Abstract
Interleukin-10 (IL-10) and interferon-γ (IFN-γ) double producer is found in a subpopulation of T regulatory type 1 (Tr1) and T helper type 1 (Th1) cells. Consequently, it is of interest how IL-10 and IFN-γ influence the immune system. However, few studies have addressed the co-operative action of these ‘immunosuppressive’ and ‘immunostimulatory’ cytokines. Here, we examine the effect of IL-10 combined with IFN-γ on dendritic cell (DC) functions. Murine bone marrow-derived conventional DCs were stimulated with IL-10 and/or IFN-γ for 24 hr. Tumour necrosis factor-α and IL-12 p40 production by DCs treated with both IL-10 and IFN-γ was significantly lower than that by DCs treated with IL-10 or IFN-γ alone. Major histocompatibility complex class II expression on DCs treated with both cytokines was attenuated compared with that on DCs treated with either cytokine alone. In contrast, levels of inducible nitric oxide synthase and indoleamine 2,3-dioxygenase, which appear to suppress T-cell responses and promote tolerance, in DCs treated with both cytokines were higher than those in DCs treated with IL-10 or IFN-γ alone. Simultaneous treatment with IL-10 and IFN-γ significantly suppressed the ability of DCs to activate CD4+ T cells compared with treatment with either cytokine. Therefore, IL-10 and IFN-γ co-operatively suppress the immunostimulatory functions of DCs.
Keywords: activation, cytokines, dendritic cells
Introduction
Interferon-γ (IFN-γ) plays a decisive role in T helper type 1 (Th1) polarization and promotes the cell-mediated immune responses involving natural killer cells and CD8+ T cells, ultimately leading to the elimination of intracellular pathogens and tumour cells.1 On the other hand, it has been reported that IFN-γ exhibits not only immunostimulatory properties but also immunoregulatory functions in certain animal models of autoimmune disease and human autoimmune diseases. Indeed, the susceptibility and severity of experimental autoimmune encephalomyelitis and collagen-induced arthritis were increased in susceptible mouse strains deficient in IFN-γ or the IFN-γ receptor.2–4 However, the paradoxical roles of IFN-γ have not been taken into full consideration, but rather have been underestimated compared with the prominent role of IFN-γ as a Th1 cytokine.
Interleukin-10 (IL-10) is a potent immunoregulatory cytokine that inhibits undesirable innate and acquired immune responses.5,6 It can be produced by various cell types including B cells, monocytes/macrophages, dendritic cells (DCs), Th2 cells and T regulatory (Treg) cells.5–9 Interleukin-10 is responsible for the limitation and eventual termination of inflammatory responses, which reduces the damage of self tissues in the process of pathogen eradication.5,6 In addition, IL-10 plays an important role in immune tolerance to protect self organs from autoimmunity.5 It antagonizes Th1 responses by inhibiting the production of IFN-γ.5,6 Interleukin-10 inhibits IFN-γ-induced monocyte/macrophage activation, subsequent cytokine production and the upregulation of costimulatory molecules.5,6 In contrast, it has been reported that IFN-γ suppresses Toll-like receptor-mediated induction of IL-10 expression.10 It seems that IL-10 and IFN-γ are entirely opposing factors in the regulation of immune responses.
The Treg cells are efficient regulators of excessive inflammation and autoimmunity. At least three distinct types of Treg cells have been identified; Foxp3-positive Treg cells, Foxp3-negative Th3 cells and T regulatory type 1 (Tr1) cells.9 The Tr1 cells mainly produce IL-10 and thereby suppress various inflammatory responses. It has been shown that a subset of Tr1 cells produce substantial levels of IFN-γ as well as IL-10.11 However, little attention has been paid to the biological and immunological significance of the simultaneous production of these apparently opposing, ‘immunosuppressive’ and ‘immunostimulatory’, cytokines.
Dendritic cells are potent professional antigen-presenting cells (APCs) that are primarily responsible for the initiation and regulation of immune responses against various antigens.12–14 It has been reported that a variety of extracellular stimuli such as cytokines, chemical mediators and pathogen-associated molecular patterns modulate the ability of DCs to induce T-cell activation and Th1, Th2 and Th17 differentiation.15–20 However, no report has demonstrated the co-operative action of IL-10 and IFN-γ on DC functions. We examined the effect of IL-10 combined with IFN-γ on DC functions using murine bone marrow-derived conventional DCs. Here, we demonstrate that these cytokines co-operatively suppress the immunostimulatory functions of DCs.
Materials and methods
Mice
C57BL/6 (B6) and BALB/c mice were purchased from Japan SLC Inc. (Hamamatsu, Shizuoka, Japan) and maintained in the specific pathogen-free conditions of our animal facility at Hokkaido University. All experiments were followed the regulations of and were approved by the Hokkaido University Animal Care and Use Committee.
Reagents and antibodies
Murine recombinant granulocyte–macrophage colony-stimulating factor (GM-CSF) was purchased from PeproTech (London, UK). Fluorescein isothiocyanate (FITC) -conjugated anti-mouse CD86 monoclonal antibody (mAb; GL1), FITC-conjugated anti-mouse CD69 mAb (H1.2F3), biotin-conjugated anti-I-Ab mAb (AF6-120.1) and streptavidin Per-CP™ were obtained from BD Biosciences (San Diego, CA). As a control immunoglobulin G (IgG), FITC-conjugated rat IgG2a and phycoerythrin (PE) -conjugated hamster IgG were purchased from BioLegend (San Diego, CA). Biotin-conjugated mouse IgG2a was purchased from Immunotech (Marseille, France). Low endotoxin and azide-free purified anti-mouse CD210 (IL-10R) mAb (1B1.3a) and rat IgG1 (isotype control) were obtained from BioLegend. Purified anti-phospho-signal transducers and activators of transcription (STAT1; Tyr701) antibody, anti-phospho-STAT3 (Tyr705) mAb (3E2) and anti-glyceraldehyde 3-phosphate dehydrogenase mAb (14C10) were purchased from Cell Signaling Technology (Beverly, MA). Purified anti-inducible nitric oxide synthase (iNOS) mAb (6/iNOS/NOS Type II) was obtained from BD Biosciences. Purified anti-indoleamine 2,3-dioxygenase (IDO) mAb (mIDO-48) was obtained from BioLegend.
DC culture
Murine bone marrow-derived DCs (BMDCs) were generated as previously described21,22 with a minor modification. Bone marrow cells were prepared from femur and tibial bone marrow of B6 mice. After lysis of erythrocytes, major histocompatibility complex (MHC) class II, CD45R (B220), CD4 and CD8 positive cells were removed by killing with mAbs (1E4, RA3-6B2, GK1.5 and 53.4.9) and rabbit complement. The cells were cultured in complete medium (CM): RPMI-1640 medium (Sigma Chemical, St Louis, MO) containing 5% fetal calf serum, 20 ng/ml GM-CSF, 50 μm 2-mercaptoethanol, 100 IU/ml penicillin and 100 μg/ml streptomycin, at a density of 1 × 106 cells/ml/well using a 24-well plate (tissue-culture-treated, FALCON®; Becton Dickinson Labware, Franklin Lakes, NJ). On day 2, the medium was gently exchanged for fresh CM. On day 4, non-adherent granulocytes were removed without dislodging clusters of developing DCs and fresh CM was added. On day 6, free-floating and loosely adherent cells were collected and cultured in CM at a density of 1·5 × 106 cells/3 ml/35-mm dish (non-treated, NUNC™; Nalge Nunc International, Rochester, NY). On day 7, suspended cells and adherent cells detached with 3 mm ethylenediaminetetraacetic acid (EDTA) were collected and used as conventional DCs (> 97% CD11c+ B220−). Culture of DCs was performed using CM in the whole experiments.
Measurement of tumour necrosis factor (TNF) and IL-12
Dendritic cells were cultured with IL-10 (20 ng/ml) and/or IFN-γ (20 ng/ml) for 24 hr at a density of 1 × 105 cells/200 μl/well using a 96-well plate (tissue-culture-treated, FALCON) and the culture supernatants were subjected to quantification of the protein level of TNF-α and IL-12 p40 by enzyme-linked immunosorbent assay using OptEIA™ Set (BD Biosciences Pharmingen, San Diego, CA). The dose of each cytokine was determined on the basis of our preliminary dose–response study (data not shown).
Measurement of cell surface expression of CD86 and I-Ab
Dendritic cells were cultured with IL-10 (20 ng/ml) and/or IFN-γ (20 ng/ml) for 24 hr at a density of 1·5 × 106 cells/3 ml/35-mm dish (non-treated, NUNC). The cells were detached with 3 mm EDTA, collected and washed three times. The cells were incubated with 2.4G2 (rat anti-mouse Fcγ III/II receptor, CD16/CD32) supernatant to prevent the binding of specific mAb to Fcγ III/II receptor and were then stained with FITC-conjugated or biotin-conjugated mAb and peridinin chlorophyll protein-conjugated streptavidin. Expression of cell surface markers was analysed by flow cytometry on EPICS® XL (Beckman Coulter, Miami, FL). Mean fluorescence intensity subtracted from the level of isotype-matched control antibody is shown.
Nitric oxide (NO) measurement
Production of NO was assayed by measurement of the nitrite concentration with the Griess–Romijin assay as previously described.23 Dendritic cells were cultured with IL-10 (20 ng/ml) and/or IFN-γ (20 ng/ml) for 24 hr at a density of 1 × 105 cells/200 μl/well using a 96-well plate (tissue culture treated, FALCON). Supernatants (100 μl) were added to 100 μl of 6 mg/ml Griess–Romijin reagent and incubated at room temperature for 10 min. Absorbance at 540 nm was measured with a microplate reader. Nitrite concentrations were calculated using a sodium nitrite standard curve as reference.
Immunoblotting
The DCs were cultured with IL-10 (20 ng/ml) and/or IFN-γ (20 ng/ml) for the indicated time period at a density of 1 × 106 cells/2 ml/well or 5 × 105 cells/1 ml/well using a 24-well or 48-well plate (tissue-culture-treated, FALCON). Reactions were halted by rapidly cooling on ice and these DCs were washed with ice-cold phosphate-buffered saline. The whole cell lysates were prepared using cell lysis buffer (Cell Signaling Technology). The cell lysates were separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis, then blotted onto a polyvinylidene difluoride membrane (Immobilon™-P transfer membrane; Millipore, Bedford, MA). The membrane was probed with primary antibody, and developed with horseradish-peroxidase-conjugated secondary antibody by enhanced chemiluminescence. The luminescence intensity was quantified using a luminescent image analyser LAS 1000 (Fujifilm, Tokyo, Japan) and a software program image gauge version 3·4 (Fujifilm) as described elsewhere.24 The intensity relative to the mean of all band intensities in each experiment is shown as a relative intensity.
DC activation of allogeneic CD4+ T cells
Dendritic cell activation of allogeneic CD4+ T cells was performed using purified CD4+ T cells from BALB/c mouse lymph nodes. CD4+ T cells were purified from axillary, mesenteric and inguinal lymph node cells using anti-CD4 (L3T4) MicroBeads and a magnetic-activated cell-sorting column (Miltenyi Biotec, Bergisch Gladbach, Germany). Purity of the CD4+ T cells (CD4+ TCRβ+ cells) was more than 98%.
The BMDCs from B6 mice were cultured with IL-10 (20 ng/ml) and/or IFN-γ (20 ng/ml) for 24 hr at a density of 1·5 × 106 cells/3 ml/35-mm dish (non-treated, NUNC). The cells were detached with 3 mm EDTA, collected and extensively washed three times. The cytokine-primed DCs (2 × 104 cells) were cocultured with CD4+ T cells (2 × 105 cells) for 4 or 5 days on a U-bottom 96-well plate (FALCON®) in triplicate wells. The number of CD69+ activated T cells in the culture of each group was counted by flow cytometry using comparison to a known number of beads as an internal standard (Flow-Count; Beckman Coulter).
Statistical analysis
Student’s t-test was used to analyse data for significant differences and P-values less than 0·05 were regarded as significant.
Results
STAT3 and STAT1 activation in DCs upon IL-10 and IFN-γ stimulation
Signalling via haematopoietic cytokine receptors is largely mediated via the Janus kinase–STAT pathway;25 STAT3 is a key mediator of IL-10 responses, while STAT1 plays a major role in IFN-γ signalling.25 We first examined the effects of IL-10 and IFN-γ on STAT1 and STAT3 activity in murine BMDCs that are positive for CD11b and negative for CD8 and B220 (data not shown), a pattern typical of conventional DCs.
Dendritic cells were stimulated with IL-10, IFN-γ or both for 15 or 30 min and intracellular protein levels of the active forms of STAT1 and STAT3, phospho-STAT1 (pSTAT1) and phospho-STAT3 (pSTAT3), were determined (Fig. 1a,b). Neither pSTAT1 nor pSTAT3 was detected in unstimulated DCs. Interleukin-10 markedly increased pSTAT3 in DCs 15 and 30 min after stimulation, whereas no influence on the level of pSTAT1 was recorded. Interferon-γ induced substantial levels of pSTAT1 and lower level of pSTAT3 at 15 and 30 min. These findings are consistent with previous studies.25 Simultaneous stimulation of DCs with IL-10 and IFN-γ resulted in marked activation of both STAT1 and STAT3. It should be noted that neither IFN-γ nor IL-10 had a significant effect on the IL-10-induced STAT3 activation or the IFN-γ-induced STAT1 activation, respectively.
Figure 1.
Signal transducers and activators of transcription 1 (STAT1) and STAT3 activation in dendritic cells (DCs) upon interleukin-10 (IL-10) and interferon-γ (IFN-γ) stimulation. Bone marrow-derived DCs were treated with IL-10 and IFN-γ for 15 or 30 min. Levels of phospho-STAT1 (pSTAT1) and phospho-STAT3 (pSTAT3) were determined by immunoblotting. (a) Representative immunoblot from three independent experiments is shown. (b) The relative intensity of the specific band is shown. Each column represents the mean ± SE of three independent experiments.
The bands of pSTAT1 and pSTAT3 at 30 min appeared to be weak after the double cytokine culture compared with those after culture with IFN-γ alone (pSTAT1) and with IL-10 alone (pSTAT3), respectively, in the immunoblot shown in Fig. 1(a). However, these differences were not observed in the two other separate experiments and the mean of intensities was not different among these three groups (Fig. 1b).
Effects of IL-10 and IFN-γ on cytokine production by DCs
Cytokine production by DCs was crucial in regulating innate and adoptive immunity. We examined the effects of IL-10 and IFN-γ on cytokine production by DCs. Murine BMDCs were treated with IL-10, IFN-γ, or both cytokines for 24 hr and TNF-α and IL-12 levels in the supernatants were calculated. Treatment with IL-10 alone slightly attenuated spontaneous DC production of TNF-α, while significantly decreasing that of IL-12 p40 compared with untreated control DCs (Fig. 2). It has been reported that IFN-γ alone shows little or no effect on cytokine production by DCs unlike macrophages.26 As reported previously, IFN-γ alone showed little or no effect on TNF-α and IL-12 p40 productions by DCs. This low IL-12 p40 production contrasts markedly with that by Toll-like receptor ligand-stimulated DCs.27,28 Treatment with IL-10 combined with IFN-γ more significantly decreased DC production of TNF-α and IL-12 p40 than treatment with IL-10 alone, demonstrating that IFN-γ augments the suppressive function of IL-10 in cytokine production by DCs.
Figure 2.
Effects of interleukin-10 (IL-10) and interferon-γ (IFN-γ) on cytokine production by dendritic cells (DCs). Bone marrow-derived DCs were treated with IL-10 and/or IFN-γ for 24 hr. The amounts of tumour necrosis factor-α (TNF-α) and IL-12 p40 in the culture supernatants were measured by enzyme-linked immunosorbent assay. Each column represents the mean ± SE of three independent experiments. Statistical significance was calculated by Student’s t-test (*P<0·05).
Although we attempt to quantify the production of biologically active IL-12p70, no detectable IL-12 p70 was obtained in any of the cultures tested (data not shown). Increased cell concentration and potent stimulators may be required to detect IL-12p70 production by DCs.29
Effects of IL-10 and IFN-γ on the cell surface expression of CD86 and MHC class II on DCs
We next evaluated the expression of cell surface markers on DCs. Murine BMDCs were treated with IL-10, IFN-γ or both cytokines for 24 hr, and the cell surface expression of CD86 and MHC class II (I-Ab) were analysed by flow cytometry. Interleukin-10 alone showed little effect on CD86 expression on DCs but IFN-γ markedly increased CD86 expression on DCs compared with that on untreated (control) DCs. The IL-10 completely blocked the IFN-γ-induced elevation of CD86 expression on DCs (Fig. 3). Treatment with IL-10 alone showed little effect on I-Ab expression on DCs, while IFN-γ slightly increased the I-Ab expression on DCs compared with control DCs. Notably, the combination of IL-10 with IFN-γ significantly decreased I-Ab expression on DCs compared with expression on DCs treated with IL-10, IFN-γ or medium alone.
Figure 3.
Effects of interleukin-10 (IL-10) and interferon-γ (IFN-γ) on CD86 and I-Ab expressions on dendritic cells (DCs). Bone marrow-derived DCs were treated with IL-10 and/or IFN-γ for 24 hr. (a) Representative histogram from three independent experiments. (b) Each column represents the mean ± SE of three independent experiments. Statistical significance was calculated by Student’s t-test (*P<0·05; **P<0·01).
Synergistic effect of IL-10 and IFN-γ on NO production and iNOS expression by DCs
Induction of iNOS and subsequent NO production by macrophages are responsible for efficient eradication of microbes.30 On the other hand, it has been reported that DC-produced NO negatively regulates T-cell proliferation during the antigen presentation.31 Consequently, NO functions appear to be different depending on the cell type and assay system used. Although the mechanism underlying iNOS induction and NO production has been extensively investigated in the monocyte/macrophage lineage, the regulatory system of NO production and iNOS induction in DCs has not been well documented.
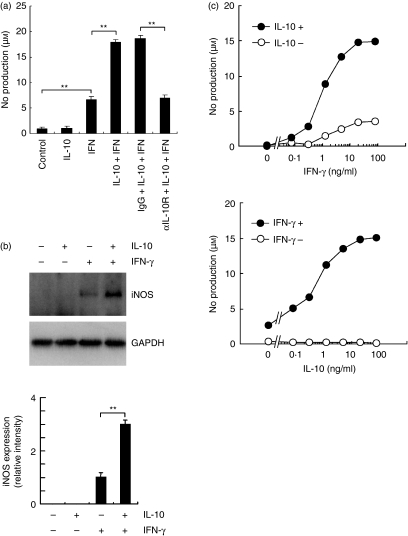
We then examined the effects of IL-10 and IFN-γ on NO production and iNOS induction in DCs. Interferon-γ significantly increased NO production and iNOS expression by DCs in agreement with a previous study (Fig. 4).31 Interleukin-10 alone showed no effects on NO production and iNOS expression by DCs (Fig. 4a,b). Of note, treatment with both IFN-γ and IL-10 considerably augmented iNOS expression and NO production. Therefore, IL-10 acted in synergy with IFN-γ in NO production and iNOS expression by DCs. The IL-10-mediated synergy in IFN-γ-induced NO production was completely blocked by treatment with anti-IL-10R (CD210) mAb (Fig. 4a).
Figure 4.
Effects of interleukin-10 (IL-10) and interferon-γ (IFN-γ) on nitric oxide (NO) production and inducible nitric oxide synthase (iNOS) expression by dendritic cells (DCs). Bone marrow-derived DCs were treated with IFN-γ and IL-10 for 24 hr in the presence or absence of anti-IL-10 receptor monoclonal antibody (mAb; αIL-10R) or isotype-matched control immunoglobulin G (IgG). (a) NO production. Cytokines were treated at 20 ng/ml. Each column represents the mean ± SE of three independent experiments. (b) Levels of iNOS and glyceraldehyde 3-phosphate dehydrogenase were determined by immunoblotting. Cytokines were treated at 20 ng/ml. Representative immunoblot from three independent experiments is shown (upper). The relative intensity of the specific band is shown (lower). Each column represents the mean ± SE of three independent experiments. Statistical significance was calculated by Student’s t-test (**P<0·01). (c) Dose–response study in NO production. The DCs were treated with IFN-γ (top) or IL-10 (bottom) at 0·078, 0·313, 1·25, 5, 20, or 80 ng/ml in the presence or absence of IL-10 (20 ng/ml) (top) or IFN-γ (20 ng/ml) (bottom). Each symbol represents the mean of triplicate wells. The analysis was repeated twice with essentially the same results.
We also performed a dose–response study of NO production by DCs (Fig. 4c). The IFN-γ alone increased NO production by DCs in a dose-dependent manner and the effect reached a peak at 20 ng/ml. In the presence of IL-10 (20 ng/ml), IFN-γ synergistically augmented the NO production in a dose-dependent manner similar to that produced by DCs stimulated with IFN-γ alone. On the other hand, IL-10 alone showed no effects on NO production by DCs up to 80 ng/ml (Fig. 4c). In contrast, IL-10 combined with IFN-γ (20 ng/ml) markedly increased NO production by DCs in a dose-dependent manner and the effect reached a peak at 20 ng/ml IL-10. It was demonstrated that the maximum effect of these cytokines was obtained at 20 ng/ml concentration.
Effects of IL-10 and IFN-γ on IDO expression by DCs
Indoleamine 2,3-dioxygenase is an enzyme that degrades the essential amino acid tryptophan. The IDO produced by DCs appears to suppress T-cell responses and promote tolerance.32 We next compared the effects of IFN-γ, IL-10 and IFN-γ plus IL-10 on IDO expression in DCs. A certain level of IDO was detected in untreated (control) DCs (Fig. 5). Treatment with IL-10 alone never affected the IDO expression. By contrast, IFN-γ treatment markedly upregulated IDO expression in DCs. Of note, IL-10 significantly enhanced the IFN-γ-induced IDO expression.
Figure 5.
Effects of interleukin-10 (IL-10) and interferon-γ (IFN-γ) on indoleamine 2,3-dioxygenase (IDO) expression in dendritic cells (DCs). Bone marrow-derived DCs were treated with IL-10 and/or IFN-γ for 24 hr. Levels of IDO were determined by immunoblotting. A representative immunoblot from three independent experiments is shown (left). The relative intensity of the specific band is shown (right). Each column represents the mean ± SE of three independent experiments. Statistical significance was calculated by Student’s t-test (*P<0·05; **P<0·01).
Ability of DCs treated with IFN-γ and IL-10 to activate CD4+ T cells
To evaluate the effects of IFN-γ and IL-10 on the APC ability of DCs to activate CD4+ T cells, DCs from B6 mice were pretreated with IFN-γ and/or IL-10, extensively washed and then cocultured with CD4+ T cells (> 98%) from BALB/c mice. On day 4 and 5, the number of CD69+ activated T cells in the culture was counted by flow cytometry. Treatment with IFN-γ or IL-10 alone showed no significant effect on DC ability to activate CD4+ T cells (Fig. 6). In contrast, simultaneous treatment of DCs with IL-10 and IFN-γ significantly suppressed the APC ability to activate CD4+ T cells.
Figure 6.
Ability of cytokine-primed dendritic cells (DCs) to activate allogeneic CD4+ T cells. Bone marrow-derived DCs (B6) were untreated (control DC) or treated with interleukin-10 (IL-10) and/or interferon-γ (IFN-γ) for 24 hr (IL-10 DC, IFN DC, or IL-10 + IFN DC). Each group of DCs (2 × 104 cells) was cocultured with CD4+ T cells (2 × 105 cells) (BALB/c) and the number of expanded CD69+ activated T cells was counted on day 4 and 5. Each symbol represents the mean ± SE of three (day 4) or five (day 5) independent experiments (*P<0·05, **P<0·01 versus control DC).
Discussion
The Tr1 cells produce large amounts of IL-10. It has been reported that a subpopulation of the Tr1 cells also produce substantial levels of IFN-γ but no IL-4.11 The Tr1 cells suppress T-cell responses in vitro and in vivo. In a colitis model, administration of Tr1 cells has been shown to ameliorate the disease manifestation.33 Although it seems clear that the IL-10 produced by Tr1 cells is responsible for their immunosuppressive activity, the role of IFN-γ simultaneously produced by a certain subset of Tr1 cells remains elusive. The IL-10 and IFN-γ double producer cells have also been found in T-bet+ Foxp3+ Th1 cells from mice infected with Toxoplasma gondii or Leishmania major.34 The IL-10 produced by the Th1 cells in these infected mice appeared to moderate the inflammatory responses. It seems that Th1 cells control themselves by producing IL-10 to minimize the tissue damage. However, coordinated action of IL-10 and IFN-γ produced by certain subsets of Tr1 and Th1 cells has not been taken into consideration in these previous studies.
In the present study, we examined influences of simultaneous signalling from these ‘immunosuppressive’ and ‘immunostimulatory’ cytokines on DC function. As previously reported, IL-10 treatment suppressed the DC production of TNF-α and IL-12. Of note, treatment of DCs with both IL-10 and IFN-γ co-operatively induced a considerable down-modulation of not only these inflammatory cytokines but also MHC class II expression. In contrast, expressions of iNOS and IDO, which suppress T-cell responses,31,32 were significantly increased by the simultaneous treatment of DCs with IL-10 and IFN-γ. In addition, the APC ability of these DCs to activate CD4+ T cells was markedly suppressed. A single treatment with either cytokine exhibited no such suppression. From these findings, we concluded that IL-10 and IFN-γ cooperatively suppressed the immunostimulatory functions of murine conventional DCs.
It has been reported that IFN-γ markedly increased TNF-α and NO production by macrophages and IL-10 decreased the IFN-γ-induced NO production.30,35,36 However, IL-10 showed little or no effect on the IFN-γ-induced NO production in the presence of excessive amounts of exogenous TNF-α. These findings suggest that IL-10 decreases the macrophage NO production by inhibiting TNF-α synthesis following IFN-γ stimulation. In this way, the autocrine production of TNF-α appears to be responsible for accelerating NO production by macrophages.
On the other hand, the regulation system of NO production by DCs has not been well characterized compared with that by macrophages. In the present study, we found that IFN-γ increased NO production and iNOS expression by DCs, while showing no effects on TNF-α production. It was also shown that IL-10 markedly enhanced the IFN-γ-induced NO production by DCs, but significantly decreased TNF-α production. Consequently, it seems that TNF-α is not involved in the IL-10-induced enhancement of NO production by DCs. This finding stands in marked contrast to that obtained with macrophage analysis.
It has been reported that IFN-γ-induced iNOS expression was synergistically enhanced by IL-22, a newly described member of the IL-10 cytokine family, in human DLD-1 colon carcinoma cells.37 Interleukin-22 induced marked phosphorylation of STAT3 in these cells and short interfering RNA technology identified that STAT3 was crucial for the synergy in iNOS expression by DLD-1 cells. In the present study, IL-10 induced marked phosphorylation of STAT3 and synergistically enhanced IFN-γ-induced iNOS expression in DCs. The IL-10-induced STAT3 activation may be responsible for the synergistic augmentation of IFN-γ-induced iNOS expression. So far, we have been unable to apply short interfering RNA technology for STAT3 knockdown in our DC culture system.
It is known that IDO degrades the essential amino acid tryptophan and shows immune suppressive properties via tryptophan starvation in the microenvironment.32 It also induces production of pro-apoptotic metabolites, kynurenines, from the tryptophan catabolism. Indeed, IDO produced by DCs during the antigen presentation suppresses T-cell proliferation and promotes T-cell tolerance. Expression of IDO by DCs is induced by IFN-γ via the STAT1 pathway. However, the definitive role of IL-10 in the IFN-γ-induced IDO expression by DCs has been unclear. We demonstrated herein that IL-10 significantly enhanced the IFN-γ-mediated IDO expression by DCs without apparent augmentation of STAT1 activation. It seems that IL-10 co-operatively acts with IFN-γ for the expression of IDO, an immunosuppressive factor in DCs, by a STAT1-independent pathway.
It has been reported that IL-10 suppresses DC functions including cytokine production, cell surface marker expression and ability to induce T-cell proliferation. However, IL-10 alone showed no significant effects on the cell surface marker expression on DCs and DC ability to induce T-cell proliferation in our present study. In previous studies, IL-10 was added during the DC development from the precursor cells38 or DC were treated with IL-10 for a long time (i.e. 2 days)39,40 compared with the present study (24 hr). It seems that short-term treatment with IL-10 alone is not sufficient to modify DC functions.
On the other hand, it has been reported that IL-10 clearly suppressed DC activation by simultaneous or subsequent stimulation with potent stimulators such as lipopolysaccharide and a cytokine cocktail, but IL-10 alone showed limited effects on DC functions.41–43 Similarly, IL-10 alone showed limited effects in most responses in our present study. However, a considerable effect of IL-10 on DC functions was observed upon simultaneous treatment with IFN-γ. Hence, the effects of IL-10 on DC functions were clearly revealed upon simultaneous or subsequent stimulation with another factor(s).
In the present study, we demonstrated a novel regulation system through co-operative action of IFN-γ and IL-10 in DC functions. This regulation may contribute to suppress excessive undesirable immunity or terminate the immune responses after pathogen eradication in response to the IFN-γ and IL-10 double producer of Tr1 or Th1 cells. Elucidation of the complex pathways of DC regulation via IFN-γ and IL-10 may lead to the development of clinical applications exploiting this new regulation system for the treatment of various infectious diseases and immune disorders.
Acknowledgments
This study was supported in part by a Grant-in-Aid for Scientific Research (B) from the Japan Society for the Promotion of Science (JSPS).
Glossary
Abbreviations:
- APC
antigen-presenting cell
- BMDC
bone marrow-derived dendritic cell
- CM
complete medium
- DC
dendritic cell
- EDTA
ethylenediaminetetraacetic acid
- FITC
fluorescein isothiocyanate
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- IDO
indoleamine 2,3-dioxygenase
- IFN
interferon
- IgG
immunoglobulin G
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- mAb
monoclonal antibody
- MHC
major histocompatibility complex
- NO
nitric oxide
- PE
phycoerythrin
- STAT
signal transducers and activators of transcription
- Th1
T helper type 1
- TNF
tumour necrosis factor
- Tr1
T regulatory type 1
- Treg
T regulatory
References
- 1.Gately MK, Renzetti LM, Magram J, Stern AS, Adorini L, Gubler U, Presky DH. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 2.Ferber IA, Brocke S, Taylor-Edwards C, Ridgway W, Dinisco C, Steinman L, Dalton D, Fathman CG. Mice with a disrupted IFN-γ gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) J Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- 3.Vermeire K, Heremans H, Vandeputte M, Huang S, Billiau A, Matthys P. Accelerated collagen-induced arthritis in IFN-γ receptor-deficient mice. J Immunol. 1997;158:5507–13. [PubMed] [Google Scholar]
- 4.Manoury-Schwartz B, Chiocchia G, Bessis N, et al. High susceptibility to collagen-induced arthritis in mice lacking IFN-γ receptors. J Immunol. 1997;158:5501–6. [PubMed] [Google Scholar]
- 5.Couper KN, Blount DG, Riley EM. IL-10: the master regulator of immunity to infection. J Immunol. 2008;180:5771–7. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- 6.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 7.Li C, Corraliza I, Langhorne J. A defect in interleukin-10 leads to enhanced malarial disease in Plasmodium chabaudi chabaudi infection in mice. Infect Immun. 1999;67:4435–42. doi: 10.1128/iai.67.9.4435-4442.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–95. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bettelli E, Oukka M, Kuchroo VK. TH-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–50. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 10.Hu X, Paik PK, Chen J, Yarilina A, Kockeritz L, Lu TT, Woodgett JR, Ivashkiv LB. IFN-γ suppresses IL-10 production and synergizes with TLR2 by regulating GSK3 and CREB/AP-1 proteins. Immunity. 2006;24:563–74. doi: 10.1016/j.immuni.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 11.Awasthi A, Carrier Y, Peron JP, et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–9. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 12.Steinman R. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–96. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 13.Hart DNJ. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood. 1997;90:3245–87. [PubMed] [Google Scholar]
- 14.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–51. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 15.Ramirez-Pineda JR, Frohlich A, Berberich C, Moll H. Dendritic cells (DC) activated by CpG DNA ex vivo are potent inducers of host resistance to an intracellular pathogen that is independent of IL-12 derived from the immunizing DC. J Immunol. 2004;172:6281–9. doi: 10.4049/jimmunol.172.10.6281. [DOI] [PubMed] [Google Scholar]
- 16.Agrawal S, Agrawal A, Doughty B, et al. Cutting edge: different Toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. J Immunol. 2003;171:4984–9. doi: 10.4049/jimmunol.171.10.4984. [DOI] [PubMed] [Google Scholar]
- 17.Dillon S, Agrawal A, Van Dyke T, et al. A Toll-like receptor 2 ligand stimulates Th2 responses in vivo, via induction of extracellular signal-regulated kinase mitogen-activated protein kinase and c-Fos in dendritic cells. J Immunol. 2004;172:4733–43. doi: 10.4049/jimmunol.172.8.4733. [DOI] [PubMed] [Google Scholar]
- 18.Dillon S, Agrawal S, Banerjee K, et al. Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. J Clin Invest. 2006;116:916–28. doi: 10.1172/JCI27203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kikuchi K, Yanagawa Y, Aranami T, Iwabuchi C, Iwabuchi K, Onoé K. Tumour necrosis factor-α but not lipopolysaccharide enhances preference of murine dendritic cells for Th2 differentiation. Immunology. 2003;108:42–9. doi: 10.1046/j.1365-2567.2003.01537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krishnamoorthy N, Oriss TB, Paglia M, Fei M, Yarlagadda M, Vanhaesebroeck B, Ray A, Ray P. Activation of c-Kit in dendritic cells regulates T helper cell differentiation and allergic asthma. Nat Med. 2008;14:565–73. doi: 10.1038/nm1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clingan JM, Yanagawa Y, Iwabuchi K, Onoé K. Effect of T helper 1 (Th1)/Th2 cytokine on chemokine-induced dendritic cell functions. Cell Immunol. 2006;242:72–9. doi: 10.1016/j.cellimm.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Zhang M, Tang H, Guo Z, et al. Splenic stroma drives mature dendritic cells to differentiate into regulatory dendritic cells. Nat Immunol. 2004;5:1124–33. doi: 10.1038/ni1130. [DOI] [PubMed] [Google Scholar]
- 24.Czuchra A, Wu X, Meyer H, van Hengel J, Schroeder T, Geffers R, Rottner K, Brakebusch C. Cdc42 is not essential for filopodium formation, directed migration, cell polarization, and mitosis in fibroblastoid cells. Mol Biol Cell. 2005;16:4473–84. doi: 10.1091/mbc.E05-01-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baker SJ, Rane SG, Reddy EP. Hematopoietic cytokine receptor signaling. Oncogene. 2007;26:6724–37. doi: 10.1038/sj.onc.1210757. [DOI] [PubMed] [Google Scholar]
- 26.Frasca L, Nasso M, Spensieri F, Fedele G, Palazzo R, Malavasi F, Ausiello CM. IFN-γ arms human dendritic cells to perform multiple effector functions. J Immunol. 2008;180:1471–81. doi: 10.4049/jimmunol.180.3.1471. [DOI] [PubMed] [Google Scholar]
- 27.Hirata N, Yanagawa Y, Ebihara T, et al. Selective synergy in anti-inflammatory cytokine production upon cooperated signaling via TLR4 and TLR2 in murine conventional dendritic cells. Mol Immunol. 2008;45:2734–42. doi: 10.1016/j.molimm.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 28.Yanagawa Y, Onoé K. Enhanced IL-10 production by TLR4- and TLR2-primed dendritic cells upon TLR restimulation. J Immunol. 2007;178:6173–80. doi: 10.4049/jimmunol.178.10.6173. [DOI] [PubMed] [Google Scholar]
- 29.Khayrullina T, Yen JH, Jing H, Ganea D. In vitro differentiation of dendritic cells in the presence of prostaglandin E2 alters the IL-12/IL-23 balance and promotes differentiation of Th17 cells. J Immunol. 2008;181:721–35. doi: 10.4049/jimmunol.181.1.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gazzinelli RT, Oswald IP, James SL, Sher A. IL-10 inhibits parasite killing and nitrogen oxide production by IFN-γ-activated macrophages. J Immunol. 1992;148:1792–6. [PubMed] [Google Scholar]
- 31.Lu L, Bonham CA, Chambers FG, Watkins SC, Hoffman RA, Simmons RL, Thomson AW. Induction of nitric oxide synthase in mouse dendritic cells by IFN-γ, endotoxin, and interaction with allogeneic T cells: nitric oxide production is associated with dendritic cell apoptosis. J Immunol. 1996;157:3577–86. [PubMed] [Google Scholar]
- 32.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–74. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 33.Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–42. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 34.O’Garra A, Vieira P. TH1 cells control themselves by producing interleukin-10. Nat Rev Immunol. 2007;7:425–8. doi: 10.1038/nri2097. [DOI] [PubMed] [Google Scholar]
- 35.Bogdan C, Vodovotz Y, Nathan C. Macrophage deactivation by interleukin 10. J Exp Med. 1991;174:1549–55. doi: 10.1084/jem.174.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oswald IP, Wynn TA, Sher A, James SL. Interleukin 10 inhibits macrophage microbicidal activity by blocking the endogenous production of tumor necrosis factor α required as a costimulatory factor for interferon γ-induced activation. Proc Natl Acad Sci USA. 1992;89:8676–80. doi: 10.1073/pnas.89.18.8676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ziesché E, Bachmann M, Kleinert H, Pfeilschifter J, Mühl H. The interleukin-22/STAT3 pathway potentiates expression of inducible nitric-oxide synthase in human colon carcinoma cells. J Biol Chem. 2007;282:16006–15. doi: 10.1074/jbc.M611040200. [DOI] [PubMed] [Google Scholar]
- 38.Allavena P, Piemonti L, Longoni D, Bernasconi S, Stoppacciaro A, Ruco L, Mantovani A. IL-10 prevents the differentiation of monocytes to dendritic cells but promotes their maturation to macrophages. Eur J Immunol. 1998;28:359–69. doi: 10.1002/(SICI)1521-4141(199801)28:01<359::AID-IMMU359>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 39.Steinbrink K, Wölfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol. 1997;159:4772–80. [PubMed] [Google Scholar]
- 40.Kaliński P, Schuitemaker JH, Hilkens CM, Kapsenberg ML. Prostaglandin E2 induces the final maturation of IL-12-deficient CD1a+CD83+ dendritic cells: the levels of IL-12 are determined during the final dendritic cell maturation and are resistant to further modulation. J Immunol. 1998;161:2804–9. [PubMed] [Google Scholar]
- 41.Haase C, Jørgensen TN, Michelsen BK. Both exogenous and endogenous interleukin-10 affects the maturation of bone-marrow-derived dendritic cells in vitro and strongly influences T-cell priming in vivo. Immunology. 2002;107:489–99. doi: 10.1046/j.1365-2567.2002.01529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Faulkner L, Buchan G, Baird M. Interleukin-10 does not affect phagocytosis of particulate antigen by bone marrow-derived dendritic cells but does impair antigen presentation. Immunology. 2000;99:523–31. doi: 10.1046/j.1365-2567.2000.00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perona-Wright G, Anderton SM, Howie SE, Gray D. IL-10 permits transient activation of dendritic cells to tolerize T cells and protect from central nervous system autoimmune disease. Int Immunol. 2007;19:1123–34. doi: 10.1093/intimm/dxm084. [DOI] [PubMed] [Google Scholar]