Abstract
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease of the central nervous system and a defect in the regulatory T-cell subset seems to be involved in the pathogenesis of the disease. Foxp3 is a transcription factor that is selectively expressed in CD4+ CD25+ regulatory T cells and is required for their development and function. T-bet is a key transcription factor for the development of T helper 1 (Th1) cells. We found that both the percentage of circulating CD4+ CD25+ Foxp3+ cells and Foxp3 expression were lower in relapsing-remitting (RR) MS patients during relapses than during remission. Otherwise, the percentage of CD4+ T-bet+ T cells and T-bet expression in CD4+ T cells were higher in relapsing than in remitting RRMS patients. CD4+ CD25+ T cells both from relapsing and from remitting RRMS patients showed significantly less capacity than corresponding cells from healthy subjects to suppress autologous CD4+ CD25− T-cell proliferation, despite a similar Foxp3 expression level. CD4+ CD25+ T cells from healthy subjects and patients in remission clearly reduced T-bet mean fluorescence intensity (MFI) in CD4+ CD25− T cells up to a ratio of 1:10, whereas CD4+ CD25+ T cells from patients in relapse were able to reduce T-bet expression only at a high ratio. Our data indicate that the increased number of regulatory T (T-reg) cells and the increased Foxp3 expression in circulating CD4+ CD25+ T cells may contribute to the maintenance of tolerance in the remission phase of MS. Moreover, the inhibitory capacity of CD4+ CD25+ T cells seems to be impaired in relapsing patients under inflammatory conditions, as shown by the high levels of T-bet expression in CD4+T cells.
Keywords: Foxp3, multiple sclerosis, relapse, remission, T-bet
Introduction
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease of the central nervous system (CNS).1 Important steps in the pathogenesis of MS are the generation of autoaggressive brain-specific T cells and their invasion of the target organ.2 According to the current concept, the autoaggressive T cells are activated in the peripheral immune organs (e.g. by cross-reacting with microbial antigen) before they are capable of trans-migrating across the blood–brain barrier.2,3 Within the brain, the autoaggressive T cells are re-activated and trigger inflammation, resulting in myelin and neuronal damage.4
The differentiation of T helper (Th) cells into the different subsets is determined by several factors, including cytokines, strength of T-cell receptor (TCR) signal, dose and form of antigens, antigen-presenting cells, co-stimulator molecules engagement and genetic background. However, the main regulation of T-cell differentiation and cytokine pathways seems to be provided by cytoplasmic and nuclear transcription factors.5 T-bet has been identified as a key transcription factor for the development of Th1 cells and the induction of interferon-γ (IFN-γ) production.6 T-bet is induced during T-cell activation by the IFN-γ–signal transducer and activator of transcription 1 (STAT1) signalling pathway7 and once expressed amplifies the production of IFN-γ.8 Mice lacking T-bet are protected from the development of experimental autoimmune encephalomyelitis (EAE)9 and in vivo administration of T-bet-specific antisense oligonucleotide inhibits EAE.10 CD4+ Th1 cells producing IFN-γ were previously thought to mediate EAE, but new data have shown that interleukin (IL)-17-producing cells play a crucial role in the pathogenesis of the disease.11 A recent study showed that T-bet is also necessary for the expression of IL-23 receptor (IL-23R) and the survival of Th17 cells.12 Foxp3 is a transcription factor that is expressed in CD4+ CD25+ regulatory T (T-reg) cells,13 and it is required for T-reg cell development and function.14,15 Ectopic expression of Foxp3 is sufficient to confer suppressive activity and to induce a T-reg cell phenotype in conventional CD4+ CD25− T cells repressing the production of cytokines and up-regulating the expression of CD25, glucocorticoid-induced tumour necrosis factor receptor (GITR) and cytotoxic T-lymphocyte antigen-4 (CTLA-4).16 Mutations in Foxp3 result in autoimmune lymphoproliferative diseases in both humans and mouse.17,18 CD4+ CD25+ Foxp3+ T-reg cells limit inflammation and tissue damage in disease models of organ-specific autoimmune diseases such as EAE,19 and a decreased expression of Foxp3 protein20,21 and mRNA20,22 was found in CD4+ CD25+ T cells from relapsing remitting MS (RRMS) patients but not in secondary progressive MS patients.22
In our previous study we found a strong up-regulation of T-bet in circulating CD4+, CD8+ T cells and monocytes from the peripheral blood of RRMS patients in relapse compared with patients in remission and controls.23 In the present study we evaluated the number of circulating CD4+ T-bet+ cells and the mean T-bet expression in CD4+ lymphocytes and the number of circulating T-reg cells and the mean Foxp3 expression in CD4+ CD25+ T cells from RRMS patients in different phases of disease and from healthy subjects. We also studied the capacity of T-reg cells to suppress CD4+ CD25− cell proliferation and T-bet expression.
Materials and methods
Patients
Patients with RRMS defined by McDonald’s criteria1 and healthy subjects were included in our study. No patients had received immunosuppressive drugs or disease-modifying agents. No corticosteroid treatment was used for 6 months before inclusion in the study and patients in relapse were bled before starting high-dose corticosteroid treatment. Clinical examination and brain and spinal cord magnetic resonance imaging (MRI) were performed in all patients before starting the enrolment. Patients were considered in relapse (relapsing patients) when they showed an episode of new neurological disturbance lasting at least 24 hr and MRI activity [≥ 1 gadolinium-diethylenetriaminepenta-acetic acid (Gd-DTPA)-enhancing lesion]. Patients were considered in remission (remitting patients) when neither new neurological symptoms nor MRI activity was registered. Disability degree was assessed using the Expanded Disability Status Scale (EDSS). MRI data were acquired using a high-resolution 1·5 Tesla system (5-mm slice thickness). Scanning sessions included acquisition of proton-density [echo time (TE) 20/repetition time (TR) 2500], T2-weighted (TE 80/TR 2500) and T1-weighted (TE 17/TR 600) images. The T1-weighted images were acquired before and 10 min after an intravenous injection of Gd-DTPA (0·1 mmol/kg). This study was approved by the ethic committee of the Catholic University (Rome, Italy), and all the participants gave written informed consent before enrolment.
Isolation of peripheral blood mononuclear cells
Peripheral blood mononuclear cells (PBMC) were isolated from venous blood by density gradient centrifugation (2500 g, 30 min) over a Ficoll–Hypaque density gradient (Pharmacia, Uppsala, Sweden). PBMC were then harvested by pipetting cells from the Ficoll/serum interface and washed twice.
Flow cytometry
For the detection of Foxp3 expression, PBMC were analyzed by three-colour intracellular flow cytometry using anti-CD4–phycoerythrin-cyanine 5 (PC5) conjugate (Beckman Coulter, Miami, FL), anti-CD25–fluorescein isothiocyanate (FITC) conjugate (Beckman Coulter) and anti-Foxp3–phycoerythrin (PE) conjugate monoclonal antibody (mAb) (clone 236A/E7; eBioscience, San Diego, CA). In particular, isolated PBMC were washed once in culture medium (Dulbecco) containing fetal calf serum (FCS) and once in phosphate-buffered saline (PBS) and incubated with both anti-CD4–PC5 and anti-CD25–FITC. After fixation, cells were permeabilized using a commercially available permeabilization/wash kit (BD Biosciences/Pharmingen, Franklin Lakes, NJ). Upon permeabilization, 5 × 105 cells were resuspended in 100 μl of PBS and incubated for 30 min with the anti-Foxp3–PE conjugate. Cells were washed again with cold PBS and resuspended in PBS for flow cytometry (EPICS XL™; Beckman Coulter). For the detection of T-bet expression, a double-labelling procedure was performed using anti-CD4–PC5 staining, followed by fixation, permeabilization and incubation with anti-T-bet (4B10)–PE (Santa Cruz Biotechnology, Santa Cruz, CA). Either Foxp3 or T-bet mean fluorescence intensity (MFI) and the percentage of CD4+ CD25+ Foxp3+ or CD4+ T-bet+ cells were evaluated using two different flow cytometry protocols. Each analysis was performed using at least 50 000 cells that were gated in the region of the lymphocyte population, as determined by light scatter properties (forward scatter versus side scatter). In order to analyse the expression of transcription factors in lymphocytes (CD4+ T cells), cells were gated in both the lymphocyte and CD4+ regions. Quadrants of dot-plots were set using appropriate isotype controls for each intracellular and extracellular antibody. Appropriate fluorochrome-conjugated isotype-matched mAbs (Beckman Coulter) were used as a control for background staining in each flow acquisition. In these assays, careful colour compensation was performed before cell analysis.
Separation of CD4+ CD25+, CD4+ CD25− T cells and monocytes
PBMC CD4+ T cells were purified by positive selection with magnetic beads conjugated with anti-CD4 (Miltenyi Biotec, Bergisch Gladbach, Germany). CD4+ CD25+ T cells were purified from sorted CD4+ T cells by positive selection with magnetic beads conjugated with anti-CD25 (Miltenyi Biotec). The purity of separated CD4+ CD25+, CD4+ CD25− T cells, evaluated by flow cytometry, was 90–95%. For monocyte separation, CD4+ T cell-depleted PBMC were incubated in RPMI-1640 containing 2n-glutamine and 5% AB+ human serum in 96-well plates for 1 hr. Supernatants with non-adherent cells were collected while the remaining adherent monocytes were used as antigen-presenting cells (APC) for the suppressive assay.
Detection of Foxp3 expression by western blotting
For immunoblotting, isolated cell populations (CD4+ CD25+, CD4+ CD25−) were directly lysed in radioimmunoprecipitation assay (RIPA) buffer, separated by electrophoresis through 12% sodium dodecyl sulphate–polyacrylamide gels and then transferred to nitrocellulose membrane. Filters were blocked for 4 hr in Tris-buffered saline containing 0.1% Tween 20 plus 5% freeze-dried milk, then incubated with polyclonal goat anti-(human Foxp3), overnight at 4°C in the same buffer. Immunoreactive bands were detected by chemiluminescence using an enhanced chemiluminescence (ECL) kit.
Immunocytochemistry
For detection of Foxp3, the bead-sorted CD4+ CD25+ cells were fixed with 4% paraformaldehyde for 10 min, rinsed in PBS, permeabilized using a commercially available permeabilization buffer (BD Biosciences/Pharmingen) and then incubated with purified anti-(human Foxp3) (eBioscience) diluted 1:200. After incubation with the appropriate FITC-labelled secondary antibody, anti-(mouse IgG) (Jackson ImmunoResearch Laboratories West Grove, PA), cells were examined using a fluorescence microscope.
Suppression assay
To examine the inhibition rate of purified CD4+ CD25+ T cells, co-cultures were established in 96-well U-bottom plates. CD4+ CD25− T cells and CD4+ CD25+ T cells (5 × 104 cells/well) were cultured in RPMI-1640 supplemented with 5% AB+ human serum, 2 mm l-glutamine, 100 U/ml penicillin and 100 μg/ml of streptomycin, in different suppressor/responder ratios (1:1, 1:2, 1:5, 1:10 and 0:1). Therefore, every well contained 3 × 105 APC responder and suppressor cells (bead-selected CD4+ CD25−) at different ratios and 2 μg/ml of anti-CD3 (PharMingen; San Diego, CA). For the carboxy fluorescein diacetate succinimidyl ester (CFSE) suppression assay, freshly harvested CD4+ CD25− T cells were resuspended in PBS and then labelled with CFSE (CellTrace; Invitrogen Life Technologies, Carlsbad, CA). CFSE-labelled CD4+ CD25− responder cells were then washed and co-cultured with CD4+ CD25+ T cells at different ratios. After 5 days of stimulation with anti-CD3, cells were harvested and the CFSE signal of gated lymphocytes was analysed by flow cytometry. In particular, the suppressive capacity (percentage) of CD4+ CD25+ T cells towards responder cells in co-culture (at each mixed cell ratio) was calculated relative to the maximal proliferation of responder cells alone after anti-CD3 stimulation [100 × (1−number of cells in co-culture that divide/number of responder cells that divide)]. For T-bet assays, T-bet expression in responder and suppressor cells was evaluated using intracellular flow cytometry following fixation, permeabilization and incubation with anti-[T-bet (4B10)]–PE conjugate.
Cytokine measurement
The spontaneous production of IFN-γ was measured by enzyme-linked immunosorbent assay (ELISA) using commercial kits (R&D Systems, Minneapolis, MN) following the manufacturer’s instructions. IFN-γ concentrations were determined from the regression line for a standard curve generated by using highly purified recombinant cytokine at various concentrations performed contemporaneously with each assay. The intra-assay and inter-assay coefficients of variation were 6% and 7%, respectively. The standard curve also served as an internal control over the sensitivity and range of each assay. All samples were assayed in duplicate.
Statistical analysis
Differences in variables among groups were tested by two-way analysis of variance (anova). Results are expressed as mean ± standard error of the mean (SEM) and were considered to be statistically significant at a P-value of < 0·05. Group comparisons (in the suppression assay) were performed using the Student’s t-test, and a P-value of < 0·05 was taken as significant.
Results
Patients
We studied 40 untreated RRMS patients in different phases of the disease [20 in remission (remitting patients) and 20 in relapse (relapsing patients)] and 25 gender- and age-matched controls. There were no significant differences in clinical features such as age, gender and disease duration between the two groups of patients. The EDSS score was similar in remitting patients and relapsing patients before the last relapse, while the EDSS score was higher in relapsing patients during relapse than in remitting patients (P = 0·019) (Table 1). Five patients were studied both in the relapse and in the remission phases of the disease.
Table 1.
Demographic and clinical features of relapsing-remitting multiple sclerosis (RRMS) patients and healthy subjects included in the study
| Relapsing patients | Remitting patients | Controls | |
|---|---|---|---|
| Number | 20 | 20 | 25 |
| Age (years) | 28·5 ± 11·5 | 32·9 ± 8·7 | 27·8 ± 6·2 |
| Gender (male/female) | 8/12 | 8/12 | 11/14 |
| Disease duration (months) | 34·7 ± 28·4 | 42·7 ± 34·3 | NA |
| Annualized relapse rate | 1·22 ± 0·76 | 1·03 ± 0·65 | NA |
| EDSS score (pre-relapse) | 3·2 ± 1·6* (1·8 ± 1·4) | 1·6 ± 1·1* | NA |
| Gd-enhancing lesions (number) | 1·72 ± 1·28 | 0 | NA |
EDSS, Expanded Disability Status Scale; Gd, gadolinium; NA, not applicable.
All data are mean ± standard deviation (SD).
Group comparisons were performed using the Student’s t-test.
A P-value of < 0·05 was taken as significant.
Foxp3 expression in circulating CD4+ CD25+ T cells from MS patients in different phases of disease and in controls
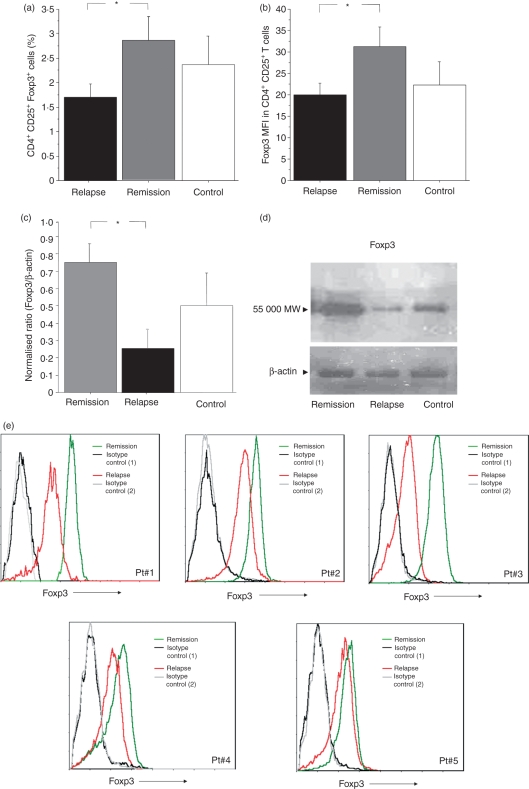
Foxp3 was mainly expressed in CD4+ CD25+ T cells in both relapsing and remitting patients and in controls. Few CD4+ CD25− T cells expressed Foxp3 at low levels in relapsing and remitting patients and in controls (0·8%, 0·6% and 0·8%, respectively). There was no difference in the frequency of CD4+ CD25+ Foxp3+ cells between all patients and controls (data not shown). A lower percentage of circulating CD4+ CD25+ Foxp3+ T cells was observed in relapsing patients than in remitting patients (1·69% versus 2·78% of CD25+ FoxP3+ T cells gated on CD4+ T cells, P = 0·047), while the number of CD4+ CD25+ Foxp3+ T cells was lower in relapsing patients than in healthy subjects, without reaching significance (1·69% versus 2·3%, P = 0·147) (Fig. 1a). No significant differences were observed in the percentage of CD4+ CD25+ Foxp3+ T cells between remitting patients and healthy subjects (2·78% versus 2·3%, P = 0·882) (Fig. 1a).
Figure 1.
Frequency of circulating CD4+ CD25+ Foxp3+ cells and mean expression of Foxp3 [mean fluorescence intensity (MFI)] in relapsing-remitting multiple sclerosis (RRMS) patients in remission and in relapse and in age- and gender-matched healthy controls. (a) The percentage of total regulatory T (T-reg) cells (CD25+ Foxp3+ cells gated on CD4+ T cells) from the peripheral blood of RRMS patients and controls was significantly higher in remitting than in relapsing RRMS patients. (b) The Foxp3 MFI was higher in CD4+ CD25+ T cells from remitting than from relapsing RRMS patients. (c) Foxp3 protein levels assessed by western blot analysis of CD4+ CD25+ T cells were significantly higher in remitting RRMS patients than in relapsing RRMS patients and controls. Data are reported as mean ± standard error of the mean (SEM). *P < 0·05. (d) Representative western blot of the same patient during remission and relapse (3 months after remission) and of one control. The same membranes were reprobed with anti-actin IgG immunoglobulin as a control for loading. MW, molecular weight. (e) Representative histograms showing Foxp3 expression in CD4+ CD25+ T cells from five RRMS patients during remission (green line) and relapse (red line). The black and grey lines indicate the fluorescence intensity of isotype controls in remission and relapse phases, respectively. The x-axis of each histogram represents specific fluorescence of Foxp3 on a four-decade logarithmic scale, and the y-axis represents the total number of events.
Foxp3 was expressed in a significantly higher percentage of CD4+ CD25+ T cells from remitting MS patients than from relapsing MS patients (23·01 ± 5·14% versus 13·42 ± 5·73% of FoxP3+ T cells gated on CD4+ CD25+ T cells, P = 0·0223), whereas no significant difference in the percentage of FoxP3-expressing cells was observed in CD4+ CD25+ T cells from controls compared with CD4+ CD25+ T cells from relapsing MS patients (18·31 ± 6·43% versus 13·42 ± 5·73%, P = 0·1811) and with CD4+ CD25+ T cells from remitting MS patients (18·31 ± 6·43% versus 23·01 ± 5·14%, P = 0·2528).
Foxp3 MFI was lower in circulating CD4+ CD25+ T cells from relapsing patients than in CD4+ CD25+ T cells from remitting patients (P = 0·039). There was no significant difference in the Foxp3 MFI of CD4+ CD25+ T cells between remitting or relapsing patients and controls (P = 0·768 and P = 0·236 respectively; Fig. 1b).
Representative flow cytometric analyses and immunocytochemistry of Foxp3 in CD4+ CD25+ T cells from the same RRMS patients during remission and relapse phases are shown in Fig. 2.
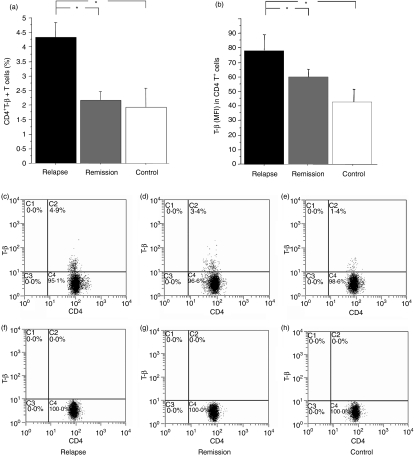
Figure 2.
Mean expression of T-bet in CD4+ T cells and percentage of CD4+ T-bet+ T cells in relapsing-remitting multiple sclerosis (RRMS) patients in different phases of disease and in healthy subjects. (a) The percentage of CD4+ T-bet+ T cells was significantly higher in relapsing RRMS patients than in remitting RRMS patients and controls (P = 0·009 and P = 0·008 respectively). (b) Mean T-bet expression, assessed by flow cytometric analysis as fluorescence intensity, was significantly higher in CD4+ cells from relapsing RRMS patients than in the CD4+ cells from remitting patients and controls (P = 0·034 and P = 0·018 respectively). Data are reported as mean ± standard error of the mean (SEM). *P < 0·05. (c–h) Representative two-parameter plots showing only cells gated on CD4+ lymphocyte populations (c–e) and respective phycoerythrin (PE) isotype controls for intracellular antibody (f–h). The y-axis of each plot represents specific fluorescence of T-bet–PE conjugate; the x-axis represents specific fluorescence of CD4–phycoerythrin-cyanine 5 (PC5) on four-decade logarithmic scales. These representative two-parameter plots are obtained from the same RRMS patient in relapse (c) and in remission (d) and from healthy controls. Quadrants were set by using appropriate isotype controls for each intracellular and extracellular antibody. Values in each plot are the percentage of CD4+ T-bet+ cells.
In order to confirm the increased expression of Foxp3 in bead-sorted CD4+ CD25+ cells from RRMS patients during remission compared with patients during relapse, we longitudinally studied five patients in both relapse and remission phases of the disease and in five controls by western blot analysis. Foxp3 protein levels in CD4+ CD25+ T cells were higher during remission than during relapse (P = 0·046; Fig. 1c). Patients in relapse showed lower Foxp3 levels than controls, but this was not significant (P = 0·198; Fig. 1c). There was no significant difference in Foxp3 expression between patients in remission and controls (P = 0·652; Fig. 1c). A representative western blot of the same patient during remission and relapse and of one control is described in Fig. 1d. Representative histograms showing Foxp3 expression in CD4+ CD25+ T cells from five RRMS patients during remission and relapse are shown in Fig. 1e.
T-bet expression in circulating CD4+ T cells from MS patients in different phases of disease and in controls
T-bet was mainly expressed in CD4+ CD25− T cells in both relapsing and remitting patients and in controls. The T-bet MFI was higher in CD4+ T cells from relapsing patients than in CD4+ T cells from remitting patients and controls (Fig. 2a; P = 0·034 and P = 0·018 respectively). The percentage of CD4+T-bet+ T cells was significantly higher in relapsing RRMS patients than in remitting patients and controls (Fig. 2b; P = 0·009 and P = 0·008 respectively). No significant differences were observed in the T-bet MFI in CD4+ T cells or in the percentage of CD4+ T-bet+ cells between patients in remission and healthy subjects (P = 0·589; Fig. 2). Representative two-parameter plots showing CD4+ T-bet+ cells are described in Fig. 2c).
CD4+ CD25+ T-cell capacity to suppress CD4+ CD25− T-cell proliferation
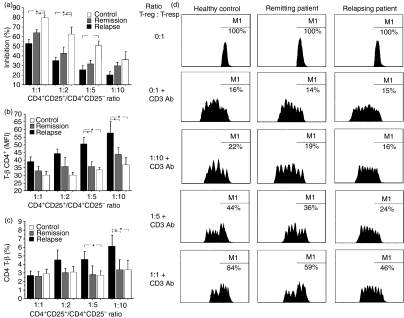
In the suppression assay, circulating CD4+ CD25+ T cells from healthy subjects in a suppressor/responder ratio of 1:1 induced an 80% reduction of CD4+ CD25− T-cell proliferation, whereas CD4+ CD25+ T cells from patients in remission showed lower suppressive capacity than controls, despite the similar Foxp3 MFI, because they induced, at the same ratio, a 63% reduction of CD4+ CD25− T-cell proliferation. CD4+ CD25+ T cells from patients in relapse, showing significantly lower Foxp3 MFI in comparison to patients in remission, clearly had less suppressive capacity than those from remitting patients and controls and, at a suppressor/responder ratio of 1:1, induced a 53% reduction of CD4+ CD25− T-cell proliferation. A higher suppressive capacity was observed in CD4+ CD25+ T cells from healthy subjects compared with CD4+ CD25+ T cells from relapsing RRMS patients until a suppressor/responder ratio of 1:5 was reached (P = 0·008 at a ratio of 1:1, P = 0·011 at a ratio of 1:2 and P = 0·038 at a ratio of 1:5) and with CD4+ CD25+ T cells from remitting RRMS patients up to a ratio of 1:2 (P = 0·026 at a ratio of 1:1 and P = 0·031 at a ratio of 1:2; Fig. 3a). CFSE histograms from one representative patient in relapse and in remission phases and from one control are shown in Fig. 3d).
Figure 3.
In vitro study aimed at evaluating the suppressive activity of bead-separated CD4+ CD25+ T cells isolated from relapsing-remitting multiple sclerosis (RRMS) patients and controls, and the effects on T-bet expression and on the percentage of CD4+ T-bet+ cells. (a) A higher suppressive capacity was observed in CD4+ CD25+ T cells from healthy subjects than from relapsing RRMS patients up to a suppressor/responder ratio of 1:5 (P = 0·008 at a suppressor/responder ratio of 1:1, P = 0·011 at a suppressor/responder ratio of 1:2 and P = 0·038 at a suppressor/responder ratio of 1:5) and from remitting RRMS patients up to a suppressor/responder ratio of 1:2 (P = 0·026 at a suppressor/responder ratio of 1:1 and P = 0·031 at a suppressor/responder ratio of 1:2). Suppression of proliferation of CD4+ CD25− T cells is shown at CD4+ CD25+/CD4+ CD25− ratios 1:1, 1:2, 1:5 and 1:10; the mean inhibition for the different study groups is also presented. Data from all controls, and from relapsing and remitting RRMS patients, are shown as the mean of triplicate wells. (b) Effects of regulatory T (T-reg) cells on decreasing the expression of T-bet in responder T cells. A higher expression of T-bet was found in responder cells from relapsing RRMS patients (at suppressor/responder ratios of 1:5 and 1:10) than from remitting RRMS patients and from controls (P = 0·028 and P = 0·015 at a suppressor/responder ratio of 1:5, and P = 0·031 and P = 0·011 at a suppressor/responder ratio of 1:10, respectively). (c) Effects of T-reg cells on reducing the percentage of CD4+ T-bet+ T cells. A higher percentage of T-bet+ cells was observed in responder cells from relapsing RRMS patients than from remitting RRMS patients (P = 0·018 at a suppressor/responder ratio of 1:10) and from controls (P = 0·021 at a suppressor/responder ratio of 1:5 and P = 0·019 at a suppressor/responder ratio of 1:10). Data are reported as mean ± standard error of the mean (SEM). *P<0·05. (d) Carboxy fluorescein diacetate succinimidyl ester (CFSE) histograms from one representative patient in relapse and in remission phase and from one control. The CFSE signal was measured as mean fluorescence intensity. Cells under the gated region M1 (%) represent no-divided CFSE-labeled responder cells. Ab, antibody; T-resp, responder T cell.
CD4+ CD25+ T-cell capacity to reduce T-bet expression in CD4+ T cells and the percentage of CD4+T-bet+ cells
Circulating CD4+ CD25+ T cells from healthy subjects and from patients in remission clearly reduced T-bet MFI in CD4+ CD25− T cells to a suppressor/responder ratio of 1:10, whereas CD4+ CD25+ T cells from patients in relapse were able to reduce T-bet expression only at a high ratio but failed to decrease T-bet MFI in autologous CD4+ CD25+ T cells at a low ratio (Fig. 3b). In particular, a higher expression of T-bet was found in responder cells from relapsing RRMS patients at suppressor/responder ratios of 1:5 and 1:10 than in those from remitting RRMS patients and controls (P = 0·028 and P = 0·015 at a suppressor/responder ratio of 1:5, P = 0·031 and P = 0·011 at a suppressor/responder ratio of 1:10, respectively; Fig. 3b).
A higher percentage of CD4+ T-bet+ cells was observed in responder cells from relapsing RRMS patients than in those from remitting RRMS patients at a suppressor/responder ratio of 1:10 (P = 0·018), whereas controls showed a lower percentage of CD4+ T-bet+ cells compared with relapsing patients, both at 1:5 (P = 0·021) and at 1:10 (P = 0·019; Fig. 3c) suppressor/responder ratios. To verify if T-bet reduction in responder T cells in the suppressive assay was associated with a decrease of Th1 functional activity, we measured IFN-γ levels in the supernatants of T-reg cell/T-responder cell co-cultures from healthy subjects. We observed a progressive reduction of IFN-γ levels from a suppressor/responder ratio of 1:10 up to 1:1, with a parallel decrease of T-bet expression in CD4+ T cells (data not shown).
Discussion
T-reg cells seems to play a major role in limiting the development of autoimmune diseases, but where and when they exert their suppressive function on effector cells is still controversial.24,25 Some studies of EAE suggest that T-reg cells mainly act in the lymph nodes by limiting the priming of naïve T cells25,26 while other studies have shown that T-reg cells can proliferate and inhibit effector cells mainly at sites of inflammation.19,27–29 Inflammatory cytokines secreted by effector cells seem to limit the suppressive function of T-reg cells in inflammatory lesions,26,29–31 whereas some studies suggest that ‘effector/memory-like’ T-reg cells have enhanced suppressive capacity and ability to migrate to inflammatory sites.32
Several studies have reported numeric or functional deficiencies of T-reg cells in various autoimmune diseases, including MS.13,22,33–36
The mechanisms responsible for the generation of T-reg cell defects in MS are still unclear. A reduced thymic-dependent de novo generation of T-reg cells that are both less sensitive to apoptosis and more effective in suppression seems to be critical for the T-reg cell dysfunction that occurs in MS patients.36
In agreement with other investigators,21,22,35 in this study we found no difference in the frequency of circulating CD4+ CD25+ Foxp3+ T cells between RRMS patients and controls.
To date there are no data about the frequency of circulating CD4+ CD25+ Foxp3+ cells and the levels of expression of Foxp3 in untreated RRMS patients in different phases of disease (relapse and remission). We observed that the percentage of CD4+ CD25+ Foxp3+ cells was lower in relapsing patients than in remitting patients. In addition, Foxp3 MFI and protein expression was significantly lower in CD4+ CD25+ T cells from relapsing patients than in CD4+ CD25+ T cells from remitting patients. Likewise, in EAE the expression level of Foxp3 on a per cell basis was lower in CNS-derived T-reg cells at the peak of disease as compared with recovery.26 We did not find significant differences in CD4+ CD25+ Foxp3+ T-cell frequency and Foxp3 MFI in CD4+ CD25+ cells between remitting or relapsing patients and healthy subjects. Venken et al. showed decreased Foxp3 expression at the single-cell level in circulating in CD4+ CD25high cells from untreated RRMS patients in the stable phase of the disease compared with controls. Some other authors suggest that not only CD4+ CD25high T cells, but also a portion of Foxp3+ cells present within the CD4+ CD25low T-cell subset showing suppressive activity on effector T cells in a transforming growth factor-β (TGF-β)-dependent manner, should be considered regulatory T cells.37 However, in humans there are a considerable number of activated, non-regulatory CD4+ T cells that express CD25 38 and it must be noted that the CD4+ CD25+ T cells used in our suppressive assay might also include non-regulatory activated T cells that might be more frequent in RRMS patients than in controls. Recent data have suggested that not only is the presence of Foxp3 important for the function of T-reg cells, but the level of its expression is also crucial.39 Foxp3 is transiently up-regulated in human CD4+ CD25− T cells upon stimulation, but at lower levels than in T-reg cells,40,41 and a reduced expression of Foxp3 seems to correlate with decreased T-reg-cell suppressive function.42,43 In our population of untreated RRMS patients the loss of functional suppression by CD4+ CD25+ T cells was not strictly correlated with the Foxp3 protein levels expressed by CD4+ CD25+ T cells. RRMS patients and controls showed a similar single-cell level of Foxp3 in CD4+ CD25+ T cells, but CD4+ CD25+ T cells from RRMS patients showed less suppressive capacity than those from controls. These data are in agreement with a previous study34 showing that the impaired suppressive function of CD4+ CD25+ T cells from RRMS patients may be caused by a defect in the regulatory subset. In addition, we found similar levels of Foxp3 in circulating CD4+ CD25+ cells from RRMS patients and from controls, and consistently with other studies40,44 our data suggest that factors other than Foxp3 may be involved in the suppressive function of CD4+ CD25+ Foxp3+ T cells. A recent report showed that IL-35, a novel inhibitory cytokine specifically produced by T-reg cells and a downstream target of Foxp3, is required for maximal T-reg cell suppressive activity.45
We found an inverse association between Foxp3 MFI in CD4+ CD25+ T cells and T-bet MFI in CD4+ T cells from RRMS patients in different phases of the disease. Relapsing patients showed lower Foxp3 expression in CD4+ CD25+ T cells and higher T-bet expression in CD4+ T cells than remitting patients. In contrast, the increase of Foxp3 expression in CD4+ CD25+ T cells in remitting patients was associated with a reduced T-bet expression in CD4+ T cells. In a suppressive assay, T-bet expression of autologous CD4+ CD25− cells progressively decreased in the presence of an increasing number of CD4+ CD25+ T cells from remitting patients; in contrast, CD4+ CD25+ T cells from relapsing patients failed to reduce the high level of T-bet expression in autologous CD4+ CD25− cells at a low suppressor/responder ratio. Our data suggest that, in RRMS patients, the inhibitory function of circulating CD4+ CD25+ Foxp3+ cells is mainly impaired during relapse, in the presence of a higher inflammatory burden. Several mechanisms of suppression, which are not mutually exclusive, have been proposed: inhibition by cell–cell contact between T-reg cells and target T cells or APC, consumption of limiting growth factors such as IL-2 and consequent apoptosis of target T cells,46 and secretion of inhibitory cytokines.45 We hypothesize that in RRMS patients during relapse, in contrast to what has been shown in EAE studies,47 not only CNS-derived CD4+ CD25− T cells but also circulating CD4+ CD25− T cells expressing high levels of T-bet, may produce soluble factors and/or excessive amounts of inflammatory cytokines that impair T-reg-cell-mediated suppression. Conversely, during remission, circulating CD4+ CD25− cells expressing low levels of T-bet still seem to be susceptible to T-reg cell regulation.
In conclusion, our data confirm a defective function of T-reg cells in MS even if peripheral CD4+ CD25+ Foxp3+ regulatory T cells play an important role in the natural extinction of autoimmune inflammation in MS. The increased number of circulating CD4+ CD25+ cells expressing higher levels of Foxp3 seems to contribute to the maintenance of tolerance during the remission phase of RRMS, while, in the relapse phase of the disease, the small number of CD4+ CD25+ cells expressing low levels of Foxp3 are unable to inhibit the activated effector T cells that express a high level of T-bet. Further studies are necessary to clarify in greater detail the role of T-reg cell dysfunction on the complex mechanisms determining ongoing inflammation in MS.
Acknowledgments
This work was supported by a grant from FISM 2003.
References
- 1.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121–7. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 2.Wekerle H, Linington C, Lassmann H, Meyermann R. Cellular immune reactivity within the CNS. Trends Neurosci. 1986;9:271–7. [Google Scholar]
- 3.Hickey WF, Hsu BL, Kimura H. T lymphocyte entry into the central nervous system. J Neurosci Res. 1991;28:254–60. doi: 10.1002/jnr.490280213. [DOI] [PubMed] [Google Scholar]
- 4.Kawakami N, Lassmann S, Li Z, Odoardi F, Bonhoeffer T, Wekerle H, Flugel A. The activation status of neuroantigen-specific T cells in the target organ determines the clinical outcome of autoimmune encephalomyelitis. J Exp Med. 2004;199:185–97. doi: 10.1084/jem.20031064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bettelli E, Sullivan B, Szabo SJ, Sobel RA, Glimcher LH, Kuchroo WK. Loss of T-bet but not STAT-1 prevents the development of EAE. J Exp Med. 2004;200:79–87. doi: 10.1084/jem.20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–69. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 7.Afkarian M, Sedy JR, Yang J, Jacobson NG, Cereb N, Yang SY, Murphy TL, Murphy KM. T-bet is a STAT1-induced regulator of IL-12R receptor in naïve CD4+ cells. Nat Immunol. 2002;3:549–57. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- 8.Lighvani AA, Frucht DM, Jankovic D, et al. T-bet is rapidly induced by interferon-gamma in lymphoid and myeloid cells. Proc Natl Acad Sci U S A. 2001;98:15137–42. doi: 10.1073/pnas.261570598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mullen AC, High FA, Hutchins AS, et al. Role of T-bet in commitment of TH1 cells before IL12-dependent selection. Science. 2001;292:1907–10. doi: 10.1126/science.1059835. [DOI] [PubMed] [Google Scholar]
- 10.Lovett-Racke AE, Rocchini AE, Choy J, Northrop SC, Hussain RZ, Ratts RB, Sikder D, Racke MK. Silencing T-bet defines a critical role in the differentiation of autoreactive T lymphocytes. Immunity. 2004;21:719–31. doi: 10.1016/j.immuni.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Bettelli E, Oukka M, Kuchroo VK. TH17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–50. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 12.Gocke AR, Cravens PD, Ben LH, Hussain RZ, Northrop SC, Racke MK, Lovett-Racke AE. T-bet regulates the fate of Th1 and Th17 lymphocytes in autoimmunity. J Immunol. 2007;178:1341–8. doi: 10.4049/jimmunol.178.3.1341. [DOI] [PubMed] [Google Scholar]
- 13.Sakaguchi S, Ono M, Setoguchi R, et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 14.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 15.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 16.Von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005;6:338–44. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- 17.Brunkow ME, Jeffery EW, Hjermid KA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 18.Bennett CL, Christie J, Ramsdell F, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–1. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 19.McGeachy MJ, Stephens LA, Anderton SM. Natural recovery and protection from autoimmune encephalomyelitis: contribution of CD4+CD25+ regulatory cells within the central nervous system. J Immunol. 2005;175:3025–32. doi: 10.4049/jimmunol.175.5.3025. [DOI] [PubMed] [Google Scholar]
- 20.Huan J, Culbertson N, Spencer L, et al. Decreased FOXP3 levels in multiple sclerosis patients. J Neurosci Res. 2005;8:45–52. doi: 10.1002/jnr.20522. [DOI] [PubMed] [Google Scholar]
- 21.Venken K, Hellings N, Thewissen M, et al. Compromised CD4(+) CD25(high) regulatory T-cell function in patients with relapsing-remitting multiple sclerosis is correlated with a reduced frequency of FOXP3-positive cells and reduced FOXP3 expression at the single-cell level. Immunology. 2007;123:79–89. doi: 10.1111/j.1365-2567.2007.02690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venken K, Hellings N, Hensen K, et al. Secondary progressive in contrast to relapsing-remitting multiple sclerosis patients show a normal CD4+CD25+ regulatory T-cell function and FOXP3 expression. J Neurosci Res. 2006;83:1432–46. doi: 10.1002/jnr.20852. [DOI] [PubMed] [Google Scholar]
- 23.Frisullo G, Angelucci F, Caggiula M, et al. pSTAT1, pSTAT3, and T-bet expression in peripheral blood mononuclear cells from relapsing-remitting multiple sclerosis patients correlates with disease activity. J Neurosci Res. 2006;84:1027–36. doi: 10.1002/jnr.20995. [DOI] [PubMed] [Google Scholar]
- 24.Rudensky AY, Campbell DJ. In vivo sites and cellular mechanisms of T reg cell-mediated suppression. J Exp Med. 2006;203:489–92. doi: 10.1084/jem.20060214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korn T, Oukka M. Dynamics of antigen-specific regulatory T-cells in the context of autoimmunity. Semin Immunol. 2007;19:272–8. doi: 10.1016/j.smim.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Kohm AP, Carpentier PA, Anger HA, Miller SD. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J Immunol. 2002;169:4712–6. doi: 10.4049/jimmunol.169.9.4712. [DOI] [PubMed] [Google Scholar]
- 27.Chen Z, Herman AE, Matos M, Mathis D, Benoist C. Where CD4+CD25+ T reg cells impinge on autoimmune diabetes. J Exp Med. 2005;202:1387–97. doi: 10.1084/jem.20051409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu W, Putnam AL, Xu-Yu Z, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–11. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Connor RA, Malpass KH, Anderton SM. The inflamed central nervous system drives the activation and rapid proliferation of Foxp3+ regulatory T cells. J Immunol. 2007;179:958–66. doi: 10.4049/jimmunol.179.2.958. [DOI] [PubMed] [Google Scholar]
- 30.Sutmuller RP, den Brok MH, Kramer M, et al. Toll-like receptor 2 controls expansion and function of regulatory T cells. J Clin Invest. 2006;116:485–94. doi: 10.1172/JCI25439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.King IL, Segal BM. Cutting edge: IL-12 induces CD4+CD25- T cell activation in the presence of T regulatory cells. J Immunol. 2005;175:641–5. doi: 10.4049/jimmunol.175.2.641. [DOI] [PubMed] [Google Scholar]
- 32.Huehn J, Siegmund K, Lehmann JC, et al. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J Exp Med. 2004;199:303–13. doi: 10.1084/jem.20031562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baecher-Allan C, Hafler DA. Human regulatory T cells and their role in autoimmune disease. Immunol Rev. 2006;212:203–16. doi: 10.1111/j.0105-2896.2006.00417.x. [DOI] [PubMed] [Google Scholar]
- 34.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patiens with multiple sclerosis. J Exp Med. 2004;199:971–9. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haas J, Hug A, Viehöver A, et al. Reduced suppressive effect of CD4+CD25high regulatory T cells on the T cell immune response against myelin oligodendrocyte glycoprotein in patients with multiple sclerosis. Eur J Immunol. 2005;35:3343–52. doi: 10.1002/eji.200526065. [DOI] [PubMed] [Google Scholar]
- 36.Haas J, Fritzsching B, Trübswetter P, et al. Prevalence of newly generated naive regulatory T cells (Treg) is critical for Treg suppressive function and determines Treg dysfunction in multiple sclerosis. J Immunol. 2007;179:1322–30. doi: 10.4049/jimmunol.179.2.1322. [DOI] [PubMed] [Google Scholar]
- 37.You S, Leforban B, Garcia C, Bach JF, Bluestone JA, Chatenoud L. Adaptive TGF-beta-dependent regulatory T cells control autoimmune diabetes and are a privileged target of anti-CD3 antibody treatment. Proc Natl Acad Sci USA. 2007;104:6335–40. doi: 10.1073/pnas.0701171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baecher-Allen C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–53. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 39.Campbell DJ, Ziegler SF. FOXP3 modifies the phenotypic and functional properties of regulatory T cells. Nat Rev Immunol. 2007;7:305–10. doi: 10.1038/nri2061. [DOI] [PubMed] [Google Scholar]
- 40.Allan SE, Crome SQ, Crellin NK, Passerini L, Steiner TS, Bacchetta R, Roncarolo MG, Levings MK. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol. 2007;19:345–54. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- 41.Passerini L, Allan SE, Battaglia M, Di Nunzio S, Alstad AN, Levings MK, Roncarolo MG, Bacchetta R. STAT5-signaling cytokines regulate the expression of Foxp3 in CD4+CD25+ regulatory T cells and CD4+CD25− effector T cells. Int Immunol. 2008;20:421–31. doi: 10.1093/intimm/dxn002. [DOI] [PubMed] [Google Scholar]
- 42.Banerjee DK, Dhodapkar MV, Matayeva E, Steinman RM, Dhodapkar KM. Expansion of FOXP3high regulatory T cells by human dendritic cells (DCs) in vitro and after injection of cytokine-matured DCs in myeloma patients. Blood. 2006;108:2655–61. doi: 10.1182/blood-2006-03-011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hill JA, Feuerer M, Tash K, et al. Foxp3 transcription-factor-dependent and -independent regulation of the regulatory T cell transcriptional signature. Immunity. 2007;27:786–800. doi: 10.1016/j.immuni.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 44.Ohata J, Miura T, Johnson TA, Hori S, Ziegler SF, Kohsaka H. Enhanced efficacy of regulatory T cell transfer against increasing resistance, by elevated Foxp3 expression induced in arthritic murine hosts. Arthritis Rheum. 2007;56:2947–56. doi: 10.1002/art.22846. [DOI] [PubMed] [Google Scholar]
- 45.Collison LW, Workman CJ, Kuo TT. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–9. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 46.Scheffold A, Murphy KM, Höfer T. Competition for cytokines: T(reg) cells take all. Nat Immunol. 2007;8:1285–7. doi: 10.1038/ni1207-1285. [DOI] [PubMed] [Google Scholar]
- 47.Korn T, Reddy J, Gao W, et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13:423–31. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]