INTRODUCTION
The Joint United Nations Program on HIV/AIDS (UNAIDS) estimates that 1.5 million HIV-infected women in low- and middle-income countries gave birth in 2006.1 With expansion of prevention of mother-to-child transmission (PMTCT) interventions, the majority of their infants will be HIV-uninfected. Reliable diagnostic testing must be available to confirm or deny infection in these exposed infants. Diagnosis of HIV in infants is complicated by transplacental transfer of maternal antibody, which is greatest in the third trimester,2 making it difficult to distinguish between maternal and infant infection using antibody-based testing. In settings where early HIV diagnosis by DNA or RNA polymerase chain reaction (PCR) tests is not available, HIV enzyme-linked immunoassay (EIA) testing has been recommended by the World Health Organization (WHO) from as early as nine months of age to rule-out infection in non-breastfeeding infants.3, 4 This recommendation is based on earlier data showing that ~95% of uninfected children serorevert by 12 months of age.4 It is unclear whether this is the case in Asian cohorts with different viral subtypes. In order to assess whether EIA testing algorithms apply to Vietnamese infants, we conducted a prospective study of EIA testing starting at 12 months of age in a cohort of HIV-exposed, uninfected infants.
METHODS
Study patients and data collection
HIV-exposed, uninfected infants enrolled in a parent study of diagnostic and monitoring testing in Ho Chi Minh City between February 2005 to August 2006 were eligible for the EIA study. Eligible infants for the parent study were ≤2 months of age, born to HIV-infected women at ≥36 weeks gestation, and lacked evidence of severe HIV disease at study entry. Pregnancy and delivery-related information were based on obstetric records and maternal recall at initial enrollment; antenatal maternal antiretroviral days and postnatal infant antiretroviral days after delivery were based on obstetric hospital records. Antiretroviral exposure was further categorized based on inclusion of protease inhibitors in the antenatal regimen. Infant age, height, and weight were collected at the time of follow-up blood testing. Height-for-age and weight-for-age Z-scores were calculated using WHO 2006 Child Growth Standards.5
PMTCT regimens
Over the course of the study, the available and recommended PMTCT regimens in Vietnam varied. Women identified as HIV-infected earlier in pregnancy initially were offered zidovudine alone or zidovudine with lamivudine, with or without a single-dose of nevirapine at labor, from approximately 34 weeks gestation. From the Fall of 2005 to the end of the enrollment period, women with CD4 counts greater than 250 cells/mm3 were offered zidovudine, lamivudine, and nelfinavir from 36 weeks gestation. Women with lower CD4 counts received zidovudine, lamivudine, and nevirapine. Throughout the enrollment period, women identified at labor were given single-dose nevirapine. The infant regimen was single-dose nevirapine after birth until December 2005, after which they were given seven days of zidovudine and lamivudine. Around March 2006 the regimen was switched to zidovudine for seven to 28 days with single-dose nevirapine, depending on maternal antiretroviral history.
Confirmation of HIV infection status
Diagnostic testing was initiated at two months of age. Samples were obtained at Children’s Hospital 1, Ho Chi Minh City, and submitted to the Pasteur Institute within six hours for processing and testing using HIV DNA PCR on whole blood (in-house assay, Pasteur Institute, Ho Chi Minh City). Positive tests were confirmed with a second DNA PCR on a new sample taken two to four weeks later. Infants with indeterminate or negative PCR tests at two months were retested at six months of age. Those with ≥2 negative DNA PCR results were considered HIV-uninfected. Uninfected infants who continued follow-up through 12 months of age were eligible for participation in the EIA study.
HIV EIA testing
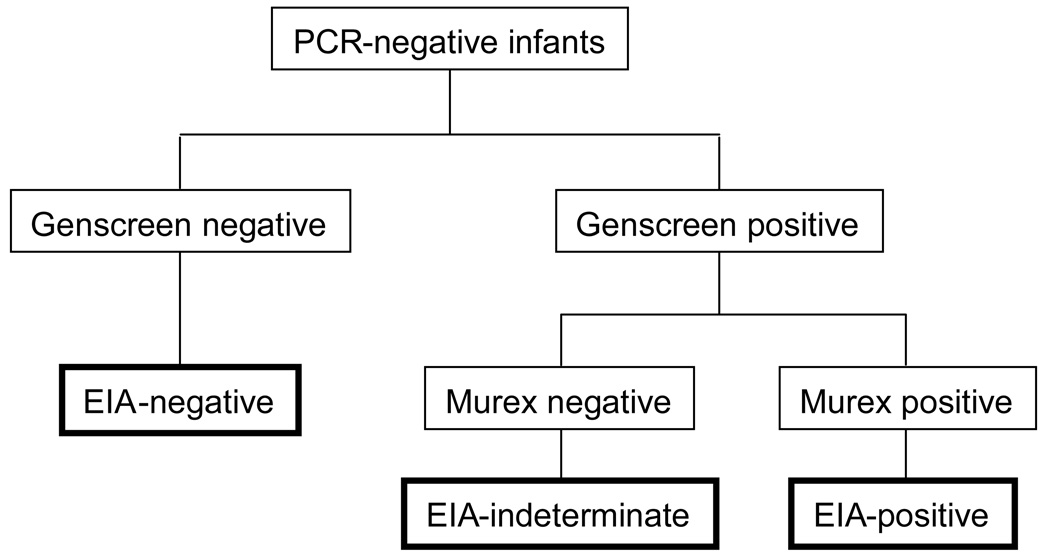
Antibody testing was conducted at 12 months of age using a two-step EIA protocol with two different third-generation assays: Genscreen HIV 1/2 version 2 (Bio-Rad, France) and Murex 1.2.0 (Murex Biotech, UK). Samples were collected at Children’s Hospital 1 and delivered within 24 hours to the Pasteur Institute, where specimen processing and testing were conducted. Optical density readings that exceeded the intra-assay cut-off value were designated as positive; readings at or below the cut-off were designated as negative. Infants with negative Genscreen tests were considered to have seroreverted and were designated as EIA-negative (Figure 1). Samples with positive Genscreen tests were retested with the Murex assay. Those with a positive Genscreen but a negative Murex test were considered EIA-indeterminate. Those with both positive Genscreen and Murex tests were considered EIA-positive. Infants who had not seroreverted at 12 months (i.e., EIA-indeterminate or EIA-positive) were retested at 18 months, and again at 24 months if necessary.
Figure 1.
EIA testing algorithm for HIV-uninfected infants
Analysis
Descriptive data were examined on the basis of the 12-month EIA result. For univariate analyses, infants with negative EIA tests were compared to the combined group of those with indeterminate and positive results (i.e., non-negative). Chi-square and Student t-test were used for categorical and continuous variables, respectively. The confidence interval (CI) for the specificity was calculated using Greenwood's formula for the log survival function.
A logistic regression model was used for multivariate analysis that included variables with p<0.2 on univariate analysis. The model was checked using graphic plots for influential points and the Hosmer-Lemeshow goodness of fit test. The univariate results and regression model were assessed at a level of significance of p<0.05. All analyses were performed in Stata Version 9.2 (College Station, Texas).
Human subjects protections
The study protocol was approved by the Ho Chi Minh City AIDS Committee Institutional Review Board and the Committee on Human Research at the University of California, San Francisco. Informed consent was obtained from the parent or legally-recognized guardian.
RESULTS
Of 303 HIV-uninfected infants enrolled in the parent study, 273 (90%) remained in follow-up through 12 months of age and were included in the EIA study. Of these 273 infants, 230 (86%) had maternal and infant perinatal antiretroviral exposure, with 11 (4.0%) infants ever breastfed for a median of three [interquartile range (IQR) 1, 7] days (Table 1).
Table 1.
Patient characteristics by 12-month EIA result*
| Variables | Total N=273 |
EIA negative N=59 (21.6) |
EIA indeterminate N=131 (48.0) |
EIA positive N=83 (30.4) |
p-value** |
|---|---|---|---|---|---|
| Male | 144 (52.8) | 29 (49.2) | 72 (55.0) | 43 (51.8) | 0.53 |
| Gestational age, median weeks | 40 (39, 40) | 40 (39, 40) | 40 (39, 40) | 40 (39, 40) | 0.45 |
| Birthweight, median kg | 2.90 (2.60, 3.18) | 2.90 (2.60, 3.20) | 2.80 (2.60, 3.10) | 2.90 (2.70, 3.20) | 0.82 |
| Maternal age, median years | 24 (21, 26) | 24 (21, 26) | 24 (21, 27) | 24 (22, 26) | 0.30 |
| Maternal antenatal ARVs, median days | 5 (0, 273) | 1 (0, 56) | 7 (0, 90) | 14 (0, 273) | 0.15 |
| Maternal ARV regimen | |||||
| sdNVP | 85 (31.1) | 20 (33.9) | 41 (31.3) | 24 (28.9) | -- |
| AZT+3TC+NFV | 72 (26.4) | 11 (18.6) | 36 (27.5) | 25 (30.1) | |
| AZT+3TC | 55 (20.2) | 12 (20.3) | 25 (19.2) | 18 (21.7) | |
| Other | 18 (6.6) | 5 (8.5) | 7 (5.3) | 6 (7.2) | |
| None | 37 (13.6) | 9 (15.3) | 21 (16.0) | 7 (8.4) | |
| Unknown | 6 (2.2) | 2 (3.4) | 1 (0.8) | 3 (3.6) | |
| Maternal protease inhibitor-containing regimen*** | 75 (32.6) | 11 (22.9) | 37 (33.9) | 27 (37.0) | 0.11 |
| Infant postnatal antiretrovirals, median days | 1 (0, 28) | 1 (0, 28) | 1(0, 28) | 4 (0, 28) | 0.77 |
| Infant ARV regimen | |||||
| sdNVP | 136 (49.8) | 30 (50.9) | 67 (51.6) | 39 (47.0) | -- |
| AZT+sdNVP | 71 (26.0) | 14 (23.7) | 31 (23.7) | 26 (31.3) | |
| AZT+3TC | 39 (14.3) | 9 (15.3) | 18 (13.7) | 12 (14.5) | |
| Other | 9 (3.3) | 3 (5.1) | 4 (3.1) | 2 (2.4) | |
| None | 12 (4.4) | 1 (1.7) | 10 (7.6) | 1 (1.2) | |
| Unknown | 6 (2.2) | 2 (3.4) | 1 (0.8) | 3 (3.6) | |
| Weight Z score at 12 months, mean +/− SD | −0.14 +/− 1.01 | −0.20 +/− 0.95 | −0.10 +/− 1.09 | −0.14 +/− 0.93 | 0.57 |
| Height Z score at 12 months, mean +/− SD | −0.30 +/− 1.24 | −0.10 +/− 1.20 | −0.41 +/− 1.26 | −0.29 +/− 1.24 | 0.15 |
| Age at EIA seroconversion, median months | 18.30 (12.87, 18.40) | 12.20 (12.17, 12.23) | 18.33 (18.27, 18.40) | 18.40 (18.30, 18.97) | -- |
Results presented as N (%) or median (interquartile range), unless otherwise noted. Abbreviations: ARV=antiretroviral, EIA=HIV enzyme-linked immunoassay, SD=standard deviation.
Univariate analyses conducted between EIA-negative vs. EIA-indeterminate and EIA-positive patients
Percentages based on the denominator of mothers who received ARVs.
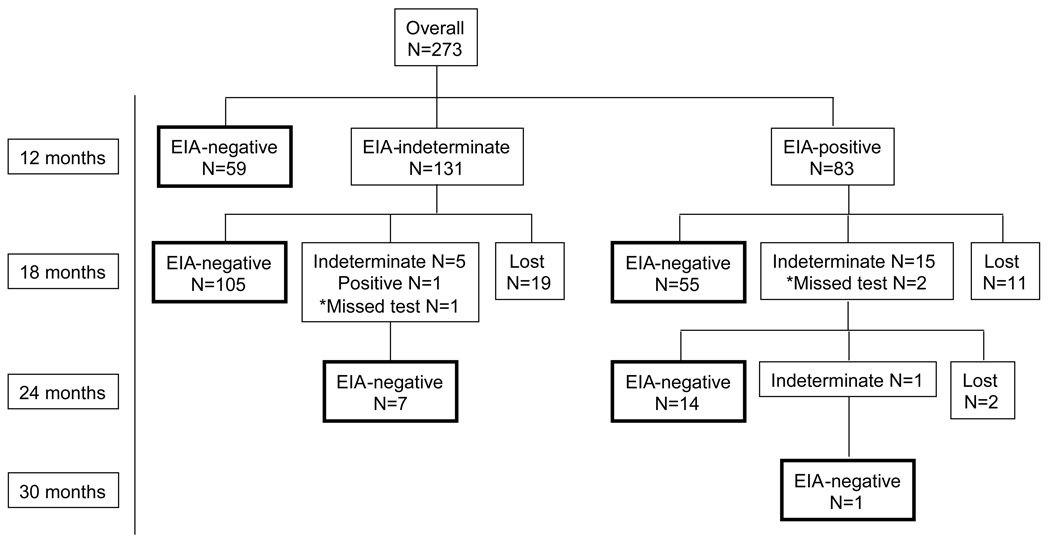
Initial EIA testing was conducted at a median of 12.20 (IQR 12.17, 12.23) months of age. Fifty-nine infants (22%) were negative by EIA, 131 (48%) were indeterminate, and 83 (30%) were EIA-positive; specificity 21.6 (95% CI 16.6, 26.3). Seroreversion was subsequently documented in 112 (85%) indeterminate and 70 (84%) positive infants; 32 were lost to follow-up (Figure 2). Infants with positive EIA tests at 12 months were 74% more likely than EIA-indeterminate infants to test indeterminate or positive at 18 months [risk ratio (RR) 1.74, 95% CI 1.15, 2.64; p=0.03].
Figure 2.
EIA testing results by age at test
*Patients who were >20 months at their second EIA
By our criteria, none of the covariates was associated with testing negative at 12 months of age on univariate analysis (Table 1). Although 30% of all mothers of non-negative infants were on protease inhibitor-containing PMTCT regimens for a median of 22.5 days (IQR 19, 28) compared with 19% of all mothers of seroreverted infants who received a median of 18 days of antiretrovirals (IQR 5, 28), this difference was not significant (RR 0.61, CI 0.33, 1.14; p=0.11).
The logistic regression model included three covariates: maternal antiretroviral history, maternal protease inhibitor exposure, and infant height Z-score. Although not significant by t-test, the model showed that height-for-age Z-score was associated with increased odds of seroreversion at 12 months of age [adjusted odds ratio (AOR) 1.33 per unit increase, 95% CI 1.01, 1.76; p=0.045]. Goodness of fit testing did not reveal grossly poor fit (p=0.44). In an exploratory analysis we found weak evidence (p=0.08) for interaction between days of maternal antiretroviral use and protease inhibitor exposure. Among infants of mothers using protease inhibitors, days of antiretroviral use was associated with lower odds of seroreversion at 12 months (AOR 0.93 per day of use, 95% CI 0.87–0.99; p=0.03), but not among infants of mothers not using protease inhibitors (AOR 0.99 per day of use, 95% CI 0.97–1.01; p=0.29).
DISCUSSION
Previously reported seroreversion rates as high as 94% at 12 months of age led to recommendations to use EIA testing in older infants as a way to rule-out infection in asymptomatic, non-breastfeeding infants.4, 6 Although a recent study in Malawi reported that seroreversion using standard EIA tests at 15 months of age had fallen from 99% in 1993-6 to 95% in 2000-3, the absolute percentages strongly support the use of EIA in their setting.7 However, the specificity of third-generation standard EIAs was only 22% in our cohort of HIV-uninfected Vietnamese infants at 12 months of age.
The reasons for our false non-negative rate of almost 80% are unclear. Current EIAs can reliably identify infection with a variety of HIV-1 subtypes, but a few studies have found subtype-related variations in assay performance.8, 9 Thai and US investigators using a less sensitive EIA (Vironostika-LS EIA, bioMérieux, France) to estimate the window periods of adults with recent infection, found significant differences between patients with subtype B and circulating recombinant form 1 (CRF01_AE).9 Comparing our results from Ho Chi Minh City, where the dominant subtype is CRF01_AE,10, 11 with the much higher seroreversion rates observed in previous studies from South Africa and Malawi, where subtype C predominate,4, 7 raises questions about when the EIA would be a reliable tool to rule-out infection in HIV-exposed but uninfected Vietnamese infants.
With regards to what factors might influence seroreversion on an individual level, our analysis found an association with increased height at 12 months. Height is preserved relative to weight during periods of malnutrition, and takes longer to recover.12, 13 Higher height-for-age Z scores may indicate stable growth and development over time, but the connection to HIV antibody titers is unclear. There was some question of whether maternal protease inhibitor use had an impact on delaying seroreversion, potentially through greater reductions in peripartum viral load. Moreover, mothers who received protease inhibitors were more likely to have higher CD4 counts and less advanced clinical disease. However, US and South African studies have not shown an association between maternal antibody titers and false-positive infant antibody testing.14,15 The kinetics of HIV antibody in an uninfected child are likely to be related to a combination of maternal peripartum and early infant health factors.
Recent studies have demonstrated that rapid HIV tests of serum and oral fluid have lower relative sensitivity in infants than adults, which is an advantage when used to rule-out disease.15, 16 In Uganda, the Determine HIV 1/2 rapid test (Abbott, The Netherlands) had a negative predictive value of 100% among 3–18 month olds, and a positive predictive value of 83% among 9–12 month olds.17 A study of 9-month old infants in Kenya observed specificities of 90% with the Determine and 96% with the Bioline (Standard Diagnostics, Republic of Korea) rapid test kits.18 In South Africa, the OraQuick Rapid HIV 1/2 Antibody test (Orasure Technologies, Inc., United States) had a positive predictive value of 68% using an oral fluid sample compared with 15% for a standard serum EIA test in a cohort of 11–18 month old infants.15 However, there are no published data on the use of these assays in Asian infants.
Our study was limited by not including rapid tests in our algorithm. In addition, our study population was composed of full-term infants who had stable access to nutritional supplements. Because prematurity or malnutrition can affect waning of maternal antibody, our results may not apply to preterm infants and those in more resource-limited areas. We also applied strict criteria for categorizing non-negative EIA test results. In practice, laboratories may consider samples close to the optical density threshold as indeterminate rather than positive. With regards to our analysis, we were unable to include variables related to the mother’s HIV disease (e.g., maternal viral load) to examine their potential association with prolonged time to seroreversion.
Low-cost and easily accessible diagnostic tools are needed to identify infected children for care and treatment, and provide reassurance to the families of the larger, uninfected proportion of the HIV-exposed infant population. In our setting of low rates of breastfeeding, indeterminate EIAs at these ages could be reliable evidence for waning maternal antibody and lack of infant infection. However, our results show that we may need to shift expectations of providers and policy-makers regarding HIV seroreversion by standard EIAs to reflect potential cross-regional differences in their performance.
ACKNOWLEDGMENTS
This study was supported by a grant to Dr. Sohn by the National Institute of Child Health and Human Development, NIH (K23 HD047166). Authors participated in the study design and protocol development (AS, LT, LG, TL), laboratory testing (AS, TT, LT, HT, TL), clinical follow-up (AS, TK), analysis (AS, TT, HT), and manuscript preparation (all). The authors thank Dr. Eric Vittinghoff for his assistance with the statistical analysis, and the patients and families who participated in the study.
Source of support: National Institute of Child Health and Human Development, NIH (K23 HD047166).
REFERENCES
- 1.UNAIDS, UNICEF, WHO. Geneva: UNAIDS; Children and AIDS: Second stocktaking report. 2008
- 2.Farquhar C, Nduati R, Haigwood N, et al. High maternal HIV-1 viral load during pregnancy is associated with reduced placental transfer of measles IgG antibody. J Acquir Immune Defic Syndr. 2005 Dec 1;40(4):494–497. doi: 10.1097/01.qai.0000168179.68781.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. Antiretroviral therapy of HIV infection in infants and children in resource-limited settings: Towards universal access, Recommendations for a public-health approach. 2006 [PubMed]
- 4.Moodley D, Bobat RA, Coutsoudis A, Coovadia HM. Predicting perinatal human immunodeficiency virus infection by antibody patterns. Pediatr Infect Dis J. 1995 Oct;14(10):850–852. doi: 10.1097/00006454-199510000-00006. [DOI] [PubMed] [Google Scholar]
- 5.WHO. [Accessed May 23, 2008];WHO Child Growth Standards. http://www.who.int/childgrowth/standards/en/
- 6.Creek TL, Sherman GG, Nkengasong J, et al. Infant human immunodeficiency virus diagnosis in resource-limited settings: issues, technologies, and country experiences. Am J Obstet Gynecol. 2007 Sep;197(3 Suppl):S64–S71. doi: 10.1016/j.ajog.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Gulia J, Kumwenda N, Li Q, Taha TE. HIV seroreversion time in HIV-1-uninfected children born to HIV-1-infected mothers in Malawi. J Acquir Immune Defic Syndr. 2007 Nov 1;46(3):332–337. doi: 10.1097/QAI.0b013e3181576860. [DOI] [PubMed] [Google Scholar]
- 8.Apetrei C, Loussert-Ajaka I, Descamps D, et al. Lack of screening test sensitivity during HIV-1 non-subtype B seroconversions. AIDS. 1996 Dec;10(14):F57–F60. doi: 10.1097/00002030-199612000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Young CL, Hu DJ, Byers R, et al. Evaluation of a sensitive/less sensitive testing algorithm using the bioMerieux Vironostika-LS assay for detecting recent HIV-1 subtype B' or E infection in Thailand. AIDS Res Hum Retroviruses. 2003 Jun;19(6):481–486. doi: 10.1089/088922203766774522. [DOI] [PubMed] [Google Scholar]
- 10.Caumont A, Lan NT, Uyen NT, et al. Sequence analysis of env C2/V3, gag p17/p24, and pol protease regions of 25 HIV type 1 isolates from Ho Chi Minh City, Vietnam. AIDS Res Hum Retroviruses. 2001 Sep 1;17(13):1285–1291. doi: 10.1089/088922201750461357. [DOI] [PubMed] [Google Scholar]
- 11.Lan NT, Recordon-Pinson P, Hung PV, et al. HIV type 1 isolates from 200 untreated individuals in Ho Chi Minh City (Vietnam): ANRS 1257 Study. Large predominance of CRF01_AE and presence of major resistance mutations to antiretroviral drugs. AIDS Res Hum Retroviruses. 2003 Oct;19(10):925–928. doi: 10.1089/088922203322493111. [DOI] [PubMed] [Google Scholar]
- 12.Dewey KG, Hawck MG, Brown KH, Lartey A, Cohen RJ, Peerson JM. Infant weight-for-length is positively associated with subsequent linear growth across four different populations. Matern Child Nutr. 2005 Jan;1(1):11–20. doi: 10.1111/j.1740-8709.2004.00004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waterlow JC. Relationship of gain in height to gain in weight. Eur J Clin Nutr. 1994 Feb;48 Suppl 1:S72–S73. discussion S73–74. [PubMed] [Google Scholar]
- 14.Chantry CJ, Cooper ER, Pelton SI, Zorilla C, Hillyer GV, Diaz C. Seroreversion in human immunodeficiency virus-exposed but uninfected infants. Pediatr Infect Dis J. 1995 May;14(5):382–387. doi: 10.1097/00006454-199505000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Sherman GG, Jones SA. Oral fluid human immunodeficiency virus tests: improved access to diagnosis for infants in poorly resourced prevention of mother to child transmission programs. Pediatr Infect Dis J. 2005 Mar;24(3):253–256. doi: 10.1097/01.inf.0000154325.85754.a3. [DOI] [PubMed] [Google Scholar]
- 16.Claassen M, van Zyl GU, Korsman SN, Smit L, Cotton MF, Preiser W. Pitfalls with rapid HIV antibody testing in HIV-infected children in the Western Cape, South Africa. J Clin Virol. 2006 Sep;37(1):68–71. doi: 10.1016/j.jcv.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Homsy J, Downing R, Finkbeiner T, et al. Rapid HIV testing prior to DNA-PCR for early screening of HIV infection in infants in Uganda. Abstract 668; 14th Conference on Retroviruses and Opportunistic Infections; Los Angeles, California. February 24–28, 2007.2007. [Google Scholar]
- 18.Opoku-Anane J, Kinuthia J, F NJ, et al. Validity and Acceptability of Rapid HIV-1 Tests in 9-Month-old Infants in Kenya. Abstract 613a; 15th Conference on Retroviruses and Opportunistic Infections; Boston, Massachusetts. February 2–6, 2008.2008. [Google Scholar]