Abstract
Lipopolysaccharide (LPS) stimulates exocytosis in neutrophils. The signalling molecules involved in the regulation of this mechanism are currently unknown. Using neutrophils from interleukin-1-receptor-associated kinase (IRAK)-4- and Toll/IL-1 receptor (TIR) domain-containing adaptor inducing IFN-β (TRIF)-deficient mice, we dissected the signalling pathways that control exocytosis. We analysed exocytosis of peroxidase-negative and azurophilic granules by following the mobilization of the β2-integrin subunit CD11b and myeloperoxidase (MPO)-containing granules, respectively. IRAK-4-null neutrophils showed marked defects in both peroxidase-negative and azurophilic granule exocytosis in response to LPS. In contrast, the exocytic response to LPS of TRIF-deficient neutrophils was not different from that of wild-type cells. No differences were observed in the exocytosis of secretory organelles between IRAK-4-null and wild-type neutrophils when they were stimulated with the phorbol ester phorbol 12-myristate 13-acetate (PMA). Electron microscopy analysis showed that no morphological abnormalities were present in the granules of IRAK-4-deficient neutrophils, suggesting that the lack of exocytic response to LPS is not attributable to developmental abnormalities. Using pharmacological inhibitors, we found that p38 mitogen-activated protein kinase (p38MAPK) is essential for the exocytosis of all neutrophil secretory organelles in response to LPS. Interestingly, we found that phosphatidylinositol 3-kinase (PI3K) is essential for azurophilic granule exocytosis but not for the mobilization of other neutrophil granules in response to LPS. Azurophilic granule exocytosis in response to Listeria monocytogenes was dependent on PI3K but not IRAK-4 activity, suggesting that alternative signalling pathways are activated in IRAK-4-deficient neutrophils exposed to whole bacteria. Our results identified IRAK-4, p38MAPK and PI3K as important regulatory components with different roles in the signalling pathways that control Toll-like receptor ligand-triggered neutrophil exocytosis.
Keywords: granulocyte, inflammation, MyD88, p38 mitogen-activated protein kinase, secretion
Introduction
Neutrophils are the first line of cellular defense in the innate immune system. They kill micro-organisms in the phagosome or by releasing microbicidal products into the extracellular space. In resting neutrophils, these microbicidal molecules are segregated in vesicles to protect the host from uncontrolled activation. Mature neutrophils contain several types of exocytosable organelles that hold a battery of molecules that contribute to the precise execution of many neutrophil functions.1,2 Azurophilic (primary) granules contain myeloperoxidase (MPO) and elastase, in addition to other antimicrobial factors.3 Specific (secondary) granules contain lactoferrin and matrix metalloproteinase 9 (MMP-9),4–6 both of which are modulators of the inflammatory response. Gelatinase (tertiary) granules also contain MMP-9 but lack lactoferrin, and secretory vesicles are readily releasable organelles containing plasma proteins including tetranectin.1 Neutrophil granules also contain membrane proteins that are translocated during activation to replenish the plasma membrane with diverse molecules that are essential for neutrophil functions.7 This includes the membrane component of the NADPH oxidase, flavocytochrome b558 (gp91phox/p22phox), which mainly localizes in the membrane of specific and gelatinase granules in resting neutrophils. Other examples are the β2-integrin Mac-1 (CD11b/CD18), which is stored in the membranes of secretory vesicles, tertiary granules and specific granules,1 and the tetraspanin molecule lysosome-associated membrane protein 3 (LAMP-3), which is a marker of azurophilic granules.8 The mobilization of neutrophil granules is hierarchical. The secretory vesicles are mobilized first, while tertiary granules, specific granules and azurophilic granules are sequentially mobilized in response to increasingly strong stimuli.1 This correlates with the different roles the granule proteins play in neutrophil functions.
During microbial infections, neutrophils are exposed, often simultaneously, to a variety of stimuli that can differentially modulate the microbicidal activity of these cells. Neutrophils recognize pathogen-associated molecular patterns (PAMPs) through membrane receptors including Toll-like receptors (TLRs).9 Human and murine neutrophils express all TLRs except TLR3.9 Stimulation of human neutrophils by TLR ligands has been shown to prime or activate a subset of neutrophil functions including the production of reactive oxygen species, phagocytosis and cytokine production.9 Previous studies demonstrated that exposure of human neutrophils to the Gram-negative bacterial cell wall component lipopolysaccharide (LPS) increases the number of flavocytochrome b558 subunits available for activation in response to a subsequent stimulus, in a p38 mitogen-activated protein kinase (p38MAPK)-dependent manner,10 indicating that TLR ligands induce the mobilization of neutrophil intracellular organelles. Recently, TLR4 has been identified as the receptor for LPS,11 and the signalling pathways activated by this ligand are starting to be elucidated. Upon LPS recognition, many proteins are recruited to the cytoplasmic domain of TLR4, the Toll/IL-1 receptor (TIR) domain. This includes the adaptor protein MyD88 which then recruits interleukin-1-receptor-associated kinases (IRAKs). One of these kinases, IRAK-4, plays a central role in TLR signalling,12 and its deficiency leads to immunodeficiency in mice13 and humans.14 The binding of LPS to TLR4 also activates a signalling pathway that is independent of IRAK-4. It involves the adaptor proteins TRIF-related adaptor molecule (TRAM) and TIR domain-containing adaptor inducing IFN-β (TRIF).15,16 TLR agonists other than LPS also activate the MyD88-dependent pathway but not the TRIF-dependent pathway17 (with the exception of TLR3 ligands). These include the Gram-positive bacteria wall component lipoteichoic acid (LTA; a TLR2/TLR6 ligand), the synthetic diacylated lipopeptide Pam2CSK4 (TLR2), flagellin (TLR5), single-stranded polyuridine oligonucleotides (polyU; a murine TLR7 ligand and human TLR8 ligand) and non-methylated CpG DNA (TLR9). Activation of p38MAPK is a central event initiated by TLR signalling.18 Results from different studies are in agreement that p38MAPK activation in response to LPS is bimodal, with an initial phase triggered by the MyD88-dependent pathway and a late phase initiated by the TRIF-dependent pathway.15 Phosphatidylinositol 3-kinase (PI3K) is also activated in response to LPS stimulation of neutrophils.19 Although the participation of p38MAPK in the regulation of glycoprotein 91 [gp91phox; phagocyte oxidase (phox)] mobilization has been demonstrated,10 the signalling pathways that mediate the exocytosis of neutrophil secretory organelles in response to LPS are poorly understood. In particular, the roles of PI3K and p38MAPK as well as the differential involvement of the MyD88-dependent and MyD88-independent pathways in the exocytosis of the different subsets of neutrophil secretory organelles remain elusive and are the subject of analysis in the present work. Here, using IRAK-4- and TRIF-deficient mice, as well as pharmacological approaches, we dissect the signalling pathways that control the exocytosis of secretory organelles in neutrophils.
Materials and methods
Animals
The IRAK-4 knockout (KO) mouse in a C57BL/6J background was described previously.13 The TRIF-deficient mice (TRIFlps2/lps2) have been generated by random germline mutagenesis in a C57BL/6J background by Dr B. Beutler using the alkylating agent N-ethyl-N-nitrosourea (ENU).15 TRIFlps2/lps2 mice were maintained as a homozygous stock for use in these studies. C57BL/6J control mice were obtained from the animal resource centre at Scripps. In some experiments, inbred C57BL/6J colony controls were employed for experiments using IRAK-4−/−. All studies were performed using 6- to 8-week-old mice and conducted according to National Institutes of Health (NIH) and institutional guidelines and with approval of the institutional animal review board.
Materials
LPS (Escherichia coli, serotype R515) was obtained from Alexis Biochemicals (San Diego, CA). Pam2CSK4 and heat-killed Listeria monocytogenes were obtained from Invivogen (San Diego, CA). LY294002, wortmannin, cytochalasin D and SB203580 were from Calbiochem (San Diego, CA). Paraformaldehyde was from Electron Microscopy Sciences (Hatfield, PA).
Antibodies
The antibodies used were: anti-human and anti-mouse CD11b-phycoerythrin (PE) (BD Pharmingen, Franklin Lakes, NJ); anti-mouse PerCP- or fluorescein isothiocyanate (FITC)-conjugated Gr-1 (Ly-6G and Ly-6C) (BD Pharmingen); FITC-conjugated mouse anti-human gp91phox (MBL); FITC-conjugated anti-human LAMP-3 (Santa Cruz Biotechnology Inc., Santa Cruz, CA); PE-conjugated anti-human MPO (GeneTex Inc., San Antonio, TX); FITC-conjugated anti-mouse MPO (HyCult Biotechnology, Uden, the Netherlands); anti-phospho p38MAPK (Thr180/Tyr182) and anti-p38MAPK (Cell Signaling, Danvers, MA).
Isolation of human neutrophils
Human neutrophils were isolated from blood from normal donors by dextran sedimentation and Ficoll density centrifugation as previously described.20
Isolation of murine neutrophils
Murine neutrophils were obtained from blood collected by cardiac puncture in K3EDTA MiniCollect tubes (Greiner Bio-One, Monroe, NC). Erythrocytes were removed by lysis using a solution consisting of 168 mm NH4Cl, 10 mm KHCO3 and 0·097 mm ethylenediaminetetraacetic acid (EDTA). In some studies, neutrophils were further isolated using a Percoll-gradient fractionation system previously described.21 For the isolation of mouse peritoneal neutrophils, animals were injected intraperitoneally with 1 ml of a sterile 4% thioglycollate solution. Polymorphonuclear cells were harvested 4 hr after injection by lavage of the peritoneal cavity with RPMI medium. After isolation, cells were kept on ice until they were used. Purity was analysed by morphology, Giemsa staining, and fluorescence-activated cell sorting (FACS) using the anti-Ly6G (Gr-1) monoclonal antibody (mAb).
Gel electrophoresis and western blotting
Proteins were separated by gel electrophoresis using NuPAGE gels and MOPS buffer (Invitrogen, Carlsbad, CA). Proteins were transferred onto nitrocellulose membranes for 120 min at 60 V and 4°. The membranes were blocked with phosphate-buffered saline (PBS) containing 5% [weight/volume (w/v)] blotting grade non-fat dry milk blocker (BioRad Laboratories, Hercules, CA) and 0·05% (w/v) Tween-20. The proteins were detected by probing the membranes with the indicated primary antibodies at appropriate dilutions and using a detection system consisting of horseradish peroxidase (HRP)-conjugated secondary antibodies (BioRad Laboratories) and the LumiGlo chemiluminescence substrate system (Upstate Biotechnology, Lake Placid, NY). Transferred proteins were visualized using Hyperfilm (Amersham Bioscience, Piscataway, NJ).
Analysis of secretory organelle mobilization in human neutrophils
Exocytosis was measured as changes in cell surface expression of granule markers by flow cytometry. Human neutrophils (1 × 106 cells/ml) were re-suspended in serum-free RPMI and stimulated with TLR ligands for 1 hr at 37°. Where indicated, cells were pretreated with an inhibitor of actin polymerization, cytochalasin D (10 μg/ml), for 15 min prior to the addition of stimuli to facilitate the exocytosis of azurophilic granules. In some experiments, inhibitors of p38MAPK (5 μm SB203580) or PI3K (20 μm LY294002 or 150 nm wortmannin) were added for 30 min before stimulation. Unless otherwise indicated, the stimuli were utilized at the following final concentrations: LPS (100 ng/ml), heat-killed Listeria monocytogenes (HKLM) (1 × 108 particles/ml), Pam2CSK4 (50 ng/ml) and PMA (100 ng/ml). After stimulation, cells were washed with PBS containing 0·5% bovine serum albumin (BSA) and incubated with specific antibodies or isotype controls (BD Biosciences, San Jose, CA) at 4° for 40 min in a final volume of 50 μl. Next, cells were washed and re-suspended in 1% paraformaldehyde. Data were collected using a FACSCalibur flow cytometer (BD Biosciences) and analysed using cellquest software (BD Biosciences).
Granule mobilization in murine neutrophils
To evaluate the mobilization of CD11b-containing organelles in murine neutrophils, blood was obtained by cardiac puncture and cells were stimulated and immunostained in whole blood (50 μl). Murine erythrocytes were lysed using a hypotonic solution consisting of 168 mm NH4Cl, 10 mm KHCO3 and 0·097 mm EDTA for 2 min and then fixed. Neutrophils were gated based on staining with the granulocyte marker Gr-1 (Ly6G) and CD11b-specific staining was measured in Gr-1-positive cells. The stimuli were utilized at the concentrations given above. In these experiments, inbred colony controls were employed for experiments using IRAK-4−/−. For experiments using TRIFlps2/lps2, C57BL/6J control mice were obtained from the animal resource centre at Scripps.
To follow azurophilic granule mobilization we used murine peritoneal neutrophils. To this end, we took advantage of the ability of some neutrophil granule proteins, including MPO, to bind to the outer cellular surface after exocytosis.22 This property has also been described for elastase23 and MMP-9.24 Murine peritoneal neutrophils were isolated, stimulated and double labelled with anti-mouse MPO and anti-mouse Gr-1 specific antibodies. Next, the cells were fixed using 1% paraformaldehyde and the increase in MPO in Gr-1-positive cells was detected by flow cytometry. The analysis of MPO release by flow cytometry correlated with the data obtained using an anti-murine MPO enzyme-linked immunosorbent assay (ELISA) kit (HyCult Biotechnology).
Measurement of reactive oxygen species (ROS)
ROS production was determined by a luminol-enhanced chemiluminescence assay using a Labsystem Luminoscan (Thermo Fisher Scientific Inc., Waltham, MA). Experiments were performed in serum-free RPMI medium in the presence of 10 μm luminol and in the absence of exogenous peroxidase. Murine neutrophils were incubated at a density of 1 × 106 cells/well and stimulated with the indicated ligands, and chemiluminescence was continuously monitored for 3 hr at 37°.
Transmission electron microscopy
Murine blood was obtained by cardiac puncture and red blood cells were lysed. Murine leucocytes were fixed in 2·5% glutaraldehyde in 0·1 m Na cacodylate buffer (pH 7·3), washed with buffer and fixed in 1% osmium tetroxide in 0·1 m Na cacodylate buffer. They were subsequently treated with 0·5% tannic acid followed by 1% sodium sulphate. The pellets were dehydrated in an ethanol series, treated with propylene oxide and embedded in Epon/Araldite. Thin sections (70 nm) of the samples were cut on a Reichert Ultracut E (Leica, Deerfield, IL) using a diamond knife (Diatome; Electron Microscopy Sciences), mounted on parlodion-coated copper slot grids and stained in uranyl acetate and lead citrate. Sections were examined on a Philips CM100 transmission electron microscope (FEI, Hillsbrough, OR).
Bacterial killing assay
Bacterial killing assays in whole blood were performed as previously described25 with modifications. Briefly, bacteria [Staphylococcus aureus American Type Culture Collection (Rockville, MD) 49521™ or E. coli DH5α (a rough, non-virulent strain susceptible to whole-blood bactericidal activity26)] were subcultured at 37° to logarithmic growth from an overnight culture and subsequently diluted in sterile PBS. The bacterial concentration was adjusted spectrophotometrically. Bacteria re-suspended in 100 μl of sterile PBS were incubated with 100 μl of blood obtained from wild-type or IRAK-4 KO mice. The samples were incubated for 2 hr with rotation at 37°. In some experiments, lysostaphin (5 U/ml) was added to the samples after 1 hr of incubation to kill extracellular S. aureus and samples were incubated for an extra period of 1 hr. Dilutions in tryptic soy broth with 0·05% saponin were plated on tryptic soy agar plates for enumeration of surviving colony-forming units (CFU). Controls, including bacteria incubated in tryptic soy broth in the absence of blood and blood incubated in the absence of bacteria, were run in parallel. Plate images were collected using a Fluor-S MultiImager (BioRad Laboratories) and colonies were quantified using the quantityone 4.2.1 quantification software (BioRad Laboratories).
Statistical analysis
Unless otherwise indicated, results are presented as mean ± standard error of the mean (SEM). Statistical analysis was performed using Student’s t-test. A value of P < 0·05 was considered statistically significant.
Results
IRAK-4, but not TRIF, is a key component of the signalling pathway mediating neutrophil exocytosis
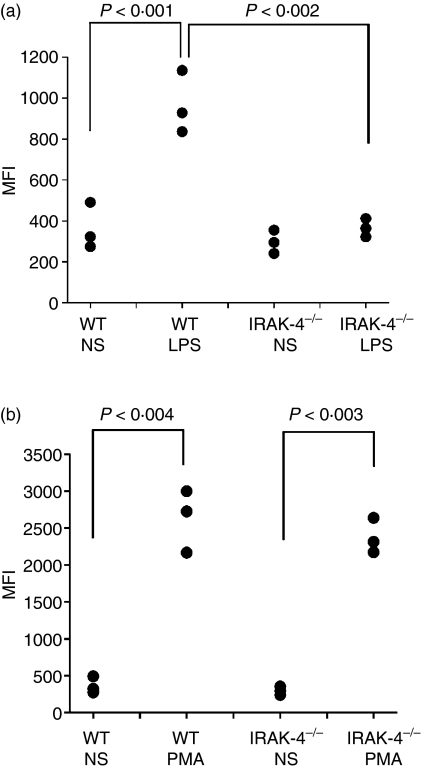
LPS stimulates the mobilization of neutrophil secretory organelles.10 Yet, upstream mediators that link TLR4 ligand recognition with the signalling pathway that regulates exocytosis are currently unknown. p38MAPK activity has been shown to participate in the regulation of the exocytosis of some neutrophil secretory organelles in response to LPS.10 As both the MyD88- and the TRIF-dependent pathways are necessary to elicit sustained p38MAPK activity,15,27 it was important to determine whether the IRAK-4 signalling pathway, the TRIF signalling pathway or both are necessary to regulate neutrophil exocytosis. To dissect the signalling pathways activated downstream of TLR4, we utilized mice nullizygous for IRAK-4 or TRIF. Exocytosis in neutrophils from these mice was analysed by following the appearance of granule markers on the cellular surface upon stimulation. First, we detected the appearance of CD11b on murine neutrophils in response to LPS stimulation (Fig. 1a). To avoid activation during isolation, whole blood was stimulated by LPS and neutrophils were simultaneously labelled for the granule marker CD11b and for the granulocyte marker Gr-1. In Fig. 1a, we show that the mobilization of CD11b-containing organelles in response to LPS is absolutely dependent on IRAK-4. Importantly, CD11b-containing organelles were efficiently mobilized in IRAK-4−/− neutrophils when PMA was used as a stimulus (Fig. 1b). These results suggest that lack of exocytic response to LPS by neutrophils deficient in IRAK-4 is attributable not to developmental defects that alter granule composition but rather to signalling abnormalities intrinsic to the deficiency of IRAK-4.
Figure 1.
Interleukin-1-receptor-associated kinase-4 (IRAK-4) is a key signalling molecule for the mobilization of neutrophil CD11b-containing organelles in response to lipopolysaccharide (LPS). The surface expression of CD11b was measured in granulocytes from wild-type (WT) or IRAK-4 knockout (IRAK-4−/−) mice. Cells were stimulated with 100 ng/ml LPS (a) or 0·1 μg/ml phorbol 12-myristate 13-acetate (PMA) (b) or incubated in the absence of stimuli (NS) for 1 hr at 37°. The surface expression of CD11b was determined in Gr-1-positive cells by flow cytometry as described in the Materials and methods section. Each symbol represents the result obtained with an individual mouse. Statistical analysis was performed using a one-tailed paired Student’s t-test except for WT/LPS versus IRAK-4−/−/LPS, where a one-tailed unpaired Student’s t-test was used. MFI, mean fluorescence intensity.
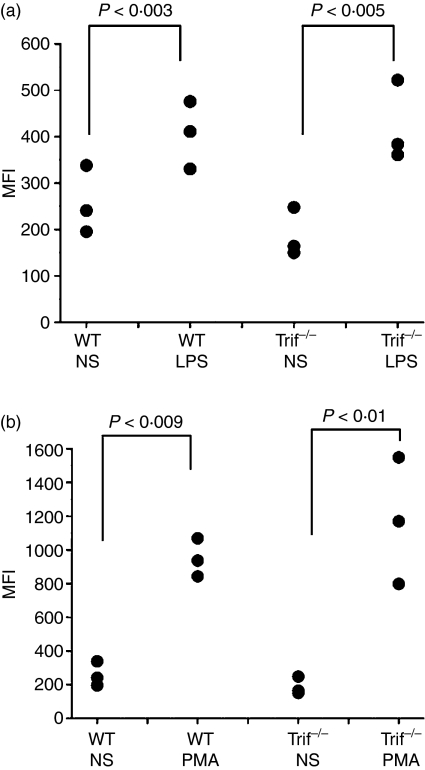
Our results suggested that the MyD88-dependent signalling pathway plays a dominant role in the exocytosis of CD11b-containing vesicles in response to TLR ligands. However, it was possible that the TRIF-dependent pathway amplifies or complements signalling initiated by the MyD88-dependent pathway. This possibility is supported by the observation that p38MAPK, a key component in TLR ligand-triggered exocytosis of neutrophil secretory organelles, can be activated in a TRIF-dependent manner.15,27,28 To explore whether the MyD88-independent signalling pathway is involved in the mobilization of CD11b-containing vesicles in response to LPS, we evaluated exocytosis in neutrophils that are deficient in the adaptor protein TRIF. We found that TRIF-deficient neutrophils mobilize CD11b in response to LPS as efficiently as wild-type neutrophils (Fig. 2a). Similarly, the secretory response of TRIF−/− neutrophils to PMA was not significantly different from that of control granulocytes (Fig. 2b). These results suggest that the IRAK-4-dependent but not the TRIF-dependent signalling pathway is necessary for the mobilization of CD11b-containing organelles in response to the TLR4 ligand in neutrophils.
Figure 2.
Toll/IL-1 receptor (TIR) domain-containing adaptor inducing IFN-β (TRIF) does not play a significant role in the regulation of lipopolysaccharide (LPS)-dependent mobilization of CD11b-containing organelles in neutrophils. The surface expression of CD11b was measured in granulocytes from wild-type (WT) or Triflps2/lps2 (Trif−/−) mice. Cells were stimulated with 100 ng/ml LPS (a) or 0·1 μg/ml phorbol 12-myristate 13-acetate (PMA) (b) or incubated in the absence of stimuli (NS) for 1 hr at 37°. The surface expression of CD11b was determined in Gr-1-positive cells by flow cytometry as described in Fig. 1 and in the Materials and methods section. Each symbol represents the result obtained with an individual mouse. Statistical analysis was performed using a one-tailed paired Student’s t-test. MFI, mean fluorescence intensity.
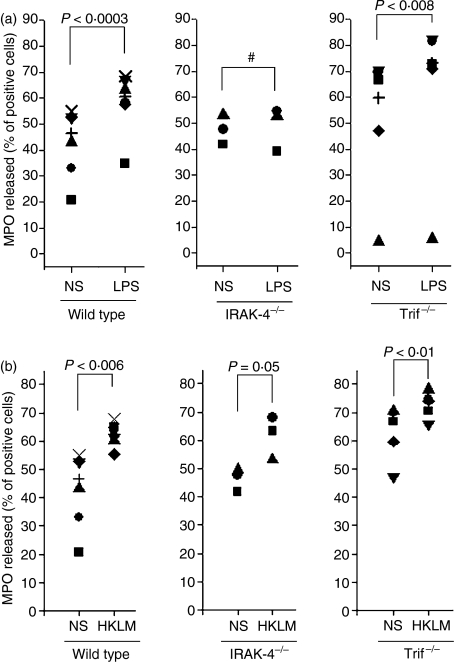
In human neutrophils, exocytosis of azurophilic granules in response to TLR ligands is only observed under experimental conditions in which the cytoskeleton is disrupted using cytochalasin D (see below). Interestingly, we found that peritoneal but not peripheral mouse neutrophils undergo azurophilic granule exocytosis in response to LPS even in the absence of cytochalasin D. This suggests that a priming mechanism is involved during neutrophil migration to the peritoneal cavity in the mouse model of thioglycolate-induced peritonitis. Thus, to explore the participation of the MyD88-dependent and the TRIF-dependent signalling pathways in the mechanism of azurophilic granule exocytosis, we used IRAK-4−/− and TRIF-deficient peritoneal neutrophils and LPS as the stimulus. Our results show that IRAK-4-deficient neutrophils have impaired mobilization of MPO-containing granules in response to LPS compared with wild-type cells (Fig. 3a). IRAK-4-deficient neutrophils also failed to exocytose after stimulation with the TLR2 agonist Pam2CSK4 (data not shown). In contrast, TRIF-deficient neutrophils showed an azurophilic granule secretory response similar to that observed in wild-type cells when stimulated with LPS (Fig. 3a). These data suggest that the mobilization of peroxidase-positive organelles is controlled by the MyD88-dependent signalling pathway and proceeds independently of TRIF-mediated mechanisms. As expected, TRIF-deficient neutrophils responded to Pam2CSK4 as efficiently as wild-type cells (data not shown), further confirming that IRAK-4 but not TRIF is a central signalling molecule in the activation of azurophilic granule exocytosis in response to TLR ligands. In contrast to their response to LPS, IRAK-4-deficient neutrophils showed a significant exocytic response of azurophilic granules to Gram-positive L. monocytogenes (Fig. 3b). These results suggest that alternative signalling pathways operate to trigger primary granule exocytosis in IRAK-4-deficient neutrophils when they are stimulated by whole bacteria, thus compensating for the absence of IRAK-4. Finally, it is worth mentioning that primary granule mobilization was significantly increased in TRIF-deficient neutrophils as compared with wild type (P < 0·01). Although this may indicate that the TRIF-dependent signalling pathway plays an inhibitory rather than a stimulatory role in this particular cellular function, further experimental analysis is necessary to test this hypothesis.
Figure 3.
Interleukin-1-receptor-associated kinase-4 (IRAK-4)-deficient but not Toll/IL-1 receptor (TIR) domain-containing adaptor inducing IFN-β (TRIF)-deficient neutrophils have impaired mobilization of azurophilic granules in response to lipopolysaccharide (LPS). Peritoneal neutrophils from wild-type (WT), IRAK-4 knockout (IRAK-4−/−) or TRIF-deficient (Trif−/−) mice were stimulated with LPS (a) or heat-killed Listeria monocytogenes (HKLM), (b), or incubated in the absence of stimuli (NS) for 1 hr at 37°. Cells were analysed for myeloperoxidase (MPO) release as described in the Materials and methods section. Statistical analysis was performed using a one-tailed, paired Student’s t-test. #, not significant.
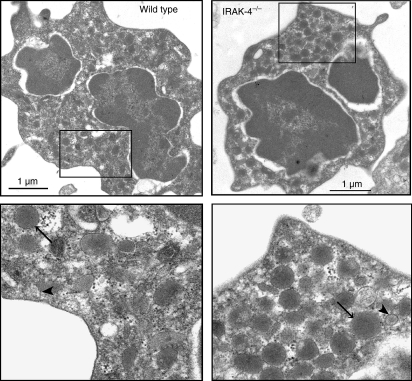
Because IRAK-4-knockout neutrophils undergo exocytosis in response to PMA, it seemed unlikely that morphological abnormalities in neutrophil granules would be responsible for the deficient secretion of these cells in response to TLR ligands. To explore this in detail, we analysed the granular morphology of IRAK-4-deficient and wild-type neutrophils using transmission electron microscopy. Our results indicate that no gross morphological abnormalities are present in granules from IRAK-4-deficient neutrophils compared with wild-type controls (Fig. 4). Moreover, no apparent differences in granule composition, heterogeneity or number were observed between neutrophils from IRAK-4-deficient and wild-type mice. These results further support a role for IRAK-4 as a signalling regulator in the process of LPS-induced exocytosis and argue against a possible role of IRAK-4 in neutrophil granular biogenesis.
Figure 4.
Interleukin-1-receptor-associated kinase-4 (IRAK-4)-deficient neutrophil granules have normal morphology. Neutrophils from wild-type or IRAK-4-deficient mice were analysed by transmission electron microscopy as described in the Materials and methods section. Large, electron-dense granules (azurophilic granules; arrows) and smaller, less electron-dense granules (presumably specific or gelatinase granules; arrowheads) were observed in both wild-type and IRAK-4-deficient neutrophils.
p38MAPK and PI3K play a significant role in the signalling pathways that control the exocytosis of neutrophil organelles in response to LPS
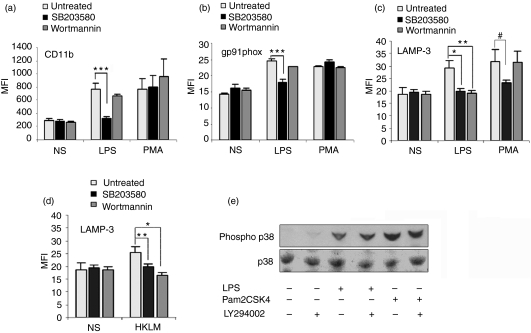
Activation of p38MAPK is a central event initiated by TLR signalling.18 Evidence has been presented by various groups supporting the theory that the MyD88-dependent pathway is necessary for early p38MAPK activation and that the MyD88-independent pathway elicits a sustained p38MAPK activation phase (late phase).15,27 It is also clear that PI3K activity is induced upon TLR activation in a MyD88-dependent manner.19,29,30 Furthermore, in a recent report, Elbim and collaborators showed that human neutrophils deficient in IRAK-4 can signal through TLR9 in a PI3K-dependent manner.31 As both p38MAPK and PI3K have been postulated as regulatory molecules of exocytosis,10,32 we sought to investigate the differential roles of p38MAPK and PI3K in the regulation of neutrophil exocytosis initiated by TLR ligands. Here, we designed experimental procedures to comparatively analyse the participation of these signalling pathways in the mobilization of various neutrophil secretory organelles after TLR stimulation. Using the pharmacological inhibitor SB203580 at concentrations specific for p38MAPK, we showed that p38MAPK activity is required for the mobilization of CD11b-containing organelles as well as for the exocytosis of azurophilic granules in response to LPS (Fig. 5a and c). We also showed that the mobilization of flavocytochrome b558 through exocytosis of intracellular granules is regulated by p38MAPK (Fig. 5b), confirming previous observations by another group.10 Taken together, our data demonstrate that p38MAPK regulates exocytosis of all neutrophil secretory organelles upon TLR timulation.
Figure 5.
Role of p38 mitogen-activated protein kinase (p38MAPK) and phosphatidylinositol 3-kinase (PI3K) in the exocytosis of neutrophil secretory organelles triggered by lipopolysaccharide (LPS). (a–c) Human neutrophils were stimulated with LPS or phorbol 12-myristate 13-acetate (PMA) in the presence of 5 μm SB203580 (black columns), 150 nm wortmannin (dark grey columns) or vehicle (light columns). The cells were analysed for the surface expression of CD11b (a), gp91phox (b), or lysosome-associated membrane protein 3 (LAMP-3) (c). Results represent the mean ± standard error of the mean (SEM) of three independent experiments. *P= 0·027, **P = 0·018, ***P < 0·003, #P = 0·085 (one-tailed, unpaired Student’s t-test). (d) PI3K plays an essential role in the regulation of azurophilic granule exocytosis in neutrophils stimulated with heat-killed Listeria monocytogenes (HKLM). Human neutrophils were incubated in the presence of the indicated inhibitors or vehicle and then stimulated with HKLM or incubated in the absence of stimulus (NS). The cells were analysed for the surface expression of LAMP-3. Results represent the mean ± SEM of three independent experiments. *P < 0·01 and **P =0·034 (one-tailed unpaired Student’s t-test). (e) Human neutrophils were incubated in the presence of the PI3K inhibitor LY294002 (20 μm) and stimulated with LPS or Pam2CSK4 for 30 min and the level of phosphorylation of p38MAPK was analysed by western blot. The data are representative of three independent experiments with similar results. MFI, mean fluorescence intensity.
Previous studies demonstrated that stimulation of human neutrophils with TLR ligands induces PI3K activity.19 To evaluate the possible participation of PI3K in the signalling pathway that controls neutrophil granule exocytosis initiated by TLR ligands, we pharmacologically inhibited PI3K in human neutrophils and measured their secretory response to LPS. Our results indicate that PI3K activity is essential for the exocytosis of azurophilic granules in response to this TLR ligand (Fig. 5c). Interestingly, inhibition of PI3K did not significantly decrease the mobilization of CD11b-containing vesicles or gp91phox-containing granules in response to LPS (Fig. 5a and b). These results indicate that different signalling mechanisms regulate the exocytosis of the various sets of neutrophil secretory organelles in neutrophils.
As PI3K seemed to play a central role in the control of azurophilic granule exocytosis and we observed that IRAK-4-deficient neutrophils have a normal azurophilic granule exocytic response to Gram-positive L. monocytogenes, it was of interest to determine whether PI3K played an important role in the signalling pathway that controls azurophilic granule secretion in response to L. monocytogenes. In Fig. 5d, we show that pharmacological inhibition of PI3K significantly decreased azurophilic granule exocytosis in response to this stimulus. These data confirm that PI3K is a key component of the signalling pathway that modulates primary granule exocytosis in neutrophils and suggest that alternative compensatory signalling pathways can lead to the activation of PI3K in the absence of IRAK-4 in response to L. monocytogenes.
The observations that PI3K inhibitors did not prevent the mobilization of secondary granules in response to the TLR4 ligand LPS while p38MAPK inhibitors blocked exocytosis suggest that p38MAPK is not activated downstream of PI3K in response to LPS, as previously suggested for other TLR ligands,30 and indicate that p38MAPK activation after LPS stimulation is independent of PI3K activity in neutrophils. To confirm this, we performed western blot analysis to detect phosphorylation of p38MAPK in the presence or absence of PI3K inhibitors. We observed that the phosphorylation of p38MAPK in response to LPS is not affected by inhibition of PI3K by the specific inhibitor LY294002, indicating that p38MAPK is activated independently of PI3K (Fig. 5e). Similar results were obtained when the TLR2 agonist Pam2CSK4 was used (Fig. 5e).
The impaired exocytic response in IRAK-4-deficient neutrophils correlates with a decreased oxidative response
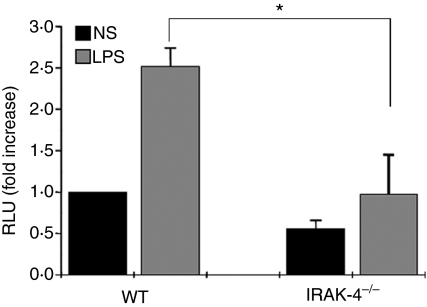
The neutrophil oxidative response is an essential mechanism for the effective bactericidal activity of neutrophils. This response is dependent on both the NADPH oxidase complex and MPO. During neutrophil activation, ROS are generated in the phagosome during phagocytosis or released to the extracellular milieu in response to soluble stimuli. As individuals with IRAK-4 deficiency suffer from recurrent bacterial infections, it was of interest to analyse whether the decreased mobilization of MPO-containing granules in IRAK-4 deficiency correlates with defects in the oxidative response. To this end, we evaluated the luminol-dependent chemiluminescence response of IRAK-4-deficient neutrophils. Using luminol, both intracellular and extracellular ROS production can be detected. To maximize the participation of endogenous MPO in the reaction, we performed our experiments in the absence of exogenous peroxidase, which is frequently added to the reaction to enhance the chemiluminescence response of neutrophils. We found that murine neutrophils lacking IRAK-4 had a marked impairment in their oxidative response to LPS (Fig. 6). We suggest that this defect is caused by both the previously shown impairment in NADPH oxidase activity in IRAK-4 deficiency,14 and the decreased secretion of MPO observed in IRAK-4-null granulocytes shown here.
Figure 6.
Interleukin-1-receptor-associated kinase-4 (IRAK-4) knockout mice have a deficient oxidative response to lipopolysaccharide (LPS). The oxidative response of neutrophils from wild-type (WT) or IRAK-4-deficient mice was analysed using the luminol-dependent chemiluminescence assay. Neutrophils were stimulated with LPS or left untreated (NS) and the chemiluminescence response was followed for 3 hr. Total relative light units (RLU) are reported relative to the wild-type control incubated in the absence of stimulus, which is designated as 1·0. The data shown represent the mean ± standard error of the mean for total RLU (n= 3). *P = 0·020 (one-tailed, unpaired Student’s t-test).
Staphylococcus aureus and Escherichia coli are susceptible to killing by whole-blood from IRAK-4-deficient mice
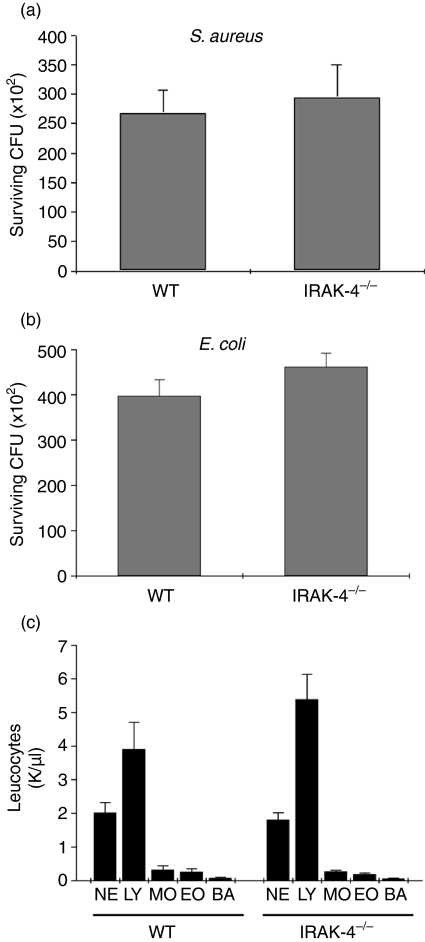
We next sought to investigate whether bacterial killing is impaired in IRAK-4-deficient murine leucocytes. To this end, we used a whole-blood killing assay to evaluate S. aureus and E. coli survival in blood from IRAK-4-deficient or wild-type mice. In Fig. 7a, we show that the rate of S. aureus survival in whole blood from IRAK-4-deficient mice was not significantly different from that observed for control wild-type mice. Similarly, no significant differences were observed in the abilities of IRAK-4-deficient and wild-type leucocytes to kill Gram-negative E. coli (Fig. 7b). These results cannot be explained by putative leucocytosis in the IRAK-4 KO mice because, similar to observations in IRAK-4-deficient patients,33 no statistically significant differences were detected in the number of any leucocyte population between IRAK-4 and wild-type mice (Fig. 7c). The observations that IRAK-4-deficient neutrophils have impaired luminol-detected oxidative response to LPS, but normal bactericidal ability, suggest that extracellular but not intracellular ROS production is impaired in IRAK-4 deficiency, probably associated with the exocytic defects present in IRAK-4 KO neutrophils.
Figure 7.
Bacterial killing analysis in whole blood from Interleukin-1-receptor-associated kinase-4 (IRAK-4)-deficient mice. Staphylococcus aureus (a) and Escherichia coli (b) killing assays in whole blood from IRAK-4-deficient (IRAK-4−/−) or wild-type (WT) mice were performed as described in the Materials and methods. Results shown are mean ± standard error of the mean (SEM) for four (a) and six (b) individual mice evaluated in duplicate or triplicate. (c) Differential leucocyte counts in IRAK-4-deficient and control mice were performed using a HEMAVET-950 Hematology system (Drew Scientific Inc., Dallas, TX). NE, neutrophils; LY, lymphocytes; MO, monocytes; EO, eosinophils; BA, basophils. The results shown are mean ± SEM (n = 10). No statistical differences were observed between IRAK-4 and wild-type mice for any of the experiments shown in this figure (unpaired Student’s t-test).
Discussion
In this work, we analysed the signalling pathways that regulate the mechanism of neutrophil exocytosis in response to LPS and characterized IRAK-4, PI3K and p38MAPK but not TRIF as essential molecular components of this mechanism.
IRAK-4 is considered a central TIR signalling mediator in innate immunity.12,34 However, infections in IRAK-4-deficient patients are caused by a narrow spectrum of micro-organisms, mainly pyogenic bacteria.14,35 This selectivity in the susceptibility to pathogens in IRAK-4 deficiency is generally explained by the fact that many micro-organisms activate alternative signalling pathways in IRAK-4-deficient cells. In particular, the TRIF-dependent pathway, which triggers an interferon-mediated immune response and is activated by TLR3 and TLR4 ligands, was held responsible for the normal immune response to certain micro-organisms observed in the absence of IRAK-4.33,35 However, some Gram-negative pyogenic bacteria have been associated with infections in patients with IRAK-4 deficiency even though the TRIF-dependent pathway is functional in this scenario. One possible explanation is that some cellular responses to the TLR4 ligand LPS are exclusively regulated by the MyD88-dependent pathway. To investigate this, we focused our analysis on neutrophil exocytosis. This process is directly associated with the mechanisms of adhesion, migration, chemotaxis and production of ROS and, therefore, of central importance to the innate immune response. Here, we have shown that IRAK-4-deficient neutrophils have a profound defect in the exocytosis of CD11b-containing secretory organelles in response to soluble TLR ligands. We have also demonstrated that the secretion of azurophilic granules in response to LPS is impaired in IRAK-4−/− neutrophils. In contrast, TRIF-deficient neutrophils showed a normal secretory response to LPS, suggesting that the MyD88-independent signalling pathway does not play any significant role in the activation of neutrophil exocytosis. Taken together, our data suggest that IRAK-4 is a key regulator of the neutrophil response and that the TRIF-dependent signalling pathway cannot overcome the neutrophil defects manifested in IRAK-4 immunodeficiency.
A previous study has shown that exposure of neutrophils to the Gram-negative bacterial wall component LPS induces the surface expression of neutrophil secondary granule membrane proteins in a p38MAPK-dependent manner.10 Here, we have further demonstrated that p38MAPK is necessary to activate the exocytosis of all neutrophil secretory organelles upon LPS stimulation. As MyD88-deficient cells are capable of activating p38MAPK in response to LPS,27 our data suggest that the delayed phase of p38MAPK phosphorylation initiated by the TRIF signalling pathway does not participate in the regulation of the exocytic response in neutrophils. In this work, we have shown that p38MAPK is an essential regulator of LPS-induced exocytosis of not only specific granules but also azurophilic granules. In contrast, our results indicate that PI3K activity is essential for azurophilic granule exocytosis but plays a minor role in regulating the exocytosis of other secretory organelles. These results suggest that the ability of LPS to stimulate the mobilization of azurophilic granules depends on its ability to activate PI3K. Interestingly, we observed that azurophilic granule exocytosis in response to L. monocytogenes proceeds independently of IRAK-4, suggesting that MyD88-independent signalling pathways are activated in response to whole bacteria. We also observed that L. monocytogenes-dependent azurophilic granule mobilization is absolutely dependent on PI3K activity. These data suggest that PI3K can be activated in a MyD88-independent manner upon neutrophil recognition of whole bacteria. In support of this theory, Elbim and collaborators have recently shown that human neutrophils deficient in IRAK-4 can signal through TLR9 in a PI3K-dependent manner.31 It is therefore reasonable to propose that TLR9-initiated compensatory signalling pathways may be responsible for the PI3K-dependent activation of azurophilic granule exocytosis in the absence of IRAK-4, although the participation of non-TLR signalling pathways in the activation of the PI3K-dependent exocytic pathway by L. monocytogenes cannot be ruled out. These mechanisms may help to explain why the infections in IRAK-4-deficient patients are caused by such a narrow spectrum of micro-organisms.
Our observations establish clear differences between the signalling pathways that control exocytosis of peroxidase-negative and peroxidase-positive granules and suggest that the molecular components of the secretory machineries that control exocytosis of these secretory organelles are different. Azurophilic granules are characterized by a low tendency to undergo exocytosis when compared with the exocytic response of other neutrophil granules in reaction to a given stimulus.1 Furthermore, priming is usually necessary for azurophilic granules to undergo detectable exocytosis.36 In this work, we used the actin depolymerizing agent cytochalasin D to facilitate exocytosis of peroxidase-positive granules from human neutrophils in response to LPS. Treatment with cytochalasin D is thought to induce actin depolymerization favouring the trafficking of vesicles towards the docking domain at the plasma membrane.37,38 Interestingly, we found that LPS-dependent azurophilic granule exocytosis in cytochalasin D-treated cells is blocked when PI3K is inhibited, suggesting that both actin depolymerization and PI3K activation are independent steps necessary for LPS to efficiently stimulate the release of azurophilic granule cargo proteins. This may indicate that PI3K has a function that is subsequent to the trafficking of azurophilic granules from the site of storage towards the plasma membrane induced by cytochalasin D, possibly regulating docking or fusion. Whether LPS induces the formation of phosphoinositide-rich domains to facilitate the docking of azurophilic granules to the plasma membrane through interaction with specific molecular linkers, as demonstrated for synaptotagmins,39 is currently unknown and should be investigated. The observations that the small GTPase Rab27a plays a key role in the regulation of azurophilic granule exocytosis and that Rab27a-deficient mice have impaired MPO secretion in response to LPS40 raise the question as to whether Rab27a effectors regulate this interaction. In support of this hypothesis, the Rab27a effector JFC1/Slp1 (synaptotagmin-like protein 1) was demonstrated to specifically bind to 3′-phosphoinositides through its C2 domains.41 This hypothesis is currently being tested in our laboratory.
Humans with IRAK-4 deficiency are highly susceptible to S. aureus infections.42 Similarly, mice deficient in IRAK-4 or in the adaptor protein MyD88 are also susceptible to infections by this micro-organism.13,43 However, in this work we found that the rate of survival of S. aureus as well as that of Gram-negative E. coli in whole blood from IRAK-4-deficient mice was not significantly different from that observed in blood from control mice. These data, together with the observation that neutrophils from IRAK-4 KO mice showed impaired exocytosis in response to TLR ligands, in principle suggest that the systemic response to infections rather than the direct ability of neutrophils to kill is affected in IRAK-4 deficiency. Thus, although our results suggest that bacterial killing is unaltered in IRAK-4 deficiency, lack of mobilization of adhesion molecules including the β2-integrin CD11B in response to TLR4 or TLR2 ligands in neutrophils will probably contribute to a defective innate immune response in IRAK-4 deficiency by preventing the mobilization of neutrophils to the site of infection. In support of this idea, studies using a model of Enterococcus faecium peritonitis showed that reduced neutrophil recruitment but normal bacterial killing occurs in the absence of the adaptor protein MyD88.44 These defects in neutrophil function would be further underscored in patients with deficiencies of the TLR-signalling pathway caused by the impaired production of neutrophil chemoattractants44 and other inflammatory mediators, including IL-6-inducible molecules,42 as well as by the defects in the secretion of granular proteins which may play a role in extracellular killing at the site of infection.
Taken together, the results presented here identify some of the molecular components that participate in the signalling pathways regulating neutrophil exocytosis in response to TLR ligands and indicate that IRAK-4, p38MAPK and PI3K play key roles in this mechanism. Our data also suggest that severe neutrophil dysfunctions associated with the process of exocytosis accompany the already known abnormalities of the innate immune system found in IRAK-4 immunodeficiency.
Acknowledgments
We thank Dr Wen-Chen Yeh who contributed the IRAK-4 knockout mouse model, and Dr Xin Du and Dr Bruce Beutler who contributed the Triflps2/lps2 mouse. We also thank Dr Malcolm Wood for assistance with the electron microscopy analysis. The work was supported by US Public Health Service Grant AI-024227 to SDC and by the Sam and Rose Stein Endowment Fund.
Glossary
Abbreviations:
- Cyt D
cytochalasin D
- IRAK
interleukin-1-receptor-associated kinase
- LAMP-3
lysosome-associated membrane protein 3
- MPO
myeloperoxidase
- PAMP
pathogen-associated molecular pattern
References
- 1.Borregaard N, Cowland JB. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood. 1997;89:3503–21. [PubMed] [Google Scholar]
- 2.Lehrer RI. Primate defensins. Nat Rev Microbiol. 2004;2:727–38. doi: 10.1038/nrmicro976. [DOI] [PubMed] [Google Scholar]
- 3.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710–20. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 4.Legrand D, Elass E, Carpentier M, Mazurier J. Lactoferrin: a modulator of immune and inflammatory responses. Cell Mol Life Sci. 2005;62:2549–59. doi: 10.1007/s00018-005-5370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Opdenakker G, Van den Steen PE, Van DJ. Gelatinase B: a tuner and amplifier of immune functions. Trends Immunol. 2001;22:571–9. doi: 10.1016/s1471-4906(01)02023-3. [DOI] [PubMed] [Google Scholar]
- 6.Chakrabarti S, Patel KD. Regulation of matrix metalloproteinase-9 release from IL-8-stimulated human neutrophils. J Leukoc Biol. 2005;78:279–88. doi: 10.1189/jlb.1004612. [DOI] [PubMed] [Google Scholar]
- 7.Sengelov H, Kjeldsen L, Kroeze W, Berger M, Borregaard N. Secretory vesicles are the intracellular reservoir of complement receptor 1 in human neutrophils. J Immunol. 1994;153:804–10. [PubMed] [Google Scholar]
- 8.Kuijpers TW, Tool AT, van der Schoot CE, Ginsel LA, Onderwater JJ, Roos D, Verhoeven AJ. Membrane surface antigen expression on neutrophils: a reappraisal of the use of surface markers for neutrophil activation. Blood. 1991;78:1105–11. [PubMed] [Google Scholar]
- 9.Hayashi F, Means TK, Luster AD. Toll-like receptors stimulate human neutrophil function. Blood. 2003;102:2660–9. doi: 10.1182/blood-2003-04-1078. [DOI] [PubMed] [Google Scholar]
- 10.Ward RA, Nakamura M, McLeish KR. Priming of the neutrophil respiratory burst involves p38 mitogen-activated protein kinase-dependent exocytosis of flavocytochrome b558-containing granules. J Biol Chem. 2000;275:36713–9. doi: 10.1074/jbc.M003017200. [DOI] [PubMed] [Google Scholar]
- 11.Poltorak A, Smirnova I, He X, et al. Genetic and physical mapping of the Lps locus: identification of the toll-4 receptor as a candidate gene in the critical region. Blood Cells Mol Dis. 1998;24:340–55. doi: 10.1006/bcmd.1998.0201. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki N, Suzuki S, Yeh WC. IRAK-4 as the central TIR signaling mediator in innate immunity. Trends Immunol. 2002;23:503–6. doi: 10.1016/s1471-4906(02)02298-6. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki N, Suzuki S, Duncan GS, et al. Severe impairment of interleukin-1 and Toll-like receptor signalling in mice lacking IRAK-4. Nature. 2002;416:750–6. doi: 10.1038/nature736. [DOI] [PubMed] [Google Scholar]
- 14.Picard C, Puel A, Bonnet M, et al. Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science. 2003;299:2076–9. doi: 10.1126/science.1081902. [DOI] [PubMed] [Google Scholar]
- 15.Hoebe K, Du X, Georgel P, et al. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature. 2003;424:743–8. doi: 10.1038/nature01889. [DOI] [PubMed] [Google Scholar]
- 16.Fitzgerald KA, Rowe DC, Barnes BJ, et al. LPS-TLR4 signaling to IRF-3/7 and NF-kappaB involves the toll adapters TRAM and TRIF. J Exp Med. 2003;198:1043–55. doi: 10.1084/jem.20031023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 18.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 19.Detmers PA, Thieblemont N, Vasselon T, Pironkova R, Miller DS, Wright SD. Potential role of membrane internalization and vesicle fusion in adhesion of neutrophils in response to lipopolysaccharide and TNF. J Immunol. 1996;157:5589–96. [PubMed] [Google Scholar]
- 20.Markert M, Andrews PC, Babior BM. Measurement of O2− production by human neutrophils. The preparation and assay of NADPH oxidase-containing particles from human neutrophils. Methods Enzymol. 1984;105:358–65. doi: 10.1016/s0076-6879(84)05048-5. [DOI] [PubMed] [Google Scholar]
- 21.Luo Y, Dorf ME. Isolation of mouse neutrophils. In: Coligan JE, editor. Current Protocols in Immunology. 1997 edn. New York: John Wiley & Sons; 1991. pp. 30.20.1–6. [Google Scholar]
- 22.Schreiber A, Luft FC, Kettritz R. Membrane proteinase 3 expression and ANCA-induced neutrophil activation. Kidney Int. 2004;65:2172–83. doi: 10.1111/j.1523-1755.2004.00640.x. [DOI] [PubMed] [Google Scholar]
- 23.Cai TQ, Wright SD. Human leukocyte elastase is an endogenous ligand for the integrin CR3 (CD11b/CD18, Mac-1, alpha M beta 2) and modulates polymorphonuclear leukocyte adhesion. J Exp Med. 1996;184:1213–23. doi: 10.1084/jem.184.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stefanidakis M, Ruohtula T, Borregaard N, Gahmberg CG, Koivunen E. Intracellular and cell surface localization of a complex between alphaMbeta2 integrin and promatrix metalloproteinase-9 progelatinase in neutrophils. J Immunol. 2004;172:7060–8. doi: 10.4049/jimmunol.172.11.7060. [DOI] [PubMed] [Google Scholar]
- 25.Liu GY, Essex A, Buchanan JT, Datta V, Hoffman HM, Bastian JF, Fierer J, Nizet V. Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. J Exp Med. 2005;202:209–15. doi: 10.1084/jem.20050846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shankar-Sinha S, Valencia GA, Janes BK, Rosenberg JK, Whitfield C, Bender RA, Standiford TJ, Younger JG. The Klebsiella pneumoniae O antigen contributes to bacteremia and lethality during murine pneumonia. Infect Immun. 2004;72:1423–30. doi: 10.1128/IAI.72.3.1423-1430.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–22. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 28.Neumann D, Lienenklaus S, Rosati O, Martin MU. IL-1beta-induced phosphorylation of PKB/Akt depends on the presence of IRAK-1. Eur J Immunol. 2002;32:3689–98. doi: 10.1002/1521-4141(200212)32:12<3689::AID-IMMU3689>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 29.Ojaniemi M, Glumoff V, Harju K, Liljeroos M, Vuori K, Hallman M. Phosphatidylinositol 3-kinase is involved in Toll-like receptor 4-mediated cytokine expression in mouse macrophages. Eur J Immunol. 2003;33:597–605. doi: 10.1002/eji.200323376. [DOI] [PubMed] [Google Scholar]
- 30.Strassheim D, Asehnoune K, Park JS, et al. Phosphoinositide 3-kinase and Akt occupy central roles in inflammatory responses of Toll-like receptor 2-stimulated neutrophils. J Immunol. 2004;172:5727–33. doi: 10.4049/jimmunol.172.9.5727. [DOI] [PubMed] [Google Scholar]
- 31.Hoarau C, Gerard B, Lescanne E, et al. TLR9 activation induces normal neutrophil responses in a child with IRAK-4 deficiency: involvement of the direct PI3K pathway. J Immunol. 2007;179:4754–65. doi: 10.4049/jimmunol.179.7.4754. [DOI] [PubMed] [Google Scholar]
- 32.Nanamori M, Chen J, Du X, Ye RD. Regulation of leukocyte degranulation by cGMP-dependent protein kinase and phosphoinositide 3-kinase: potential roles in phosphorylation of target membrane SNARE complex proteins in rat mast cells. J Immunol. 2007;178:416–27. doi: 10.4049/jimmunol.178.1.416. [DOI] [PubMed] [Google Scholar]
- 33.Yang K, Puel A, Zhang S, et al. Human TLR-7-, -8-, and -9-mediated induction of IFN-alpha/beta and -lambda is IRAK-4 dependent and redundant for protective immunity to viruses. Immunity. 2005;23:465–78. doi: 10.1016/j.immuni.2005.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janssens S, Beyaert R. Functional diversity and regulation of different interleukin-1 receptor-associated kinase (IRAK) family members. Mol Cell. 2003;11:293–302. doi: 10.1016/s1097-2765(03)00053-4. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki N, Saito T. IRAK-4 – a shared NF-kappaB activator in innate and acquired immunity. Trends Immunol. 2006;27:566–72. doi: 10.1016/j.it.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Ottonello L, Barbera P, Dapino P, Sacchetti C, Dallegri F. Chemoattractant-induced release of elastase by lipopolysaccharide (LPS)-primed neutrophils; inhibitory effect of the anti-inflammatory drug nimesulide. Clin Exp Immunol. 1997;110:139–43. doi: 10.1046/j.1365-2249.1997.5011400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valentijn K, Valentijn JA, Jamieson JD. Role of actin in regulated exocytosis and compensatory membrane retrieval: insights from an old acquaintance. Biochem Biophys Res Commun. 1999;266:652–61. doi: 10.1006/bbrc.1999.1883. [DOI] [PubMed] [Google Scholar]
- 38.Jog NR, Rane MJ, Lominadze G, Luerman GC, Ward RA, McLeish KR. The actin cytoskeleton regulates exocytosis of all neutrophil granule subsets. Am J Physiol Cell Physiol. 2007;292:C1690–700. doi: 10.1152/ajpcell.00384.2006. [DOI] [PubMed] [Google Scholar]
- 39.Schiavo G, Gu QM, Prestwich GD, Sollner TH, Rothman JE. Calcium-dependent switching of the specificity of phosphoinositide binding to synaptotagmin. Proc Natl Acad Sci USA. 1996;93:13327–32. doi: 10.1073/pnas.93.23.13327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Munafo DB, Johnson JL, Ellis BA, Rutschmann S, Beutler B, Catz SD. Rab27a is a key component of the secretory machinery of azurophilic granules in granulocytes. Biochem J. 2007;402:229–39. doi: 10.1042/BJ20060950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Catz SD, Johnson JL, Babior BM. The C2A domain of JFC1 binds to 3′-phosphorylated phosphoinositides and directs plasma membrane association in living cells. Proc Natl Acad Sci USA. 2002;99:11652–7. doi: 10.1073/pnas.172382799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ku CL, von BH, Picard C, et al. Selective predisposition to bacterial infections in IRAK-4-deficient children: IRAK-4-dependent TLRs are otherwise redundant in protective immunity. J Exp Med. 2007;204:2407–22. doi: 10.1084/jem.20070628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takeuchi O, Hoshino K, Akira S. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J Immunol. 2000;165:5392–6. doi: 10.4049/jimmunol.165.10.5392. [DOI] [PubMed] [Google Scholar]
- 44.Leendertse M, Willems RJ, Giebelen IA, et al. TLR2-dependent MyD88 signaling contributes to early host defense in murine Enterococcus faecium peritonitis. J Immunol. 2008;180:4865–74. doi: 10.4049/jimmunol.180.7.4865. [DOI] [PubMed] [Google Scholar]