Abstract
Autoantigen-presenting immunomodulatory dendritic cells (DCs) that are used for adoptive transfer have been shown to be a promising therapy for a number of autoimmune diseases. We have previously demonstrated that enteroantigen-pulsed DCs treated with interleukin-10 (IL-10) can partly protect severe combined immunodeficient (SCID) mice adoptively transferred with CD4+ CD25− T cells from the development of wasting disease and colitis. We therefore established an in vitro test that could predict the in vivo function of DCs and improve strategies for the preparation of immunomodulatory DCs in this model. Based on these in vitro findings, we here evaluate three methods for DC generation including short-term and long-term IL-10 exposure or DC exposure to dexamethasone in combination with vitamin D3 (Dex/D3). All DCs resulted in lower CD4+ CD25− T-cell enteroantigen-specific responses in vitro, but Dex/D3 DCs had the most prominent effect on T-cell cytokine secretion. In vivo, Dex/D3 DCs most efficiently prevented weight loss and gut pathology upon CD4+ CD25− T-cell transfer in SCID mice, although the effect on gut pathology was antigen independent. Our data in the SCID T-cell transfer model illustrate some correlation between in vitro and in vivo DC function and document that prevention of experimental inflammatory bowel disease by transfer of immunosuppressive DCs is possible.
Keywords: colitis, dendritic cells, dexamethasone, interleukin-10, severe combined immunodeficiency, tolerance
Introduction
The in vitro generation of immunomodulatory or tolerogenic dendritic cells (DCs) for therapeutic use is a major focus in autoimmunity research and transplantation immunology, and stimulation of immature DCs with interleukin-10 (IL-10), tumour necrosis factor-α or other immunomodulatory reagents1–4 has proved successful in a number of murine disease models. These models include allotransplantation,5–7 experimental autoimmune encephalomyelitis (EAE),1,4 myasthenia gravis3 and type 1 diabetes8 and recently we have shown that short-term IL-10-treated DCs pulsed with enteroantigen can suppress the wasting disease and development of colitis in the severe combined immunodeficiency (SCID) T-cell transfer model.9 In this model adoptively transferred CD4+ T cells repopulate the spleen and the gut-associated lymphoid tissue and lead to the development of chronic colitis.10 The transferred CD4+ T cells have been shown to react in an antigen-specific, H-2-restricted way against enterobacterial antigens.11 In this sense, the model resembles human inflammatory bowel disease (IBD).12,13
In our previous study,9 we used bone-marrow-derived DCs (BM-DCs) that were exposed to a short 2-day IL-10 culture before being pulsed with enteroantigen. Injection of these DCs prevented both the weight loss observed in SCID mice upon CD4+ CD25− T-cell transfer and, partly, the gut pathology. We aimed to improve strategies for the generation of immunomodulatory DCs that would exert an improved effect in this model and to develop assays to predict in vitro the function of DCs in vivo. We established an in vitro assay that allowed for the screening of immunomodulatory DCs with an ability to reduce enteroantigen-specific CD4+ CD25− T-cell responses and based on this, we aimed to compare DCs exposed to short-term IL-10 (s-IL-10 DC) with alternative protocols reported to be optimal for the generation of immunomodulatory DCs, in particular DCs exposed to long-term IL-10 (l-IL-10 DC)2 or a combination of dexamethasone and vitamin D3 (Dex/D3 DC).14 We compared phenotype, DC cytokine secretion, T-cell differentiation and cytokine secretion together with the in vivo effect in prophylactic experiments in the SCID T-cell transfer model of colitis. Based on these in vitro experiments, Dex/D3 DCs were shown to be potent candidates for the inhibition of enteroantigen responses. Here, we demonstrate that transfer of Dex/D3 DCs partly prevents weight loss in the SCID T-cell transfer model of colitis, whereas prevention of gut pathology is seen after transfer of Dex/D3 DCs unexposed to enteroantigen, demonstrating the need for further enteroantigen characterization to fully understand DC-mediated regulation in this model of IBD.
Materials and methods
Mice
Conventional 6- to 8-week-old female BALB/c mice and SCID mice were purchased from Taconic Europe (Ry, Denmark) and kept under controlled microbial conditions at the local animal facility of the Panum Institute. All animal experiments were performed in accordance with the permissions obtained from the Animal Ethical Committee, Copenhagen, The Department of Justice.
Generation of BM-DC
Dendritic cells were generated from BM cells derived from BALB/c mice. The BM cells obtained from femurs and tibias were washed and cultured overnight in six-well plates (TPP, Trasadingen, Schwitzerland) at 2 × 106 cells/ml in 3 ml culture medium/well. Culture medium (CM) was RPMI-1640 with Glutamax supplemented with 10% fetal calf serum (FCS; Harlan Sera-Lab Ltd, Hillcrest, UK) and antibiotics. The next day, non-adherent cells were harvested and resuspended in CM containing 10 ng/ml granulocyte–macrophage colony-stimulating factor plus 20 ng/ml IL-4 (both from Peprotech, Rocky Hill, NJ) and cultured at 1 × 106 cells/ml in 3 ml CM/well. Fresh cytokines and medium were added on day 3. For different subsets of DCs, 20 ng/ml IL-10 was added from day 1 or 40 ng/ml IL-10 (Peprotech) was added on day 6, 1 μm dexamethasone (Sigma-Aldrich, St Louis, MO) was added on day 6, 10 nm vitamin D3 (BIOMOL Research Labs, Butler Pike, PA) was added on day 7 as published previously14 and 133 μg/ml enterobacterial extract on day 7 in different combinations. Cells were harvested on day 8 using phosphate-buffered saline (PBS)/trypsin/ethylenediaminetetraacetic acid solution (Gibco, Paisley, UK). Dendritic cells were typically 70% CD11c-positive, as published elsewhere.9
Induction of colitis
The CD4+ T cells were isolated from mesenteric lymph nodes or spleens of BALB/c mice with anti-CD4 Dynabeads and Detach-a-Bead (Dynal, Oslo, Norway) according to the manufacturer’s instructions. These cells were subsequently depleted of CD25+ cells with phycoerythrin (PE)-conjugated anti-CD25 antibody (cat. no. 130-091-013) and Anti-PE microbeads (cat. no. 130-048-801) kit from Miltenyi Biotec (Bergisch Gladbach, Germany). The CD4+ CD25− T cells were resuspended in PBS/1% bovine serum albumin (BSA) and transferred intraperitoneally (i.p.) to SCID mice, 3 × 105/mouse. Mice treated with DCs received 1 × 106 DCs i.p. 2–3 hr in advance. Mice were then monitored once or twice per week for weight loss, loose stools, bloody diarrhoea and rectal prolapse.
Immunohistochemistry
For histological examinations, the anal 2 cm of the gut were fixed in 4% paraformaldehyde. The samples were embedded in paraffin, cut by serial microtomy and stained with periodic acid Schiff/haematoxylin/eosin. The specimens were all examined in a blinded fashion by one pathologist. Histopathological changes were evaluated as described previously.15 Score 0, no signs of inflammation; score 1, slight infiltrations in lamina propria by mononuclear cells (scores 0 to 1 are present in non-transplanted SCID mice); score 2, moderate mononuclear cell infiltration of lamina propria, increased mitotic activity in the epithelial layer; score 3, as in 2 but patchy mononuclear cell infiltration in the submucosal layer, elongation of the crypts with depletion of goblet cells; score 4, as in 3, but some crypt destruction and epithelial metaplasia; score 5, as in 4, but including increased crypt destruction and microabscesses, polymorphonuclear infiltration of epithelial layer and surrounding infiltration with mononuclear cells in the submucosal layer, epithelial ulcerative lesions.
Enterobacterial extract
The extract was prepared by removing the fecal content of colon and caecum into ice-cold PBS followed by sonication, centrifugation, filtration at 4° and protein concentration measurement as described previously.11 The extract was kept at −80° until use.
Enteroantigen specific CD4+ T-cell proliferation
For primary proliferation assays, titrated day 8 enteroantigen-pulsed or unpulsed DCs were cultured together with 1 × 105 CD4+ CD25− T cells purified from mesenteric lymph nodes of BALB/c mice. The cells were cultured for 5 days including 18 hr incorporation of 0·5 μCi/well [3H]thymidine (Amersham, Little Chalfont, UK) for the measurement of proliferation and supernatant was collected for analysis of secreted cytokines. For secondary proliferation assays, 1 × 105 total cells from primary cultures were harvested and cocultured with 1 × 105 enteroantigen-pulsed or unpulsed antigen-presenting cells (APCs; unfractionated spleen cells). These stimulator APCs are prepared from splenocytes (8 × 106 cells/well in 2 ml CM in 24-well plates pulsed overnight with 200 μg/ml enterobacterial extract, washed and irradiated) from BALB/c mice as previously described.11 Unpulsed spleen cells from C57/BL6 mice served as the positive control in the secondary proliferation assay. The cells were cultured for 5 days, including 18 hr incorporation of 0·5 μCi/well [3H]thymidine (Amersham) for the measurement of proliferation, and supernatant was collected from these secondary cultures for the analysis of cytokine secretion.
Measurement of cytokines
Murine interferon-γ (IFN-γ), IL-2, IL-4, IL-5, IL-10 and IL-12p40/p70, were analysed in supernatants of DCs and mixed lymphocyte reactions with a T helper type 1 (Th1)/Th2 cytokine 6-Plex and IL-17 with a singleplex for Luminex (Biosource Cat. No LMC0002 and LMC0171, respectively) according to the manufacturer’s protocol. Measurement was carried out using the Luminex 100 IS (Luminexcorp, Austin, TX). More than 100 events were acquired per bead set. starstation version 2·0 software (Applied Cytometry Systems, Sheffield, UK) was used for cytokine quantification analysis.
Expression of notch ligands measured with QuantiGene Plex
Levels of messenger RNA (mRNA) for Jagged 1, Jagged 2 and Delta 4 were compared with and expressed as % of mRNA of the housekeeping gene hypoxanthine guanine phosporibosyl transferase 1. Measurement was performed with a customized QuantiGene® Plex Reagent System (Panomics, Fremont, CA) from Panomics. In short, cell lysates were hybridized with mRNA target-specific probes conjugated to fluorescent microspheres. Amplification was then performed by hybridization with biotinylated label probes with the ability to bind streptavidin-conjugated R-PE, allowing determination of target bead and mRNA quantification simultaneously on a flow cytometer. Measurement was carried out using the Luminex 100 IS (Luminexcorp). Approximately 100 events were acquired per bead set. starstation version 2·0 software (Applied Cytometry Systems) was used for cytokine quantification analysis.
Statistics
Significant differences between sample means were determined with the two-tailed Student’s t-test for independent samples, and results were considered significant when P < 0·05. A two-way analysis of variance (anova) was used to determine the significant differences between weight curves of untreated and treated SCID mice. Significant differences between histopathology scores were determined with the Fisher’s exact test.
Fluorescence-activated cell sorting analysis
The BM-DCs were harvested as described above and stained for 0·5 hr in PBS with 0·25% BSA, using allophycocyanin-, fluorescein isothiocyanate- or PE-coupled antibodies against CD11c, CD40, CD80, CD86, CD273 (PD-L1), CD274 (PD-L2) and I-A/E (all from BD Pharmingen, San Jose, CA). For the measurement of cytokine secretion, 4 × 105 cells/well were set up in round-bottom microculture plates at the concentration of 2 × 106/ml in complete CM together with 50 ng/ml phorbol 12-myristate 13-acetate and 750 ng/ml ionomycin to activate the cytokine-secreting T cells. Monensin was supplied and cells were incubated for 3 hr, incubated with Fc blocking antibodies, with antibodies against surface markers, fixated, permeabilized and stained with antibodies for intracellular cytokine staining, all according to the protocol of a BD Cytofix/Cytoperm Plus Fixation/Permeabilization kit (BD Bioscience cat. no. 554715). Antibodies against IFN-γ and IL-17 were used (all from BD Pharmingen). Subsequently, cells were washed once and cells were analysed on a FACSCalibur (BD) and data were analysed using BD CellQuest software. At least 10 000 cells were collected, using a live gate based on forward scatter/side scatter properties.
Results
In vitro reactivity of enteroantigen-specific CD4+ CD25− T cells
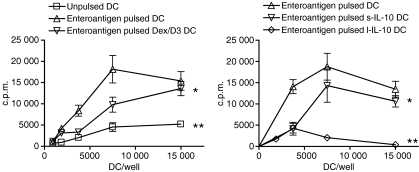
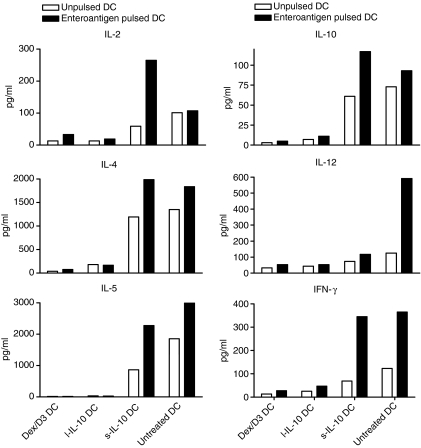
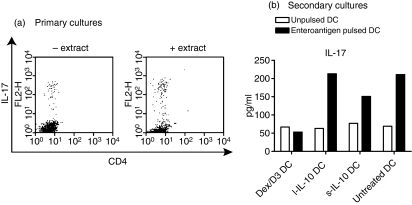
We examined long-term and short-term exposure of DCs to IL-10 as well as Dex/D3 treatment in relation to proliferation of enteroantigen-specific CD4+ CD25− T cells from normal mice in vitro. From these studies both l-IL-10 DCs and Dex/D3 DCs induced significantly lower responses of enteroantigen-specific CD4+ T-cell proliferation [P = 0·004 and P < 0·0001 respectively (two-way anova)] in particular at relevant DC numbers (7500 DC/well) whereas reduction observed with s-IL-10 DCs was less significant [P = 0·01 (two-way anova)] (Fig. 1). In accordance with these data, secretion of IL-4, IL-5, IL-10 and IFN-γ from DC–T-cell cultures was reduced when Dex/D3-treated or long-term IL-10-treated enteroantigen-exposed DCs were used as stimulator cells as compared with short-term IL-10-treated or untreated enteroantigen-exposed DCs (Fig. 2). In contrast, IL-12 was reduced in DC–T-cell cultures with enteroantigen-exposed DCs in response to pretreatment with Dex/D3 or IL-10 when compared with cultures using untreated enteroantigen-exposed DCs (Fig. 2). In addition, we tested the induction of Th17 cells in the primary DC–T-cell cocultures and observed a small enteroantigen-specific increase in the number of these cells from 5·8% to 8·4% (Fig. 3a), which was not reduced when enteroantigen-exposed Dex/D3-treated or IL-10-treated DCs were used as stimulator cells (data not shown). The antigen-specific IL-17 secretion was more pronounced when we measured IL-17 in secondary cultures where normal or treated enteroantigen-pulsed DCs were used as primary stimulators and cells from these cultures were re-exposed to enteroantigen-pulsed spleen cells in secondary cultures (Fig. 3b) where the number of enteroantigen-specific Th17 cells also increased from a background of 13–23%. Here, initial stimulation with enteroantigen-pulsed Dex/D3 DC resulted in lower IL-17 secretion in secondary cultures (Fig. 3b). In addition, secretion of IL-2, IL-4, IL-12 and IFN-γ was lower whereas IL-5 secretion was unchanged and IL-10 secretion was increased in similar secondary cultures. However, these changes were not antigen-specific and enteroantigen-specific proliferation was not suppressed in secondary cultures (data not shown).
Figure 1.
Dendritic cell (DC) -mediated suppression of enteroantigen-specific CD4+ CD25− T cells in vitro; 1 × 105 CD4+ CD25− from naïve BALB/c mice were cocultured with titrated number of unpulsed or enteroantigen-pulsed (133 μg/ml) bone-marrow-derived dendritic cells exposed to dexamethasone (1 μm) + vitamin D3 (10 nm) (Dex/D3 DC) or short-term interleukin-10 (40 ng/ml; sIL-10 DC) or long term IL-10 (20 ng/ml; l-IL-10 DC). Cultures were left for 5 days including 18 hr incorporation of [3H]thymidine for the measurement of proliferation. (a) The effect of Dex/D3 DC; (b) the effect of s-IL-10 and l-IL-10 DCs. Data are shown as one selective experiment of three performed with similar findings. Curves that significantly different from curves for enteroantigen-pulsed DCs are indicated (*P < 0·05, **P < 0·001, two-way anova).
Figure 2.
Dendritic cell (DC)-mediated suppression of cytokine secretion; 1 × 105 CD4+ CD25− from naïve BALB/c mice were cocultured with 1 × 104 enteroantigen unpulsed or pulsed bone-marrow-derived DCs exposed to dexamethasone (1 μm) + vitamin D3 (10 nm) (Dex/D3 DC) or short-term interleukin-10 (40 ng/ml; sIL-10 DC) or long-term IL-10 (20 ng/ml; l-IL-10 DC). Cytokines were analysed from the supernatant after 5 days of DC–T-cell cultures. Data are shown as one selective experiment out of two performed with similar findings.
Figure 3.
Induction of T helper type 17 (Th17) cells. (a) 1 × 105 CD4+ CD25− from naïve BALB/c mice were cocultured with 1 × 104 enteroantigen-pulsed or unpulsed bone-marrow-derived dendritic cells and Th17 cells were analysed by flow cytometry after 5 days. (b) Alternatively, total cells from cocultures were re-exposed to enteroantigen-pulsed antigen-presenting cells (unfractionated spleen cells) and interleukin-17 (IL-17) secretion was analysed from supernatants from these secondary cultures. Data are shown as one selective experiment out of two performed with similar findings.
DC phenotype
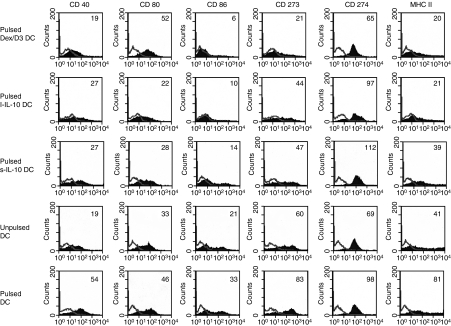
In accordance with previous results, untreated DCs had the phenotype of immature DCs with low expression of costimulatory molecules CD40, CD80 and CD86 as well as CD273, CD274 and major histocompatibility complex (MHC) class II. The expression of all these surface molecules was upregulated upon addition of enterobacterial extract (Fig. 4) as a consequence of DC maturation. The Dex/D3 DCs exhibited downregulation of CD40, CD86, CD273 and CD274 to levels comparable with or lower than unpulsed DCs whereas downregulation of MHC class II molecules was similar to the downregulation observed after long-term culture with IL-10 (i.e. l-IL-10 DC). Long-term culture with IL-10 resulted in downregulation of CD80, CD86 and CD273 and MHC class II to levels comparable with or lower than unpulsed DCs, whereas short-term culture with IL-10 (i.e. s-IL-10 DC) resulted in downregulation of CD80, CD86 and CD273 to levels comparable with or lower than unpulsed DCs (Fig. 4). Downregulation of CD40, CD86 and MHC class II was seen with both dexamethasone and vitamin D3 alone, whereas major downregulation of CD273 and CD274 was only seen with the two reagents in combination (data not shown).
Figure 4.
Dendritic cell (DC) phenotype. Bone-marrow-derived DCs (BM-DCs) were stimulated as indicated, harvested on day 8 and analysed by flow cytometry for expression of various surface molecules. Enteroantigen-unpulsed or pulsed BM-DCs and enteroantigen-pulsed Dex/D3 DC, short-term IL-10 DCs (s-IL-10 DC) and long-term IL-10 DCs (l-IL-10 DC) stained for CD40, CD80, CD86, CD273, CD274 and MHC class II molecules (filled curves) or isotype control antibodies (open line) (1 × 106 cells pr. sample) are shown. DCs are gated as large granular cells. Numbers indicate mean fluorescence intensity (MFI) values. Data are shown as one selective experiment of two performed with similar findings.
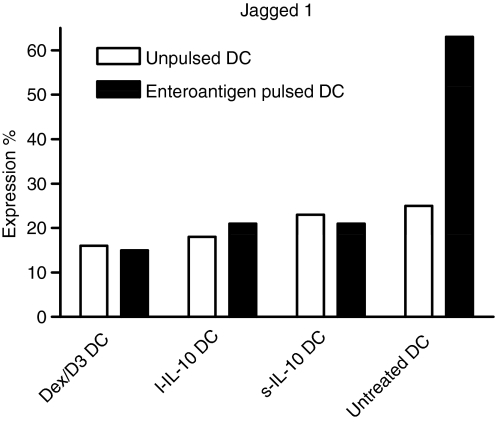
In addition, we quantified mRNA levels for the Notch ligands Jagged 1, Jagged 2 and Delta 4 because the relative expression of these are important in determination of Th1 (Delta 4) or Th2 (Jagged 1) responses.16,17 Only Jagged 1 expression was detectable (Fig. 5) and was upregulated after DC pulsing with enteroantigen. The Dex/D3 DC and both types of IL-10-exposed DCs did not upregulate Jagged 1 after enteroantigen pulsing.
Figure 5.
Dendritic cell (DC) expression of Jagged 1. Bone marrow-derived DCs (BM-DCs) were unpulsed or enteroantigen-pulsed exposed to dexamethasone (1 μm) + vitamin D3 (10 nm) (Dex/D3 DC) or short-term interleukin-10 (40 ng/ml; sIL-10 DC) or long-term IL-10 (20 ng/mll l-IL-10 DC) as indicated. At day 8, cell lysates were made from 4 × 105 cells/sample and messenger RNA was analysed with the QuantiGene Plex Reagent System. Relative expression as compared to expression of the housekeeping gene hypoxanthine guanine phosporibosyl transferase 1 is shown. Data are shown as one selective experiment out of two performed with similar findings.
DC secretion of IL-10 and IL-12
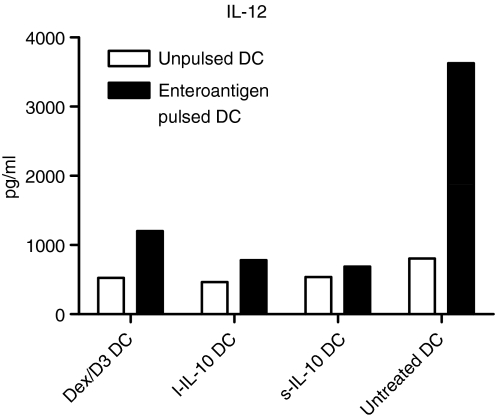
We next tested the levels of secreted of IL-10 and IL-12p40/p70 in DC supernatants. Dex/D3-treated DCs did not have an increased secretion of IL-10 compared with untreated DCs pulsed with enteroantigen (data not shown). However, Dex/D3-treated DCs and those DCs undergoing long-term and short-term treatments with IL-10 led to suppression of IL-12 as compared with untreated DCs pulsed with enteroantigen (Fig. 6). The suppressive effect of Dex/D3-treated DCs was exclusively caused by dexamethasone (data not shown).
Figure 6.
Dendritic cell (DC) -mediated interleukin-12 (IL-12) p40/p70 secretion. Bone marrow-derived DCs (BM-DCs) were stimulated as indicated and supernatants (from approximately 1 × 106 cells/sample) were harvested on day 8 for analysis of cytokine secretion. Unpulsed or enteroantigen-pulsed BM-DCs exposed to dexamethasone (1 μm) + vitamin D3 (10 nm) (DEX/D3 DC) or short-term IL-10 (40 ng/ml) (sIL-10 DC) or long-term IL-10 (20 ng/ml) (l-IL-10 DC) are indicated. Data are shown as one selective experiment out of two performed with similar findings.
Enteroantigen-pulsed Dex/D3 DC ameliorate colitis development
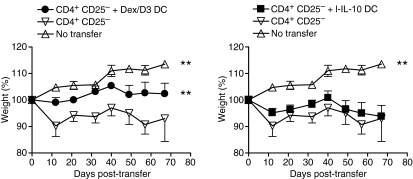
Adoptive transfer of CD4+ CD25− cells into SCID mice leads to a chronic colitis with weight loss, loose stools, bloody diarrhoea and rectal prolapse.10,11,18 We have previously shown that enteroantigen-pulsed s-IL-10 DCs injected i.p. into SCID mice 2–3 hr before adoptive transfer of CD4+ CD25− T cells can prevent weight loss.9 We here tested the effect of enteroantigen-pulsed Dex/D3 DCs and demonstrate that these DCs are also able to reduce the development of weight loss in colitic mice (P < 0·0001, two-way anova) whereas l-IL-10 DC were not effective (Fig. 7). The Dex/D3 DCs without enteroantigen exposure were without effect. Also, the observed effect was primarily mediated by dexamethasone (data not shown) and was dependent on DC generation in FCS. Finally, therapeutic experiments where DCs were injected after the establishment of colitis were generally unsuccessful (data not shown).
Figure 7.
Enteroantigen-pulsed dexamethasone/vitamin D3 (Dex/D3) dendritic cells (DCs) ameliorate weight loss in severe combined immunodeficiency (SCID) mice adoptively transferred with CD4+ CD25− T cells. Enteroantigen-pulsed BM-DCs were exposed to dexamethasone (1 μm) + vitamin D3 (10 nm) (Dex/D3 DC) or long-term IL-10 (20 ng/ml; l-IL-10 DC) as indicated, harvested on day 8 and injected intraperitoneally into SCID mice (1 × 106/mouse) before CD4+ CD25− T-cell transfer (3 × 105/mouse). Mouse weight was registered regularly, and mice were killed around day 70. Data represent mean ± SEM of mice in each treatment group (n = 6). Curves that are significantly different from curves derived from SCID mice without transfer are indicated (**P < 0·001, two-way anova).
Histopathology
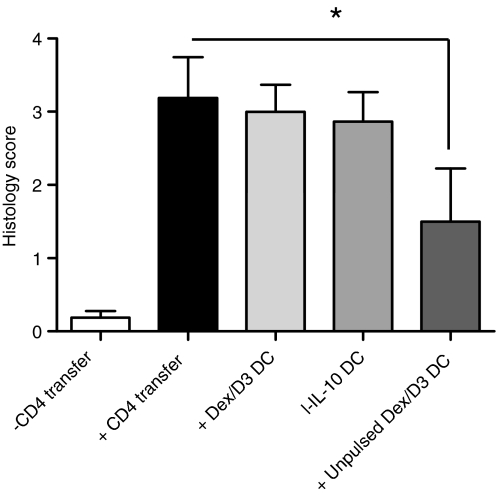
Figure 8 shows the colitis score after adoptive transfer of CD4+ CD25− T cells with or without injection of enteroantigen-pulsed Dex/D3 DCs or l-IL-10 DCs. However, no significant reduction in histology score was observed in contrast to pretreatment with s-IL-10 DC, which was previously shown to induce a minor reduction.9 However, suppression of the immunopathology was possible with Dex/D3 DCs in the absence of enteroantigen exposures [mean score 1·5, P = 0·04 (Fisher’s exact test)] (Fig. 8) which were superior to other combinations tested (data not shown).
Figure 8.
Histology score in severe combined immunodeficiency (SCID) mice. Enteroantigen-pulsed or unpulsed bone marrow-derived dendritic cells (BM-DCs) were exposed to dexamethasone (1 μm) + vitamin D3 (10 nm) (Dex/D3 DC) or short-term IL-10 (40 ng/ml; sIL-10 DC) or long-term IL-10 (20 ng/ml; ;l-IL-10 DC) as indicated, harvested on day 8 and injected intraperitoneally into SCID mice (1 × 106/mouse) before CD4+ CD25− T-cell transfer (3 × 105/mouse). When mice were killed around day 70, the distal 2 cm of the gut was stained with haematoxylin & eosin and histopathology scoring was performed. Data represents mean ± SEM of mice in each treatment group (n = 6 to n = 12). (*P < 0·05, Fischer’s exact test).
T cells in vivo
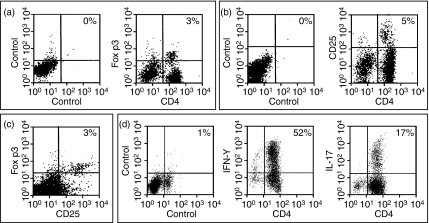
Finally, we analysed the change in proinflammatory Th1 and Th17 and regulatory T cells in vivo in relation to DC treatment. Mesenteric lymph nodes from colitic animals contained a mixture of IFN-γ-producing Th1 and Th17 in mesenteric lymph nodes (Fig. 9d). Also, regulatory T cells identified as CD4+ CD25+ Foxp3+ T cells were identified (Fig. 9a–c), and a similar distribution was observed in the spleen (data not shown). However, we did not observe any significant changes in levels of Th1, Th17 or CD4+ CD25+ Foxp3+ T cells after treatment with immunomodulatory DCs (data not shown).
Figure 9.
T-cell profile in colitic mice. Severe combined immunodeficiency (SCID) mice were injected intraperitoneally with 3 × 105 CD4+ CD25− T cells. When mice were killed around day 70, mesenteric lymph nodes were harvested and analysed for the presence of CD4+ Foxp3+ cells (a), CD4+ CD25+ cells (b), CD25+ Foxp3+ cells (c), and interferon-γ (IFN-γ) or interleukin-17 (IL-17) -secreting CD4+ T cells (d). Data are presented as one selective experiment out of two experiments performed with similar findings. Pools of lymphoid cells from four animals were used.
Discussion
DCs can be manipulated to induce either effector T-cell responses or T-cell anergy and regulatory T-cell activity depending on the maturation level.1,5–7,19,20 Recently, immunomodulatory DCs have been generated that in allotransplantation settings and several murine models of autoimmune diseases have been shown to reduce graft-rejection or to ameliorate chronic inflammation.1,3–8,19,20 Previously, we demonstrated for the first time that this therapy can also be used to modify the disease severity of experimental IBD.9 Based on the SCID T-cell transfer model of colitis, we demonstrated that s-IL-10 DCs pulsed with enterobacterial extract were able to prevent the weight loss observed in SCID mice after CD4+ CD25− T-cell transfer, and ameliorate the immunopathology of the gut wall.
Interleukin-10 pretreatment has been shown to induce immunosuppressive DCs, which can inhibit CD4+ Th1, CD4+ Th2 and CD8+ T-cell responses in an antigen-specific manner, but different protocols for IL-10 exposure exist and can be separated into protocols that use either long-term or short-term exposure.19–21 Also, immunosuppressive DCs have been established with the use of dexamethasone, vitamin D3 or both.14,22,23 Here, based on the identification of DCs with the ability to induce low enteroantigen-specific CD4+ CD25− T-cell responses in vitro, we compared s-IL-10 DCs, l-IL-10 DCs and Dex/D3 DCs in terms of immunomodulatory effect on colitogenic T-cell responses in vitro and on in vivo development of IBD in the SCID T-cell transfer model of colitis.
All three methods tested seemed to suppress DC secretion of proinflammatory cytokines and to inhibit CD40, CD86 and MHC class II expression upon exposure to enteroantigen. We also tested PD-L1 and PD-L2 expression of DCs. PD-1 ligation on T cells by PD-L1 or PD-L2 induces an inhibitory signal mediated by the immunoreceptor tyrosine-based inhibitory motif (ITIM) and immunoreceptor tyrosine-based switch motif (ITSM) and has shown to play a critical role in immunological tolerance.24–27 Consequently, the downregulation of these surface molecules was unexpected, but not necessarily contradictive for immunosuppression of T cells which is likely to be a result of further modulation of additional costimulatory molecules, inhibitory molecules and cytokines. In addition we analysed the mRNA expression of Notch ligands Jagged 1, Jagged 2 and Delta 4. Here only Jagged 1 was expressed upon exposure to enteroantigen. Since Jagged 1 expression in contrast to Delta 4 expression has been shown to be involved in the induction of Th2 cells this is in conflict with the cytokine profile observed, demonstrating that additional DC–T-cell interactions other than the balance between Jagged 1 and Delta 4 determine the final outcome of Th1 and Th2 responses.16,17,28
The in vitro DC effect on CD4+ CD25 T cells primarily correlated with reduction of clinical symptoms such as weight loss in vivo, which is an early symptom in colitic mice and which is related to the large cytokine production induced when mice are reconstituted with T cells. Long-term IL-10 culture or exposure to Dex/D3 was, compared with short-term IL-10 culture, in general more efficient in terms of in vitro reduction of CD4+ CD25− T-cell responses against enteroantigen where lower Th1- and Th2-related cytokines were observed in primary cultures. We did not see DC-dependent changes in induction of CD4+ CD25+ Foxp3+ T cells which generally reached a level of 2–4% during culture (data not shown). However, although all three types showed potent in vitro reduction of T-cell responses, only s-IL-10 DCs and Dex/D3 DCs had an effect on weight loss in vivo whereas, l-IL-10 DCs had no effect. Also, no correlation was observed in terms of gut pathology, and the in vitro predictions must therefore be interpreted with caution.
The minor effect on the in vivo development of colitis, could be linked to the lack of true tolerance in responding T cells. Indeed, no suppression of the proliferative response was observed when cells from primary cultures were set up in secondary cultures against enteroantigen-pulsed spleen cells (data not shown). However, in these secondary cultures we did observe an enteroantigen-specific suppression of IL-2, IL-4, IL-12 and IFN-γ secretion whereas IL-5 secretion was unchanged and IL-10 secretion was increased when cells from primary cultures were cultured together with enteroantigen-pulsed APCs as compared with unpulsed APCs. However, this pattern was independent of the DC type used for primary stimulation (data not shown).
An alternative explanation for the minor effects in vivo could be that Th17 cells were only partly affected. The Th17 cells are a unique subset of CD4+ T cells driven by the transcription factor RORγt after induction by the cytokines TGF-β and IL-6 or IL-21 and they are maintained by IL-23.29–38 They are known to be important for a number of experimental autoimmune diseases such as EAE and collagen-induced arthritis. We here demonstrate that these cells are differentiated in vitro and react with enteroantigen specificity after repeated stimulations (in particular when enteroantigen-pulsed DCs are used as primary stimulators followed by restimulation with enteroantigen-pulsed spleen cells) and could therefore be involved in the immunopathology induced against enteroantigens seen in experimental and clinical IBD. Interestingly, only Dex/D3 DCs induced full suppression of IL-17 secretion in secondary cultures, which might reflect the effect on clinical symptoms in vivo of these DCs as compared with the other DCs tested. Also, we demonstrate that they are induced in vivo after CD4+ CD25− transfer into SCID mice, but in contrast to in vitro experiments, treatment with immunosuppressive DCs neither changed the number of Th17 nor the number of Th1 cells or regulatory T cells (Treg cells) present in relevant lymphoid organs in vivo. Also, immunomodulatory enteroantigen-pulsed DCs were unable to suppress in vitro the Th17 cells from mice with established colitis (data not shown). However, although Th17 cells might still be important for driving inflammation, the exact role of IL-17 cells in pathogenesis seems to be less important for the induction of experimental colitis39,40 as compared with EAE or collagen-induced arthritis, since transfer of IL-17a−/− or cotreatment with neutralizing antibodies does not prevent colitis induction, but controversy on this still remains.
In the SCID transfer model of colitis, no single well-defined antigen has been discovered except for Helicobacter species, which in certain models have been associated with the immune pathology of colitis.41,42 In the present study we therefore used an enterobacterial extract. Exposure to this extract clearly delivers a maturation signal to DCs, as shown by upregulation of CD80, CD86 as well as CD40, and MHC class II molecules, and the increased secretion of IL-1α, IL-6, IL-12 p40/70 and tumour necrosis factor-α.9 It is likely that DCs exposed to our enterobacterial extract are more unstable as immunosuppressive cells in vivo and may be converted to proinflammatory DCs after injection i.p., which explains the lack of effect on gut pathology and Th17 and Treg T-cell numbers induced in vivo. Indeed, we did observe an effect of Dex/D3 DCs when these were not pulsed with enteroantigen extract. In contrast, a clinical effect was seen on weight loss observed in SCID mice after T-cell transfer when enteroantigen-pulsed Dex/D3 DCs were used. Therefore, the effect on weight loss is probably more sensitive to even small and temporary changes in T-cell function compared with gut pathology. However, the reduction in weight loss was dependent on DC generation in FCS which is known to induce a CD4+ T-cell-dependent Th2 response to FCS-derived antigens including BSA and thereby enhances the systemic production of IL-4, IL-5 and IL-10 as an adjuvant effect.21,43–45
In conclusion, our data show that DCs pretreated with dexamethasone and vitamin D3 inhibit enteroantigen-specific T-cell differentiation, prevent the weight loss and ameliorate gut pathology observed in SCID mice after CD4+ CD25− T-cell transfer with improved capacity compared with both long-term and short-term IL-10-treated DCs. However, in particular the effect on gut pathology was shown to be antigen-independent. If antigen-specific tolerance is to be induced, which would make DC-based therapy superior to conventional immunosuppressive treatment, the use of such immunomodulatory DCs in experimental and clinical IBD awaits the inclusion of appropriate enteroantigens without unspecific immunostimulatory functions which indeed seem to abolish the inhibitory function of the Dex/D3 DCs used in this study.
Acknowledgments
This work was supported by grants from ‘Augustinusfonden’ the ‘Colitis-Crohn Foreningen’ and ‘The Lundbeck Foundation’. We would also like to thank Ane Rulykke and Trine Lind Devantier for excellent technical performance.
Glossary
Abbreviations:
- APC
antigen-presenting cell
- BM
bone marrow
- BSA
bovine serum albumin
- DC
dendritic cell
- EAE
experimental autoimmune encephalomyelitis
- FCS
fetal calf serum
- IBD
inflammatory bowel disease
- IFN
interferon
- IL
interleukin
- i.p.
intraperitoneally
- MHC
major histocompatibility complex
- PBS
phosphate-buffered saline
- PE
phycoerythrin
- SCID
severe combined immunodeficiency
- SEM
standard error of the mean
- TGF
transforming growth factor
References
- 1.Menges M, Rössner S, Voigtländer C, et al. Repetitive injections of dendritic cells matured with tumor necrosis factor alpha induce antigen-specific protection of mice from autoimmunity. J Exp Med. 2002;195:15–21. doi: 10.1084/jem.20011341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haase C, Jorgensen TN, Michelsen BK. Both exogenous and endogenous interleukin-10 affects the maturation of bone-marrow-derived in vitro and strongly influences T cell priming in vivo. Immunology. 2002;107:489–99. doi: 10.1046/j.1365-2567.2002.01529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yarilin D, Duan R, Huang YM, Xiao BG. Dendritic cells exposed in vitro to TGF-beta1 ameliorate experimental autoimmune myasthenia gravis. Clin Exp Immunol. 2002;127:214–9. doi: 10.1046/j.1365-2249.2002.01748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zinser E, Lechmann M, Golka A, Lutz MB, Steinkasserer A. Prevention and treatment of experimental autoimmune encephalomyelitis by soluble CD83. J Exp Med. 2004;200:345–51. doi: 10.1084/jem.20030973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lutz MB, Suri RM, Niimi M, et al. Immature dendritic cells generated with low doses of GM-CSF in the absence of IL-4 are maturation-resistant and prolong allograft survival in vivo. Eur J Immunol. 2000;30:1813–22. doi: 10.1002/1521-4141(200007)30:7<1813::AID-IMMU1813>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 6.Lutz MB, Kukutsch NA, Menges M, Rössner S, Schuler G. Culture of bone marrow cells in GM-CSF plus high doses of lipopolysaccharide generates exclusively immature dendritic cells which induce alloantigen-specific CD4 T cell anergy in vitro. Eur J Immunol. 2000;30:1048–52. doi: 10.1002/(SICI)1521-4141(200004)30:4<1048::AID-IMMU1048>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 7.Fu F, Li Y, Qian S, et al. Costimulatory molecule-deficient dendritic cell progenitors (MHC class II+ CD80dim CD86−) prolong cardiac allograft survival in nonimmunosuppressed recipients. Transplantation. 1996;62:659–65. doi: 10.1097/00007890-199609150-00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steptoe RJ, Ritchie JM, Jones LK, Harrison LC. Autoimmune diabetes is suppressed by transfer of proinsulin-encoding Gr-1+ myeloid progenitor cells that differentiate in vivo into resting dendritic cells. Diabetes. 2005;54:434–42. doi: 10.2337/diabetes.54.2.434. [DOI] [PubMed] [Google Scholar]
- 9.Pedersen AE, Gad M, Kristensen NN, Haase C, Nielsen CH, Claesson MH. Tolerogenic dendritic cells pulsed with enterobacterial extract suppress development of colitis in the severe combined immunodeficiency transfer model. Immunology. 2007;121:526–32. doi: 10.1111/j.1365-2567.2007.02600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Claesson MH, Bregenholt S, Bonhagen K, et al. Colitis-inducing potency of CD4+ T cells in immunodeficient, adoptive hosts depends on their state of activation, IL-12 responsiveness, and CD45RB surface phenotype. J Immunol. 1999;162:3702–10. [PubMed] [Google Scholar]
- 11.Brimnes J, Reimann J, Nissen M, Claesson M. Enteric bacterial antigens activate CD4(+) T cells from SCID mice with inflammatory bowel disease. Eur J Immunol. 2001;31:23–31. doi: 10.1002/1521-4141(200101)31:1<23::aid-immu23>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 12.Duchmann R, Neurath M, Meyer zum Buschenfelde KH. Responses to self and non-self intestinal microflora in health and inflammatory bowel disease. Res Immunol. 1997;148:589–94. doi: 10.1016/s0923-2494(98)80154-5. [DOI] [PubMed] [Google Scholar]
- 13.Duchmann R, May E, Heike M, Knolle P, Neurath M, Meyer zum Buschenfelde KH. T cell specificity and cross reactivity towards enterobacteria, bacteroides, bifidobacterium, and antigens from resident intestinal flora in humans. Gut. 1999;44:812–8. doi: 10.1136/gut.44.6.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pedersen AE, Gad M, Walter MR, Claesson MH. Induction of regulatory dendritic cells by dexamethasone and 1alpha,25-dihydroxyvitamin D(3) Immunol Lett. 2004;91:63–9. doi: 10.1016/j.imlet.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Moller PL, Paerregaard A, Gad M, Kristensen NN, Claesson MH. Colitic SCID mice fed Lactobacillus spp. show an ameliorated gut histopathology and an altered cytokine profile by local T cells. Inflamm Bowel Dis. 2005;11:814–9. doi: 10.1097/01.mib.0000175906.77340.15. [DOI] [PubMed] [Google Scholar]
- 16.Amsen D, Blander JM, Lee GR, Tanigaki K, Honjo T, Flavell RA. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell. 2004;117:515–26. doi: 10.1016/s0092-8674(04)00451-9. [DOI] [PubMed] [Google Scholar]
- 17.Yvon ES, Vigouroux S, Rousseau RF, et al. Overexpression of the Notch ligand, Jagged-1, induces alloantigen-specific human regulatory T cells. Blood. 2003;102:3815–21. doi: 10.1182/blood-2002-12-3826. [DOI] [PubMed] [Google Scholar]
- 18.Gad M, Kristensen NN, Kury E, Claesson MH. Characterization of T-regulatory cells, induced by immature dendritic cells, which inhibit enteroantigen-reactive colitis-inducing T-cell responses in vitro and in vivo. Immunology. 2004;113:499–508. doi: 10.1111/j.1365-2567.2004.01977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinbrink K, Wolf M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol. 1997;159:4772–80. [PubMed] [Google Scholar]
- 20.Steinbrink K, Jonuleit H, Muller G, Schuler G, Knop J, Enk AH. Interleukin-10-treated human dendritic cells induce a melanoma-antigen specific anergy in CD8(+) T cells resulting in a failure to lyse tumor cells. Blood. 1999;93:1634–42. [PubMed] [Google Scholar]
- 21.Haase C, Ejrnaes M, Juedes AE, Wolfe T, Markholst H, von Herrath MG. Immunomodulatory dendritic cells require autologous serum to circumvent nonspecific immunosuppressive activity in vivo. Blood. 2005;106:4225–33. doi: 10.1182/blood-2005-03-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piemonti L, Monti P, Sironi M, et al. Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J Immunol. 2000;164:4443–51. doi: 10.4049/jimmunol.164.9.4443. [DOI] [PubMed] [Google Scholar]
- 23.Piemonti L, Monti P, Allavena P, et al. Glucocorticoids affect human dendritic cell differentiation and maturation. J Immunol. 1999;162:6473–81. [PubMed] [Google Scholar]
- 24.Kroner A, Mehling M, Hemmer B, et al. A PD-1 polymorphism is associated with disease progression in multiple sclerosis. Ann Neurol. 2005;58:50–7. doi: 10.1002/ana.20514. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen C, Hansen D, Husby S, Jacobsen BB, Lillevang ST. Association of a putative regulatory polymorphism in the PD-1 gene with susceptibility to type 1 diabetes. Tissue Antigens. 2003;62:492–7. doi: 10.1046/j.1399-0039.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Yoshida T, Nakaki F, Hiai H, Okazaki T, Honjo T. Establishment of NOD-Pdcd1−/− mice as an efficient animal model of type I diabetes. Proc Natl Acad Sci USA. 2005;102:11823–8. doi: 10.1073/pnas.0505497102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–51. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 28.van Duivenvoorde LM, Han WG, Bakker AM, et al. Immunomodulatory dendritic cells inhibit Th1 responses and arthritis via different mechanisms. J Immunol. 2007;179:1506–15. doi: 10.4049/jimmunol.179.3.1506. [DOI] [PubMed] [Google Scholar]
- 29.Betelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 30.Mangan PR, Harrington LE, O’Quinn DB, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–4. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 31.Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–32. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 32.Ivanov II, McKenzie BS, Zhou L, et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 33.Korn T, Bettelli E, Gao W, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–7. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–40. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nurieva R, Yang XO, Martinez G, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–3. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 36.Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–41. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Veldhoen M, Hocking RJ, Flavell RA, Stockinger B. Signals mediated by transforming growth factor-beta initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nat Immunol. 2006;7:1151–6. doi: 10.1038/ni1391. [DOI] [PubMed] [Google Scholar]
- 38.Zhou L, Ivanov II, Spolski R, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–74. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 39.Ogawa A, Andoh A, Araki Y, Bamba T, Fujiyama Y. Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clin Immunol. 2004;110:55–62. doi: 10.1016/j.clim.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 40.Izcue A, Hue S, Buonocore S, et al. Interleukin-23 restrains regulatory T cell activity to drive T cell-dependent colitis. Immunity. 2008;28:559–70. doi: 10.1016/j.immuni.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Franklin CL, Riley LK, Livingston RS, et al. Enteric lesions in SCID mice infected with “Helicobacter typhlonicus,” a novel urease-negative Helicobacter species. Lab Anim Sci. 1999;49:496–505. [PubMed] [Google Scholar]
- 42.Schomer NH, Dangler CA, Schrenzel MD, Fox JG. Helicobacter bilis-induced inflammatory bowel disease in SCID mice with defined flora. Infect Immunol. 1997;65:4858–64. doi: 10.1128/iai.65.11.4858-4864.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Papaccio G, Nicoletti F, Pisanti FA, Bendtzen K, Galdieri M. Prevention of spontaneous autoimmune diabetes in NOD mice by transferring in vitro antigen-pulsed syngeneic dendritic cells. Endocrinology. 2000;141:1500–5. doi: 10.1210/endo.141.4.7437. [DOI] [PubMed] [Google Scholar]
- 44.Feili-Hariri M, Dong X, Alber SM, Watkins SC, Salter RD, Morel PA. Immunotherapy of NOD mice with bone marrow-derived dendritic cells. Diabetes. 1999;48:2300–8. doi: 10.2337/diabetes.48.12.2300. [DOI] [PubMed] [Google Scholar]
- 45.Feili-Hariri M, Falkner DH, Morel PA. Regulatory Th2 response induced following adoptive transfer of dendritic cells in prediabetic NOD mice. Eur J Immunol. 2002;32:2021–30. doi: 10.1002/1521-4141(200207)32:7<2021::AID-IMMU2021>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]