Abstract
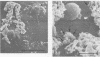
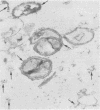
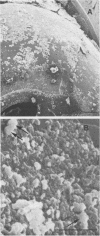
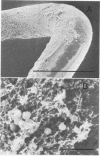
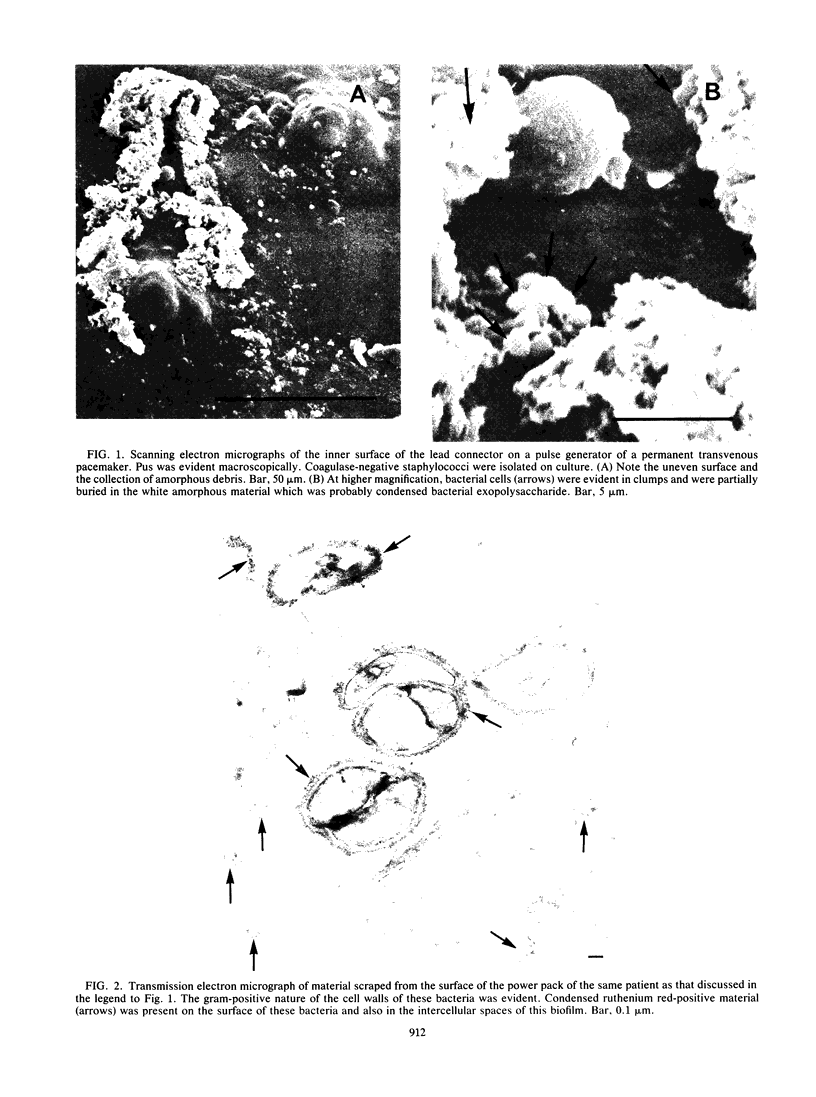
The mode of growth of the bacteria adherent to the surfaces of various components of cardiac pacemakers infected with coagulase-negative staphylococci was examined by scanning and transmission electron microscopy. Within developing adherent microcolonies, coccoid cells could be clearly seen to be enveloped by an amorphous material that condensed radically upon dehydration for electron microscopy, producing an amorphous residue in scanning electron micrographs and a fibrous anionic ruthenium red-staining residue in transmission electron micrographs. The differences in morphologies of the condensed residues may reflect differences in exopolysaccharides elaborated by the microorganisms and the degree to which materials of host origin are incorporated. The coccoid cells of coagulase-negative staphylococci were less clearly seen in thick, mature, adherent biofilms on heavily colonized surfaces because the condensed residue of their exopolysaccharide glycocalyces covered, and sometimes occluded, the bacteria cells. These coagulase-negative staphylococcal strains appeared to produce more exopolysaccharide material than did the strains infecting various intravascular catheters.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Christensen G. D., Simpson W. A., Bisno A. L., Beachey E. H. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect Immun. 1982 Jul;37(1):318–326. doi: 10.1128/iai.37.1.318-326.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Ingram J. M., Cheng K. J. Structure and function of the cell envelope of gram-negative bacteria. Bacteriol Rev. 1974 Mar;38(1):87–110. doi: 10.1128/br.38.1.87-110.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Irvin R. T., Cheng K. J. The role of bacterial surface structures in pathogenesis. Crit Rev Microbiol. 1981;8(4):303–338. doi: 10.3109/10408418109085082. [DOI] [PubMed] [Google Scholar]
- Malick L. E., Wilson R. B. Modified thiocarbohydrazide procedure for scanning electron microscopy: routine use for normal, pathological, or experimental tissues. Stain Technol. 1975 Jul;50(4):265–269. doi: 10.3109/10520297509117069. [DOI] [PubMed] [Google Scholar]
- Marrie T. J., Costerton J. W. Scanning and transmission electron microscopy of in situ bacterial colonization of intravenous and intraarterial catheters. J Clin Microbiol. 1984 May;19(5):687–693. doi: 10.1128/jcm.19.5.687-693.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrie T. J., Nelligan J., Costerton J. W. A scanning and transmission electron microscopic study of an infected endocardial pacemaker lead. Circulation. 1982 Dec;66(6):1339–1341. doi: 10.1161/01.cir.66.6.1339. [DOI] [PubMed] [Google Scholar]
- Peters G., Locci R., Pulverer G. Adherence and growth of coagulase-negative staphylococci on surfaces of intravenous catheters. J Infect Dis. 1982 Oct;146(4):479–482. doi: 10.1093/infdis/146.4.479. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]