Abstract
Vδ2+ T cells, the major circulating T-cell receptor-γδ-positive (TCR-γδ+) T-cell subset in healthy adults, are involved in immunity against many microbial pathogens including Mycobacterium tuberculosis. Vδ2+ T cells recognize small phosphorylated metabolites (phosphoantigens), expand in response to whole M. tuberculosis bacilli, and complement the protective functions of CD4+ T cells. CD4+ CD25high Foxp3+ T cells (Tregs) comprise 5–10% of circulating T cells and are increased in patients with active tuberculosis (TB). We investigated whether, in addition to their known role in suppressing TCR-αβ+ lymphocytes, Tregs suppress Vδ2+ T-cell function. We found that depletion of Tregs from peripheral blood mononuclear cells increased Vδ2+ T-cell expansion in response to M. tuberculosis (H37Ra) in tuberculin-skin-test-positive donors. We developed a suppression assay with fluorescence-activated cell sorting-purified Tregs and Vδ2+ T cells by coincubating the two cell types at a 1 : 1 ratio. The Tregs partially suppressed interferon-γ secretion by Vδ2+ T cells in response to anti-CD3 monoclonal antibody plus interleukin-2 (IL-2). In addition, Tregs downregulated the Vδ2+ T-cell interferon-γ responses induced by phosphoantigen (BrHPP) and IL-2. Under the latter conditions there was no TCR stimulus for Tregs and therefore IL-2 probably triggered suppressor activity. Addition of purified protein derivative (PPD) increased the suppression of Vδ2+ T cells, suggesting that PPD activated antigen-specific Tregs. Our study provides evidence that Tregs suppress both anti-CD3 and antigen-driven Vδ2+ T-cell activation. Antigen-specific Tregs may therefore contribute to the Vδ2+ T-cell functional deficiencies observed in TB.
Keywords: regulatory T cells, tuberculosis, Vδ2+ T cells
Introduction
Development of protective immunity against Mycobacterium tuberculosis depends on the interplay between various T-lymphocyte subsets and professional antigen-presenting cells (APCs). Conventional CD4+ and CD8+ T-cell receptor-αβ-positive (TCR-αβ+) T cells play a major role in adaptive immunity against M. tuberculosis.1–6 Among unconventional T cells, human γδ T cells have also been implicated in protective immunity against M. tuberculosis.7,8 In adults, γδ T cells normally constitute between 2 and 5% of peripheral blood T cells. The majority of circulating human γδ T cells expresses the Vγ9Vδ2 TCR (also termed Vγ2Vδ2 TCR). Vγ9Vδ2+ T cells (Vδ2+ T cells) are readily activated by mycobacterial extracts in vitro and expand greatly in response to systemic infections.9–11 In non-human primates, Vδ2+ T-cell responses increase during primary mycobacterial infection and after challenge with bacillus Calmette–Guérin (BCG).7 The BCG vaccination of human adults enhances Vδ2+ T-cell responses in vitro suggesting the development of a memory-like phenotype.12 Although the role of Vδ2+ T cells in immune defence against mycobacterial infections remains poorly characterized, Vδ2+ T cells may contribute to early immune responses to M. tuberculosis and serve as a bridge between innate and adaptive immune responses.13,14 Unlike TCR-αβ+ T cells, human Vδ2+ T cells do not recognize peptides presented by classical or non-classical major histocompatibility complex molecules. Instead Vδ2+ T cells recognize a group of non-peptidic prenyl pyrophosphate antigens known as phosphoantigens.15–17 While Vδ2+ T cells and αβ TCR+ T cells display similar functional properties, their differential contribution to the immune defence against M. tuberculosis may be linked to the type of antigen they recognize and the mode of recognition.11,18
Regulation of Vδ2+ T-cell function during M. tuberculosis infection is poorly understood. In contrast to acute systemic infections such as malaria, in tuberculosis (TB) the number and function of Vδ2+ T cells are downregulated.19–22 A variety of mechanisms have been proposed for the decrease in Vδ2+ T-cell function in TB, including redistribution to the lung parenchyma, lack of CD4+ T helper activity, activation-induced cell death and immune suppression.14,23 Regulatory T cells (Tregs) have a major role in suppressing other immune cells such as CD4+ TCR-αβ+ T cells, CD8+ TCR-αβ+ T cells, natural killer T (NKT) cells, monocytes and dendritic cells.24 The role of Tregs in suppression of Vδ2+ T-cell function has not been studied.
Although the primary role of Tregs in the immune system is to prevent autoimmunity, Tregs have also been shown to suppress immune responses against persistent pathogens.25–29 In the murine model of TB two studies demonstrated that decreased Treg numbers correlated with lower mycobacterial load.29,30 More importantly, increased Treg numbers have been demonstrated both in blood and at sites of infection in patients with active TB.31–33 Two main subsets of Tregs have been described: naturally occurring CD4+ CD25+ Tregs (nTregs) and inducible Tregs (iTregs). The nTregs develop in the thymus and constitutively express the α-chain of the interleukin-2 (IL-2) receptor (CD25) and the transcription factor Foxp3. The iTregs develop in the periphery from conventional CD4+ T cells and, upon TCR triggering under tolerogenic conditions, they express CD25 and, in humans, Foxp3.34–36 There is no single marker that distinguishes these two Treg subsets in humans and it remains unclear if they are two completely distinct cell populations. Our current study focuses on the role of Tregs that constitutively express high levels of CD25 and Foxp3 (CD4+ CD25high Foxp3+) referred to as Tregs throughout this manuscript.
Here, we demonstrate that CD4+ CD25high Foxp3+ T cells suppress both anti-CD3 and phosphoantigen-triggered Vδ2+ T-cell responses. Taking advantage of the different antigen specificities of αβ and Vδ2+ T cells we developed an assay for simultaneous stimulation of Tregs and Vδ2+ T cells with purified protein derivative (PPD) and phosphoantigen. We establish that PPD triggers Treg suppression of Vδ2+ T-cell function. Our data suggest that antigen-specific Tregs contribute to the Vδ2+ T-cell functional deficiencies observed in TB. This constitutes the first report on the suppression of phosphoantigen-reactive Vδ2+ T cells by antigen-specific Tregs.
Materials and methods
Monoclonal antibodies and antigens
The following monoclonal antibodies (mAbs) were used for T-cell purification and fluorescence-activated cell sorting (FACS): fluorescein isothiocyanate (FITC)-conjugated anti-Vδ2 TCR (clone B6.1); phycoerythrin (PE)-conjugated CD25, peridinin chlorophyll protein (PerCP)-labelled anti-CD4 and allophycocyanin-labelled anti-CD3. The anti-CD3 mAb clone Hit3a was used for T-cell stimulation. All mAbs and corresponding isotypic controls were purchased from BD Pharmingen, San Diego, CA.
Mycobacterium tuberculosis H37Ra (American Type Culture Collection, Manassas, VA) was cultured in Middlebrook 7H9 with albumin dextrose catalase enrichment and frozen stocks were prepared as described previously.9 Bacterial counts and viability were performed by light microscopy and by counting colony-forming units on 7H10 medium. Before use in T-cell assays, mycobacteria were washed three times in RPMI-1640, sonicated for 40 seconds, passed multiple times through a 25-gauge needle to disrupt clumps, and diluted in non-heat-inactivated serum-containing media.
BrHPP (Phosphostim) was kindly provided by Christian Belmant, Innate Pharma, Marseille, France.37 The PPD from M. tuberculosis (PPD) was obtained from Wyeth Lederle Vaccines, Pearl River, NY.
Cell culture reagents
Cells were cultured in RPMI-1640 supplemented with 10% pooled human serum (SeraCare Life Sciences, Milford, MA), 20 mm HEPES, 2 mm l-glutamine, 1 mm sodium pyruvate, non-essential amino acids, 100 U/ml penicillin and 100 μg/ml streptomycin (all from BioWhittaker, Walkersville, MD) in 96-well U-bottomed plates (Becton Dickinson, Franklin Lakes, NJ).
Isolation of peripheral blood mononuclear cells and monocytes
Peripheral blood mononuclear cells (PBMC) were isolated from 240 cm3 of blood from six healthy tuberculin-skin-test-positive (TST+) donors (18–45 years old) recruited from among laboratory staff. All protocols were approved by Case Western Reserve University’s institutional review board. Informed written consent was obtained from all participants. PBMC were isolated by density gradient centrifugation over sodium diatrizoate/Hypaque (GE HealthCare, Uppsala, Sweden). For monocyte isolation, PBMC were incubated on plastic tissue culture dishes (Falcon, Becton Dickinson) precoated with pooled human serum for 1 hr at 37°; non-adherent cells were removed, dishes were extensively washed and adherent cells were collected by scraping with a plastic policeman. Monocytes were resuspended in culture media and irradiated with 3000 rads before adding to 96-well plates in the suppression assays.
CD25+ T-cell depletion assay
CD25+ T cells were depleted from PBMC using the MACS CD25 Microbeads magnetic cell sorting kit and LS columns according to the manufacturer’s instructions (Miltenyi Biotec, Bergisch Gladbach, Germany). Depletion was confirmed by staining with anti-CD25 mAbs and FACS analysis. Depleted and not depleted PBMC (2 × 106 cells/well) were cultured at 37° in 12-well tissue culture plates with or without H37Ra (2 × 105 CFU/ml). Cells were harvested on day 7 and viable cells were determined using the Trypan blue exclusion method. Baseline (day 1) and day 7 samples were stained with FITC-anti-Vδ2TCR and allophycocyanin-anti-CD3 mAbs. Cells were acquired using a FACSCalibur flow cytometer (BD, San Jose, CA) and analysed with the flowjosoftware (Tree Stop, Stanford University, CA). Ten thousand events were recorded for each cell surface marker. The cut-off lines for positive and negative fluorescence were set manually based on the distribution of cells stained with FITC- and PE-conjugated isotypic controls, and were kept constant within each experiment.
Purification of CD4+ CD25high (Tregs), CD4+ CD25−, Vδ2+ T cells
Non-adherent cells were labelled with the following cocktail of mAbs: FITC-conjugated anti-Vδ2 TCR, PerCP-conjugated anti-CD4 and PE-conjugated-anti-CD25 plus or minus allophycocyanin-conjugated anti-CD3. CD4+ CD25−, CD4+ CD25high and Vδ2+ T cells (purity > 95% for all three groups) were isolated by FACS using a FACSAria flow cytometer (BD Biosciences, San Jose, CA). The analysis and sort gates were restricted to the population of lymphocytes by means of their forward and side scatter properties. Foxp3 staining was performed with the human regulatory T-cell staining kit (eBioscience, San Diego, CA) following the manufacturer’s instructions.
In vitro suppression assay
To analyse Treg-mediated suppression by polyclonal stimulation, 96-well U-bottom plates (Becton Dickinson) were coated overnight at 4° with anti-CD3 mAbs (10 μg/ml) in 0·1 m Tris–HCl pH 9·5, and washed three times with PBS. The FACS-purified CD4+ CD25− or Vδ2+ T cells (2·5 × 104 cells/well) were cultured in the absence or presence of CD4+ CD25high cells at a 1 : 1 ratio. IL-2 (25 U/ml) was added as costimulatory stimulus.
To measure suppression by antigen-specific Tregs, FACS-purified CD4+ CD25− or Vδ2+ T cells (2·5 × 104 cells/well) were cultured in 96-well U-bottom plates with or without CD4+ CD25high T cells at a 1 : 1 ratio. Irradiated monocytes (2·5 × 104 cells/well) were used as APCs. For stimulation of the Vδ2+ T cells, IL-2 (25 U/ml) plus BrHPP (10 μm) was added. For antigenic stimulation of the CD4+ T-cell subsets PPD was added at 10 μg/ml.
Interferon-γ enzyme-linked immunosorbent assay
Plates were incubated at 37° for 5 days and 100 μl of cell culture supernatant was harvested and stored at −20°. Interferon-γ (IFN-γ) was determined in culture supernatants by sandwich enzyme-linked immunosorbent assay with the anti-IFN-γ antibody pairs M-700A and biotinylated M-701B (Endogen, Cambridge, MA).
Statistical analysis
Statistical analyses were performed using the paired Student’s t-test. P values < 0·05 were considered significant. Results are expressed as means ± SEM.
Results
Depletion of peripheral blood CD25+ T cells increases M. tuberculosis-induced Vδ2+ T-cell expansion
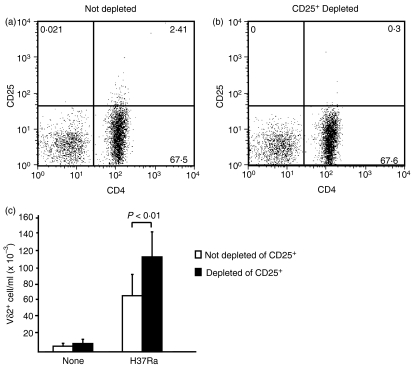
To explore the role of Tregs on suppression of γδ T-cell function, we depleted CD25+ T cells from PBMC of TST+ donors (Fig. 1a,b). We then compared the expansion of Vδ2+ T cells in response to M. tuberculosis in CD25+ T-cell-depleted and non-depleted PBMC. As shown in Fig. 1(c), Vδ2+ T-cell expansion in response to M. tuberculosis was higher in CD25+-depleted compared to non-depleted PBMC (P < 0·01). After stimulation with M. tuberculosis, Vδ2+ T cells expanded from 6705 cells/ml (range 864–12 935 cells/ml) to 66 050 cells/ml (range 15 318–134 160 cells/ml) in PBMC cultures containing CD25+ T cells and from 9795 cells/ml (range 1026–21 158 cells/ml) to 111 700 cells/ml (range 67 200–197 763 cells/ml) in CD25+-depleted cultures (n = 4). On average there was a 42% greater increase in Vδ2+ T-cell expansion in cultures depleted of CD25+ T cells compared to non-depleted cultures (n = 4, P < 0·01). Our results suggest that CD25+ T cells are activated by M. tuberculosis and suppress the expansion of mycobacteria reactive-Vδ2+ T cells.
Figure 1.
Depletion of CD25+ T cells increases Vδ2+ T-cell expansion in response to Mycobacterium tuberculosis. Peripheral blood mononuclear cells (PBMC) isolated from tuberculin-skin-test-positive (TST+) donors were not depleted (a) or CD25+ depleted with anti-CD25-labelled magnetic beads (b). Not depleted or CD25+-depleted PBMC (2 × 106 cells/well) were stimulated for 7 days with or without whole M. tuberculosis (H37Ra, 0.2 × 105 CFU/ml) (c). Total cell numbers were determined by the trypan blue exclusion method and the percentage of Vδ2+ T cells was determined by flow cytometry. Means ± SEM are shown for four experiments.
CD4+ CD25high Foxp3+ T cells suppress anti-CD3-triggered Vδ2+ T-cell responses
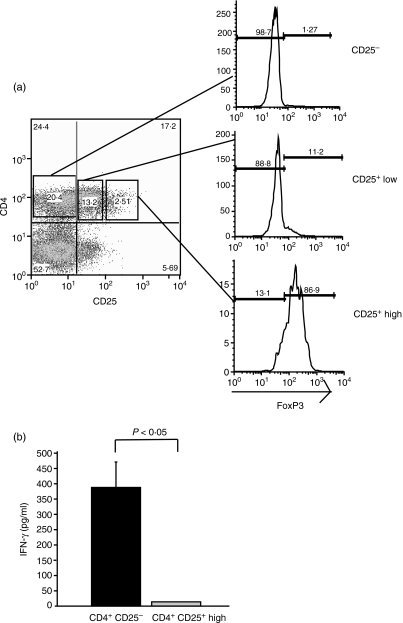
The results described above indicate that CD25+ T cells suppress Vδ2+ T-cell expansion in response to M. tuberculosis. However, inhibition may be indirect, resulting from suppression of CD4+ T cells and reduced T-cell helper activity in the form of IL-2. In addition, because only CD4+ T cells expressing high levels of CD25 are suppressor cells, depletion of all CD25+ cells decreases non-Treg subsets.38 As shown in Fig. 2(a), only CD4+ CD25high T cells coexpressed Foxp3 (87%), a specific marker for Tregs. To better characterize the effect of Tregs on Vδ2+ T cells we developed a suppression assay using FACS-purified CD4+ CD25high, CD4+ CD25− and Vδ2+ T cells. The average purity was 96 ± 2·94% for CD4+ CD25high, 95 ± 2·28% for CD4+ CD25− and 96 ± 2·95% for Vδ2+ T cells (n = 6).
Figure 2.
Purification and functional characterization of CD4+ CD25high Foxp3+ T cells. (a) Peripheral blood mononuclear cells (PBMC) were labelled with anti-CD4 and anti-CD25 monoclonal antibodies and purified by fluorescence-activated cell sorting based on the level of CD25 expression. CD25high gate was drawn around the events with > 102 mean fluorescence on the CD25 axis. Foxp3 expression was determined by intracellular staining in the sorted populations. (b) Flow-sorted CD4+ CD25− and CD4+ CD25high T cells were stimulated with plate-bound anti-CD3 monoclonal antibody plus interleukin-2. Interferon-γ (IFN-γ) was measured by enzyme-linked immunosorbent assay in 5-day culture supernatants. One representative experiment of three is shown.
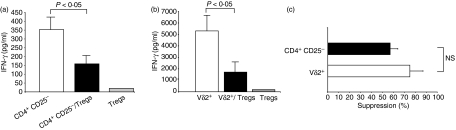
Upon stimulation with plate-bound anti-CD3 plus IL-2, Tregs suppressed the IFN-γ responses of both CD4+ CD25− and Vδ2+ T cells (Fig. 3a,b). The magnitude of Treg-mediated inhibition of Vδ2+ T-cell responses (75 ± 12%; range 52–100%) was not significantly different from that of CD4+ CD25− T-cell responses (57 ± 7%; range 48–71%; P > 0·05) (Fig. 3c). As expected, Tregs were anergic when stimulated with anti-CD3 plus IL-2.
Figure 3.
CD4+ CD25high T cells suppress polyclonally stimulated CD4+ CD25− and Vδ2+ T cells. Fluorescence-activated cell sorted CD4+ CD25− (a) and Vδ2+ T cells (b) (2.5 × 104 cells/well) were stimulated with plate-bound anti-CD3 monoclonal antibody (10 μg/ml) and interleukin-2 (25 U/ml) in the presence or absence of CD4+ CD25high regulatory T cells (Tregs) in a 1 : 1 effector to suppressor ratio. Tregs were also stimulated without effector cells. Cell culture supernatants were harvested on day 5 and interferon-γ (IFN-γ) was determined by enzyme-linked immunosorbent assay. Shown are mean values ± SEM of three independent experiments. (c) Percentage suppression of IFN-γ production was determined as follows: (IFN-γ in cultures with CD4+ CD25high)/(IFN-γ in cultures without CD4+ CD25high) × 100. Means ± SEM of three independent experiments are shown.
Therefore, CD4+ CD25high T cells purified by flow cytometry had the Treg phenotype, i.e. they expressed Foxp3 and were anergic and suppressive upon polyclonal stimulation. Thus CD4+ CD25− and Vδ2+ T cells were equally susceptible to suppression by polyclonally stimulated Tregs.
Antigen-specific Tregs partially suppress phosphoantigen-triggered IFN-γ secretion by Vδ2+ T cells
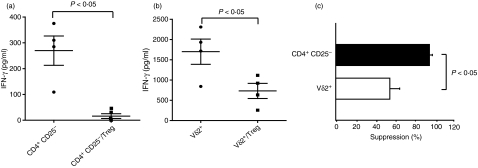
In addition to polyclonal stimulation, antigen-specific stimulation can trigger the suppression of CD4+ T cells by Tregs.39,40 We hypothesized that Tregs can be rendered suppressive by antigen stimulation and inhibit Vδ2+ T-cell function. Taking advantage of the different antigen repertoire of ΤCR-αβ+ and ΤCR-γδ+ T cells, we developed a suppression assay using PPD as CD4 T-cell antigen and BrHPP plus IL-2 as Vδ2+ T-cell stimulus. As shown before,39 CD4+ CD25− T-cell responses to PPD were almost completely abolished in most donors in the presence of Tregs (mean suppression 95 ± 3%, n = 4) (Fig. 4a). Vδ2+ T-cell responses to BrHPP plus IL-2 were also significantly inhibited by coincubation with Tregs (mean suppression 55 ± 10%, range 42–73%, n = 4) (Fig. 4b). Suppression by Tregs of Vδ2+ T responses to phosphoantigen was significantly less than Treg suppression of CD4+ CD25− responses to PPD (P < 0·05) (Fig. 4c). In summary, our data suggest that PPD triggers antigen-specific Tregs that completely suppress CD4+ CD25− T-cell responses and partially inhibit phosphoantigen-reactive Vδ2+ T cells.
Figure 4.
CD4+ CD25high T cells suppress antigen-specific CD4+ CD25− and Vδ2+ T-cell responses. (a) Fluorescence-activated cell sorting (FACS) sorted CD4+ CD25− (2.5 × 104 cells) isolated from tuberculin-skin-test-positive (TST+) donors were stimulated with purified protein derivatve (PPD; 10 μg/ml) in the presence or absence of CD4+ CD25high T cells (Tregs) at a 1 : 1 effector : suppressor ratio. Interferon-γ (IFN-γ) in 5-day culture supernatants was determined by enzyme-linked immunosorbent assay (ELISA). Mean values ± SEM of four independent experiments are shown. (b) FACS-sorted Vδ2+ T cells (2.5 × 104 cells) isolated from TST+ donors were stimulated with BrHPP (10 μm) plus interleukin-2 (25 U/ml) and PPD (10 μg/ml) in the presence or absence of CD4+ CD25high T cells (Tregs) at 1 : 1 effector : suppressor ratio. IFN-γ in 5-day culture supernatants was determined by ELISA. Mean values ± SEM of four independent experiments are shown. (c) Percentage suppression was determined as follows: (IFN-γ in cultures with CD4+ CD25high)/(IFN-γ in cultures without CD4+ CD25high) × 100. Means ± SEM are shown for four independent experiments.
Treg suppression can be triggered in the absence of TCR stimulation
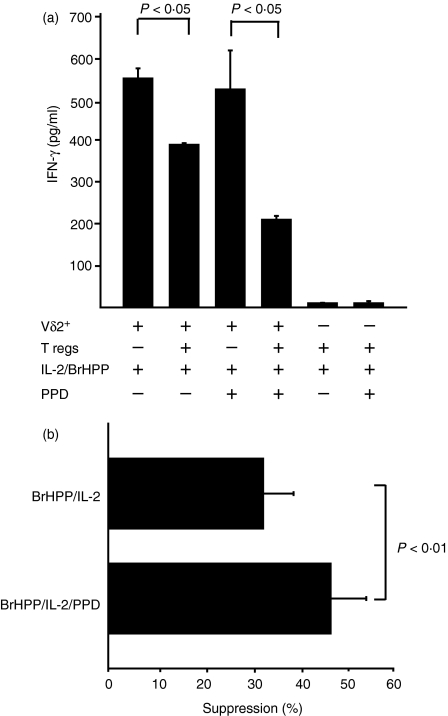
Tregs have not previously been shown to suppress human effector cells in the absence of TCR stimulation. To determine if TCR triggering of Tregs is an absolute requirement for suppression of Vδ2 T-cell function, we compared suppression in the presence or absence of TCR-αβ stimulus, i.e. PPD. IFN-γ production by Vδ2+ T cells stimulated with BrHPP and IL-2 was partially inhibited when cocultured with Tregs in the absence of PPD (P < 0·05) (Fig. 5a). Addition of PPD to these cocultures significantly increased Treg-mediated suppression presumably as the result of TCR triggering of Tregs (Fig. 5b) (P < 0·01). These results suggest that exogenous IL-2 alone can trigger Tregs to suppress Vδ2+ T cells significantly; and that providing Tregs with a TCR stimulus such as PPD increases the suppression induced by IL-2 alone.
Figure 5.
CD4+ CD25high T cells can suppress Vδ2+ T cells in the absence of TCR-αβ triggering. (a) Fluorescence-activated cell-sorted Vδ2+ T cells (2.5 × 104 cells) isolated from tuberculin-skin-test-positive donors were stimulated with BrHPP (10 μm) plus interleukin-2 (IL-2; 25 U/ml) with or without purified protein derivative (PPD; 10 μg/ml) in the presence or absence of CD4+ CD25high T cells (Tregs) at 1 : 1 effector : suppressor ratio. Interferon-γ (IFN-γ) in 5-day culture supernatants was determined by enzyme-linked immunosorbent assay. Mean values ± SEM are shown of triplicates of one representative experiment. (b) Percentage suppression was determined as follows: (IFN-γ in cultures with CD4+ CD25high)/(IFN-γ in cultures without CD4+CD25high) × 100. Means ± SEM of seven independent experiments are shown.
Discussion
Vδ2+ T cells complement the protective role of αβ T cells against M. tuberculosis. We have previously demonstrated that patients with TB have depressed Vδ2+ T-cell responses to phosphoantigen.22 The mechanisms of Vδ2+ T-cell-depressed responses during TB are poorly understood. Here we investigated the role of Tregs as suppressors of Vδ2+ T cells. We made use of the different antigenic repertoire of Vδ2+and CD4+αβ T cells to elucidate if mycobacterial antigens can trigger Treg suppression.
We demonstrated that CD4+ CD25high Foxp3+ T cells isolated from human blood suppressed IFN-γ secretion by both CD4+ and Vδ2+ T cells. While previous studies have shown Treg suppression of a variety of cell targets including CD4+ and CD8+ T cells, monocytes and dendritic cells, this is the first report of Tregs suppressing Vδ2+ T cells.38–44
Mycobacterium tuberculosis induces both CD4+ and Vδ2+ T-cell responses in PBMC isolated from TST+ individuals. Our observation that depletion of CD4+ CD25+ T cells increased Vδ2+ T-cell expansion in response to M. tuberculosis supports the idea that Tregs are triggered in an antigen-specific manner. Elegant studies by Suffia et al. have demonstrated that nTregs not only recognize self-antigens but also respond to foreign antigens.45 In this context, our results suggest M. tuberculosis antigens are recognized by and activate Tregs, which in turn suppress Vδ2+ expansion.
Because Vδ2+ T-cell activation is known to depend on IL-2 secreted by CD4+ T cells,46 decreased Vδ2+ T-cell expansion in the presence of CD25+ T cells could be secondary to the suppression of CD4+ T-cell helper activity. Therefore we developed a system to directly study the effect of Tregs on Vδ2+ T cells independent of fluctuations in CD4+ T-cell help or IL-2. In addition, we focused on CD4+ CD25high Foxp3+ Tregs as this population has been shown to exhibit the true suppressor phenotype. In our experimental model Tregs suppressed polyclonal responses to anti-CD3 mAb plus IL-2 in both CD4 and Vδ2+ T cells. As described before for suppression of CD4+ CD25− T cells, suppression of Vδ2+ T cells by Tregs was dependent on cell–cell contact (data not shown).38,42,47 Even though Vδ2+ T cells secreted 10 times more IFN-γ than CD4+ T cells, both T-cell subsets were equally susceptible to inhibition by Tregs. Therefore Tregs can downregulate potent IFN-γ responses by Vδ2+ T cells.
It has been recently proposed that Tregs function in the immune system beyond control of self-reactive T cells. Tregs may be critical in regulating the responses to microbial infections by limiting the collateral damage caused by excessive inflammation. On the other hand, Tregs could represent a mechanism for pathogens to evade the immune response. Two pieces of evidence point to a role of Tregs in human TB: first, TB patients have higher numbers of CD4+ CD25+ Foxp3+ T cells both in peripheral blood and at sites of infection;31–33 and second, Tregs expand in vitro in response to whole M. tuberculosis bacilli or mycobacterial ManLAM (Hirsch et al., unpublished data).48 Our results demonstrate that mycobacterial antigen-reactive Tregs suppress antigen-specific Vδ2+ T-cell responses and this may explain Vδ2+ T-cell deficits in TB. Therefore activation of Tregs by M. tuberculosis may represent a mechanism to suppress a broad range of protective T-cell responses, including Vδ2+ T cells, in M. tuberculosis infection.
While both Vδ2+ and CD4+ CD25− polyclonal responses were equally downregulated by Tregs, antigen-specific Vδ2+ T-cell responses were less susceptible to inhibition than antigen-specific CD4+ T-cell responses. There are at last three possible explanations for this difference. First, phosphoantigen-stimulated Vδ2+ T cells may be intrinsically more resistant to suppression by Tregs than mycobacterial peptide-reactive CD4+ CD25− T cells. Second, in the case of antigen-specific stimulation, only a few clones among the CD4+ CD25− T cells recognize PPD antigens. On the other hand, because of the restricted antigenic repertoire of Vδ2+ T cells, a large proportion will respond to phosphoantigens. Therefore the effective ratio of Tregs to antigen-specific CD4+ CD25− T cells is higher than the ratio of Tregs to antigen-specific Vδ2+ T cells. This allows better suppressor cell to effector cell contact and suppression of the CD4+ CD25− T cells compared to the Vδ2+ T cells. Third, as reported by Misra et al.49 and Taams et al.,50 Tregs downregulate APC function and, as a consequence, they may decrease the processing and presentation of peptide antigen to CD4+ T cells. Although Vδ2+ T-cell responses are higher in the presence of APC,51 phosphoantigen recognition by the Vδ2+ TCR is independent of processing and presentation by APC. Therefore inhibited APC function would have a greater impact on CD4+ T-cell responses than on Vδ2+ T-cell responses.
Activation through the TCR is thought to be an absolute requirement for Treg function. Most studies of Tregs have been performed using polyclonal stimuli such as anti-CD3 or allogeneic APC, with antigen-specific activation with tetanus toxoid, HSP65, PPD or leishmania antigens.25,39 In these experiments Tregs and the cells they affect are stimulated at the same time. Therefore, it is difficult to separate TCR stimulation of Tregs from that of the cells being suppressed. Dieckmann et al. overcame these limitations by using Tregs and APC from syngeneic and allogeneic donors and provided evidence that stimulation of human Tregs through the TCR induces suppression of CD4+ T cells.42 However, these studies do not rule out the possibility that suppression could be triggered without activation through the TCR, by cytokines for example. Our experimental system allowed concomitant stimulation of effector and suppressor cells with different antigens, and suggested that some Tregs can be suppressed in the absence of αβ TCR stimulation. We hypothesize that IL-2 may activate TCR-independent suppression.
While we have not directly investigated the mechanism of suppression of Vδ2+ T cells by Tregs, previous reports have demonstrated that CD4+ CD25+ Foxp3+ Treg suppression of human CD4+ T cells is contact dependent and independent of IL-10 or surface molecules such as cytotoxic T-lymphocyte antigen-4, programmed death-1 ligand and glucocorticoid-induced tumour necrosis factor receptor.52 Therefore it is likely that suppression of Vδ2+ T cells responses by Tregs will use a similar mechanism. In addition, mouse nTregs isolated from lymph node or spleen suppress mouse, δ T cells in a contact-dependent manner and independently of IL-10 or transforming growth factor-β (Silva-Santos B. M., personal communication).
In summary, our study represents the first report on the suppression of Vδ2+ T-cell function by circulating CD4+ CD25high Foxp3+ T cells. In addition, we confirmed that mycobacterial antigen-reactive suppressor cells are part of the CD4+ CD25high Foxp3+ T-cell repertoire in human peripheral blood.
Acknowledgments
This work was supported by grants from the National Institutes of Health (AI AI27243 and AI70022 to W.H.B) and contract no. HHSN266200700022C/NO1-AI-70022 for the Tuberculosis Research Unit (TBRU). C.S.M. was supported by T32-HL07889 and R.E.R. was supported by the American Lung Association (RG48786N). We thank Christian Belmant (Innate Pharma) for kindly providing bromohydrin pyrophosphate (BrHPP).
References
- 1.Pedrazzini T, Hug K, Louis JA. Importance of L3T4+ and Lyt-2+ cells in the immunologic control of infection with Mycobacterium bovis strain bacillus Calmette–Guérin in mice. Assessment by elimination of T cell subsets in vivo. J Immunol. 1987;139:2032–7. [PubMed] [Google Scholar]
- 2.Orme IM, Collins FM. Adoptive protection of the Mycobacterium tuberculosis-infected lung. Dissociation between cells that passively transfer protective immunity and those that transfer delayed-type hypersensitivity to tuberculin. Cell Immunol. 1984;84:113–20. doi: 10.1016/0008-8749(84)90082-0. [DOI] [PubMed] [Google Scholar]
- 3.Ladel CH, Daugelat S, Kaufmann SH. Immune response to Mycobacterium bovis bacille Calmette–Guérin infection in major histocompatibility complex class I- and II-deficient knock-out mice: contribution of CD4 and CD8 T cells to acquired resistance. Eur J Immunol. 1995;25:377–84. doi: 10.1002/eji.1830250211. [DOI] [PubMed] [Google Scholar]
- 4.Mogues T, Goodrich ME, Ryan L, LaCourse R, North RJ. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J Exp Med. 2001;193:271–80. doi: 10.1084/jem.193.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 6.North RJ, Jung YJ. Immunity to tuberculosis. Annu Rev Immunol. 2004;22:599–623. doi: 10.1146/annurev.immunol.22.012703.104635. [DOI] [PubMed] [Google Scholar]
- 7.Shen Y, Zhou D, Qiu L, et al. Adaptive immune response of Vgamma2Vdelta2+ T cells during mycobacterial infections. Science. 2002;295:2255–8. doi: 10.1126/science.1068819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen ZW, Letvin NL. Adaptive immune response of Vgamma2Vdelta2 T cells: a new paradigm. Trends Immunol. 2003;24:213–9. doi: 10.1016/s1471-4906(03)00032-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Havlir DV, Ellner JJ, Chervenak KA, Boom WH. Selective expansion of human gamma delta T cells by monocytes infected with live Mycobacterium tuberculosis. J Clin Invest. 1991;87:729–33. doi: 10.1172/JCI115053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfeffer K, Schoel B, Plesnila N, Lipford GB, Kromer S, Deusch K, Wagner H. A lectin-binding, protease-resistant mycobacterial ligand specifically activates V gamma 9+ human gamma delta T cells. J Immunol. 1992;148:575–83. [PubMed] [Google Scholar]
- 11.Morita CT, Jin C, Sarikonda G, Wang H. Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vgamma2Vdelta2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol Rev. 2007;215:59–76. doi: 10.1111/j.1600-065X.2006.00479.x. [DOI] [PubMed] [Google Scholar]
- 12.Hoft DF, Brown RM, Roodman ST. Bacille Calmette–Guérin vaccination enhances human gamma delta T cell responsiveness to mycobacteria suggestive of a memory-like phenotype. J Immunol. 1998;161:1045–54. [PubMed] [Google Scholar]
- 13.Boom WH, Canaday DH, Fulton SA, Gehring AJ, Rojas RE, Torres M. Human immunity to M. tuberculosis: T cell subsets and antigen processing. Tuberculosis (Edinb) 2003;83:98–106. doi: 10.1016/s1472-9792(02)00054-9. [DOI] [PubMed] [Google Scholar]
- 14.Chen ZW. Immune regulation of gammadelta T cell responses in mycobacterial infections. Clin Immunol. 2005;116:202–7. doi: 10.1016/j.clim.2005.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka Y, Sano S, Nieves E, et al. Nonpeptide ligands for human gamma delta T cells. Proc Natl Acad Sci USA. 1994;91:8175–9. doi: 10.1073/pnas.91.17.8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Constant P, Davodeau F, Peyrat MA, Poquet Y, Puzo G, Bonneville M, Fournie JJ. Stimulation of human gamma delta T cells by nonpeptidic mycobacterial ligands. Science. 1994;264:267–70. doi: 10.1126/science.8146660. [DOI] [PubMed] [Google Scholar]
- 17.Thedrez A, Sabourin C, Gertner J, Devilder MC, Allain-Maillet S, Fournie JJ, Scotet E, Bonneville M. Self/non-self discrimination by human gammadelta T cells: simple solutions for a complex issue? Immunol Rev. 2007;215:123–35. doi: 10.1111/j.1600-065X.2006.00468.x. [DOI] [PubMed] [Google Scholar]
- 18.Konigshofer Y, Chien YH. Gammadelta T cells – innate immune lymphocytes? Curr Opin Immunol. 2006;18:527–33. doi: 10.1016/j.coi.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Barnes PF, Grisso CL, Abrams JS, Band H, Rea TH, Modlin RL. Gamma delta T lymphocytes in human tuberculosis. J Infect Dis. 1992;165:506–12. doi: 10.1093/infdis/165.3.506. [DOI] [PubMed] [Google Scholar]
- 20.Li B, Bassiri H, Rossman MD, et al. Involvement of the Fas/Fas ligand pathway in activation-induced cell death of mycobacteria-reactive human gamma delta T cells: a mechanism for the loss of gamma delta T cells in patients with pulmonary tuberculosis. J Immunol. 1998;161:1558–67. [PubMed] [Google Scholar]
- 21.Gioia C, Agrati C, Goletti D, et al. Different cytokine production and effector/memory dynamics of alpha beta+ or gamma delta+ T-cell subsets in the peripheral blood of patients with active pulmonary tuberculosis. Int J Immunopathol Pharmacol. 2003;16:247–52. doi: 10.1177/039463200301600310. [DOI] [PubMed] [Google Scholar]
- 22.Rojas RE, Chervenak KA, Thomas J, et al. Vdelta2+ gammadelta T cell function in Mycobacterium tuberculosis- and HIV-1-positive patients in the United States and Uganda: application of a whole-blood assay. J Infect Dis. 2005;192:1806–14. doi: 10.1086/497146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou D, Lai X, Shen Y, et al. Inhibition of adaptive Vgamma2Vdelta2+ T-cell responses during active mycobacterial coinfection of simian immunodeficiency virus SIVmac-infected monkeys. J Virol. 2003;77:2998–3006. doi: 10.1128/JVI.77.5.2998-3006.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity. 2004;21:589–601. doi: 10.1016/j.immuni.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+ CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–7. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 26.Mendez S, Reckling SK, Piccirillo CA, Sacks D, Belkaid Y. Role for CD4(+) CD25(+) regulatory T cells in reactivation of persistent leishmaniasis and control of concomitant immunity. J Exp Med. 2004;200:201–10. doi: 10.1084/jem.20040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lundgren A, Suri-Payer E, Enarsson K, Svennerholm AM, Lundin BS. Helicobacter pylori-specific CD4+CD25high regulatory T cells suppress memory T-cell responses to in infected individuals. Infect Immun. 2003;71:1755–62. doi: 10.1128/IAI.71.4.1755-1762.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kursar M, Bonhagen K, Fensterle J, Kohler A, Hurwitz R, Kamradt T, Kaufmann SH, Mittrucker HW. Regulatory CD4+ CD25+ T cells restrict memory CD8+ T cell responses. J Exp Med. 2002;196:1585–92. doi: 10.1084/jem.20011347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scott-Browne JP, Shafiani S, Tucker-Heard G, Ishida-Tsubota K, Fontenot JD, Rudensky AY, Bevan MJ, Urdahl KB. Expansion and function of Foxp3-expressing T regulatory cells during tuberculosis. J Exp Med. 2007;204:2159–69. doi: 10.1084/jem.20062105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kursar M, Koch M, Mittrucker HW, Nouailles G, Bonhagen K, Kamradt T, Kaufmann SH. Cutting edge: regulatory T cells prevent efficient clearance of Mycobacterium tuberculosis. J Immunol. 2007;178:2661–5. doi: 10.4049/jimmunol.178.5.2661. [DOI] [PubMed] [Google Scholar]
- 31.Ribeiro-Rodrigues R, Resende Co T, Rojas R, Toossi Z, Dietze R, Boom WH, Maciel E, Hirsch CS. A role for CD4+ CD25+ T cells in regulation of the immune response during human tuberculosis. Clin Exp Immunol. 2006;144:25–34. doi: 10.1111/j.1365-2249.2006.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guyot-Revol V, Innes JA, Hackforth S, Hinks T, Lalvani A. Regulatory T cells are expanded in blood and disease sites in patients with tuberculosis. Am J Respir Crit Care Med. 2006;173:803–10. doi: 10.1164/rccm.200508-1294OC. [DOI] [PubMed] [Google Scholar]
- 33.Chen X, Zhou B, Li M, et al. CD4(+)CD25(+)FoxP3(+) regulatory T cells suppress Mycobacterium tuberculosis immunity in patients with active disease. Clin Immunol. 2007;123:50–9. doi: 10.1016/j.clim.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 34.Ziegler SF. FOXP3: of mice and men. Annu Rev Immunol. 2006;24:209–26. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 35.Becker C, Stoll S, Bopp T, Schmitt E, Jonuleit H. Regulatory T cells: present facts and future hopes. Med Microbiol Immunol. 2006;195:113–24. doi: 10.1007/s00430-006-0017-y. [DOI] [PubMed] [Google Scholar]
- 36.Belkaid Y. Regulatory T cells and infection: a dangerous necessity. Nat Rev Immunol. 2007;7:875–88. doi: 10.1038/nri2189. [DOI] [PubMed] [Google Scholar]
- 37.Espinosa E, Belmant C, Pont F, et al. Chemical synthesis and biological activity of bromohydrin pyrophosphate, a potent stimulator of human gamma delta T cells. J Biol Chem. 2001;276:18337–44. doi: 10.1074/jbc.M100495200. [DOI] [PubMed] [Google Scholar]
- 38.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+ CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–53. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 39.Taams LS, Vukmanovic-Stejic M, Smith J, et al. Antigen-specific T cell suppression by human CD4+ CD25+ regulatory T cells. Eur J Immunol. 2002;32:1621–30. doi: 10.1002/1521-4141(200206)32:6<1621::AID-IMMU1621>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 40.Levings MK, Sangregorio R, Roncarolo MG. Human CD25(+)CD4(+) T regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J Exp Med. 2001;193:1295–302. doi: 10.1084/jem.193.11.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J Exp Med. 2001;193:1285–94. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G. Ex vivo isolation and characterization of CD4(+)CD25(+) T cells with regulatory properties from human blood. J Exp Med. 2001;193:1303–10. doi: 10.1084/jem.193.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahnke K, Bedke T, Enk AH. Regulatory conversation between antigen presenting cells and regulatory T cells enhance immune suppression. Cell Immunol. 2007;250:1–13. doi: 10.1016/j.cellimm.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 44.Liang B, Workman C, Lee J, et al. Regulatory T cells inhibit dendritic cells by lymphocyte activation gene-3 engagement of MHC class II. J Immunol. 2008;180:5916–26. doi: 10.4049/jimmunol.180.9.5916. [DOI] [PubMed] [Google Scholar]
- 45.Suffia IJ, Reckling SK, Piccirillo CA, Goldszmid RS, Belkaid Y. Infected site-restricted Foxp3+ natural regulatory T cells are specific for microbial antigens. J Exp Med. 2006;203:777–88. doi: 10.1084/jem.20052056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kabelitz D, Pechhold K, Bender A, Wesselborg S, Wesch D, Friese K, Janssen O. Activation and activation-driven death of human gamma/delta T cells. Immunol Rev. 1991;120:71–88. doi: 10.1111/j.1600-065x.1991.tb00588.x. [DOI] [PubMed] [Google Scholar]
- 47.Taams LS, Smith J, Rustin MH, Salmon M, Poulter LW, Akbar AN. Human anergic/suppressive CD4(+)CD25(+) T cells: a highly differentiated and apoptosis-prone population. Eur J Immunol. 2001;31:1122–31. doi: 10.1002/1521-4141(200104)31:4<1122::aid-immu1122>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 48.Garg A, Barnes PF, Roy S, et al. Mannose-capped lipoarabinomannan- and prostaglandin E2-dependent expansion of regulatory T cells in human Mycobacterium tuberculosis infection. Eur J Immunol. 2008;38:459–69. doi: 10.1002/eji.200737268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Misra N, Bayry J, Lacroix-Desmazes S, Kazatchkine MD, Kaveri SV. Cutting edge: human CD4+ CD25+ T cells restrain the maturation and antigen-presenting function of dendritic cells. J Immunol. 2004;172:4676–80. doi: 10.4049/jimmunol.172.8.4676. [DOI] [PubMed] [Google Scholar]
- 50.Taams LS, van Amelsfort JM, Tiemessen MM, Jacobs KM, de Jong EC, Akbar AN, Bijlsma JW, Lafeber FP. Modulation of monocyte/macrophage function by human CD4+ CD25+ regulatory T cells. Hum Immunol. 2005;66:222–30. doi: 10.1016/j.humimm.2004.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rojas RE, Torres M, Fournie JJ, Harding CV, Boom WH. Phosphoantigen presentation by macrophages to Mycobacterium tuberculosis– reactive Vgamma9Vdelta2+ T cells: modulation by chloroquine. Infect Immun. 2002;70:4019–27. doi: 10.1128/IAI.70.8.4019-4027.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baecher-Allan C, Hafler DA. Human regulatory T cells and their role in autoimmune disease. Immunol Rev. 2006;212:203–16. doi: 10.1111/j.0105-2896.2006.00417.x. [DOI] [PubMed] [Google Scholar]