium (DMEM) and then treated for 24 hr with SEA and various TLR ligands with matched negative controls. IL-8 levels in culture supernatants were determined by enzyme-linked immunosorbent assay (ELISA). Results from five independent experiments are displayed as fold induction (relative to unstimulated cells) ± standard error of the mean.
Figure 6.
Induction of Jagged-1 mRNA by soluble egg antigen (SEA) is not dependent upon Toll-like receptor 4 (TLR4). TLR4−/− (K/O) and wild-type bone marrow macrophages (BMMs) were treated for 2 hr with 10 ng/ml lipopolysaccharide (LPS), 10 μg/ml SEA or medium control. Jagged-1 mRNA levels were determined by real-time polymerase chain reaction (PCR) and data from six independent experiments are displayed as fold induction (relative to unstimulated cells) + standard error of the mean. *, P < 0·00001 compared with wild-type cells.
The ERK-1/2 pathway is required for Jagged-1 induction by SEA and LPS
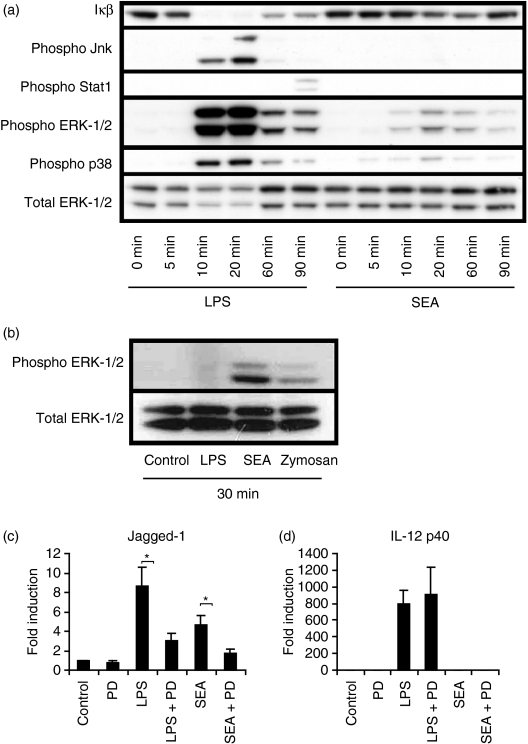
SEA reportedly activated ERK-1/2 in a TLR4-dependent manner in DCs,27 and we therefore investigated whether SEA activated this, and other TLR-regulated signalling pathways, in macrophages. Both LPS and SEA triggered ERK-1/2 and p38 phosphorylation in BMMs, with a maximal effect apparent 10–60 min post-stimulation (Fig. 7a). The effect was selective, as SEA did not trigger IκB degradation, phosphorylation of Jnk or Y701 phosphorylation on Stat1 (Fig. 7a). Selective activation of ERK-1/2 in DCs by the carbohydrate lacto-n-fucopentaose III (LNFPIII) from SEA was also reported by others.27 In that study, however, p38 was not activated, in contrast to our findings involving whole SEA on macrophages. Given that SEA was able to signal via TLR2 and TLR4 (Fig. 5c,d), both of which normally activate Jnk and NF-κB,37 it may be that SEA actively inhibits these early signalling events.
Figure 7.
Soluble egg antigen (SEA) selectively regulates Toll-like receptor (TLR) signalling pathways in bone marrow macrophages (BMMs). (a) BMMs were treated for 0, 5, 10, 20, 60 and 90 min with 10 ng/ml lipopolysaccharide (LPS) or 10 μg/ml SEA. Levels of IκB-α, phospho c-Jun N-terminal kinase (Jnk) (Thr183/Tyr185), phospho signal transducer and activator of transcription 1 (Stat1) (Tyr701), phospho extracellular signal-regulated kinase-1/2 (ERK-1/2) (Thr202/Tyr204), phospho p38 (Thr180/Tyr182) and total ERK-1/2 were visualized by immunoblotting. The results displayed are representative of three independent experiments. (b) HEK293-TLR2 cells were stimulated with 10 ng/ml LPS, 10 μg/ml SEA, 20 μg/ml zymosan or medium control for 30 min and ERK-1/2 levels visualized by immunoblotting. (c, d) BMMs were pre-incubated for 15 min with and without 30 μm PD-98059, followed by stimulation for 2 hr with 10 ng/ml LPS, 10 μg/ml SEA, or medium control. Jagged-1 (c) and interleukin (IL)-12p40 (d) mRNA levels were determined by real-time polymerase chain reaction (PCR), and data from seven independent experiments are displayed as fold induction (relative to unstimulated cells) + standard error of the mean. *, P < 0·05 compared with non-PD-98059-treated samples.
To determine the TLR dependence of ERK-1/2 activation by SEA, we used the HEK293-TLR system. Because SEA activated multiple TLRs (Fig. 5), it was not possible to address SEA signalling through individual TLRs in a natural macrophage population such as BMMs. SEA, which had negligible effects on IL-8 production from parent HEK293 cells (Table 1), triggered ERK-1/2 phosphorylation in HEK293-TLR2 cells (Fig. 7b), consistent with its effects on IL-8 production from these cells (Fig. 5c). The specificity of this system was again confirmed through the use of a TLR2 agonist (zymosan) and a TLR4 agonist (LPS) (Fig. 7b). Given this robust TLR-dependent activation of ERK-1/2 by SEA, we next investigated whether the ERK-1/2 pathway was required for SEA-induced Jagged-1 expression. The ERK inhibitor PD-98059 impaired LPS- and SEA-inducible Jagged-1 mRNA expression in BMMs (Fig. 7c) and this effect was selective as inducible expression of IL-12p40 mRNA was not affected (Fig. 7d). Thus, the ERK-1/2 pathway contributes to induction of Jagged-1 by LPS and SEA in BMMs.
Discussion
Inducible expression of Jagged-1 in APCs in response to SEA from S. mansoni provides a potential mechanism for Th2 polarization by this stimulus; however, this remains an area of some controversy. Although Jagged-1 knock-down38 and over-expression7 studies suggest a role for this cell-surface ligand in Th2 development, a recent study showed that SEA induced Jagged-2 but not Jagged-1 in DCs,39 whilst others reported that SEA did not regulate either Jagged-1 or Jagged-2 mRNA expression in DCs.40 Furthermore, neither of these studies found any evidence that Jagged-2 plays a role in DC-mediated Th2 development. In our hands, SEA did modestly induce Jagged-1 gene expression in DCs (Fig. 4a). SEA-mediated induction of Jagged-1 was, however, rapid and transient and this may account for the lack of inducible Jagged-1 expression in response to SEA in earlier studies, where measurements were made at longer time points than examined here.39,40 Nonetheless, we found that Jagged-1 induction by SEA was modest in DCs by comparison to the response observed in BMMs and HMDMs (Fig. 4, 2). The mechanisms responsible for the reduced response in DCs are unknown, but it is clearly a selective effect because IL-12p40 expression was more robustly induced in DCs than in BMMs.
Despite the strong effect of SEA on Jagged-1 in BMMs, treatment of these cells with SEA did not promote IL-4 production from splenocytes in response to anti-CD3 stimulation (Fig. 4c). We conclude that, at least in macrophages, inducible Jagged-1 expression alone is unlikely to be sufficient for SEA-mediated Th2 polarization. Whether it contributes to the SEA-induced DC signal that promotes Th2 responses remains to be determined, but a recent study found no evidence for Notch ligands, including Jagged-1, in instructing Th1 and/or Th2 cell fates.41 IL-33 is also unlikely to be involved because SEA did not affect expression of this gene, though it is still possible that other Th2-promoting helminths do. Ym1, a gene product expressed by alternatively activated macrophages in response to a number of parasitic worms,42 was also required for Th2 development in response to simvastatin,43 and thus represents a further candidate for helminth-induced Th2 development.
Given that the induction of Jagged-1 by SEA was selective (other TLR-inducible genes, e.g. IL-33 and IL-12p40, were not induced; Fig. 2) and was more pronounced in macrophages than DCs (Fig. 4), there is likely to be a macrophage-specific function for Jagged-1 in the response to SEA. Whether such a function is integral to, or independent of, macrophage-regulated adaptive immunity remains to be clarified. Macrophages do express Notch receptors,44 so one possibility is that Jagged-1 acts in a autocrine/juxtacrine manner, perhaps contributing to the development of alternatively activated (M2) macrophages, which have important functions during Th2 responses.45 M2 macrophages, for example, protect against immunopathology in schistosomiasis by down-regulating Th1 responses.30 If macrophage-expressed Jagged-1 does contribute to the Th2 phenotype, the lack of IL-4 production in splenocytes cultured with BMMs in our system (Fig. 4) may point towards such an indirect mechanism, rather than a more direct effect on Th2 development. Another possibility is that TLR-inducible Jagged-1 provides a survival signal to macrophages, given that other macrophage survival factors (including CSF-1) acutely induced Jagged-1 expression in BMMs.46 Intriguingly, Lunatic Fringe, a protein that specifically inhibits Jagged-1 signalling through Notch-1,45 was also expressed in macrophages but was down-regulated by TLR4 ligation (our unpublished microarray data). Whether LPS and SEA promote autocrine Notch-1 signalling via inducible Jagged-1 in macrophages will be addressed in future studies. There is also evidence that SEA and other helminth products can suppress Th1 responses by down-regulating LPS-induced IL-12 production in macrophages/DCs.12,13 Our findings are consistent with these studies (Fig. 3c), but whether SEA-inducible Jagged-1 is involved in this suppression remains to be determined.
Alternatively, SEA inhibition of IL-12 production may relate to more direct effects on signalling. SEA did not trigger Jnk phosphorylation in BMMs, and caused minimal degradation of IκB (Fig. 7a), despite signalling via TLR4 and TLR2 (Fig. 5c,d). As degradation of IκB and the subsequent release of NF-κB into the nucleus are involved in driving IL-12 expression,47 this finding is consistent with the inhibitory effect of SEA on LPS-induced IL-12p40 gene expression (Fig. 3c). Even the phosphorylation of ERK-1/2 and p38 was diminished compared with LPS (Fig. 7a), despite the fact that SEA was as active as LPS on HEK293-TLR4 cells (Fig. 5d) and robustly active on HEK293-TLR2 cells (Fig. 5c). The implication is that, whilst SEA signals via TLR2 and TLR4, it acts as only a partial agonist in macrophages. This might result in selective activation of a subset of TLR signalling pathways, as well as inhibition of responses to classical TLR2 and TLR4 agonists. It is possible that activation of other pathways, such as those involving C-type lectins,36 may also contribute to this selective response.
Partial agonists of TLR4 do exist. A recent study showed that monophosphoryl lipid A, which signals via TLR4 but is substantially less potent than LPS, signalled via TRIF but only weakly through the MyD88 adaptor protein.48 One possibility, therefore, is that SEA signals primarily via TRIF, in contrast to LPS, which also signals strongly via MyD88. Indeed, selective activation of MyD88-independent signalling may be associated with Th2 development: LPS primes Th2 responses in MyD88-deficient mice,22,23 and SEA has been shown to induce Th2 polarization in DCs via MyD88-independent pathways.28
What are the components of SEA that are detected by TLR2 and TLR4? The carbohydrate LNFPIII is present in SEA and has been reported to signal via TLR4 to activate ERK-1/227 and NF-κB16 in DCs. In our hands, even at very high concentrations, LNFPIII conjugated to bovine serum albumin (BSA) had only very modest effects, as compared with SEA, on Jagged-1 expression in BMMs (data not shown). Thus, we are uncertain as to whether this carbohydrate moiety contributes to the effects of SEA we have observed in macrophages. Similarly, phosphatidylserine derived from eggs and adult schistosomes has been shown to signal via TLR2 on DCs,49 although this study also reported that SEA itself did not. In our experiments, SEA was a very potent activator of signalling downstream of TLR2 (Fig. 5c). Yet another study showed that dsRNA derived from SEA activates DCs in a TLR3-dependent manner, and that live eggs also act partly via this pathway,26 although it may not be required to control infection.50 We also found that SEA could activate signalling via TLR3 (Fig. 5b and data not shown), but this effect was variable, presumably because the dsRNA in SEA preparations was susceptible to degradation. Another study showed that immature DCs internalized SEA via C-type lectins,36 raising the possibility that these receptors also regulate SEA-triggered signalling. Given the complexity of SEA, it is perhaps unsurprising that so many pathways have been implicated in its detection, and that knockout of a single TLR gene (TLR4) failed to ablate inducible Jagged-1 expression (Fig. 6).
SEA activated ERK-1/2 in a TLR-dependent manner (Fig. 7b), and pharmacological inhibition of the ERK-1/2 pathway prevented induction of Jagged-1 in response to SEA or LPS (Fig. 7c). Despite the well-recognized effects of TLR agonists in activating the ERK-1/2 pathway,51,52 there is actually very little known about functional consequences of TLR-induced ERK-1/2 activation. One function may be in constraining the development of a Th1 response. For example, inactivation of the Tpl2/Cot gene, which encodes a serine/threonine kinase required for maximal ERK-1/2 activation in response to TLR ligands, resulted in a Th1-skewed phenotype upon challenge with Leishmania major.53 TLR-induced IL-10 production is dependent on ERK-1/2,54 which may contribute to this phenotype. Our data identifies Jagged-1 as an ERK-dependent immediate TLR response gene, which may also constrain Th1 responses.
An understanding of the transcription factors involved in Jagged-1 induction would further our knowledge of SEA-mediated signalling. To date, the Jagged-1 gene has not been analysed thoroughly at the promoter level. Tcf/Lef (T cell-specific factor/lymphoid enhancer binding factor)-binding sites were found within the promoter region of human, mouse and rat Jagged-1,55,56 indicating that Jagged-1 may be a target of the Wnt signalling pathway. Although Wnt5A is a target of TLR signalling in macrophages,57 it seems unlikely that this or other TLR-inducible Wnt family members are responsible for Jagged-1 induction by SEA and LPS because of the rapid nature of the response (Fig. 1a). We have not attempted to perform Jagged-1 promoter studies in macrophages because Jagged-1 mRNA was constitutively expressed and not regulated by SEA in the RAW264 macrophage-like cell line (data not shown), which is commonly used for promoter analyses. Our own informatics analysis confirmed the existence of potential Tcf/Lef-binding sites, as well as those for the interferon regulatory factor family, upstream and downstream of the Jagged-1 transcription start site (data not shown). Whether any of these factors are responsible for SEA-inducible Jagged-1 expression remains to be clarified. In summary, we have demonstrated that SEA robustly induced expression of the Notch ligand Jagged-1 in mouse and human macrophages by an ERK-dependent mechanism. The future identification of specific functions of inducible Jagged-1 in macrophages will provide insights into the immune-modulating effects of SEA and the host response to helminth infection.
Acknowledgments
This work was supported by project grant 301210 from the National Health and Medical Research Council of Australia. FG was supported by a joint CSIRO and Institute for Molecular Bioscience scholarship. The authors wish to acknowledge the ARC Special Research Centre for Functional and Applied Genomics.
Glossary
Abbreviations:
- APC
antigen-presenting cell
- BMM
bone marrow-derived macrophage
- CSF-1
colony-stimulating factor 1
- DC
dendritic cell
- ERK
extracellular signal-regulated kinase
- HMDM
human monocyte-derived macrophage
- HPRT
hypoxanthine phosphoribosyltransferase
- Jnk
c-Jun N-terminal kinase
- LNFPIII
lacto-n-fucopentaose III
- LPS
lipopolysaccharide
- PAMP
pathogen-associated molecular pattern
- SEA
schistosome egg antigen
- TLR
Toll-like receptor
References
- 1.Kidd P. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern Med Rev. 2003;8:223–46. [PubMed] [Google Scholar]
- 2.Miyatake S, Arai N, Arai K. Chromatin remodeling and T helper subset differentiation. IUBMB Life. 2000;49:473–8. doi: 10.1080/15216540050166990. [DOI] [PubMed] [Google Scholar]
- 3.Xu DM, Chan WL, Leung BP, et al. Selective expression and functions of interleukin 18 receptor on T helper (Th) type 1 but not Th2 cells. J Exp Med. 1998;188:1485–92. doi: 10.1084/jem.188.8.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Townsend MJ, Fallon PG, Matthews DJ, Jolin HE, McKenzie AN. T1/ST2-deficient mice demonstrate the importance of T1/ST2 in developing primary T helper cell type 2 responses. J Exp Med. 2000;191:1069–76. doi: 10.1084/jem.191.6.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmitz J, Owyang A, Oldham E, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–90. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 6.Dinarello CA. An IL-1 family member requires caspase-1 processing and signals through the ST2 receptor. Immunity. 2005;23:461–2. doi: 10.1016/j.immuni.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Amsen D, Blander JM, Lee GR, Tanigaki K, Honjo T, Flavell RA. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell. 2004;117:515–26. doi: 10.1016/s0092-8674(04)00451-9. [DOI] [PubMed] [Google Scholar]
- 8.Amsen D, Antov A, Jankovic D, et al. Direct regulation of gata3 expression determines the T helper differentiation potential of notch. Immunity. 2007;27:89–99. doi: 10.1016/j.immuni.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang TC, Yashiro-Ohtani Y, Del Bianco C, Knoblock DM, Blacklow SC, Pear WS. Notch directly regulates gata3 expression during T helper 2 cell differentiation. Immunity. 2007;27:100–10. doi: 10.1016/j.immuni.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–60. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 11.Maizels RM, Balic A, Gomez-Escobar N, Nair M, Taylor MD, Allen JE. Helminth parasites – masters of regulation. Immunol Rev. 2004;201:89–116. doi: 10.1111/j.0105-2896.2004.00191.x. [DOI] [PubMed] [Google Scholar]
- 12.Kane CM, Cervi L, Sun J, McKee AS, Masek KS, Shapira S, Hunter CA, Pearce EJ. Helminth antigens modulate TLR-initiated dendritic cell activation. J Immunol. 2004;173:7454–61. doi: 10.4049/jimmunol.173.12.7454. [DOI] [PubMed] [Google Scholar]
- 13.Goodridge HS, Wilson EH, Harnett W, Campbell CC, Harnett MM, Liew FY. Modulation of macrophage cytokine production by ES-62, a secreted product of the filarial nematode Acanthocheilonema viteae. J Immunol. 2001;167:940–5. doi: 10.4049/jimmunol.167.2.940. [DOI] [PubMed] [Google Scholar]
- 14.de Jong EC, Vieira PL, Kalinski P, Schuitemaker JHN, Tanaka Y, Wierenga EA, Yazdanbakhsh M, Kapsenberg ML. Microbial compounds selectively induce Th1 cell-promoting or Th2 cell-promoting dendritic cells in vitro with diverse Th cell-polarizing signals. J Immunol. 2002;168:1704–9. doi: 10.4049/jimmunol.168.4.1704. [DOI] [PubMed] [Google Scholar]
- 15.Okano M, Satoskar AR, Nishizaki K, Harn DA. Lacto-N-fucopentaose III found on Schitosoma mansoni egg antigens functions as adjuvant for proteins by inducing Th2-type response. J Immunol. 2001;167:442–50. doi: 10.4049/jimmunol.167.1.442. [DOI] [PubMed] [Google Scholar]
- 16.Thomas PG, Carter MR, Da’dara AA, DeSimone TM, Harn DA. A helminth glycan induces APC maturation via alternative NF-kappa B activation independent of I kappa B alpha degradation. J Immunol. 2005;175:2082–90. doi: 10.4049/jimmunol.175.4.2082. [DOI] [PubMed] [Google Scholar]
- 17.Cervi L, MacDonald AS, Kane C, Dzierszinski F, Pearce EJ. Cutting edge: dendritic cells copulsed with microbial and helminth antigens undergo modified maturation, segregate the antigens to distinct intracellular compartments, and concurrently induce microbe-specific Th1 and helminth-specific Th2 responses. J Immunol. 2004;172:2016–20. doi: 10.4049/jimmunol.172.4.2016. [DOI] [PubMed] [Google Scholar]
- 18.Kiura K, Kataoka H, Yasuda M, Inoue N, Shibata K. The diacylated lipopeptide FSL-1 induces TLR2-mediated Th2 responses. FEMS Immunol Med Microbiol. 2006;48:44–55. doi: 10.1111/j.1574-695X.2006.00119.x. [DOI] [PubMed] [Google Scholar]
- 19.Redecke V, Hacker H, Datta SK, Fermin A, Pitha PM, Broide DH, Raz E. Cutting edge: activation of toll-like receptor 2 induces a Th2 immune response and promotes experimental asthma. J Immunol. 2004;172:2739–43. doi: 10.4049/jimmunol.172.5.2739. [DOI] [PubMed] [Google Scholar]
- 20.Raymond CR, Wilkie BN. Toll-like receptor, MHC II, B7 and cytokine expression by porcine monocytes and monocyte-derived dendritic cells in response to microbial pathogen-associated molecular patterns. Vet Immunol Immunopathol. 2005;107:235–47. doi: 10.1016/j.vetimm.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 21.Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002;196:1645–51. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fitzgerald KA, Rowe DC, Barnes BJ, et al. LPS-TLR4 signaling to IRF-3/7 and NF-kappa B involves the toll adapters TRAM and TRIF. J Exp Med. 2003;198:1043–55. doi: 10.1084/jem.20031023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaisho T, Hoshino K, Iwabe T, Takeuchi O, Yasui T, Akira S. Endotoxin can induce MyD88-deficient dendritic cells to support T(h)2 cell differentiation. Int Immunol. 2002;14:695–700. doi: 10.1093/intimm/dxf039. [DOI] [PubMed] [Google Scholar]
- 24.Pearce EJ. Priming of the immune response by schistosome eggs. Parasite Immunol. 2005;27:265–70. doi: 10.1111/j.1365-3024.2005.00765.x. [DOI] [PubMed] [Google Scholar]
- 25.Pearce EJ, Kane CM, Sun J. Regulation of dendritic cell function by pathogen-derived molecules plays a key role in dictating the outcome of the adaptive immune response. Chem Immunol Allergy. 2006;90:82–90. doi: 10.1159/000088882. [DOI] [PubMed] [Google Scholar]
- 26.Aksoy E, Zouain CS, Vanhoutte F, et al. Double-stranded RNAs from the helminth parasite Schistosoma activate TLR3 in dendritic cells. J Biol Chem. 2005;280:277–83. doi: 10.1074/jbc.M411223200. [DOI] [PubMed] [Google Scholar]
- 27.Thomas PG, Carter MR, Atochina O, Da’Dara AA, Piskorska D, McGuire E, Harn DA. Maturation of dendritic cell 2 phenotype by a helminth glycan uses a toll-like receptor 4-dependent mechanism. J Immunol. 2003;171:5837–41. doi: 10.4049/jimmunol.171.11.5837. [DOI] [PubMed] [Google Scholar]
- 28.Jankovic D, Kullberg MC, Caspar P, Sher A. Parasite-induced Th2 polarization is associated with down-regulated dendritic cell responsiveness to Th1 stimuli and a transient delay in T lymphocyte cycling. J Immunol. 2004;173:2419–27. doi: 10.4049/jimmunol.173.4.2419. [DOI] [PubMed] [Google Scholar]
- 29.Hayashi N, Matsui K, Tsutsui H, et al. Kupffer cells from Schistosoma mansoni-infected mice participate in the prompt type 2 differentiation of hepatic T cells in response to worm antigens. J Immunol. 1999;163:6702–11. [PubMed] [Google Scholar]
- 30.Herbert DR, Holscher C, Mohrs M, et al. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity. 2004;20:623–35. doi: 10.1016/s1074-7613(04)00107-4. [DOI] [PubMed] [Google Scholar]
- 31.Stacey KJ, Young GR, Clark F, Sester DP, Roberts TL, Naik S, Sweet MJ, Hume DA. The molecular basis for the lack of immunostimulatory activity of vertebrate DNA. J Immunol. 2003;170:3614–20. doi: 10.4049/jimmunol.170.7.3614. [DOI] [PubMed] [Google Scholar]
- 32.Sweet MJ, Campbell CC, Sester DP, Xu DM, McDonald RC, Stacey KJ, Hume DA, Liew FY. Colony-stimulating factor-1 suppresses responses to CpG DNA and expression of toll-like receptor 9 but enhances responses to lipopolysaccharide in murine macrophages. J Immunol. 2002;168:392–9. doi: 10.4049/jimmunol.168.1.392. [DOI] [PubMed] [Google Scholar]
- 33.Okano M, Satoskar AR, Nishizaki K, Abe M, Harn DA. Induction of Th2 responses and IgE is largely due to carbohydrates functioning as adjuvants on Schistosoma mansoni egg antigens. J Immunol. 1999;163:6712–7. [PubMed] [Google Scholar]
- 34.Helmby H, Grencis RK. Essential role for TLR4 and MyD88 in the development of chronic intestinal nematode infection. Eur J Immunol. 2003;33:2974–9. doi: 10.1002/eji.200324264. [DOI] [PubMed] [Google Scholar]
- 35.Jenkins SJ, Hewitson JP, Ferret-Bernard S, Mountford AP. Schistosome larvae stimulate macrophage cytokine production through TLR4-dependent and -independent pathways. Int Immunol. 2005;17:1409–18. doi: 10.1093/intimm/dxh319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Liempt E, van Vliet SJ, Engering A, Garcia Vallejo JJ, Bank CM, Sanchez-Hernandez M, van Kooyk Y, van Die I. Schistosoma mansoni soluble egg antigens are internalized by human dendritic cells through multiple C-type lectins and suppress TLR-induced dendritic cell activation. Mol Immunol. 2007;44:2605–15. doi: 10.1016/j.molimm.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 37.Takeuchi O, Akira S. Toll-like receptors; their physiological role and signal transduction system. Int Immunopharmacol. 2001;1:625–35. doi: 10.1016/s1567-5769(01)00010-8. [DOI] [PubMed] [Google Scholar]
- 38.Liotta F, Frosali F, Querci V, et al. Human immature myeloid dendritic cells trigger a T(H)2-polarizing program via Jagged-1/Notch interaction. J Allergy Clin Immunol. 2008;121:1000–5. doi: 10.1016/j.jaci.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 39.Krawczyk CM, Sun J, Pearce EJ. Th2 differentiation is unaffected by Jagged2 expression on dendritic cells. J Immunol. 2008;180:7931–7. doi: 10.4049/jimmunol.180.12.7931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Worsley AG, Leibundgut-Landmann S, Slack E, Phng LK, Gerhardt H, Sousa CR, Macdonald AS. Dendritic cell expression of the Notch ligand Jagged 2 is not essential for Th2 response induction in vivo. Eur J Immunol. 2008;38:1043–9. doi: 10.1002/eji.200737335. [DOI] [PubMed] [Google Scholar]
- 41.Ong CT, Sedy JR, Murphy KM, Kopan R. Notch and presenilin regulate cellular expansion and cytokine secretion but cannot instruct Th1/Th2 fate acquisition. PLoS ONE. 2008;3:e2823. doi: 10.1371/journal.pone.0002823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nair MG, Cochrane DW, Allen JE. Macrophages in chronic type 2 inflammation have a novel phenotype characterized by the abundant expression of Ym1 and Fizz1 that can be partly replicated in vitro. Immunol Lett. 2003;85:173–80. doi: 10.1016/s0165-2478(02)00225-0. [DOI] [PubMed] [Google Scholar]
- 43.Arora M, Chen L, Paglia M, Gallagher I, Allen JE, Vyas YM, Ray A, Ray P. Simvastatin promotes Th2-type responses through the induction of the chitinase family member Ym1 in dendritic cells. Proc Natl Acad Sci USA. 2006;103:7777–82. doi: 10.1073/pnas.0508492103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jonsson JI, Xiang Z, Pettersson M, Lardelli M, Nilsson G. Distinct and regulated expression of Notch receptors in hematopoietic lineages and during myeloid differentiation. Eur J Immunol. 2001;31:3240–7. doi: 10.1002/1521-4141(200111)31:11<3240::aid-immu3240>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 45.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–61. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 46.Nomaguchi K, Suzu S, Yamada M, Hayasawa H, Motoyoshi K. Expression of Jagged 1 gene in macrophages and its regulation by hematopoietic growth factors. Exp Hematol. 2001;29:850–5. doi: 10.1016/s0301-472x(01)00657-9. [DOI] [PubMed] [Google Scholar]
- 47.Murphy TL, Cleveland MG, Kulesza P, Magram J, Murphy KM. Regulation of interleukin 12 p40 expression through an NF-kappa B half-site. Mol Cell Biol. 1995;15:5258–67. doi: 10.1128/mcb.15.10.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, Mitchell TC. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science. 2007;316:1628–32. doi: 10.1126/science.1138963. [DOI] [PubMed] [Google Scholar]
- 49.van der Kleij D, Latz E, Brouwers J, et al. A novel host-parasite lipid cross-talk – Schistosomal lyso-phosphatidylserine activates toll-like receptor 2 and affects immune polarization. J Biol Chem. 2002;277:48122–9. doi: 10.1074/jbc.M206941200. [DOI] [PubMed] [Google Scholar]
- 50.Vanhoutte F, Breuilh L, Fontaine J, et al. Toll-like receptor (TLR)2 and TLR3 sensing is required for dendritic cell activation, but dispensable to control Schistosoma mansoni infection and pathology. Microbes Infect. 2007;9:1606–13. doi: 10.1016/j.micinf.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 51.Barton GM, Medzhitov R. Toll-like receptor signaling pathways. Science. 2003;300:1524–5. doi: 10.1126/science.1085536. [DOI] [PubMed] [Google Scholar]
- 52.Banerjee A, Gerondakis S. Coordinating TLR-activated signaling pathways in cells of the immune system. Immunol Cell Biol. 2007;85:420–4. doi: 10.1038/sj.icb.7100098. [DOI] [PubMed] [Google Scholar]
- 53.Sugimoto K, Ohata M, Miyoshi J, et al. A serine/threonine kinase, Cot/Tpl2, modulates bacterial DNA-induced IL-12 production and Th cell differentiation. J Clin Invest. 2004;114:857–66. doi: 10.1172/JCI20014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Banerjee A, Gugasyan R, McMahon M, Gerondakis S. Diverse Toll-like receptors utilize Tpl2 to activate extracellular signal-regulated kinase (ERK) in hemopoietic cells. Proc Natl Acad Sci USA. 2006;103:3274–9. doi: 10.1073/pnas.0511113103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Katoh M, Katoh M. Notch ligand, JAG1, is evolutionarily conserved target of canonical WNT signaling pathway in progenitor cells. Int J Mol Med. 2006;17:681–5. [PubMed] [Google Scholar]
- 56.Estrach S, Ambler CA, Lo Celso C, Hozumi K, Watt FM. Jagged 1 is a beta-catenin target gene required for ectopic hair follicle formation in adult epidermis. Development. 2006;133:4427–38. doi: 10.1242/dev.02644. [DOI] [PubMed] [Google Scholar]
- 57.Blumenthal A, Ehlers S, Lauber J, et al. The wingless homolog WNT5A and its receptor frizzled-5 regulate inflammatory responses of human mononuclear cells induced by microbial stimulation. Blood. 2006;108:965–73. doi: 10.1182/blood-2005-12-5046. [DOI] [PubMed] [Google Scholar]