Abstract
Lacrimal epithelial cells appear to constitutively secrete autoantigens to their underling stroma. The present experiments address the hypothesis that they also secrete soluble factors that regulate immune responses. Epithelial cells, spleen cells, and lymphocytes were obtained from rabbits or rats and cultured in various configurations. Monocytes from rat bone marrow were matured to dendritic cells (DC) ex vivo. Proliferation was measured by [3H]-thymidine incorporation; surface MHC Class II and CD86 by flow cytometry; and mRNA relative abundances by real time RT-PCR. Microporous culture inserts containing rat lacrimal cells inhibited proliferation of rabbit lymphocytes co-cultured with autologous lacrimal cells and of rat lymphocytes co-cultured with TNF-α-stimulated DC. They inhibited CD86 and MHC Class II surface expression by maturating DC and reversed surface expression of CD86 but not MHC Class II by partially-matured DC. Subsequent exposure of partially matured DC to mediators from rat lacrimal cells reversed the ability to stimulate lymphocyte proliferation. TGF-β1 and IL-10 mRNAs increased somewhat when rat lacrimal cells were isolated but decreased markedly in rabbit lacrimal cells. Antibodies to TGF-β prevented soluble factors from rat lacrimal cells from inhibiting proliferation of rabbit lymphocytes co-cultured with rabbit lacrimal cells, but recombinant TGF-β alone did not mimic the soluble factors. IL-10 immunopositivity was detected in epithelial cells of interlobular ducts and occasional interstitial cells in rabbit lacrimal gland. Rat lacrimal epithelial cells secrete TGF-β and other factors that synergize to suppress lymphocyte proliferation and regulate DC maturation. Interlobular duct epithelial cells in rabbit lacrimal glands may express similar functions.
Introduction
Immunopathophysiological processes in the lacrimal glands frequently are associated with exocrine quiescence or dysfunction, inflammation of the conjunctiva and cornea, symptoms of ocular discomfort, and visual impairment. The process in Sjögren’s syndrome is characterized by increased numbers of CD4+ T cells, IgG+- and IgM+ B cells, and dendritic cells[1,2,3] organized into ectopic, IgG autoantibody-producing lymphoid tissues[4], while the histopathological syndrome commonly associated with aging appears to occur independently of autoantibody-producing immune disorders.[5, 6]
Pathogenesis of inflammatory autoimmune lacrimal gland diseases remains poorly understood. However, several inferences can be drawn from experimental findings that dacryoadenitis can be induced by immunization with lacrimal autoantigens [7, 8, 9] and by adoptive transfer of lymphocytes that have been activated against lacrimal autoantigens [10, 11, 12, 13]. Significant numbers of lymphocytes specific for lacrimal autoantigens must normally escape thymic deletion; potentially pathogenic autoantigens must be present in the stromal space of the lacrimal gland; and antigen presenting cells must constitutively enter the stromal space, take up and process autoantigens, and present relevant epitopes to CD4+ T cells. Cytophysiological studies with ex vivo models suggest that lacrimal epithelial cells secrete the autoantigens as byproducts of the transcytotic mechanism they use to transfer secretory IgA (sIgA) from the stromal space to the lumen of the acinus - duct system [14, 15]. As has been reviewed elsewhere [16] the transcytotic secretory apparatus is comprised of subcellular compartments that are common to virtually all nucleated cells, but the traffic of membrane constituents and lumenal fluid phase contents to the stromal space is extraordinarily vigorous in lacrimal gland epithelial cells [17,18].
If lacrimal epithelial cells constitutively secrete a significant burden of autoantigens, then active mechanisms may normally operate to regulate immune responses to those autoantigens. Some time ago Pockley and Montgomery reported findings that seem, in retrospect, to support this hypothesis. They found that cultures of mechanically dispersed cells from rat lacrimal glands produce soluble factors that inhibit mitogen-stimulated spleen cell proliferation, hybridoma proliferation, and growth factor-dependent T cell proliferation [19], and they suggested that such factors might play a role in keeping the stromal spaces of the lacrimal gland populated by the mature plasmacytes that produce the dIgA that the gland’s epithelial cells secrete [20, 21].
Lacrimal plasmacytes mature from IgA+ plasmablasts that have emigrated from mucosal immune inductive sites, primarily the organized lymphoid tissues of the gut and bronchus. It is now known that inductive sites and effector sites of the mucosal immune system employ some of the same paracrine mediators to accomplish their specialized functions. These include TGF-β and IL-10. In the inductive sites, TGF-β induces antigen-specific B cells to undergo IgM-to-IgA class switching [22, 23], mature into plasmablasts, and emigrate via afferent lymph vessels [24]. In the effector sites it induces plasmablasts to undergo terminal differentiation [25, 26, 27]. Notably, TGF-β and IL-10 also are immunoregulatory cytokines. In addition to suppressing proliferation of activated T lymphocytes [28, 29], they induce naïve CD4+ T cells to differentiate as regulatory cells, which secrete TGF-β and IL-10 in response to antigenic stimulation [30] and they induce immature dendritic cells to mature as tolerogenic antigen presenting cells [31]. While not necessarily solely responsible, they contribute importantly to the local signaling milieu that allows the intestinal lamina propria to function as both a mucosal immune effector site and also an inductive site for oral tolerance [32].
Work in several laboratories has demonstrated that epithelial cells in human [33, 34] and rat lacrimal glands [35] express mRNAs for TGF-β1 and TGF-β2. Immunohistochemical, physiological [36, 37] and cytophysiological [38, 39] studies indicate that epithelial cells in the interlobular ducts of the rabbit lacrimal gland secrete various cytokines and growth factors to the lumen of the acinus-duct system, via their apparatus for exocrine protein secretion, and to the underlying stromal space, via, under normal circumstances, the same compartments that secrete autoantigens [38, 39]. Accordingly, TGF-β might be one of the several factors mediating the anti-proliferative activity documented by Pockley and Montgomery.
When epithelial cells are isolated from lacrimal gland and placed in primary culture, they begin to express MHC Class II molecules [40]. Lacrimal epithelial cells from New Zealand white rabbits stimulate peripheral blood- and spleen lymphocytes to proliferate in autologous mixed cell reactions, presumably by presenting autoantigen epitopes directly to CD4+ T cells [41]. Preliminary experiments revealed that, as might have been predicted from Pockley’s and Montgomery’s findings with cells from rat lacrimal glands [19], epithelial cells isolated from rat lacrimal glands suppress, rather than stimulate, proliferation of lymphocytes from rat lymph nodes and spleen. As described herein, the authors have found that rat lacrimal gland epithelial cells secrete soluble factors that inhibit the proliferation of rabbit lymphocytes co-cultured with autologous lacrimal epithelial cells, and that also inhibit the proliferation of rat lymphocytes co-cultured with dendritic cells matured ex vivo from rat bone marrow monocytes. Moreover, exposure to such factors inhibits induction of surface CD86 expression by dendritic cells maturing ex vivo, decreases surface CD86 expression by partially-matured dendritic cells, and prevents dendritic cells from subsequently stimulating lymphocyte proliferation. In accord with the markedly different behaviors of the ex vivo models, the relative abundances of mRNAs for TGF-β1 and IL-10 either remain unchanged or increase when epithelial cells are isolated from rat lacrimal glands and decrease markedly when epithelial cells are isolated from rabbit lacrimal glands.
Materials and Methods
Animals
Male Lewis rats (8–12 weeks old) were purchased from Harlan (Indianapolis, IN). Female New Zealand White rabbits (3.5–4 kg) were obtained from Irish Farms (Norco, CA). All animal experiments were approved by the Institutional Animal Care and Use Committee and were conducted in accordance with the American Physiological Society Guiding Principles for the Use of Animals in Research and the ARVO Statement on the Use of Animals in Ophthalmic and Vision Research. Animals were maintained in a facility accredited by the American Association for Laboratory Animal Science.
Reagents
Ham’s F12 medium, Dulbecco’s modified Eagle’s medium (DMEM), RPMI 1640 medium, fetal calf serum, and antibiotic-antimycotic mixture were purchased from Invitrogen-Gibco products (Rockville, MD). Density gradient medium for separation of mononuclear cells (Fico/Lite™-LR) from rats was from Atlanta Biologicals, Lawrenceville, GA. Bovine serum albumin, soybean trypsin inhibitor, linoleic acid, and lipopolysaccharides (LPS) were purchased from Sigma-Aldrich (St. Louis, MO). [3H]-thymidine was from DuPont NEN Research Products (Wilmington, DE). Recombinant rat GM-CSF, recombinant rat IL-4, recombinant rat TNF-α, recombinant human TGF-β1, and monoclonal anti-TGF-β1, -β2, -β3 antibody (clone 1D11) were from R & D Systems (Minneapolis, MN). Optimal cutting temperature compound (OCT) was from Miles, Inc. (Elkhart, NJ). Antibodies to rat CD86 and rat MHC Class II for flow cytometry were from eBioscience (San Diego, CA). Monoclonal antibody to human IL-10, MAB 274 was from R&D Systems (Minneapolis, MN). Biotin-labeled goat anti-mouse IgG Fc was from Chemicon International, (Temecula, CA). Formaldehyde (3.7%) and Zinc and Gold conjugate blocking solution (5% BSA + 5% normal donkey serum + 0.1% cold water fish skin gelatin) were from Electron Microscopy Sciences (Hatfield, PA). Vectastain Elite ABC kit was obtained from Vector Laboratories, Inc. (Burlingame, CA). Cell culture inserts with high pore-density membranes (0.4 µm HD Transwell™ Permeable Supports) were from Corning Incorporated Life Sciences (Acton, MA). Culture flasks and culture plates were from BD Falcon™ (San Jose, CA).
Primer and probe sequences for real time RT-PCR are presented in Table 1. They were selected with Primer Express™ software (Applied Biosystems, Foster City, CA) and were synthesized by Applied Biosystems. The 5′ reporter- and 3′ quencher dyes were, respectively, 6-carboxyfluorescein (FAM) and 6-carboxytetramethylrhodamine (TAMRA).
Table 1.
Primer and Probe Sequences for Real Time RT-PCR
| Target | Sequence | Accession # | |
|---|---|---|---|
| Rat TGF-β1 | Fwd → | 5'-AGAAGTCACCCGCGTGCTA-3' | NM021578 |
| ← Rev | 5'-TGTGATGTCTTTGGTTTTGTCATAGA-3' | ||
| Probe | 5'-TGGTGGACCGCAACAACGCA-3' | ||
| Rat IL-10 | Fwd → | 5'-AAGCTGAAGACCCTCTGGATACA-3' | NM012854 |
| ← Rev | 5'-CACTGCCTTGCTTTTATTCTCACA-3' | ||
| Probe | 5'-TGCGACGCTGTCATCGATTTCTCC-3' | ||
| Rabbit TGF-β1 | Fwd → | 5'-AAGGGCTACCACGCCAACTT-3' | AF000133 |
| ← Rev | 5'-CGGGTTGTGCTGGTTGTACA-3' | ||
| Probe | 5'-TGCCTGGGACCCTGCCCCTAC-3' | ||
| Rabbit IL-10 | Fwd → | 5'-TTGTCGGAGATGATCCAGTTTTAC-3' | AF068058 |
| ← Rev | 5'-TGGCTGGACTGTGGTTCTCA-3' | ||
| Probe | 5'-TGAAGGACGTGATGCCGCAAG-3' |
Sequences were selected with Primer Express™ software (Applied Biosystems, Foster City, CA).
Cell isolation and culture
Spleen cells and spleen lymphocytes from rats
Individual spleens were harvested, dispersed mechanically, and meshed using 70 µm Falcon cell strainers. Red blood cells were lysed with ammonium chloride buffer, and the cells were washed and resuspended in RPMI medium supplemented with 10% fetal calf serum, 10 mM HEPES, 50 µM 2-mercaptoethanol, 100 U ml−1 penicillin, and 0.1 mg ml−1 streptomycin. To enrich lymphocytes, the cell suspension (5×106 cells ml−1) was layered onto Fico/Lite™-LR in a 1:1 ratio and centrifuged at 1000 × g for 20 min at 20° C. Mononuclear cells from the interface were washed, resuspended in supplemented RPMI medium, and cultured for 48 h before further experiments.
Epithelial cells from rat lacrimal gland
Procedures for purification of epithelial cells from rat exorbital lacrimal glands were adapted from the methods previously used for epithelial cells from rabbit lacrimal glands [11], with the modifications that the collagenase concentrations were increased to 350 U ml−1 for first digestion and to 250 U ml−1 for the second digestion, and the hyaluronidase concentration was increased to 150 U ml−1 for the first and second digestions.
Epithelial cells, spleen cells, and peripheral blood lymphocytes from rabbits
Cells were obtained from rabbit tissues according to methods used in previous studies [11,12].
Dendritic cells from rat bone marrow monocytes
A standard protocol [42] was used to mature dendritic cells from rat bone marrow monocytes. Monocytes were incubated in complete lymphocyte medium supplemented with 10 ng recombinant rat GM-CSF and 10 ng recombinant rat IL-4 for 7- or 8 days, with 75% of the medium removed and replaced every 2- or 3 days. Recombinant rat TNF-α was added at a concentration of; 10 ng/ml for the last 2 days. Medium containing floating cells was removed, and lightly adherent dendritic cells were harvested by gently flushing with fresh medium.
Mixed-cell reactions
Purified LGEC and lymphocytes or spleen cells were isolated and cultured separately for 2 d. Mixed-cell reactions were performed in 12-well, 24-well or 96-well plates, using equal numbers (1 × 106 mL−1) of lymphocytes and 2500-rad γ-irradiated LGECs co-cultured for 5 d. [3H]-thymidine was added on the fourth day of co-culture, and cells were harvested 24 h later with a commercial cell harvester (Model 290 PHD, Brandel, Gaithersburg, MD). A beta scintillation counter (Model LS 6000IC, Beckman Instruments Inc., Fullerton, CA) was used to measure [3H]-thymidine incorporation. A minimum of six replicate wells for each experimental condition were counted.
Flow cytometry
Analyses of cell surface marker expression were performed in FACStarPlus flow cytometer or FACSDiVa cell sorter using CellQuest software (BD Biosciences, San Jose, CA).
Real-time polymerase chain reaction (PCR)
Table 1 lists the primer- and probe sequences used in this study and the study described in the accompanying article. Sequences were selected using Primer Express™ software (Applied Biosystems, Foster City, CA) and synthesized by Applied Biosystems. All probes incorporated the 5′ reporter dye 6-carboxyfluorescin (FAM) and the 3′ quencher dye 6-carboxytertramethylrhodamine (TAMRA). Real-time PCR analysis was performed with an ABI PRISM® 7900HT Sequence Detection System (Applied Biosystems) using TaqMan universal PCR master mix containing the internal dye, ROX, as a passive reference. The PCR reaction volume was 10 µl. It contained 1× TaqMan universal PCR master mix, 300 nM forward and reverse primers, 250 nM probe, and 1 µL of cDNA template. The FAM signal was measured against the ROX signal to normalize for non-PCR-related fluorescence fluctuations. The cycle threshold (CT) value represented the refraction cycle number at which a positive amplification reaction was measured and was set at 10 times the standard deviation of the mean baseline emission calculated for PCR cycles 3–15. The difference between the CT values for each target mRNA and for the housekeeping gene, GAPDH, in each sample were used to calculate the relative abundance of the target mRNA in that sample (Ji et al., 2003) [43]. Each sample was assayed in triplicate.
Immunohistochemical staining
OCT-embedded tissue samples were sectioned at 7 µm using a cryostat, fixed in 3.7% formaldehyde / Zinc, washed with TBS containing 10 mM glycine to inactivate residual aldehyde, and then washed with TBST. The sections were blocked with Gold Conjugate Blocking Solution. incubated with primary antibody and biotinylated secondary antibody, and counterstained with hematoxylin as in previous studies [36, 37].
Statistics
Data were expressed as means ± sems. Differences between groups were evaluated using Student’s t-test. P values ≤ 0.05 were considered significant.
Results
Inhibition of lymphocyte proliferation
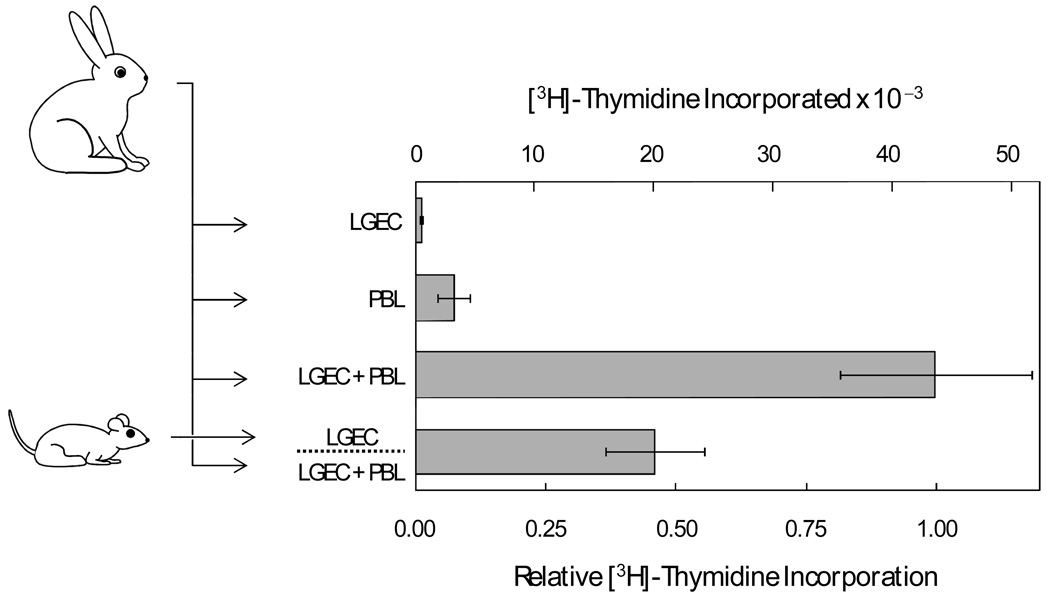
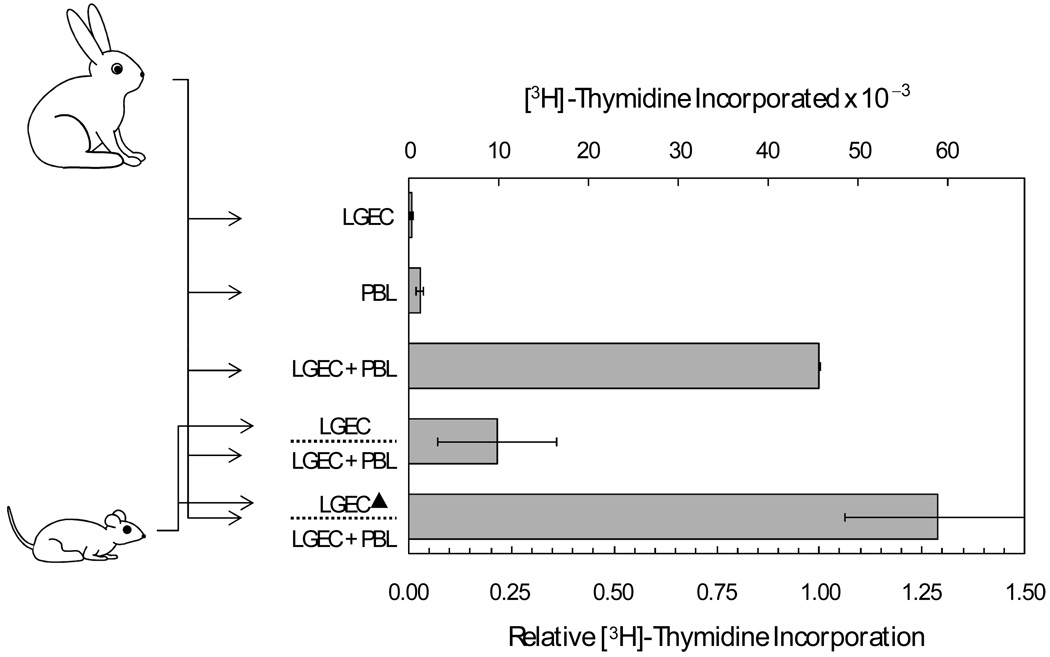
While lymphocytes from rabbit spleen and peripheral blood proliferate when they are co-cultured with autologous lacrimal gland epithelial cells [11], preliminary experiments indicated that epithelial cells from rat lacrimal glands inhibit proliferation of lymphocytes from rat spleen and lymph nodes (data not shown). Co-cultures of lymphocytes and lacrimal epithelial cells from rabbits were used to test the hypothesis that soluble factors from rat lacrimal epithelial cells suppress autoantigen-driven lymphocyte proliferation. As illustrated in Figure 1, addition of microporous inserts containing rat lacrimal epithelial cells caused a greater than 50% reduction of the proliferation of rabbit PBL co-cultured with autologous lacrimal epithelial cells.
Figure 1. Influence of soluble factors from isolated rat lacrimal epithelial cells on proliferation of rabbit peripheral blood lymphocytes (PBL) co-cultured with autologous lacrimal gland epithelial cells.
Epithelial cells (LGEC) and PBL were isolated from individual rabbits. [3H]-Thymidine cpm incorporation was measured after the two cell preparations were cultured separately, in autologous mixed cell reactions, and in autologous mixed cell reactions in the presence of presence of microporous inserts containing LGEC isolated from rats. [3H]-Thymidine cpm values were determined in four replicate wells for each condition in each of 8 separate preparations. Lower axis label presents values normalized to cpm for the paired LGEC - PBL mixed cell reaction for each preparation, and error bars are sems for the normalized values. Upper axis labels present actual cpm values‥
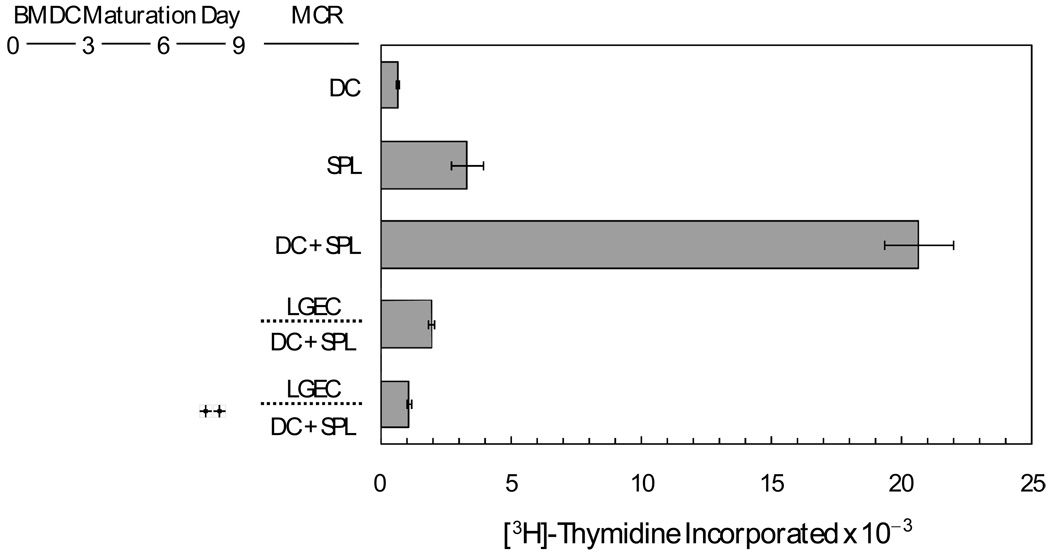
Lacrimal epithelial cells are unconventional antigen presenting cells, and, in any event, it was necessary to consider that factors secreted by rat lacrimal epithelial cells might have anomalous influences on lymphocytes from rabbits. Therefore, experiments were done with dendritic cells that had been matured ex vivo from rat bone marrow monocytes. As illustrated in Figure 2, spleen lymphocytes incorporated [3H]-thymidine at appreciable rates even when they were not co-cultured with dendritic cells, presumably because the preparations contained antigen presenting cells that had become activated in the course of the isolation procedure. Nevertheless, addition of bone marrow-derived dendritic cells markedly increased the rate of spleen lymphocyte proliferation. The presence of microporous inserts containing rat lacrimal epithelial cells inhibited spleen lymphocyte proliferation by more than 90%. Moreover, soluble factors from the lacrimal epithelial cells inhibited spleen lymphocyte proliferation even if the dendritic cells had been first stimulated with LPS to enhance surface expression of co-stimulatory molecules and secretion of inflammatory cytokines [44].
Figure 2. Influence of soluble factors from rat lacrimal epithelial cells mediators on proliferation of rat spleen lymphocytes co-cultured with ex vivo-matured, bone marrow-derived dendritic cells.
Dendritic cells (DC) were matured from rat bone marrow monocytes ex vivo for 9 days, then placed in mixed cell reactions with spleen lymphocytes (SPL) with or without microporous inserts containing rat lacrimal epithelial cells. Symbols indicate that LPS was present at a concentration of 10 µg/mL during day 8 and day 9 of DC maturation. Values presented are means ± sems from three replicate wells for each condition.
Control of dendritic cell phenotype and function
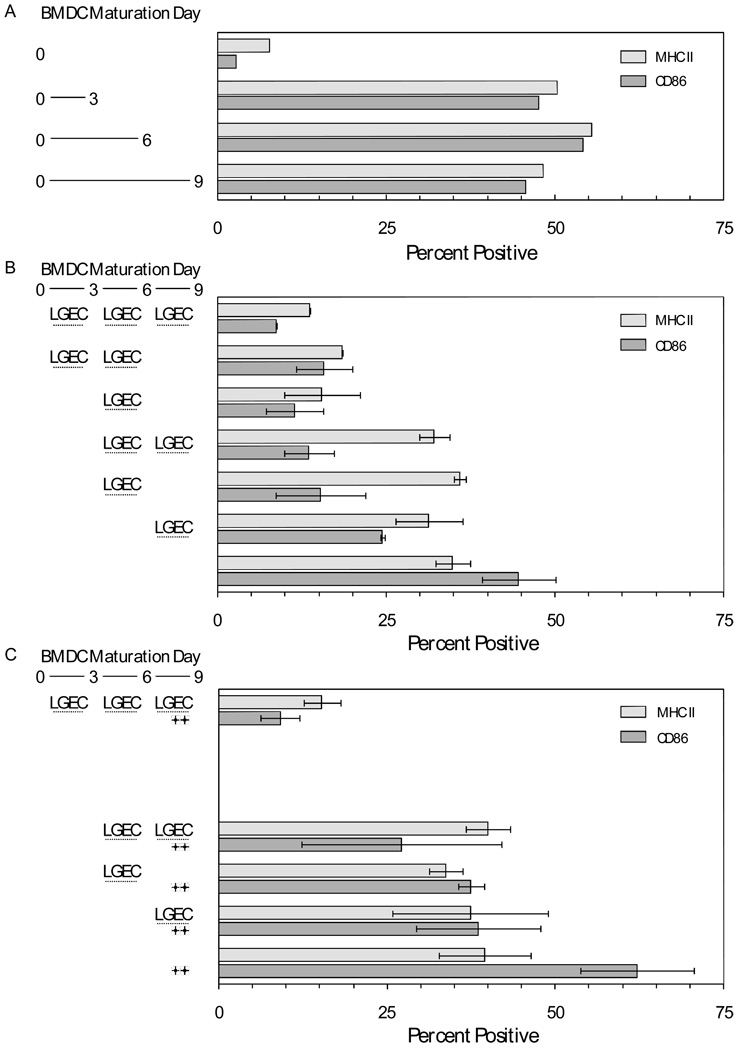
In the experiments depicted in Figure 1 and Figure 2, soluble factors secreted by rat lacrimal epithelial cells may have acted directly on lymphocytes to inhibit their proliferation; they also may have acted on the antigen presenting cells. An initial survey was done to determine the time-course of induction of surface CD86 and MHC Class II by dendritic cells maturing ex vivo from bone marrow monocytes. Cell samples were analyzed by flow cytometry upon isolation (day 0) and on day 3, day 6, and day 9. As illustrated in Figure 3A, levels of CD86 and MHC Class II surface expression were modest on day 0 but maximal by day 3. The hypothesis that soluble factors from rat lacrimal epithelial cells influence dendritic cell phenotypic expression was tested in the experiments summarized in Figure 3B and 3C. Microporous inserts containing rat lacrimal epithelial cells were added to wells containing freshly-isolated monocytes and maturing dendritic cells at different times and for varying intervals, as indicated in the figures. When rat lacrimal epithelial cells were present from day 0 through day 9, soluble factors they secreted prevented the normal increases of surface MHC Class II expression and CD86 expression. The influence of the soluble factors persisted even if the inserts were removed on day 6 or on day 3. If dendritic cells were allowed to mature in the absence of the soluble factors for 3 days, i.e., long enough to achieve maximal surface expression of MHC Class II and CD86, the subsequent addition of inserts containing rat lacrimal epithelial cells failed to reverse surface MHC Class II expression, but it decreased surface CD86 expression by roughly 60%, even if the inserts were present only on days 4, 5, and 6. If dendritic cells were allowed to mature in the absence of soluble factors for 6 days before inserts containing lacrimal epithelial cells were added, surface CD86 expression decreased by roughly 30%.
Figure 3. Ex vivo maturation of dendritic cells from rat bone marrow monocytes and influences of soluble factors from rat lacrimal epithelial cells.
A. DC were matured from rat bone marrow monocytes ex vivo for 3, 6, or 9 days. Surface expression of MHC Class II and CD86 was determined by flow cytometry. B. DC were matured from rat bone marrow monocytes for 9 days. Microporous inserts containing ratLGEC were present during the indicated intervals. Values presented are means ± ranges for two replicate cell preparations. C. DC were matured as in panel B. Symbols indicate that LPS was present at a concentration of 10 µg/mL during day 8 and day 9. Values presented are means ± ranges for two replicate cell preparations.
The results in Figure 3B suggested that soluble factors secreted by rat lacrimal epithelial cells might induce maturing dendritic cells to express stable phenotypes, with the level of MHC Class II expression depending upon the state of maturation the dendritic cells had achieved before they were exposed to the soluble factors. Experiments testing the robustness of the induced phenotype are summarized in Figure 3C. LPS did not increase surface MHC Class II expression or CD86 expression if inserts containing rat lacrimal epithelial cells were present throughout the 9 day period. If dendritic cells were allowed to mature for 3 days before inserts containing epithelial cells were added, subsequent stimulation with LPS increased surface CD86 expression, but by only half the extent as for cells that had never been exposed to soluble mediators from lacrimal epithelial cells.
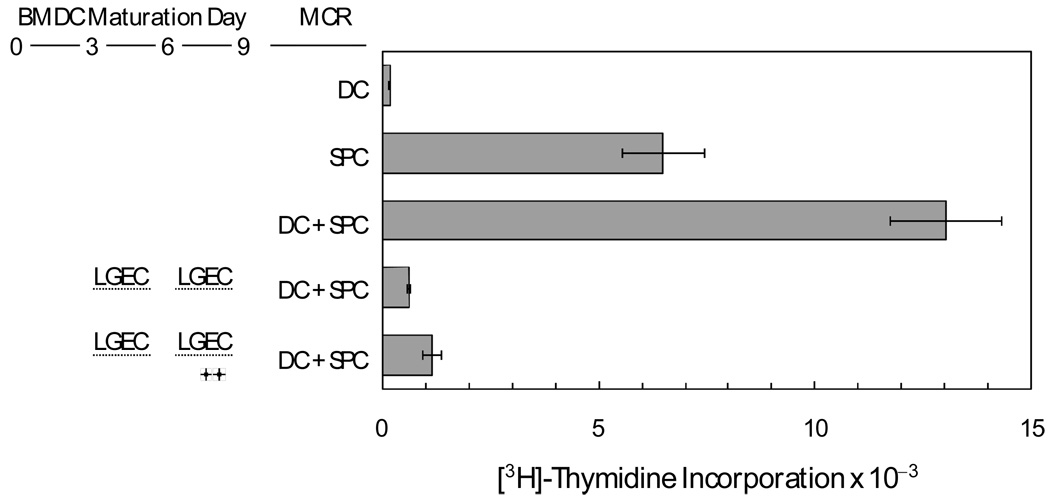
In the models that have been studied most extensively, the CD4+ T cells that are stimulated by MHC Class II - epitope complexes in the absence of the CD80 or CD86 co-stimulatory ligands are rendered anergic. Thus, it seemed reasonable to hypothesize if dendritic cells were allowed to mature sufficiently to increase surface MHC Class II expression and surface CD86 expression, then exposed to soluble factors from rat lacrimal epithelial cells causing them to down-regulate surface CD 86 expression, they would lose their ability to stimulate spleen cell proliferation. Experiments testing this hypothesis are summarized in Figure 4. As with the spleen lymphocytes used in the experiment depicted in Figure 2, dendritic cells that had been matured in the absence of soluble factors from rat lacrimal epithelial cells stimulated [3H]-thymidine incorporation by rat spleen cells. Dendritic cells that were exposed to soluble factors from day4 through day 9 suppressed [3H]-thymidine incorporation to well below the value for control spleen cells; stimulation with LPS did not abrogate this suppressive action.
Figure 4. Influence of soluble factors from rat lacrimal epithelial cells on function of ex vivo-matured dendritic cells.
DC were matured from rat bone marrow monocytes ex vivo with inserts containing isolated rat lacrimal epithelial cells LGEC and with LPS present as indicated. They were then placed in co-cultures with spleen cells (SPC) for 5 days. Values presented are means ± sems for 6 replicate wells for each condition.
Candidate immunoregulatory mediators
TGF-β is known to be expressed in lacrimal glands of humans [33,34] and rats [35], as well as rabbits [36,37]. In two separate experiments, summarized in Figure 6, neutralizing antibodies to TGF-β prevented rat lacrimal epithelial cells from inhibiting proliferation of rabbit PBL co-cultured with autologous lacrimal epithelial cells. In fact, the TGF-β antibodies enhanced [3H]-thymidine incorporation, slightly in one experiment, substantially in the other. Recombinant human TGF-β alone did not inhibit PBL proliferation in this model, suggesting that the inhibitory action of TGF-β might depend on synergy with other factors that also are secreted by the rat lacrimal epithelial cells.
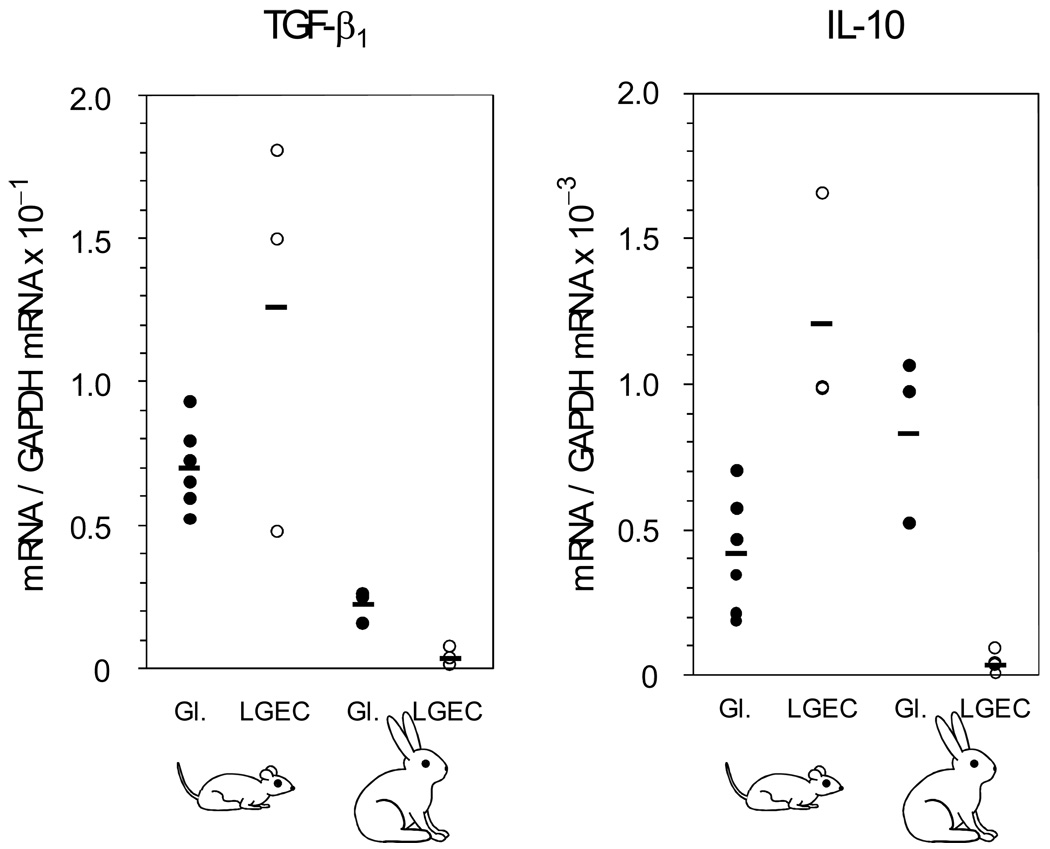
Figure 6. Relative abundances of mRNAs for TGF-β1 and IL-10 in whole lacrimal gland extracts and isolated cell preparation from rats and rabbits.
Real time RT-PCR analyses were done with species-specific primers and probe combinations. The abundance of the target mRNA in samples from separate animals were expressed relative to the abundance of GAPDH mRNA in the same sample. Individual values and means are presented.
IL-10, which also functions as a regulatory cytokine [45, 46], has recently been reported to be present in rat lacrimal glands [47], but its expression in lacrimal glands of rabbits has not been described. Real time RT-PCR analyses were performed with species-specific primer and probe combinations to evaluate the relative abundances of mRNAs for TGF-β1 and IL-10 in lacrimal gland extracts and isolated lacrimal gland epithelial cells from rat and rabbits. As illustrated in Figure 7, the mean relative abundance of TGF-β1 mRNA was roughly 3-fold greater in rat lacrimal glands than in rabbit lacrimal glands. Compared to the mean value in the gland extracts, it increased to more 2-fold in two isolated epithelial cell preparations from rat lacrimal glands and decreased somewhat in a third isolated cell preparation. In contrast, the relative abundance of TGF-β1 mRNA decreased more than 6-fold when epithelial cells were isolated from rabbit lacrimal glands.
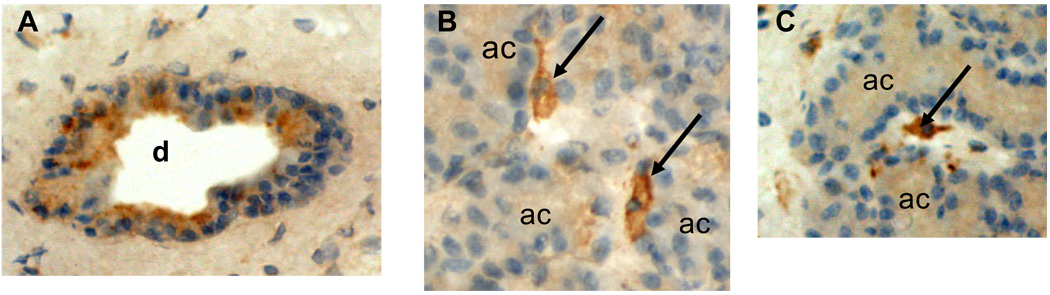
Figure 7. Immunohistochemical localization of IL-10 in rabbit lacrimal gland.
A. Immunohistochemical staining for IL-10 revealed strong immunopositivity in the epithelial cells of interlobular ducts (d), although not all cells were equally immunopositive. B and C. Strong immunopositivity also was detected in occasional cells (arrows), presumably macrophages, in interstitial connective tissue between acini, and there was light immunopositivity within acinar cells (ac).
The mean relative abundance of IL-10 mRNA was 2-fold greater in rabbit lacrimal glands than in rat lacrimal gland extracts. It decreased 25-fold when epithelial cells were isolated from rabbit lacrimal glands, and it increased 3-fold when epithelial cells were isolated from rat lacrimal glands. Immunohistochemical studies have shown that TGF-β is most prominently present in epithelial cells of the interlobular ducts of rabbit lacrimal glands [36, 37]. Therefore, the decrease of TGF-β mRNA relative abundance that occurred when epithelial cells were isolated from rabbit lacrimal glands might have reflected a preferential enrichment of acinar cells with respect to ductal cells. As shown in Figure 7, IL-10 immunopositivity in rabbit lacrimal glands was primarily localized in epithelial cells of interlobular ducts and secondarily localized in cells, presumably macrophages, scattered rather sparsely through interstitial connective tissue surrounding acini. Light immunopositivity was detected in acinar cells; it is not possible to determine the extent to which it reflected IL-1o produced within the acinar cells versus IL-10 that was secreted by interstitial cells and subsequently taken up by acinar cells. Since the procedure for isolating epithelial cells from rabbit lacrimal glands includes a filtration step to remove interstitial cells, the 4-fold greater reduction of IL-10 mRNA relative abundance may have reflected preferential enrichment of acinar epithelial cells with respect to cells in the interstitium and interlobular ducts that express IL-10 at much higher levels; however it also might to some extent have resulted from down-regulation of IL-1o expression as epithelial cells were removed from their normal in vivo signaling milieu.
Discussion
The experiments described above demonstrate that epithelial cells isolated from rat lacrimal glands secrete factors that exert potent immunosuppressive- and immunoregulatory influences. As anticipated by the study of Pockley and Montgomery [19], these factors suppress the proliferation of lymphocytes co-cultured with ex vivo-matured dendritic cells. They also regulate phenotypic expression of maturing dendritic cells, but their influences differ at different stages of the maturation process. They prevent immature dendritic cells from up-regulating surface CD86 and MHC Class II, and they cause partially matured dendritic cells to down-regulated surface CD86 expression, but not surface MHC Class II. The factors secreted by rat lacrimal epithelial cells also appear to induce partially matured dendritic cells to express an immunosuppressive- or immunoregulatory phenotype that is robust, in that it is not reversed by stimulation with LPS.
The apparent immunosuppressive- or immunoregulatory function of epithelial cells isolated from rat lacrimal glands contrasts markedly with the immunoactivating functions of epithelial cells isolated from the lacrimal glands of rabbits, which appear to function as unconventional antigen presenting cells for autologous lymphocytes. The abundances of mRNAs for TGF-β1 and IL-10 were both found to be more than 30-fold greater in epithelial cells from rat lacrimal glands than in cells from rabbit lacrimal glands. IL-10 has been shown to prevent rabbit lymphocytes from proliferating in co-culture with autologous lacrimal epithelial cells [48, 49]. The present findings concerning potential influences of TGF-β are more complicated. The addition of neutralizing antibodies to TGF-β abrogated the ability of inserts containing rat lacrimal epithelial cells to inhibit proliferation of rabbit PBL in co-culture with autologous lacrimal epithelial cells. However, recombinant human TGF-β did not mimic the influence of soluble factors from rat lacrimal epithelial cells in this model. One possible explanation is that TGF-β acts in concert with other factors rat lacrimal epithelial cells by to inhibit PBL proliferation. In contrast to the findings with PBL, TGF-β neutralizing antibodies to failed to abrogate the inhibition of rabbit spleen cell proliferation by factors from rat lacrimal epithelial cells, but recombinant TGF-β inhibited acinar cell-mediated spleen cell proliferation as effectively as factors from rat lacrimal cells (data not shown). A potential explanation is that TGF-β is one of several mediators rat lacrimal epithelial cells produce that can induce spleen cells to express an inhibitory function. While this tentative hypothesis is rather complicated, it seems to accord with the finding that soluble factors secreted by rat lacrimal epithelial cells influence maturing dendritic cells’ expression of surface CD86 and induce the matured cells to express an immunosuppressive- or immunoregulatory function.
It is not likely that TGF-β and IL-10 are the only immunosuppressive- or immunoregulatory factors that isolated rat lacrimal epithelial cells produce, since Pockley and Montgomery found that supernatant media from dispersed rat lacrimal gland cell cultures contained factors smaller than 3500 Da that also inhibited proliferation spleen cells stimulated with LPS- or Salmonella typhimurium mitogen [19]. Likewise, it may not be only their relatively lower levels of TGF-β and IL-10 expression that account for the ability of epithelial cells from rabbit lacrimal glands to stimulate the proliferation of autologous lymphocytes. Immunohistochemical staining and immunofluorescence studies have confirmed that ex vivo epithelial cell models from rabbit lacrimal glands express MHC Class II and prolactin, a potential mitogenic factor for T cells and B cells. Recent experiments in the authors’ laboratories indicate that they also express mRNAs for co-stimulatory ligands, CD86 and CD80, as well as the inflammatory cytokines, IL-1α, IL-1β, IL-6, and TNF-α; moreover, the relative abundances of the inflammatory cytokine mRNAs are greater in isolated, primary cultured epithelial cell preparations than in extracts of whole lacrimal glands.
The finding that the relative abundances of mRNAs for TGF-β1 and IL-10 increased somewhat when epithelial cells were isolated from rat lacrimal glands and decreased markedly when epithelial cells were isolated from rabbit lacrimal glands suggests that there may be fundamental differences between then networks of autocrine signals that regulate cytokine expression in the two ex vivo models. The authors have not attempted to address the question of whether such differences might also encompass regulation of the low molecular weight factors whose existence was demonstrated by Pockley and Montgomery.
The present findings suggest that immunoregulation in the intact rat lacrimal gland is, at least in part, described by the theoretical paradigm proposed earlier [16] and briefly outlined in the Introduction. That is, epithelial cells secrete paracrine mediators which induce plasmablasts entering the stromal space of the gland to undergo terminal differentiation, support the survival of the matured plasmacytes, and, at the same time, regulate immune responses to the autoantigens that the epithelial cells also secrete to the stromal space. The data so far available indicate that such mediators act both to inhibit proliferation of autoreactive lymphocytes within the gland and also to induce immature dendritic cells that have taken up the autoantigens within the gland to mature as immunosuppressive- or immunoregulatory antigen presenting cells. However, this theoretical paradigm doe not assume the epithelial cells are the only physiologically-significant source of immunoregulatory paracrine mediators in the intact lacrimal gland. Notably, the interstitial tissue surrounding the acini in the rabbit lacrimal gland contains cells the express IL-10 interstitial cells. Sensory and parasympathetic nerve endings release peptide transmitters, such as vasoactive intestinal polypeptide, that are known to play immunoregulatory roles in other systems. The work of Niederkorn et al. has shown that regulatory T cells play critical roles in preventing initiation and adoptive transfer of autoimmune dacryoadenitis in mice [50]. It now is plausible to suggest that signalling milieu within the normal lacrimal gland generates immunoregulatory antigen presenting cells that, in turn, contribute to the ongoing generation of regulatory lymphocytes.
The immunosuppressive- and immunoregulatory functions which the authors propose for the rat lacrimal gland recapitulate functions that operate in the major effector sites of the mucosal immune system, i.e., the lamina propria of the intestines [32] and airways [51, 52]. They also appear to have much in common with immunoregulatory functions that operate in certain enclosed tissue spaces within the visual system, i.e., the anterior chamber [53, 54, 55, 56] and the sub-retinal space [57, 58]. Neither the anterior chamber nor the sub-retinal space is a mucosal immune effector sites, but, notably, both are partially bounded by epithelia that secrete wide varieties of immunosuppressive- and immunoregulatory mediators [59, 60].
Despite the evident differences between the signalling networks that regulate expression of immunosuppressive- and immunoregulatory mediators in epithelial cells isolated from lacrimal glands of rats and rabbits, there is reason to predict that the same theoretical paradigm describes immunoregulation in the rabbit lacrimal gland, but that the expression of key mediators in the rabbit lacrimal gland is organized according to the gland’s histoarchitectural plan, since both TGF-β and IL-10 immunoreactivities are concentrated in the interlobular ducts, the epithelial elements most proximal to the venules through which immature plasmablasts are likely to enter the stromal space. The studies of Schechter [36] and Ding [37] further demonstrate that, in female rabbits, the reproductive hormones regulate both the level at which ductal epithelial cells express TGF-β and also the proportions in which they secrete it to the stromal space versus the fluid forming in lumen of the acinus-duct system. It is easy to imagine that this mode of regulation might have implications for the greater prevalence of inflammatory autoimmune lacrimal gland disease in women. For example, the preliminary finding (unpublished) that increasing levels of estradiol and progesterone are responsible for increasing ductal epithelial TGF-β expression during pregnancy suggests that the decreased availability of these hormones at menopause might diminish support for TGF-β expression and create a signalling milieu permissive of inflammatory autoimmune activity.
Figure 5. Influence of neutralizing antibody to TGF-β on ability of factors from rat lacrimal epithelial cells to inhibit proliferation of rabbit PBL co-cultured with autologous lacrimal acinar cells.
Co-cultures of PBL and autologous lacrimal epithelial cells from rabbits were configured with or without microporous inserts containing LGEC from rat lacrimal glands, as in Figure 1. The symbol indicates the presence of antibody to TGF-β. Values presented are means ± ranges for two separate experiments, each done with 3 replicate wells for each condition. Lower- and upper axis labels are as in Figure 1.
Acknowledgement
The authors thank Dr. J. Dixon Gray for performing flow cytometry analyses. They thank Dr. Gray and Drs. Wendy Gilmore, David A. Horwitz, Alicia A. McDonough, Steven A. Stohlman, and Deming Sun for their advice in the course of this work. This work was supported by NIH Grants EY 005801, EY 013720, EY 010550, EY 012689, EY 003040, and DK 048522, and by a Berthe-Fouassier Grant from the Foundation of France
References
- 1.Belfort R, Mendes NF. Identification of T and B lymphocytes in the human conjunctiva and lacrimal gland in ocular diseases. Br J Ophthalmol. 1980;64:217–219. doi: 10.1136/bjo.64.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pepose JS, Akata RF, Pflugfelder SC, Voigt W. Mononuclear cell phenotypes and immunoglobulin gene rearrangements in lacrimal gland biopsies from patients with Sjögren’s syndrome. Ophthalmol. 1990;97:1599–1605. doi: 10.1016/s0161-6420(90)32372-2. [DOI] [PubMed] [Google Scholar]
- 3.Ogawa Y, Kuwana M, Yamazaki K, et al. Periductal area as the primary site for T-cell activation in lacrimal gland chronic graft-versus-host disease. Invest Ophthalmol Vis Sci. 2003;44:1888–1896. doi: 10.1167/iovs.02-0699. [DOI] [PubMed] [Google Scholar]
- 4.Salomonsson S, Jonsson MV, Skarstein K, et al. Cellular basis of ectopic germinal center formation and autoantibody production in the target organ of patients with Sjögren’s syndrome. Arthritis Rheum. 2003;48:3187–3201. doi: 10.1002/art.11311. [DOI] [PubMed] [Google Scholar]
- 5.Waterhouse JP. Focal adenitis in salivary and lacrimal glands. Proc Roy Soc Med. 1963;56:911–917. doi: 10.1177/003591576305601031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whaley K, Williamson J, Wilson T, et al. Sjögren’s syndrome and autoimmunity in a geriatric population. Age Aging. 1972;1:197–206. doi: 10.1093/ageing/1.4.197. [DOI] [PubMed] [Google Scholar]
- 7.Liu SH, Prendergast RA, Silverstein AM. Experimental autoimmune dacryoadenitis. I. Lacrimal gland disease in the rat. Invest Ophthalmol Vis Sci. 1987;28:270–275. [PubMed] [Google Scholar]
- 8.Saegusa K, Ishimaru N, Yanagi K, et al. Prevention and induction of autoimmune exocrinopathy is dependent on pathogenic autoantigen cleavage in murine Sjögren’s syndrome. J Immunol. 2002;169:1050–1057. doi: 10.4049/jimmunol.169.2.1050. [DOI] [PubMed] [Google Scholar]
- 9.Jiang G, Ke Y, Sun D, et al. A new model of experimental autoimmune keratoconjunctivitis sicca (KCS) induced in Lewis rat by the autoantigen Klk1b22. Invest Ophthalmol Vis Sci. 2009 doi: 10.1167/iovs.08-1949. in press. [DOI] [PubMed] [Google Scholar]
- 10.Liu SH, Zhou DH, Hess AD. Adoptive transfer of experimental autoimmune dacryoadenitis in susceptible and resistant mice. Cell Immunol. 1993;150:311–320. doi: 10.1006/cimm.1993.1199. [DOI] [PubMed] [Google Scholar]
- 11.Guo Z, Song D, Azzarolo AM, et al. Autologous lacrimal-lymphoid mixed cell reactions induce dacryoadenitis in rabbits. Exp Eye Res. 2000;71:23–31. doi: 10.1006/exer.2000.0855. [DOI] [PubMed] [Google Scholar]
- 12.Zhu Z, Stevenson D, Schechter JE, et al. Lacrimal histopathology and ocular surface disease in a rabbit model of autoimmune dacryoadenitis. Cornea. 2003;22:25–32. doi: 10.1097/00003226-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Thomas PB, Zhu Z, Selvam S, et al. Autoimmune dacryoadenitis and keratoconjunctivitis induced in rabbits by subcutaneous injection of autologous lymphocytes activated ex vivo against lacrimal antigens. J. Autoimmun. 2008;31:116–122. doi: 10.1016/j.jaut.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie J, Qian L, Wang Y, et al. Novel biphasic traffic of endocytosed EGF to recycling and degradative compartments in lacrimal gland acinar cells. J Cell Physiol. 2004;199:108–125. doi: 10.1002/jcp.10458. [DOI] [PubMed] [Google Scholar]
- 15.Rose CM, Qian L, Hakim L, et al. Accumulation of catalytically active proteases in lacrimal gland acinar cell endosomes during chronic ex vivo muscarinic receptor stimulation. Scand J Immunol. 2005;61:36–50. doi: 10.1111/j.0300-9475.2005.01527.x. 50. [DOI] [PubMed] [Google Scholar]
- 16.Mircheff AK, Wang Y, de Saint Jean M, et al. Lacrimal epithelium mediates hormonal influences on APC and lymphocyte cycles in the ocular surface system. In: Zierhut M, Rammensee HG, Streilein JW, editors. Antigen Presenting Cells and the Eye. New York, NY: Informa; 2007. pp. 93–119. [Google Scholar]
- 17.Lambert RW, Maves CA, Gierow JP, et al. Plasma membrane internalization and recycling in rabbit lacrimal acinar cells. Invest Ophthalmol Vis Sci. 1993;34:305–316. [PubMed] [Google Scholar]
- 18.Gierow JP, Lambert RW, Mircheff AK. Fluid phase endocytosis by isolated rabbit lacrimal acinar cells. Exp Eye Res. 1995;60:511–525. doi: 10.1016/s0014-4835(05)80066-1. [DOI] [PubMed] [Google Scholar]
- 19.Pockley AG, Montomery PC. Identification of lacrimal gland associated immunomodulatory activities having different effects on T and B cell proliferative responses. Regional Immunol. 1990–1991;3:198–203. [PubMed] [Google Scholar]
- 20.Franklin RM, Kenyon KR, Tomasi TB., Jr Immunohistologic studies of human lacrimal gland: localization of immunoglobulins, secretory component, and lactoferrin. J Immunol. 1973;110:984–992. [Google Scholar]
- 21.Allansmith MR, Kajiyama G, Abelson MB, Simon MA. Plasma cell content of main and accessory lacrimal glands and conjunctiva. Am J Ophthalmol. 1976;82:819–826. doi: 10.1016/0002-9394(76)90056-8. [DOI] [PubMed] [Google Scholar]
- 22.Iwasato T, Kanari Y, Shimizu A, et al. Transforming growth factor beta induced class switch recombination may be modified during cell proliferation restored by IL-2 stimulation. Immunol Lett. 1994;39:173–178. doi: 10.1016/0165-2478(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 23.Spieker-Polet H, Yam PC, Arbieva A, et al. In vitro induction of the expression of multiple IgA isotype genes in rabbit B cells by TGF-β and IL-2. J Immunol. 1999;162:5380–5388. [PubMed] [Google Scholar]
- 24.Hargreaves DC, Hyman PL, Lu TT, et al. A coordinated change in chemokine responsiveness guides plasma cell movements. J Exp Med. 2001;194:45–56. doi: 10.1084/jem.194.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen SS, Li Q. Transforming growth factor-beta 1 (TGF-beta 1) is a bifunctional immune regulator for mucosal IgA responses. Cell Immunol. 1990;128:353–361. doi: 10.1016/0008-8749(90)90032-m. [DOI] [PubMed] [Google Scholar]
- 26.Eckmann L, Morzycka-Wrobleska E, Smith JR, Kagnoff MF. Cytokine-induced differentiation of IgA B cells: studies using an IgA expressing B-cell lymphoma. Immunol. 1992;76:235–241. [PMC free article] [PubMed] [Google Scholar]
- 27.Husband AJ. Mucosal memory-maintenance and recruitment. Vet. Immunol. Immunopath. 2002;87:131–136. doi: 10.1016/s0165-2427(02)00044-2. [DOI] [PubMed] [Google Scholar]
- 28.Vánky F, Nagy N, Hising C, et al. Human ex vivo carcinoma cells produce transforming growth factor β and thereby can inhibit lymphocyte functions in vitro. Cancer Immunol. Immunother. 1997;43:317–323. doi: 10.1007/s002620050339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Summers KL, O’Donnell JL, Heiser A, et al. Synovial fluid transforming growth factor β inhibits dendritic cell - T lymphocyte interactions in patients with chronic arthritis. Arth Rheum. 1999;42:507–518. doi: 10.1002/1529-0131(199904)42:3<507::AID-ANR16>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 30.Seder RA, Marth T, Sieve MC. Factors involved in the differentiation of TGF-beta-producing cells from naive CD4+ T cells: IL-4 and IFN-γ have opposing effects, while TGF-β positively regulates its own production. J Immunol. 1998;160:5719–5728. [PubMed] [Google Scholar]
- 31.Tompkins AB, Hutchinson P, de Kretser DM, Hedger MP. Characterization of lymphocytes in the adult rat testis by flow cytometry: effects of activin and transforming growth factor beta on lymphocyte subsets in vitro. Biol Reprod. 1998;58:43–951. doi: 10.1095/biolreprod58.4.943. [DOI] [PubMed] [Google Scholar]
- 32.Weiner HL. Induction and mechanism of action of transforming growth factor-β-secreting Th3 regulatory cells. Immunol Rev. 2001;182:207–214. doi: 10.1034/j.1600-065x.2001.1820117.x. [DOI] [PubMed] [Google Scholar]
- 33.Yoshino K, Garg R, Monroy D, et al. Production and secretion of transforming growth factor beta (TGF-beta) by the human lacrimal gland. Curr Eye Res. 1996;15:615–624. doi: 10.3109/02713689609008901. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen DH, Beuerman RW, Thompson HW, DiLoreto DA. Growth factor and neurotrophic factor mRNA in human lacrimal gland. Cornea. 1997;16:192–199. [PubMed] [Google Scholar]
- 35.Lee JJ, Kim MK, Shin KS, et al. Transforming growth factor-beta expressin in rat eyes with mechanical debridement of corneal epithelium or cornea flap. J Cataract Refract Surg. 2008;34:662–669. doi: 10.1016/j.jcrs.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 36.Schechter J, Carey M, Wallace M, Wood R. Distribution of growth factors and immune cells are altered in the lacrimal gland during pregnancy and lactation. Exp Eye Res. 2000;71:129–142. doi: 10.1006/exer.2000.0859. [DOI] [PubMed] [Google Scholar]
- 37.Ding C, Chang N, Fong YC, et al. Interacting influences of preg⌝nancy and corneal injury on rabbit lacrimal gland immunoarchitecture and function. Invest Ophthalmol Vis Sci. 2006;47:1368–1375. doi: 10.1167/iovs.05-1034. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, Chiu CT, Nakamura T, et al. Elevated prolactin redirects secretory vesicle traffic in rabbit lacrimal acinar cells. Am J Physiol Endocrinol Metab. 2007;292:E1122–E1134. doi: 10.1152/ajpendo.00381.2006. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Chiu CT, Nakamura T, et al. Traffic of endogenous, over-expressed, and endocytosed prolactin in rabbit lacrimal acinar cells. Exp Eye Res. 2007;85:749–761. doi: 10.1016/j.exer.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mircheff AK, Gierow JP, Lambert RW, et al. Class II antigen expression by lacrimal epithelial cells. An updated working hypothesis for antigen presentation by epithelial cells. Invest Ophthalmol Vis Sci. 1991;32:2302–2310. [PubMed] [Google Scholar]
- 41.Guo Z, Azzarolo AM, Schechter JE, et al. Lacrimal gland epithelial cells stimulate proliferation in autologous lymphocyte preparations. Exp Eye Res. 2000;71:11–22. doi: 10.1006/exer.2000.0856. [DOI] [PubMed] [Google Scholar]
- 42.Powell T, Major JR, MacPherson G. Chapter 18, Generation of dendritic cells from rat bone marrow. In: Robinson SP, Stagg AJ, editors. Methods in Molecular Medicine. Vol 64: Dendritic Cell Protocols. Totowa, N.J.: Humana Press; 2001. pp. 199–205. [DOI] [PubMed] [Google Scholar]
- 43.Ji Q, Chang L, VanDenBerg D, Stanczyk FZ, Stolz A. Selective reduction of AKR1C2 in prostate cancer and its role in DHT metabolism. Prostate. 2003;54:275–289. doi: 10.1002/pros.10192. [DOI] [PubMed] [Google Scholar]
- 44.Beutler B, Hoebe K, Du X, Ulevitch RJ. How we detect microbes and respond to them: the Toll-like receptors and their transducers. J. Leukoc. Biol. 2003;74:479–485. doi: 10.1189/jlb.0203082. [DOI] [PubMed] [Google Scholar]
- 45.Akdis CA, Blaser K. Mechanisms of interleukin-10-mediated immune suppression. Immunol. 2001;103:131–136. doi: 10.1046/j.1365-2567.2001.01235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol. 2001;2:725–731. doi: 10.1038/90667. [DOI] [PubMed] [Google Scholar]
- 47.Lee HI, Kim MK, Oh JY, et al. The role of cyclosporine and mycophenolate in an orthotopic porcine-to-rat corneal xenotransplantation. J Korean Med Sci. 2008;23:492–501. doi: 10.3346/jkms.2008.23.3.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu Z, Stevenson D, Schechter JE, et al. Prophylactic effect of IL-10 gene transfer on induced autoimmune dacryoadenitis. Invest Ophthalmol Vis Sci. 2004;45:1375–1381. doi: 10.1167/iovs.03-0755. [DOI] [PubMed] [Google Scholar]
- 49.Zhu Z, Stevenson D, Schechter JE, et al. Tumor necrosis factor inhibitor gene expression suppresses lacrimal gland immunopathology in a rabbit model of autoimmune dacryoadenitis. Cornea. 2003;22:343–351. doi: 10.1097/00003226-200305000-00012. [DOI] [PubMed] [Google Scholar]
- 50.Niederkorn JY, Stern ME, Pflugfelder SC, et al. Desiccating stress induces T cell-mediated Sjöfgren’s syndrome-like lacrimal keratoconjunctivitis. J Immunol. 2006;176:3950–3957. doi: 10.4049/jimmunol.176.7.3950. [DOI] [PubMed] [Google Scholar]
- 51.Tlaskalová-Hogenová H, Tučková L, Lodinová-Źádniková R, et al. Mucosal immunity: Its role in defense and allergy. Int Arch Allergy Immunol. 2002;128:77–89. doi: 10.1159/000059397. [DOI] [PubMed] [Google Scholar]
- 52.Akbari O, Stock P, DeKruyff RH, Umetsu DT. Mucosal tolerance and immunity: regulating the development of allergic disease and asthma. Int Arch Allergy Immunol. 2003;130:108–118. doi: 10.1159/000069012. [DOI] [PubMed] [Google Scholar]
- 53.Kaplan HJ, Streilein JW. Immune response to immunization via the anterior chamber of the eye. 1. F1 lymphocyte induced immune deviation. J Immunol. 1977;118:809–814. [PubMed] [Google Scholar]
- 54.Niederkorn J, Streilein JW, Shadduck JA. Deviant immune reponses to allogeneic tumors injected intracamerally and subcutaneously in mice. Invest Ophthalmol Vis Sci. 1981;20:355–363. [PubMed] [Google Scholar]
- 55.Wilbanks GA, Streilein JW. Distinctive humoral responses following anterior chamber and intravenous administration of soluble antigen. Evidence for active suppression of IgG2-secreting B cells. Immunol. 1990;71:566–572. [PMC free article] [PubMed] [Google Scholar]
- 56.Ksander BR, Streilein JW. Failure of infiltrating precursor cytotoxic T cells to acquire direct cytotoxic function in immunologically privileged sites. J Immunol. 1990;145:2057–2063. [PubMed] [Google Scholar]
- 57.Forrester JV, Lumsden L, Liversidge J, et al. Immunoregulation of uveoretinal inflammation. Prog Ret Eye Res. 1995;14:393–412. [Google Scholar]
- 58.Rezai KA, Semnani RT, Farrokh-Siar L, et al. Human fetal retinal pigment epithelial cells induce apoptosis in allogenic T-cells in a Fas ligand and PGE2 independent pathway. Curr Eye Res. 1999;18:430–439. doi: 10.1076/ceyr.18.6.430.5269. [DOI] [PubMed] [Google Scholar]
- 59.Nishida T, Taylor AW. Specific aqueous humor factors induce activation of regulatory T cells. Invest Ophthalmol Vis Sci. 1999;40:2268–2274. [PubMed] [Google Scholar]
- 60.Zamiri P, Masli S, Kitaichi N, et al. Thrombospondin plays a vital role in the immune privilege of the eye. Invest Ophthalmol Vis Sci. 2005;46:908–919. doi: 10.1167/iovs.04-0362. [DOI] [PubMed] [Google Scholar]